Abstract
CD44 is expressed by variety of cells, including glial and T cells. Furthermore, in the demyelinating lesions of multiple sclerosis (MS), CD44 expression is chronically elevated. In this study, we demonstrate that targeted deletion of CD44 attenuated MOG peptide-induced experimental autoimmune encephalitomyelitis (EAE) through novel regulatory mechanisms affecting Th differentiation. Specifically, by developing chimeras and using adoptive transfer experiments, we noted that CD44 deficiency on CD4+ T cells but not other cells, conferred protection against EAE induction. CD44 expression played a crucial role in Th differentiation, in as much as, deletion of CD44 inhibited Th1/Th17 differentiation while simultaneously enhancing Th2/Treg differentiation. In contrast, expression of CD44 promoted Th1/Th17 differentiation. When osteopontin (OPN) and hyaluronic acid (HA), the two major ligands of CD44 were tested for their role in Th differentiation, OPN but not HA promoted Th1/Th17 differentiation. Furthermore, activation of CD44+ encephalitogenic T cells with MOG peptide led to demethylation at the ifnγ/il17a promoter region while displaying hypermethylation at the il4/foxp3 gene promoter. Interestingly, similar activation of CD44-deficient encephalitogenic T cells led to increased hypermethylation of ifnγ/il17a gene and marked demethylation of il4/foxp3 gene promoter. Together, these data suggested that signaling through CD44, in encephalitogenic T cells, plays a crucial role in the differentiation of T helper cells through epigenetic regulation, specifically DNA methylation of Th1/Th17 and Th2 cytokine genes. The current study also suggests that molecular targeting of CD44 receptor to promote a switch from Th1/Th17 to Th2/Treg differentiation may provide a novel treatment modality against EAE.
Keywords: EAE/MS, CD44, Th1/Th2 cells, Cell differentiation
Introduction
MS starts with increased migration of autoreactive lymphocytes across the blood-brain barrier (1–3). As an immune-privileged site, activated lymphocytes normally enter and leave the central nervous system (CNS) in hours without causing damage. However, in MS, these cells can overcome the CNS protective microenvironment, probably through failure of suppressive function from the regulatory T cells (Treg), reside in CNS and cause inflammation, result in sclerotic plaques and neurological symptoms (4, 5). Autoreactive T cells, such as Th1 and Th17 cells and their recruited inflammatory cells, produce a variety of cytokines. Local production of cytokines in CNS varies significantly during the disease progress, and changes in discrete sets of cytokines are associated with acute response and recovery phases of the disease. In this regard, Th1-Th2-Th17 cytokines or immune responses that regulate these cytokines are especially highlighted during the disease. Their balance affects the disease progress or recovery, such as a specific Th2 accumulation in CNS or a shift from Th1-type to Th2-type immune response rendering protection against the disease. Therefore, the search for new drugs that specifically target pathogenic Th1 and Th17 cells is tremendously interesting and important. Some drugs play immunomodulatory roles in polarizing Th cells toward Th1, Th2 or Th17 effectors, such as copolymer-I and Berberine (6–10). EAE is a commonly used and well established animal model with many similarities to human MS including episodes of relapsing and remitting paralysis, which is induced by immunization of myelin antigens such as myelin oligodendrocyte glycoprotein (MOG) or a MOG peptide of amino acids 35–55 (MOG35–55) in complete Freund’s adjuvant.
CD44 is a widely distributed cell surface glycoprotein expressed by a variety of lymphoid and non-lymphoid cells. CD44 is encoded by 20 exons, 7 of which form the invariant extracellular region of the so called standard form (CD44s). By alternate splicing, up to 10 variant exons (CD44 v1–v10) can be inserted within the extracellular region (11–13). The extensive alternative splicing of CD44 is believed to contribute to its sophisticated implication in the immune response and immune regulation. Studies from our laboratory and elsewhere have shown that CD44 and its isoforms participate in lymphocyte migration, proliferation and activation not only by establishing specific transmembrane complexes but also by organizing signaling cascades through association with its partner proteins such as p185HER2 and c-Src kinase (12, 14–20). CD44 is recruited to the immunological synapse during DC and T cell interactions and affects the subsequent T cell activation, IL-2 and IFN-γ production, phosphotyrosine and protein kinase c-θ enrichment at the synapse (20). As regards Th differentiation, targeted deletion of CD44 was shown by us to induce a Th2-biased immune response to the antigens of SRBC and OVA (21). Also, Th1 and Th2 cells express CD44 and rely on CD44 for their rolling and adhesion to the endothelium (17, 18, 20–22). HA and OPN are the main ligands for CD44 molecule (12, 23).
There is strong evidence to indicate that CD44 and its ligands may play a critical role in the regulation of MS or EAE. CD44 is expressed at low levels in astrocytes and microglia; but the expression level is elevated in demyelinated lesions. Oligodendrocytes only express detectable levels of CD44 in vitro but they are induced to express CD44 in vivo during MS development (24–26). It was reported that CD44 is chronically elevated in demyelinating lesions, alters the hyaluronan-based extracellular matrix and subsequent signaling, and causes failure of remyelination (27). Also, patients with MS have been shown to have elevated levels of OPN in their serum; mice deficient in OPN exhibit milder form of EAE (28, 29). Together, such studies indicate the crucial role played by CD44 and its ligands in the regulation of neuroinflammation during MS. However, how such interactions involving CD44, influence the differentiation of encephalitogenic T cells into Th1/Th2/Th17/Treg subsets, and its consequences on the clinical disease has not been previously investigated. In this report, we provide mechanistic evidence for the role of CD44 in encephalitogenic T cell differentiation and ensuing pathogenesis.
Material and Methods
Mice and reagents
Wild-type C57BL/6 (WT or CD44+/+) mice were purchased from the National Cancer Institute. CD44 knockout (CD44 KO or CD44−/−) mice were generated at Amgen Institute (Canada) and kindly provided to us by Dr. Tak Mak. These mice are on C57BL/6 background and have been extensively characterized in our previous studies (15, 16). CD4+ T cell–deficient (CD4−/−) mice were purchased from The Jackson Laboratory. Mice were housed in the University of South Carolina Animal Facility. Animal procedures were performed according to NIH guidelines under protocols approved by the Institute Animal Care and Use Committee of the university. MOG35–55 peptide was purchased from NeoMPS. Incomplete Freund’s adjuvant (IFA) and desiccated Mycobacterium tuberculosis H37RA were purchased from DIFCO. Pertussis toxin (PTX) was purchased from List Biological Laboratories. Pep-1 peptide and scrambled control peptide were synthesized as previously described (30). OPN-specific goat IgG was purchased from R&D Systems. Isotype control for the OPN IgG was purchased from Jackson Immuno Research. PMA and ionomycin were purchase from Sigma.
EAE induction and adoptive transfer
Mice were immunized s.c. with 150 µg MOG35–55 in IFA containing 6 mg/ml heat-killed H37RA strain of Mycobacterium tuberculosis. On days 0 and 2 after immunization, mice received 200 ng and 400 ng PTX i.p.respectively. For adoptive transfer, splenocytes were prepared on day 8 of immunization and stimulated for 3 days with 30µg/ml of MOG35–55. On day 4, 10 ×106 cells were injected i.v. into irradiated (300 rads from a 137Cs source) naive recipients. Mice were given 200 ng of PTX at the day of transfer and 400 ng of PTX at day 2 post transfer. Mice were monitored and scored daily for disease progression as described previously (31).
Isolation and staining of CNS-infiltrated inflammatory cells
For isolation of infiltrating mononuclear cells (MNCs) from pooled spinal cord and brain, mice were perfused with 30 ml heparin-PBS. Single-cell suspensions were prepared, and subjected to Percoll gradient (70%/30%) centrifugation. Isolated MNCs were incubated with anti-CD3, anti-CD4 or anti-CD8 antibodies for 30 min at 4°C after blocking of non-specific staining. The staining was analyzed using a flow cytometer (Beckman Coulter, CXP FC500).
Histopathology
Brain and spinal cords were removed from mice after heparin-PBS perfusion and fixed in 10% paraformaldehyde overnight. Paraffin-embedded 10µm sections were stained with H&E or Luxol Fast Blue (LFB) (American MasterTech Scientific) and examined under light microscope. Sections were scored for the degree of inflammation as described elsewhere (32).
RNA isolation, cDNA synthesis, and real-time PCR
Total RNA was isolated from spinal cords and cDNA was synthesized from 1µg of the RNA. Real-time PCR for amplifying Opn was performed by using SYBR green on StepOne Plus cycler (Applied Biosystems). Relative fold expression values were calculated based on the expression of GAPDH gene. Opn forward primer: 5’-TGAGCATTCCAAAGAGAGCCAGGA-3’; Opn reverse primer: 5’-ACTAGCTTGTCCTTGTGGCTGTGA-3’; GAPDH forward primer: 5’-TCAACAGCAACTCCCACTCTTCCA-3’; GAPDH reverse primer: 5’-ACCCTGTTGCTGTAGCCGTATTCA-3’.
T cell restimulation and cytokine measurement
To analyze MOG-specific Th1, Th2 or Th17 cells, splenocytes or CNS-infiltrating MNCs were stimulated with 30µg/ml of MOG35–55 for 24 h, followed by stimulation with 50 ng/ml PMA and 1 µg/ml ionomycin in the presence of 2 µm monensin for 5 h. Part of the cultures were supplemented with anti-OPN antibody or isotype control goat IgG (3 µg/ml each), Pep-1 peptide or scrambled control peptide (100 µg/ml each). For intracellular staining, cells were stained for surface CD4, then fixed and permeabilized and stained for intracellular cytokines with anti-IL17, anti-IL4, or anti-IFN-γ (Cytofix/Cytoperm intracellular staining Kit, BD Pharmingen). IFN-γ production was also measured by ELISPOT assay (ELISpot kit, R&D systems). Cell supernatants were collected at 24 h of MOG35–55 stimulation. Cytokine production in the supernatants was measured by multiplexed microsphere cytokine immunoassay (Bio-Plex Cytokine Assay kit, Bio-Rad). Sera were collected between d20 to d22 post-immunization. IL-4 and IFN-γ production in sera were measured by sandwich ELISA.
Th cell dfferentiation
Naïve CD4+ T cells were isolated from spleen of naïve mice as previously described (21). Cells were stimulated for 4 days with plate-bound anti-CD3 and soluble anti-CD28 (3 µg/ml each) plus irradiated T cell-depleted WT splenocytes under Th1, Th2 or Th17-polarizing condition. Th1 condition: IL-12 (10 ng/ml) and anti-IL-4 (10 µg/ml); Th2 condition: IL-4 (2 ng/ml), anti-IL-12 (10 µg/ml), and anti-IFNγ (10 µg/ml); Th17 condition: TGFβ1 (10 ng/ml), IL-6 (20 ng/ml), IL-23 at day 3 (20 ng/ml), anti-IL-4 (10 µg/ml), anti-IL-12 (10 µg/ml), and anti-IFNγ (10 µg/ml). On day 4, cells were stimulated with PMA and ionomycin for 4–5 h in the presence of monensin and processed for intracellular cytokines staining of IL-4, IL-17 and IFN-γ as described above. TGFβ1, IL-6 and IL-23 were purchased from R&D Systems. Other cytokines and antibodies were purchased from eBioscience.
DNA methylation analysis
To analyze methylation status of ifnγ, il4, il17a or foxp3 loci, quantitative MethyLight assays were developed (33). Splenocytes from immunized or unimmunized mice were stimulated with 30 ug/ml of MOG35–55 for 24 h. CD4+ T cells were purified with magnetic beads (StemCell) from the culture. For analysis of foxp3, non-cultured splenic CD4+ T cells isolated from immunized or unimmunized mice were used. Genomic DNA was extracted and subjected to bisulfite-mediated C>T converstion. Primers were designed to amplify the bisulfite converted promoter sequences and outside of CpG sites to ensure unbiased amplification of methylated and unmethylated alleles. TaqMan real-time PCR probe pair detects simultaneously the methylated status (CG) and the unmethylated status (TG) of the locus in one sample. The methylation-specific probe was labeled with FAM; the unmethylation-specific probe was labeled with JOE. Black Hole Quencher 1 was used for both probes. The relative signals as exemplified by the cycle threshold (Ct) value specific for the mathylated state (CtCG) and the unmethylated state (CtTG) were used for calculation of methylation rates (Cmeh % = 100/[1+2{CtCG−CtTG}]). Assays were run on StepOne Plus cycler (ABI). Table 1 listed primer and probe sequences.
Table I.
Primer and TaqMan probe sequences used for quantitative Methylight assay of DNA methylation.
| ifnγ | il4 | |
|---|---|---|
| Primer: | ||
| Forward | TTAGGAGTTTTAGGAGTTTAATT | ATAGAGTTATTGATGGGTTTTAATT |
| Reverse | AAAAATTTCAACAATATCTCTATAAACC | AATCTATAAATCCACTATAACAACC |
| Probe | ||
| Methylated DNA | AGGAGGTAATACGCGGCGGTTGTTG | AGATTATCGGTATTTTGAACGAGGTTAT |
| Unmethylated DNA | AGGTAATATGTGGTGGTTGTTGATGTAG | AGAGATTATTGGTATTTTGAATGAGGTT |
| i17a | foxp3 | |
| Primer: | ||
| Forward | GTATAAAGGGTAGATAGATTTGG | GGGATTTAGGAGGGGATTTTTT |
| Reverse | ACATTTAAAACATACAATATTCCCT | GAAAAAT TTTACCTAATACCCACATTTT |
| Probe | ||
| Methylated DNA | TGTTCGTTTTTGCGTAGATATTAGGTCGTT | TCGTTTTAGGAGGCGGTAGTAGCGTTTTT |
| Unmethylated DNA | TTTGGTGGGATATGTTTGTTTTTGTGT | TTGTTTTAGGAGGTGGTAGTAGTGTTTTT |
Chimera Development
The chimeras were developed as described previously (21). Briefly, CD4−/− mice (CD44+/+) were lethally irradiated (950 rads from a 137Cs source) and reconstituted with a total of 10 × 106 bone marrow cells in a mixture consisting of 1 part CD44−/− bone marrow + 3 parts of CD4−/− (CD44+/+) bone marrow, which resulted in only CD4+ T cells being CD44−/− while all other cells were CD44+/+, as shown by us and others previously (21, 34). For the control, the mixture consisted of 1 part CD44+/+ bone marrow + 3 parts of CD4−/− (CD44+/+) bone marrow, which resulted in all cells that were CD44+/+. The level of chimerism was measured as described previously (21). Mice were allowed to reconstitute for at least 6 wk before the immunization with MOG35–55.
Statistical analysis
The differences between experimental and control groups were analyzed using the Student t test, except for comparing EAE clinical scores using the Mann-Whitney U test, with p < 0.05 being considered statistically significant.
Results
Amelioration of EAE by CD44 deletion correlates with decreased Th1 but enhanced Th2 responses
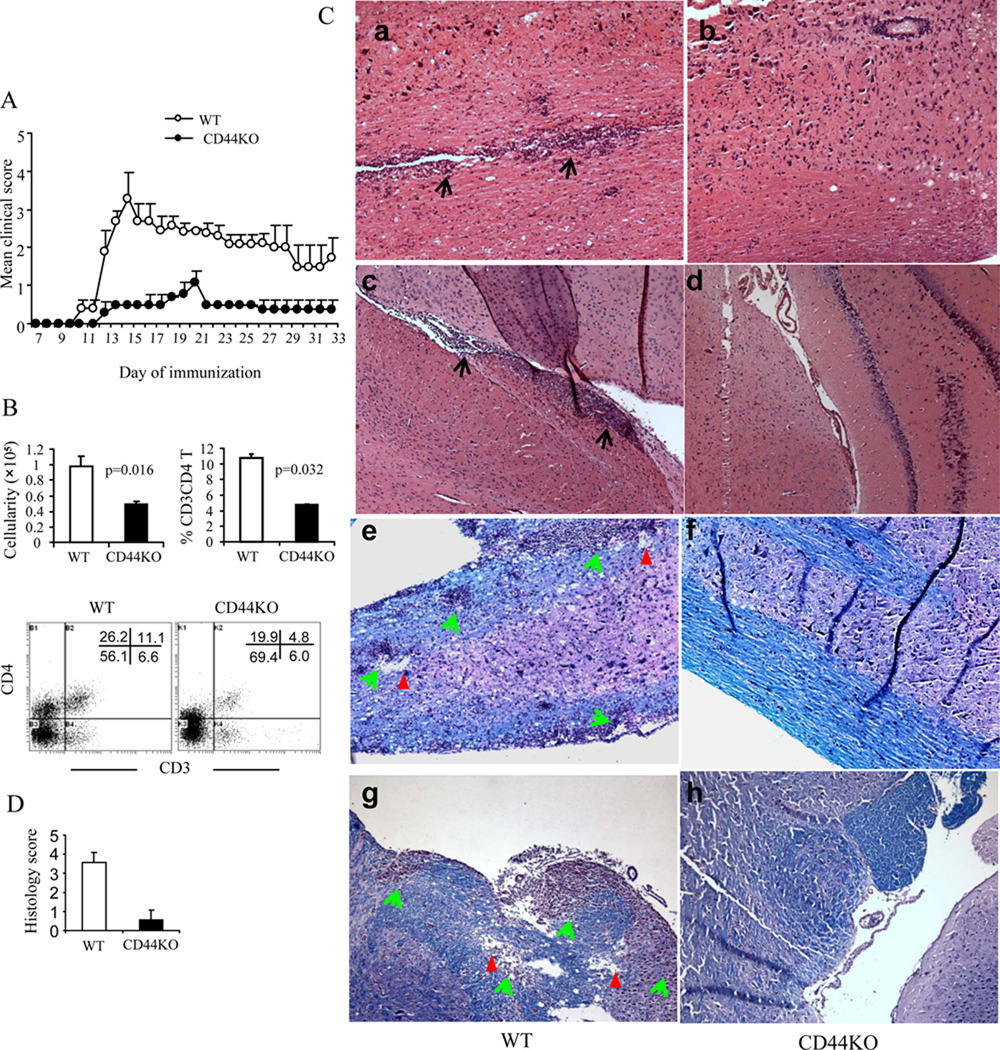
We first investigated effects of CD44 targeted deletion in the development of EAE using CD44−/− mice. CD44 deletion resulted in a significant reduction in disease severity, and it delayed the clinical onset (Fig. 1A, Table 2). In additional experiments, we noted that in EAE-induced CD44−/− mice, there was a marked reduction in the number of CNS-infiltrating MNCs; specifically, the percentage of CNS-infiltrating CD4+ T cells was significantly decreased, whereas that for CD8+ T cells was not altered (Fig. 1B, and data not shown). The histopathology showed a markedly decreased inflammation and demyelination in CNS of EAE-induced CD44−/− mice when compared to CD44+/+ mice (Fig. 1C and D). We even observed 3 relapses in MOG-immunized CD44+/+ mice but without a single one occurring in CD44−/− mice. With the stimulation ex vivo, CD4+ T cells from CD44−/− mice showed a significantly different polarization from Th1 to Th2 to the eliciting MOG35–55 peptide when compared to the CD44+/+ T cells. Thus, CD4+ T cells that were deficient in CD44 produced decreased level of Th1-cytokines, including IFN-γ but increased amounts of Th2-cytokines, including IL-4, IL-5, and IL-13 (Fig 2). These data suggested a switch in Th differentiation from Th1 to Th2 caused by CD44 deletion. Importantly, this Th1 to Th2 switch was also demonstrable in CNS-infiltrating CD44−/−CD4+ T cells (Fig. 3)
Figure 1. Amelioration of EAE by deletion of CD44.
A. EAE was induced by immunization of MOG35–55 peptide. Clinical signs of EAE were monitored daily and scored. Data are expressed as mean ± SEM. B. Cells infiltrating the CNS were enumerated as well as stained to identify CD3+CD4+, CD3+CD8+ T cells by flow cytometry. A representative dot-plot for double–staining for CD3 and CD4 is depicted. The vertical bars represent mean ± SEM from three different experiments. C. Spinal cord (a, b, e. f) and brain (c,d, g, h) sections were prepared on d 20 post-MOG immunization and analyzed for inflammation by H&E (a–d) and for degree of demyelination (e–h) by Luxol fast blue (LFB). Demyelination is indicated by loss of blue staining and vacuolation associated with mononuclear cell infiltration. Black and green arrows indicate mononuclear cell infiltrates; red arrowheads indicate demyelination and vacuolation. D. Mean ± SEM histological scores for brain sections. Original magnification, ×100.
Table II.
Clinical symptoms of EAE
| WT | CD44KO | |
|---|---|---|
| Mean day of onset | 11.4 ± 0.98 | 14.1±1.07* |
| Mean maximum score | 3.3 ± 0.34 | 0.8 ± 0.14* |
| Mean peak day | 15.6 ± 0.53 | 21 ± 0.82* |
p<0.01 comparison between WT and CD44 KO groups
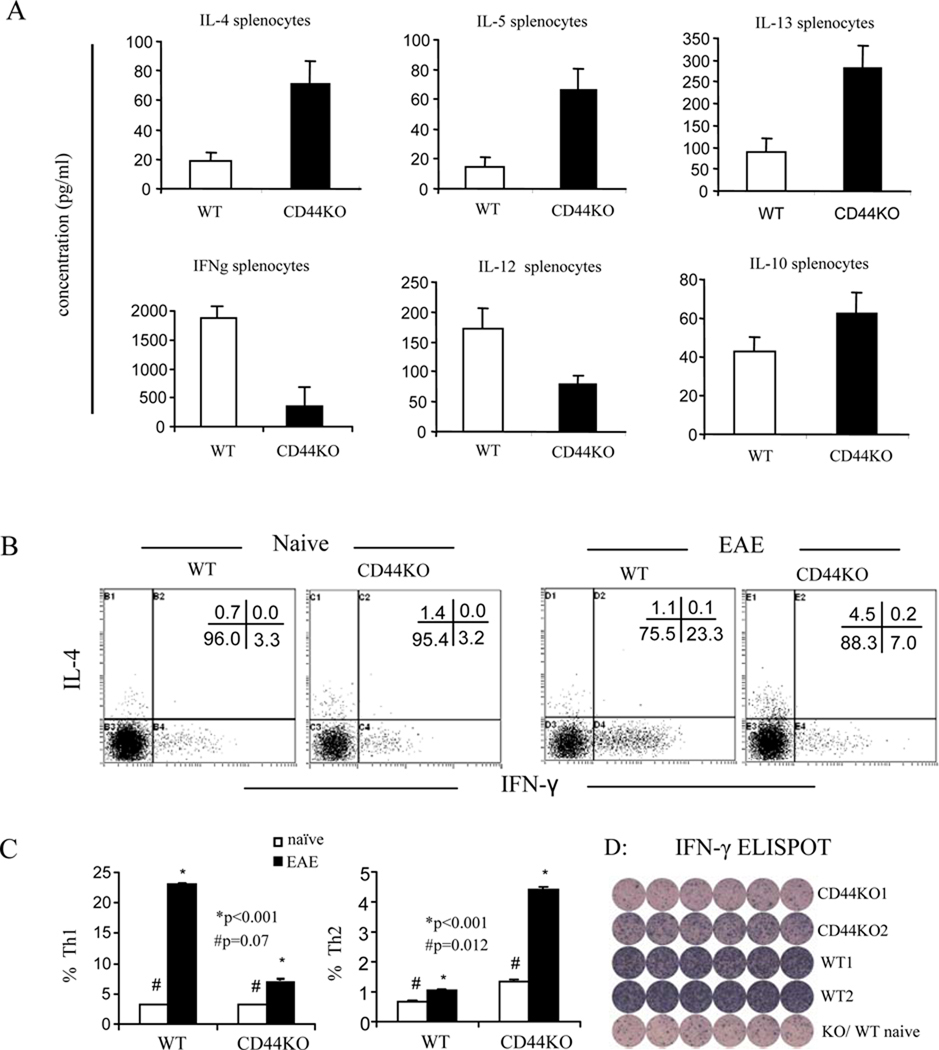
Figure 2. A switch from Th1 to Th2 cytokine production and T cell differentiation in the spleens of EAE-induced CD44 KO mice.
Splenocytes at d 14 post immunization were restimulated with MOG35–55 peptide for 24 h. Cytokines in the supernatants were detected by the multiplexed cytokine immunoassay. Th1/Th2 cells were analyzed by intracellular staining of IFN-γ and IL-4 as well as ELISpot assay of IFN-γ. A. Cytokine measurement in splenic T cell cultures; B. Representative dot-plots of the intracellular staining of CD4+ T cells; C. Percentage of Th1 (IFN-γ) and Th2 (IL-4) cells expressed as mean ± SEM from three independent experiments; D. ELISPOT detection of IFN-γ in the whole splenocytes from EAE-induced CD44 KO (two mice: CD44KO1 and CD44KO2) and WT mice (two mice: WT1 and WT2). Last row: Control spleen cells from naïve CD44KO (first 3 wells) and WT mice (last 3 wells).
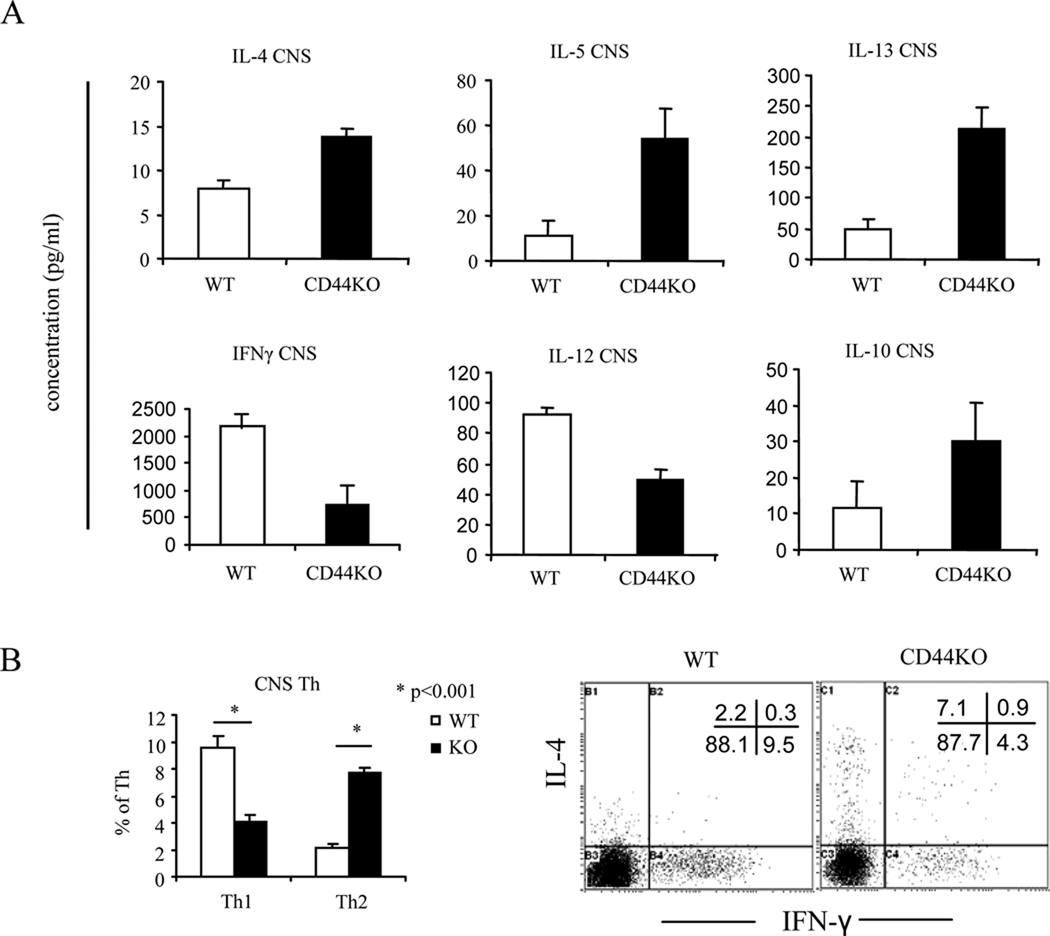
Figure 3. CNS-infiltrating mononuclear cells (MNCs): reduction in Th1 and increase in Th2 in terms of cytokines and differentiation following myelin peptide stimulation.
CNS-infiltrating MNCs were harvested on d 14 post immunization and restimulated in vitro with MOG35–55 peptide for 24 h. Cytokines in the supernatants and Th1/Th2 cells were measured as described in Fig. 2. A. Cytokine measurement in culture supernatants; B. Representative dot-plots and average percentage of Th1 and Th2 cells expressed as mean ± SEM from three independent experiments. Gates were set in the CD4+ subsets.
CD44 deletion in encephalitogenic T cells plays a critical role in EAE
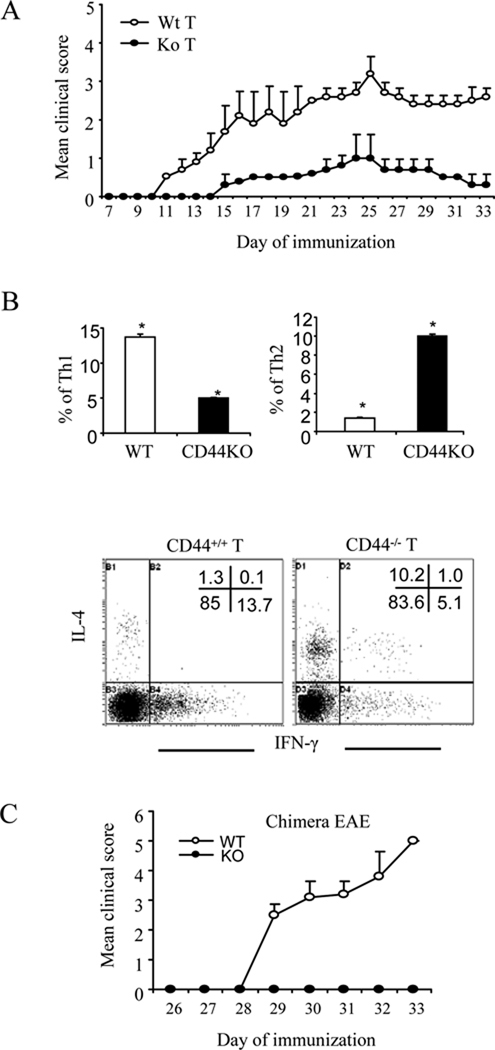
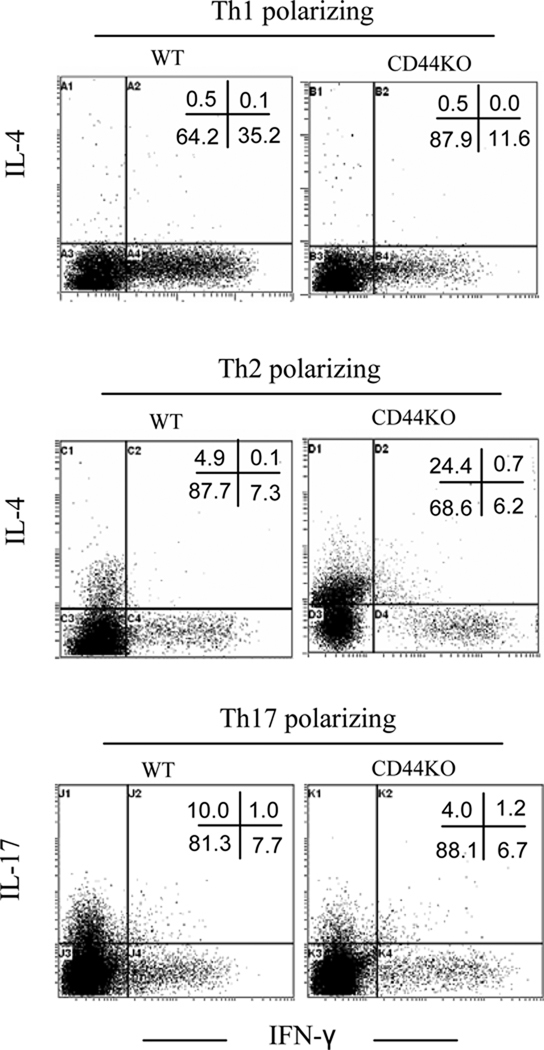
To investigate whether the signaling for such a switch arose in the encephalitogenic T cells, CD44−/− or CD44+/+ encephalitogenic T cells were induced and transferred into naive recipient CD44+/+ mice. The data indicated that transfer of CD44+/+ encephalitogenic T cells caused robust disease progression in recipient mice whereas CD44−/− encephalitogenic T cells caused markedly milder signs of EAE (Fig.4A). Again, the CNS-infiltrating CD44−/− encephalitogenic T cells showed a preference to Th2 polarization in response to the elicting MOG35–55 stimulation whereas Th1 polarization was inhibited (Fig. 4B). Furthermore, to corroborate these studies, we created chimeras in which only the CD4+ T cells were CD44−/− and noted that such mice were completely resistant to EAE when compared to mice that had CD44+/+CD4+ T cells (Fig. 4C). Collectively, these data suggested that CD44 deletion in CD4+ T cells directly promotes a switch from Th1-Th2 differentiation of encephalitogenic Th cells and ameliorates clinical disease. To further evaluate the functional consequence of CD44 deletion under varying culture conditions that promoted Th cell differentiation, naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD8 antibodies under Th1, Th2 or Th17-polarizing condition. As shown in Fig. 5, CD44 deficiency inhibited Th1 and Th17 polarization whereas Th2 polarization was enhanced. These data provided further evidence that CD44 deletion promotes Th2 differentiation while inhibiting the proinflammatory Th1 and Th17 differentiation.
Figure 4. CD44 expression on encephalitogenic T cells regulates EAE pathogenesis.
A. Splenocytes obtained from WT and CD44 KO mice on d 8 post-immunization were stimulated with MOG35–55 peptide for 3 days. Next, the cells were harvested and adoptively transferred into lightly irradiated (300 rads) C57BL/6 recipients (10 ×106 cells per mouse). Clinical signs of EAE were monitored daily and expressed as mean clinical scores ± SEM. B. CNS-infiltrating MNCs isolated from the recipients at d 30 of adoptive transfer were stimulated with MOG35–55 peptide for 24 h followed by intracellular staining for IFN-γ and IL-4. Representative dot-plots and average percentage of Th1 and Th2 are shown. Data were expressed as mean ± SEM from three independent experiments. Gates were set for CD4+ subset. *: p<0.001 comparison between WT and CD44 KO group. C. CD44−/−CD4+ T cell chimeras were created as described in the Methods. EAE was induced and clinical scores were expressed as mean ± SEM. WT represents chimeras in which the CD4+ T cells and all other cells were CD44+/+; KO represents chimeras in which only CD4+ T cells were CD44−/− while remaining cells were CD44+/+.
Figure 5. CD44−/−CD4+ T cells preferentially polarize to Th2 cells in vitro.
Naïve CD4+ T cells from WT or CD44 KO mice were stimulated in vitro with anti-CD3 and anti-CD28 antibodies accompanied with Th1-, Th2-, or Th17-polarizing condition. On day 4 of the culture, cells were stimulated with PMA and ionomycin for 4–5 h and IFN-γ, IL-4, and IL-17 production was detected by intracellular staining. Data were expressed as mean ± SEM from three independent experiments.
CD44-OPN interactions regulate epigenetic changes leading to promotion of Th2
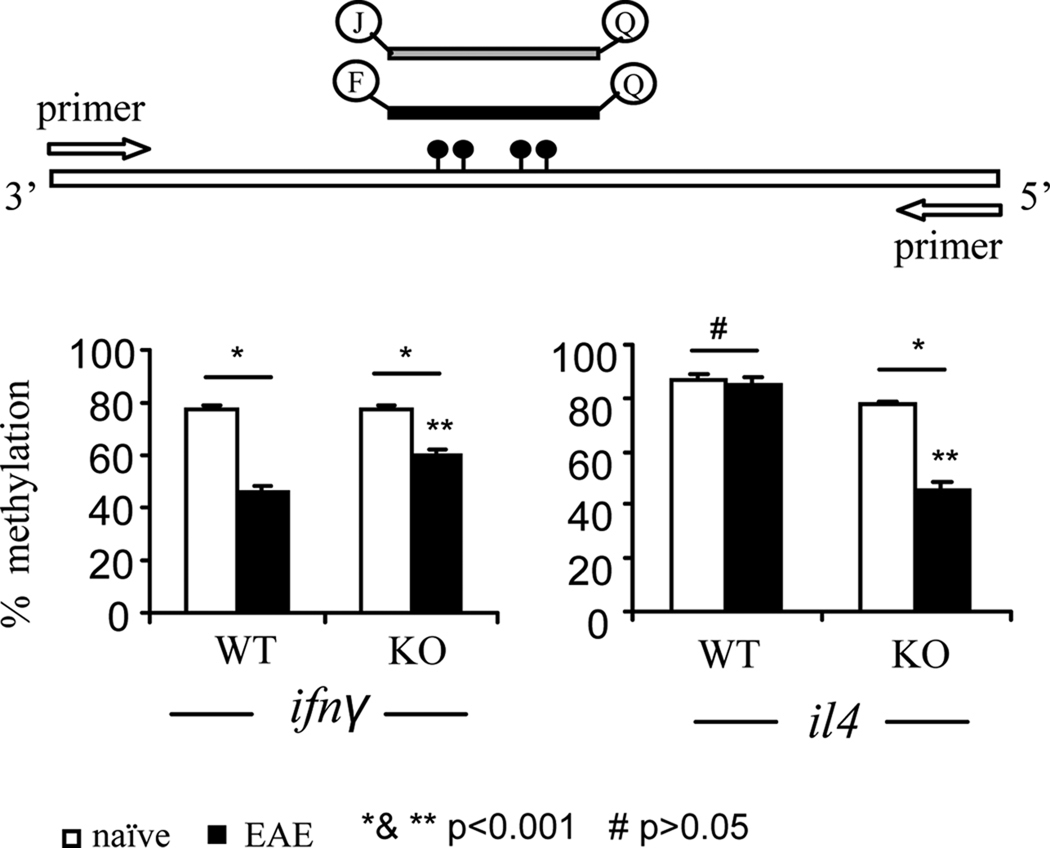
Th1 and Th2 polarization is also associated with epigenetic changes in chromatin structure and DNA methylation at the ifnγ and il4 loci (35–38). To investigate whether CD44 signals are implicated in epigenetic imprinting of the ifnγ and il4 loci, DNA methylation at the promoter of the ifnγ and il4 loci in encephalitogenic CD4+ T cells was assessed (Fig 6). In CD44−/−CD4+ T cells isolated from naive mice, following activation with MOG35–55 for 24 h, the CpG dinucleotides within both promoters were found to be hypermethylated, exhibiting 77–87% methylation. However, additional comparisons revealed that there was no difference in methylation of ifnγ promoter between naïve CD44−/−CD4+ T cells and CD44+/+CD4+ T cells, whereas less methylation of il4 promoter was noted in naïve CD44−/−CD4+ T cells when compared to CD44+/+ CD4+ T cells (77% versus 87%, p<0.01; Fig 6). In contrast, in encephalitogenic CD44−/−CD4+ T cells, methylation of ifnγ promoter was significantly more than that found in encephalitogenic CD44+/+CD4+ T cells (62% versus 45%, p<0.001). Moreover, encephalitogenic CD44−/−CD4+ T cells exhibited dramatic decrease in DNA methylation of the il4 promoter when compared to similar cells from CD44+/+CD4+ T cells (45% versus 87%). These data together demonstrated that activation of CD44 affects epigenetic imprinting by DNA hypomethylation of the ifnγ and hypermethylation of il4 promoters, thereby promoting Th1 differentiation, whereas in the absence of CD44 activation, this process is reversed thereby promoting Th2 differentiation.
Figure 6. DNA methylation analysis.
Splenocytes at d 14 post immunization were restimulated with MOG35–55 peptide for 24 h. CD4+ T cells were further purified from the culture. Genomic DNA was isolated from the CD4+ T cells and subjected to bisulfite conversion. DNA methylation at ifnγ and il4 loci was analyzed by Methylight quantitative real-time PCR. The schematic depiction of the protocol for the PCR and average percentage of DNA methylation has been shown. Data were expressed as mean ± SEM from three independent experiments. *: p<0.001 comparison between naïve and EAE group; **: p<0.001 between WT EAE and CD44 KO EAE group; #: p>0.05 comparison between naïve WT and EAE WT group for il4.
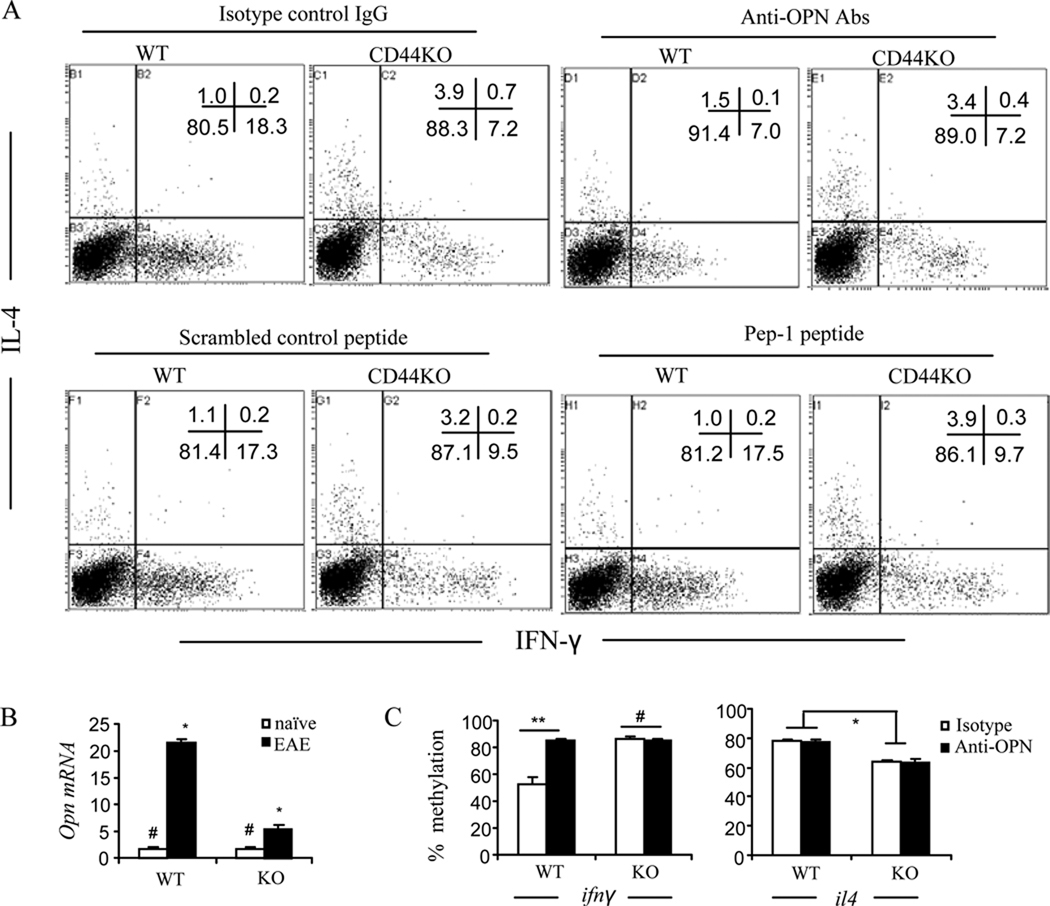
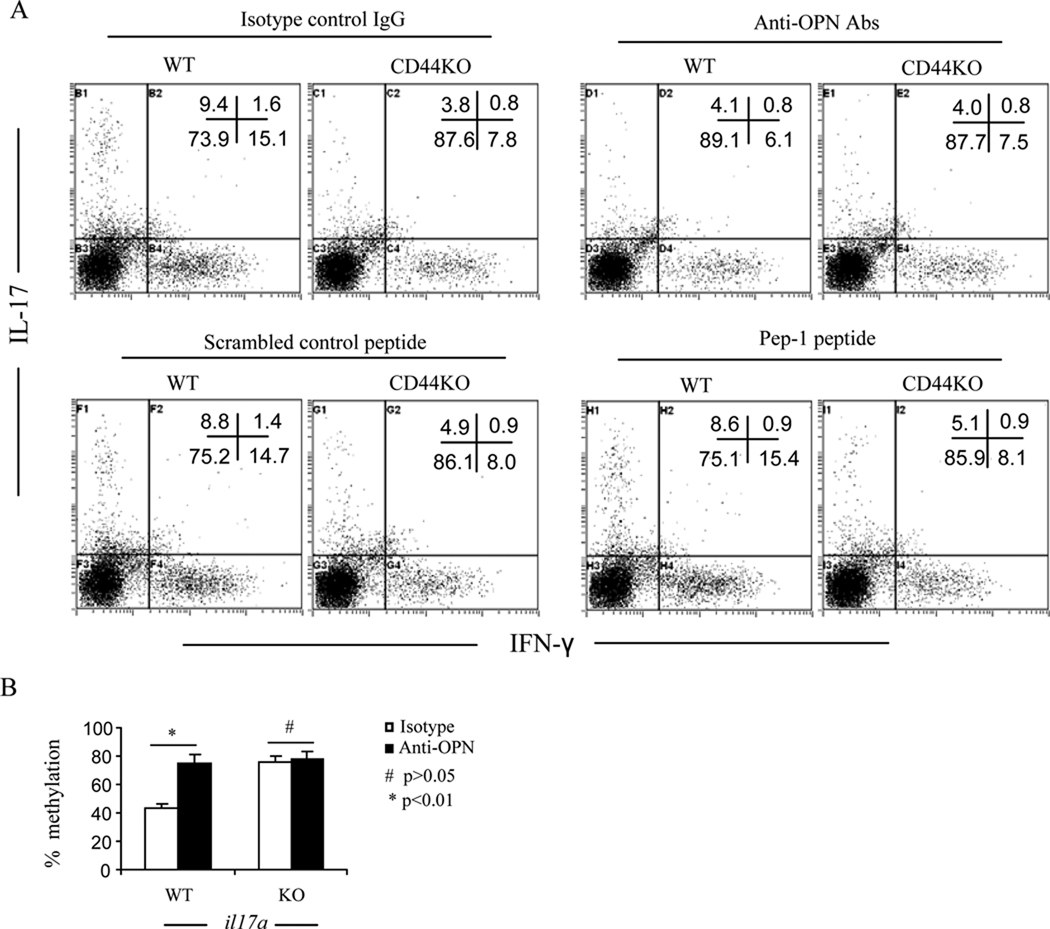
To identify which signaling pathways were involved, we targeted HA and OPN, two important ligands of CD44. Pep-1 is a HA binding peptide known to block CD44-HA interactions (30, 39). Thus, we used Pep-1 or neutralizing anti-OPN antibody in cultures of T cells activated with MOG35–55. As show in Fig. 7A, neutralization of OPN markedly inhibited IFN-γ production of CD44+/+CD4+ T cells (60% inhibition, from 18.3% to 7.2%). The addition of Abs failed to exhibit a similar effect on IFN-γ production in CD4+ T cells from CD44−/− mice thereby suggesting that these Abs were inhibiting CD44-OPN interactions. The Abs against OPN failed to exhibit a significant effect on IL-4 production. Blockade with Pep-1 did not significantly affect IFN-γ or IL-4 production in any of the CD4+ T cell cultures. It should be noted that the concentration of Pep-1 tested here was validated previously to functionally block CD44-HA interaction (30). These data demonstrated that CD44-OPN interactions may potentiate Th1 polarization. In support of this finding, when we analyzed the spinal cords for Opn mRNA levels, we found that EAE-induced CD44+/+ mice had elevated levels of Opn mRNA when compared to naïve CD44+/+ mice, thereby suggesting that there is OPN induction during EAE pathogenesis. Furthermore, EAE-induced CD44−/− mice had significantly lower levels of Opn mRNA when compared to EAE-induced WT mice (Fig. 7B). These data also suggested that OPN induction during CNS inflammation may also be regulated by CD44. Furthermore, blocking CD44-OPN interaction affected the methylation at the ifnγ promoter of the CD44+/+CD4+ T cells. As shown in Fig. 7C, consistent with the inhibition of IFNγ production, the demethylation at the ifnγ promoter of the CD44+/+CD4+ T cells was significantly remethylated. These data demonstrated that CD44-OPN interaction regulates epigenetic changes leading to promotion of Th1 differentiation whereas blockade of this pathway shuts off Th1 differentiation thereby promoting Th2 differentiation.
Figure 7. Interaction between CD44 and OPN is required for Th1 differentiation.
A. Splenocytes at d 14 post immunization were restimulated with MOG35–55 peptide for 24 h in the presence of anti-OPN antibody, Pep-1 peptide or their respective controls. Th1/Th2 cells were analyzed by intracellular staining of IFN-γ and IL4. Representative dot-plots from three independent experiments were shown. Gates were on CD4 subset. B. Expression of Opn mRNA in the spinal cords on d 14 postimmunization was detected by real-time PCR. Average of relative fold changes were expressed as mean ± SEM from three independent experiments. # p>0.05 comparison between naïve WT and naïve CD44 KO group; * p<0.001 comparison between WT EAE and CD44 KO EAE group. C. CD4+ T cells were purified from the culture and subjected to the DNA methylation analysis as described. Comparison for IFN-γ: ** p<0.001 between naïve WT and EAE WT group; # p>0.05 comparison between naïve CD44 KO and EAE CD44 KO group. Comparison for IL-4: * p < 0.01 for anti-OPN or <0.001 for isotype comparison between WT and CD44 KO group.
Status of Th17 and FOXP3+ Treg cells
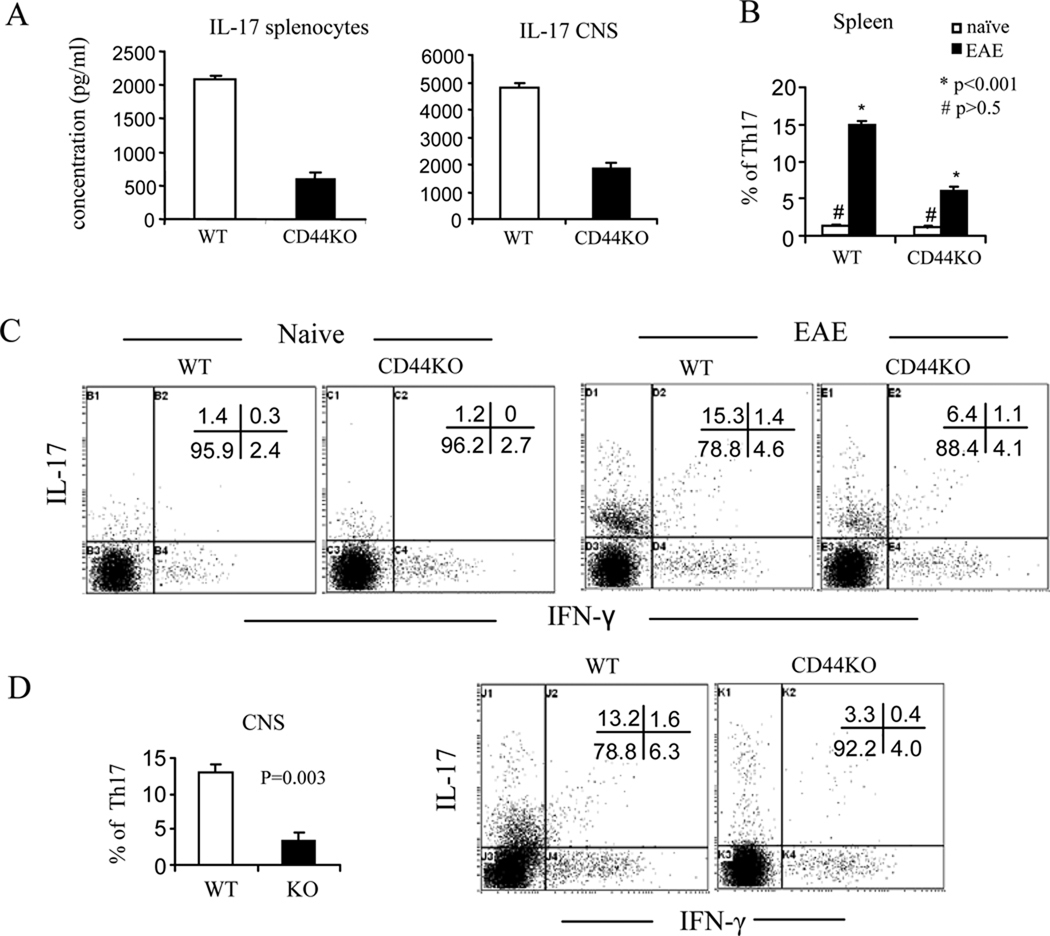
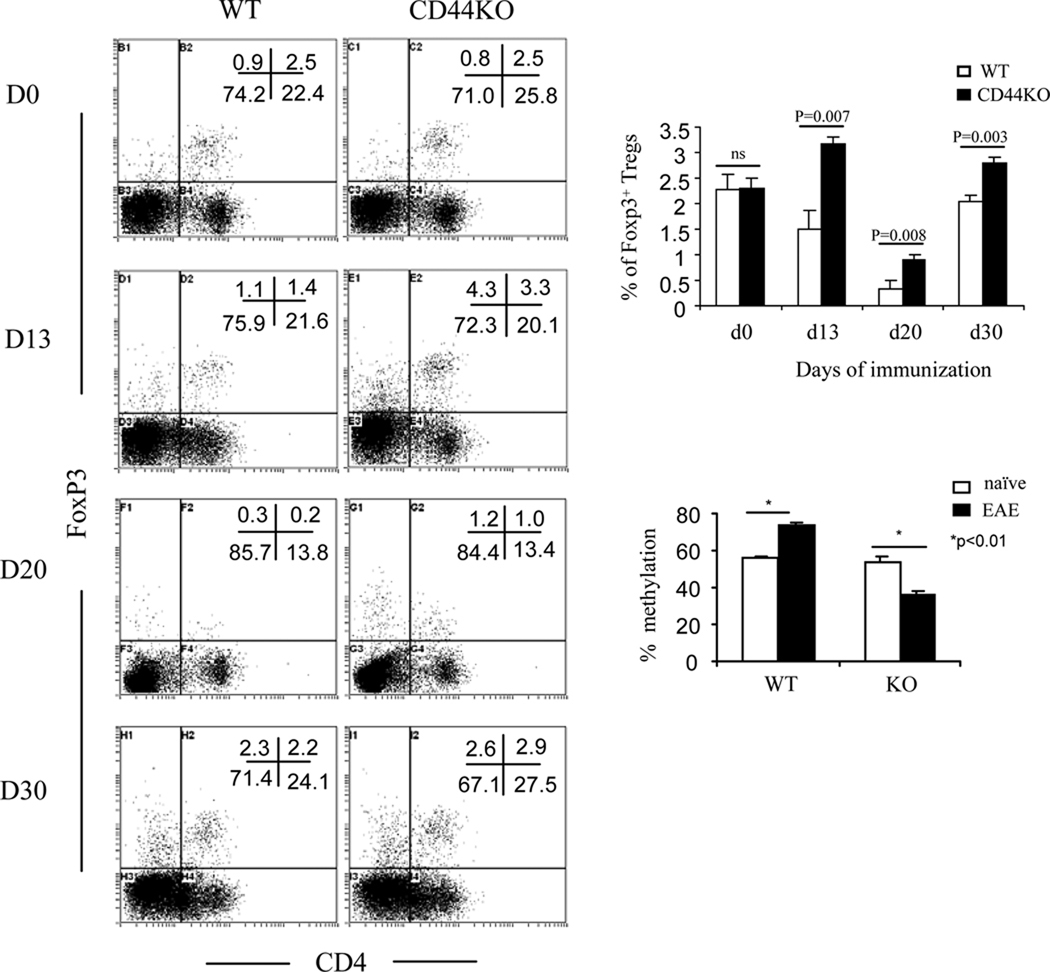
Next, we also assessed the status of Th17 and FOXP3+CD4+ Treg cells. Deletion of CD44 markedly suppressed Th17 polarization of naïve CD44−/−CD4+ T cells in vitro (Fig. 5). In EAE-induced CD44−/− mice, there was a dramatic decrease in IL-17 production in T cells restimulated with MOG35–55 both in the periphery and in the CNS (Fig. 8A). This response correlated with a significantly decreased frequency of IL-17-producing CD4+ T cells in the periphery (Fig. 8B and C) as well as in the CNS (Fig 8D). CD44-OPN rather than CD44-HA signaling pathway promoted Th17 differentiation in as much as blocking the former with anti-OPN Abs rather than the latter with Pep-1, significantly inhibited IL-17 production from CD44+/+CD4+ T cells (57% inhibition, from 9.4% to 4.1%) (Fig. 9A). When we investigated the methylation status of il17 promoter, MOG-activated T cells from CD44−/− mice displayed hypermethylation when compared to the wild-type T cells. Also, blocking CD44-OPN interaction in wild-type but not CD44−/− T cells increased the methylation at the il17 promoter (Fig. 9B). Conversely, CD44−/− mice exhibited a significant increase in the percentages of FoxP3+CD4+ Tregs in the periphery at different stages of the disease (Fig. 10). Methylation status of foxp3 promoter revealed that on day 13, T cells from CD44−/− EAE induced mice had significantly lower methylation than similar cells from wild-type mice (Fig. 10). All together, these data demonstrated that the unique promotion of Th differentiation between Th1, Th2, Th17 and/or Treg cells was induced by deletion of CD44 and regulated through epigenetic modulation.
Figure 8. Deletion of CD44 decreases generation of myelin-reactive Th17 cells.
Splenocytes and CNS-infiltration MNCs at d 22 post immunization were restimulated with MOG35–55 peptide for 24 h. Production of IL-17 in the supernatants was detected by the multiplexed cytokine immunoassay. Th1/Th17 cells were analyzed by intracellular staining of IFN-γ and IL-17. A. IL-17 measurement in the culture supernatants; B. percentage of Th17 cells in spleen; C. Representative dot-plots of Th17 cells in spleen; D. percentage of Th17 cells in CNS and representative dot-plots. All cytokine measurements and percentages are expressed as mean ± SEM from three independent experiments.
Figure 9. Interaction between CD44 and OPN promotes Th17 differentiation.
A. Splenocytes at d 18 post immunization were restimulated with MOG35–55 peptide for 24 h in the presence of anti-OPN antibody, Pep-1 peptide or their respective controls as described in Fig 7. Th1/Th17 cells were analyzed by intracellular staining of IFN-γ and IL17. Representative dot-plots from two independent experiments were shown. Gates were set on CD4 subset. B. CD4+ T cells were purified from the culture and subjected to DNA methylation analysis of il17a promoter as described in Methods. Data were expressed as mean ± SEM.
Figure 10. Deletion of CD44 induces expansion of FOXP3+ Tregs during EAE.
Splenocytes were obtained from mice before immunization or at different days postimmunization (Day 0, 13, 20, 30) and used for staining CD4 followed by intracellular staining of FOXP3. Representative dot-plots at each day and average percentage of CD4+FOXP3+ Tregs from three independent experiments were shown. Data were expressed as mean ± SEM. To study the methylation at foxp3 promoter, CD4+ T cells were isolated from d 0 (naïve) or d 13, and subject to DNA methylation analysis as described in Methods. Data were expressed as mean ± SEM.
Discussion
CD44 has previously been shown to regulate lymphocyte migration, proliferation and activation; however, its role in Th differentiation is not well understood. Our laboratory previously reported that CD44 regulates Th1-Th2 differentiation when activated with particulate or soluble antigens such as SRBC or OVA (21). In this study, we found that CD44 can regulate the differentiation and activity of Th1, Th2, Th17 and Tregs in an autoimmune disease. Overall, the current study indicated that CD44 sufficiency promotes Th1/Th17 differentiation while CD44 deficiency favors Th2/Treg differentiation. Furthermore, such a switch in T cell differentiation appears to be regulated by epigenetic mechanisms.
The role of CD44 was first demonstrated by diminished Th1 and enhanced Th2 response in EAE mice in which CD44 was genetically deleted and further corroborated by adoptive transfer experiments showing loss of encephalitogenicity of in situ deletion of CD44 in CD4+ T cells. It was evident that CD44 promoted Th1 differentiation; also, deletion of CD44 inhibited Th1 differentiation and simultaneously enhanced Th2 differentiation. The experiments in which T cells from CD44-deficient or–sufficient mice were activated with anti-CD3 plus anti-CD28 further substantiated such predisposition. Our studies also showed that CD44 confers signals to the epigenetic imprinting of the ifnγ and il4 loci that occurs during T cell differentiation, leading to promotion of IFN-γ gene expression. Moreover, we noted greater levels of demethylation of il4 locus in CD44-deficient cells thereby promoting a switch from Th1 to Th2. There is increasing evidence for the role of DNA methylation in governing cytokine expression developmentally before and after Th differentiation (36–38). A pattern of hypomethylation at the ifnγ promoter is maintained in Th1 cells, but this pattern becomes remethylated during Th2 development. Il4 remains methylated during Th1 differentiation, but it becomes highly demethylated in Th2 cells. Here, methylation of DNA silences a locus as a function of terminal differentiation. Although T-bet and GATA3 can induce chromatin remodeling at the ifnγ and il4 loci at early stages of Th1/Th2 differentiation, DNA methylation can occur later, independent of those transcription factors for epigenetic memory of ifnγ and il4 activation, once cells have firmly committed to the Th1 or Th2 lineage. Thus, DNA demethylation also potentiates heritable and stable gene expression (36–38).
The principal ligand of the CD44 is HA (23). We did not observe a significant difference in IFN-γ production with Pep-1, an HA binding peptide known to block CD44-HA interactions (30, 39). However, CD44 can interact with several additional molecules. The list of CD44 ligands is continuously expanding and so far the additional ligands include OPN, collagen, fibronectin, fibrinogen, laminin, chondroitin sulfate, mucosal vascular addressin, serglycin/gp600, the major histocompatibility complex class II invariant chain (li), L-selectin, E-selectin and galectin-8 (12, 13). A number of studies have demonstrated that OPN critically contributes to development of Th1-mediated immunity and disease. It was established that T-bet-dependent expression of OPN is essential for efficient skewing of CD4+ T and CD8+ T cells toward Th1 and Tc1 pathway, respectively. In MS patients, increased levels of OPN protein were found in the serum and plasma as well as cerebrospinal fluid. In EAE-induced mice, the sclerotic plaques contain high levels of Opn transcripts and OPN-deficient mice showed decreased development of the disease correlating with decreased Th1 response (28, 29, 40, 41). Despite such studies on the important role of OPN in EAE, previous studies have not identified the target receptor of OPN in regulating EAE. In the current study we noted that use of anti-OPN Ab in cultures caused dramatic reduction in IFN-γ production by CD44+/+CD4+ T cells but not CD44−/−CD4+ T cells. This effect also modulates the epigenetic modification at the ifnγ gene promoter. These data indicated CD44-OPN signaling participates in Th1 differentiation of encephalitogenic T cells and furthermore, deletion of CD44 may deprive Th1-polarizing signaling and promote Th2 differentiation. We also noted that the levels of OPN mRNA increased significantly during EAE in the CNS of CD44+/+ mice whereas EAE-induction in CD44−/− mice failed to increase the levels of OPN. These data suggested that OPN may play a negative role in the pathogenesis of EAE and that CD44 expression may also regulate the production of OPN, which is considered to be a cytokine, in the CNS.
In as much as other Th effectors are implicated in MS/EAE, we were also interested in evaluating the status of Th17 and Treg cells, two critical players in pathogenesis of EAE (42). It was reported that Treg can prevent EAE and this effect takes place before the disease onset (43, 44). We did observe the highest percentage of peripheral Tregs on the pre-onset stage (d13). Actually, CD44-deficiency induced an expansion of total FOXP3+ population at all three stages of EAE including pre-onset, peak, and pre-relapse (d13, d20, d30). We also noted a significant increase in FOXP3+CD4− population on day 13, which could be CD8+ Tregs. Such cells have been shown to exist and perform suppressive function in EAE (45–47). In addition to induction of Tregs, we also noted that IL-17 production during EAE and Th17 differentiation of naïve T cells as well as encephalitogenic T cells was significantly inhibited following CD44 deletion. Our studies demonstrate for the first time that CD44-OPN signal pathway may also promote encephalitogenic Th17 differentiation, and that deficiency of CD44, in contrast, may enhance Treg differentiation. Notably, these data were corroborated with epigenetic imprinting of the il17 and foxp3 loci following CD44 signaling. Together, our findings also provide mechanistic clues on how antibodies against CD44 can inhibit neuroinflammation during EAE (48–50).
Pathogenesis of MS also depends upon the balance of Th-shaping cytokines such as IL-12 and IL-10 as well as distinct activities of Th subsets. IL-12 is essential for the generation of autoreactive EAE-inducing Th1 cells, whereas IL-10 antagonizes the disease-promoting effects of IL-12 and has been associated with remission from EAE. Modulation of IL-10/IL-12 cytokine circuit by IFN-β inhibits the development of epitope spreading and disease progression in EAE (51–53). In OPN−/− EAE mice, IL-10 also assists toward Th2 skewing (28, 29). Our findings showed concomitant induction of IL-10 and down regulation of IL-12 production in CD44−/− EAE mice, which reflects decreased Th1- and skewing toward Th2-immune response as well as fortified functionality of Tregs, which together may account for reversal of the disease.
In summary, our study demonstrates that CD44 plays a critical immmunoregulatory role in EAE. Specifically, CD44 promotes Th1/Th17 differentiation; whereas deficiency of CD44 inhibits Th1/Th17 differentiation and simultaneously enhances Th2/Treg differentiation. Expression of CD44 on encephalitogenic T cells leads to potential interactions with OPN, and consequent epigenetic regulation including hypomethylation of ifnγ and il17a genes and enhanced differentiation of Th1 and Th17 cells. In contrast, CD44 deficiency leads to hypermethylation of ifnγ and il17a and hypomethylation of il4 gene, leading to Th2 cell differentiation. Our study elucidated role of CD44 and provides mechanisms of the action in EAE that will benefit the designing of therapeutic strategy by targeting CD44 in EAE or human MS. Thus, molecular targeting of CD44 receptor to promote a switch from Th1/Th17 to Th2/Treg differentiation may provide a novel treatment modality against EAE.
Acknowledgments
This work was supported in part by National Institute of Health Gants R01 AI053703, R01 AI058300, R01 HL058641, and P01 AT003961.
Abbreviations
- MS
Multiple sclerosis
- CNS
central nervous system
- MOG
myelin oligodendrocyte glycoprotein
- EAE
experimental autoimmune encephalomyelitis
- PTX
pertussis toxin
- LFB
Luxol Fast Blue
- HA
hyaluronic acid or hyaluronan
- OPN
osteopontin
- Treg
regulatory T cells
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009 doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasper LH, Shoemaker J. Multiple sclerosis immunology: The healthy immune system vs the MS immune system. Neurology. 74(Suppl 1):S2–S8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- 4.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci U S A. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 9.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 10.Qin X, Guo BT, Wan B, Fang L, Lu L, Wu L, Zang YQ, Zhang JZ. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 185:1855–1863. doi: 10.4049/jimmunol.0903853. [DOI] [PubMed] [Google Scholar]
- 11.van Weering DH, Baas PD, Bos JL. A PCR-based method for the analysis of human CD44 splice products. PCR Methods Appl. 1993;3:100–106. doi: 10.1101/gr.3.2.100. [DOI] [PubMed] [Google Scholar]
- 12.Naor D, Nedvetzki S, Walmsley M, Yayon A, Turley EA, Golan I, Caspi D, Sebban LE, Zick Y, Garin T, Karussis D, Assayag-Asherie N, Raz I, Weiss L, Slavin S, Golan I. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann N Y Acad Sci. 2007;1110:233–247. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- 13.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 14.Seth A, Gote L, Nagarkatti M, Nagarkatti PS. T-cell-receptor-independent activation of cytolytic activity of cytotoxic T lymphocytes mediated through CD44 and gp90MEL-14. Proc Natl Acad Sci U S A. 1991;88:7877–7881. doi: 10.1073/pnas.88.17.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, McKallip RJ, Zeytun A, Do Y, Lombard C, Robertson JL, Mak TW, Nagarkatti PS, Nagarkatti M. CD44-deficient mice exhibit enhanced hepatitis after concanavalin A injection: evidence for involvement of CD44 in activation-induced cell death. J Immunol. 2001;166:5889–5897. doi: 10.4049/jimmunol.166.10.5889. [DOI] [PubMed] [Google Scholar]
- 16.McKallip RJ, Fisher M, Do Y, Szakal AK, Gunthert U, Nagarkatti PS, Nagarkatti M. Targeted deletion of CD44v7 exon leads to decreased endothelial cell injury but not tumor cell killing mediated by interleukin-2-activated cytolytic lymphocytes. J Biol Chem. 2003;278:43818–43830. doi: 10.1074/jbc.M304467200. [DOI] [PubMed] [Google Scholar]
- 17.Lesley J, Hyman R. CD44 structure and function. Front Biosci. 1998;3:d616–d630. doi: 10.2741/a306. [DOI] [PubMed] [Google Scholar]
- 18.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 19.Rafi-Janajreh AQ, Nagarkatti PS, Nagarkatti M. Role of CD44 in CTL and NK cell activity. Front Biosci. 1998;3:d665–d671. doi: 10.2741/a311. [DOI] [PubMed] [Google Scholar]
- 20.Hegde VL, Singh NP, Nagarkatti PS, Nagarkatti M. CD44 mobilization in allogeneic dendritic cell-T cell immunological synapse plays a key role in T cell activation. J Leukoc Biol. 2008 doi: 10.1189/jlb.1107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan H, Nagarkatti PS, Nagarkatti M. Role of CD44 in the differentiation of Th1 and Th2 cells: CD44-deficiency enhances the development of Th2 effectors in response to sheep RBC and chicken ovalbumin. J Immunol. 2009;183:172–180. doi: 10.4049/jimmunol.0802325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonder CS, Clark SR, Norman MU, Johnson P, Kubes P. Use of CD44 by CD4+ Th1 and Th2 lymphocytes to roll and adhere. Blood. 2006;107:4798–4806. doi: 10.1182/blood-2005-09-3581. [DOI] [PubMed] [Google Scholar]
- 23.Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31:189–206. doi: 10.1385/IR:31:3:189. [DOI] [PubMed] [Google Scholar]
- 24.Girgrah N, Letarte M, Becker LE, Cruz TF, Theriault E, Moscarello MA. Localization of the CD44 glycoprotein to fibrous astrocytes in normal white matter and to reactive astrocytes in active lesions in multiple sclerosis. J Neuropathol Exp Neurol. 1991;50:779–792. doi: 10.1097/00005072-199111000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Moretto G, Xu RY, Kim SU. CD44 expression in human astrocytes and oligodendrocytes in culture. J Neuropathol Exp Neurol. 1993;52:419–423. doi: 10.1097/00005072-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Tuohy TM, Wallingford N, Liu Y, Chan FH, Rizvi T, Xing R, Bebo B, Rao MS, Sherman LS. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia. 2004;47:335–345. doi: 10.1002/glia.20042. [DOI] [PubMed] [Google Scholar]
- 27.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 28.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 29.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 30.Guan H, Nagarkatti PS, Nagarkatti M. Blockade of hyaluronan inhibits IL-2-induced vascular leak syndrome and maintains effectiveness of IL-2 treatment for metastatic melanoma. J Immunol. 2007;179:3715–3723. doi: 10.4049/jimmunol.179.6.3715. [DOI] [PubMed] [Google Scholar]
- 31.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4'-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cottrell S, Jung K, Kristiansen G, Eltze E, Semjonow A, Ittmann M, Hartmann A, Stamey T, Haefliger C, Weiss G. Discovery and validation of 3 novel DNA methylation markers of prostate cancer prognosis. J Urol. 2007;177:1753–1758. doi: 10.1016/j.juro.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Partida-Sanchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity. 2004;20:279–291. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- 35.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, Wells AD. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. J Biol Chem. 285:2545–2553. doi: 10.1074/jbc.M109.038794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 38.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 39.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med. 2000;192:769–779. doi: 10.1084/jem.192.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 41.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Cabbage SE, Huseby ES, Sather BD, Brabb T, Liggitt D, Goverman J. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J Immunol. 2007;178:887–896. doi: 10.4049/jimmunol.178.2.887. [DOI] [PubMed] [Google Scholar]
- 44.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen ML, Yan BS, Kozoriz D, Weiner HL. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol. 2009;39:3423–3435. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nigam P, Velu V, Kannanganat S, Chennareddi L, Kwa S, Siddiqui M, Amara RR. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic Simian Immunodeficiency Virus infection correlates with diminished antiviral T cell response and viral control. J Immunol. 184:1690–1701. doi: 10.4049/jimmunol.0902955. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, Vaslin B. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol. 2007;81:13444–13455. doi: 10.1128/JVI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laman JD, Maassen CB, Schellekens MM, Visser L, Kap M, de Jong E, van Puijenbroek M, van Stipdonk MJ, van Meurs M, Schwarzler C, Gunthert U. Therapy with antibodies against CD40L (CD154) and CD44-variant isoforms reduces experimental autoimmune encephalomyelitis induced by a proteolipid protein peptide. Mult Scler. 1998;4:147–153. doi: 10.1177/135245859800400312. [DOI] [PubMed] [Google Scholar]
- 49.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci U S A. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brennan FR, O'Neill JK, Allen SJ, Butter C, Nuki G, Baker D. CD44 is involved in selective leucocyte extravasation during inflammatory central nervous system disease. Immunology. 1999;98:427–435. doi: 10.1046/j.1365-2567.1999.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 52.Tuohy VK, Yu M, Yin L, Mathisen PM, Johnson JM, Kawczak JA. Modulation of the IL-10/IL-12 cytokine circuit by interferon-beta inhibits the development of epitope spreading and disease progression in murine autoimmune encephalomyelitis. J Neuroimmunol. 2000;111:55–63. doi: 10.1016/s0165-5728(00)00384-2. [DOI] [PubMed] [Google Scholar]
- 53.Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]