Abstract
Background
Aristolochic acid (AA) is a toxin found in plants of the genus Aristolochia, to which humans can be exposed either through certain Chinese herbal medicines or through inadvertent commingling with food crops. Our objective was to estimate cumulative exposures of AA associated with increased risk of end stage renal disease (ESRD), and to conduct a systematic review and meta-analysis on AA-induced upper tract urothelial carcinoma (UUC).
Methods
Using epidemiological studies on AA-related disease from multiple different world regions, a systematic review was performed in which relative risks, hazard ratios, and odds ratios were derived or extracted directly; and a meta-analysis was conducted. One study was used to estimate a benchmark dose lower confidence limit (BMDL) for AA-related ESRD.
Results
Mean values for risk ratios - odds ratios, relative risks, or hazard ratios - of UUC caused by AA ranged from 1 to 49. A meta-analysis of these studies resulted in a pooled odds ratio of 5.97 (95% CI: 2.78–12.84) for this AA-related cancer. The obtained BMDL for AA-related ESRD was 0.42 g cumulative aristolochic acid exposure.
Conclusions
Aristolochic acid exposure is significantly associated with an increased risk of UUC, and there is a dose-dependent relationship between cumulative AA exposure and end stage renal disease risk.
Impact
Individuals who use certain Chinese herbal medicines may significantly increase their risk of developing UUC and/or ESRD, as would individuals who are inadvertently exposed to aristolochic acid through commingling of Aristolochia plants with harvested food crops.
Keywords: aristolochic acid, benchmark dose, end stage renal disease, meta-analysis, upper tract urothelial carcinoma
INTRODUCTION
Aristolochic acid (AA) is a toxin found in plants of the genus Aristolochia. For millennia, Aristolochia plants have been used for medicinal purposes, particularly for women in childbirth to ease labor and delivery. Since then, these plants have been used by populations worldwide to treat diverse health conditions. Nearly 200 years ago, the toxicity of certain Aristolochia species was recognized in humans and animals (1). However, Aristolochia plants containing AA are still commonly used in certain herbal medicines, particularly specific Chinese herbal medicines that have been used worldwide (2–4). There may also be inadvertent dietary exposure to AA in certain parts of the world. Indeed, a recent commentary by researchers in the International Agency for Research on Cancer (IARC) states that AA-associated cancer may have global proportions (5).
In the 1990s, aristolochic acid was identified as the agent causing severe renal diseases in Belgian women who had consumed weight-loss supplements containing Aristolochia fangchi. In these women, rapidly progressive renal interstitial fibrosis led ultimately to chronic renal failure, and about 5% of the women developed end-stage renal disease (ESRD). Nearly all the ESRD patients who had consumed AA had developed either upper tract urothelial carcinoma (UUC) or urothelial dysplasia: an unusually high rate for ESRD patients in general (2–5). Yet these well-studied cases likely represent only a small proportion of the total number of AA nephropathy (AAN) cases worldwide. Nearly a million kilograms of Aristolochia plants are harvested each year in China alone for medicinal purposes (1). In Asian nations, they are often marketed under the medicinal names Guan mutong (MuTong, mu tong) or Guang fangchi (fangji). All herbal medicines suspected to contain aristolochic acid are listed in IARC Monograph 100A (6). IARC has classified plants containing aristolochic acid as a Group 1 human carcinogen (6).
Additionally, certain populations worldwide may suffer inadvertent AA exposure. Aristolochic acid-specific DNA adducts were found in renal tissue from Balkan Endemic Nephropathy (BEN) patients (6). BEN is a chronic, progressive renal disease found in rural communities along tributaries of the Danube River in Serbia, Bosnia, Croatia, Bulgaria, and Romania; and is strongly associated with UUC. Because of the unusual epidemiology of BEN (occurrence only in particular farming villages, and familial but not inherited pattern of disease), scientists had for decades speculated that an environmental agent could be causing the disease. Various agents, including AA, ochratoxin A (a nephrotoxic mycotoxin found in multiple agricultural commodities), heavy metals, selenium deficiency, and Pliocene coal deposits leaching aromatic hydrocarbons into well water, have all been postulated in the last 50 years as causing BEN. Most have been ruled out, such as heavy metals and selenium deficiency (7). Due to BEN’s similarities in pathophysiology to AAN in the herbal medicine cases in Belgium, aristolochic acid was suspected to be a cause of BEN. Recently, aristolactam-DNA (AL-DNA) adducts were found in the renal cortex of patients with BEN (8), lending support to the hypothesis that AA is the causal agent of this disease. As Balkan populations do not typically consume Chinese herbal medicines, the exposure route is believed to be consumption of bread in which seeds from the weed Aristolochia clematitis had commingled with wheat grain (9).
Although epidemiological data for AA-related diseases are limited in comparison to those for other dietary toxins such as aflatoxin (10–12), several key studies have assessed not only the increased risk of a particular disease due to AA exposure, but have also correlated disease incidence with different cumulative AA doses. The aims of this article were to review these epidemiological studies, to estimate AA doses associated with nontrivial risk of end stage renal disease (ESRD), and to conduct a systematic review and meta-analysis on the studies that estimate risk of AA-related upper tract urothelial carcinoma.
MATERIALS AND METHODS
We performed a literature search to June 2012 on articles in the PubMed database concerning the effects of AA exposure in human populations. Search terms used without restriction included combinations of: (aristolochic acid), (guang fangchi/fangji), (guan mutong/mu tong), (end stage renal disease), (Balkan Endemic Nephropathy), (Chinese herb[s] nephropathy), (urothelial carcinoma), (transitional cell carcinoma), and (dose response). From the retrieved articles, we reviewed reference lists to identify further relevant studies. These studies provided the basis for our dose-response assessment and meta-analysis. The systematic review and meta-analysis were conducted and reported in adherence to PRISMA standards for meta-analyses (13).
For the dose-response assessment, we identified one epidemiological study that found at least four different cumulative doses of AA and quantified their corresponding effects in the humans who consumed those doses. To these data, we applied benchmark dose modeling to estimate a point of departure for AA risk assessment purposes. Benchmark dose modeling is a methodology applied in dose-response assessment to find the lowest dose of a toxin expected to have biological significance. Compared with the former method of using No Observed Effect Levels (NOELs) as a point of departure, benchmark dose modeling involves finding a statistical model that best fits the entire dose-response curve; then, from that model, identifying the dose corresponding to a certain proportion (often 10%) of adverse response in the study population. This particular dose is the benchmark dose. The lower bound of the confidence interval around that dose, the BMDL, is often used in regulatory risk assessments as the point of departure from which to calculate a reference dose (RfD) or tolerable daily/weekly intake (TDI/TWI) for human exposure to a substance. We used the US Environmental Protection Agency (EPA) Benchmark Dose Software (BMDS v.2.2) to fit statistical models for the dose-response data in one study on AA toxicity. Multiple statistical models can be fit in BMDS. We chose the model that had the lowest Akaike Information Criterion (AIC) score and the highest P-value (corresponding to best statistical fit) to estimate the BMDL.
For the systematic review and meta-analysis, studies were included if they met the following criteria: (1) human subjects; (2) aristolochic acid, plants containing AA, or herbal medicines containing AA as the exposure of interest; (3) upper tract urothelial carcinoma (UUC) as the outcome of interest; and (4) relative risk (RR), odds ratio (OR), or hazard ratio (HR) estimates, or data to calculate these. The following data were extracted from each study: authors; publication year; study design, location, and period; participants’ gender/ages; number of participants; number of UUC cases; adjusted RRs/ORs/HRs; and variables adjusted for analysis. Because RR and OR can be used interchangeably when the disease is relatively rare (<15%; UUC rates are lower than this in the populations studied), and HRs were estimated from Cox proportional hazard models and used to approximate RRs (14), we calculated a summary OR for AA-related UUC.
If the study examined the association between AA exposure and UUC at various exposure levels, we chose the ORs reflecting the highest levels of AA exposure for the meta-analysis (12). The data synthesis was performed using both fixed-effects and random-effects models; if heterogeneity is present, the random effect model is considered more appropriate, as variation among studies can be taken into account (15, 16). Heterogeneity among the studies was assessed by the Cochran’s Q statistic and I2 statistic. Publication bias was also assessed.
RESULTS
Literature search and study characteristics
Ten studies were found in which human exposure to aristolochic acid was associated with adverse health effects. The step-by-step process of our literature search is presented in Figure 1. From 98 results, we excluded animal studies, chemical methodological studies, in vitro studies, and review articles. Using the eligibility criteria described above, six studies were selected. Four more relevant studies were identified from authority reports (17, 18). Eight of the ten studies reported odds ratios, relative risks, or hazard ratios; or data from which these risk metrics could be calculated for AA-related UUC. These eight studies were included in the meta-analysis.
Figure 1.
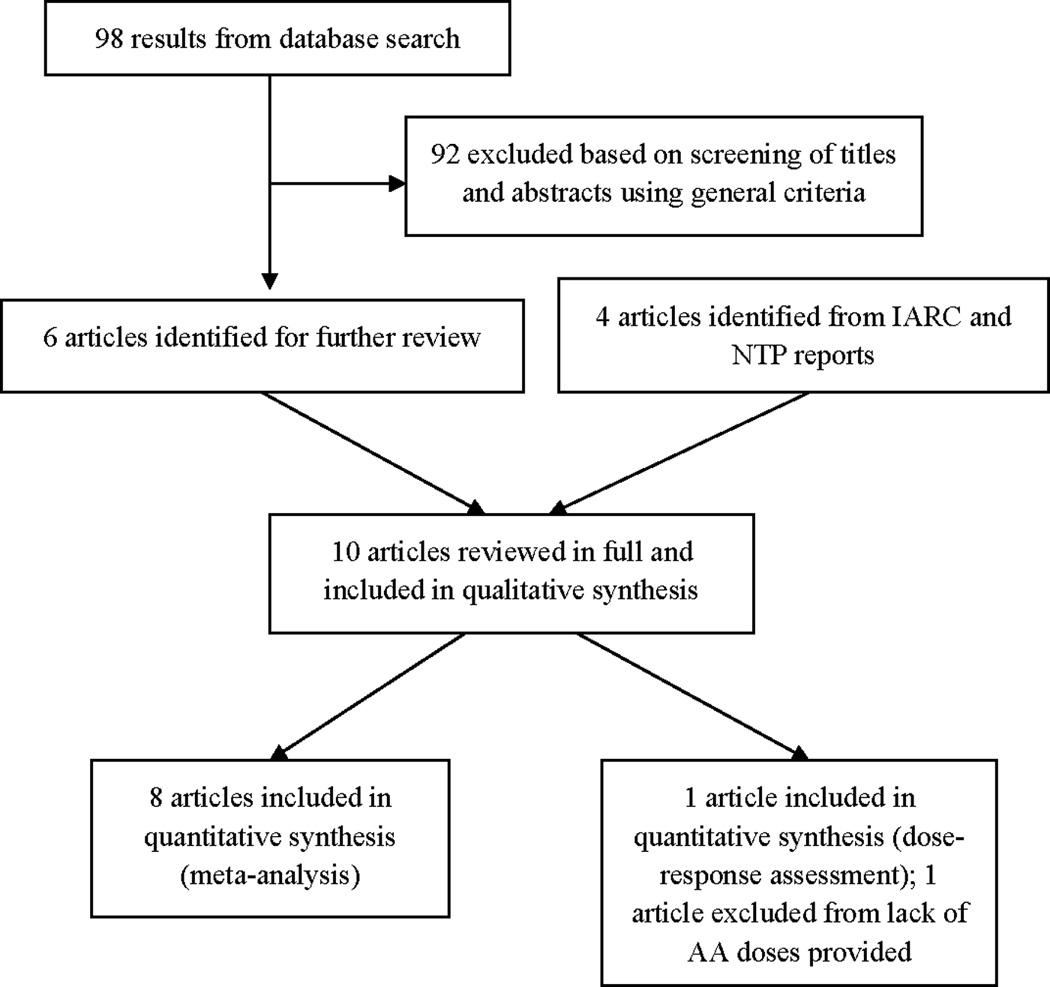
Selection of studies for inclusion in the systematic review for meta-analysis and dose-response assessment.
Table 1 provides an overview of the eligible studies. The eight studies (19–26) on AA exposure and UUC risk (one cohort, five case-control, and two survival analyses) were published between 2004–2012. Four studies were conducted in Taiwan; three in China; and one across Croatia, Bosnia, and Serbia. Four ORs and the corresponding confidence intervals were calculated using the data provided in the articles (20, 24–26). One of the case-control studies investigated the risk of UUC at two AA exposure levels (151–250 mg; >250 mg) (23). AL-DNA adducts and TP53 mutation spectra served as biomarkers of exposure in two molecular epidemiological studies (25, 26). We used the ORs from AL-DNA adducts in our meta-analysis, as AL-DNA adducts have been demonstrated to serve as a specific biomarker of effect for AA-induced UUC, as well as a robust biomarker of AA exposure (26). Other important characteristics of the eligible studies, including the study periods, numbers of participants, numbers of UUC events, measure of exposure, and covariates adjusted in the analysis are also listed in Table 1.
Table 1.
Characteristics of the eligible studies included in the systematic review.
| No | Source | Location/ Period |
Age, yrs |
Relevant doses/ Exposure |
Health effect measured |
No. of total participants |
No. of UUC events |
Measure of exposure |
Results (95% CI) |
Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Wu et al., 2004 (19) (Survival analysis, multivariate Cox proportional model) | Taiwan 1983–2003 | Chinese herbal medicines | Upper tract urothelial carcinoma | 730 | 30 | Medical records/questionnaire | HR=5.2 | Age, sex, compound analgesic usage, underground water intake | |
| 2 | Li et al., 2005 (20) (Hospital-based case control study, 283 patients) | Beijing, China 2004 | 22–88 | Chinese herbs containing aristolochic acid | Upper tract urothelial carcinoma | 264 | 24 | Questionnaire | OR=49 (11.1–215.8) | n/a |
| 3 | Chang et al., 2007 (21) (Survival analysis, Univariate Cox proportional model) | Taiwan 1993–2002 | 46–87 | Chinese herbal medicines | Upper tract urothelial carcinoma | 1537 | 26 | Medical records/questionnaire | HR=6.21 | n/a |
| 4 | Li et al., 2008 (22) (Hospital-based cohort study, 1735 patients) | Beijing, China 1996–2005 | 8–79 | Chinese herbs containing aristolochic acid | Upper tract urothelial carcinoma | 1429 | 27 | Medical records | RR=5.85 (2.4–11.1) | n/a |
| 5 | Lai et al., 2009 (23) (Population-based case control study, 179,295 subjects in total) | Taiwan 1997–2002 | Aristolochic acid >500 mg | Urinary Tract Cancer | 577 | 36 | Prescription history | OR=2.0 (1.4–2.9) | Age, sex, chronic arsenic exposure, history of chronic urinary tract infection | |
| 6 | Xiao et al., 2011 (24) (Hospital-based case control study, 3790 renal transplant recipients) | Beijing 1974–2011 | 27–71 | Aristolochic acid | Urothelial Carcinoma | 3790 | 100 | Clinical records | OR=11.6 (7.62–17.66) | n/a |
| 7 | Chen et al., 2012 (25) (Case control study) | Taiwan | Aristolochic acid | Upper tract urothelial carcinoma | 148 | 89 | AL-DNA adducts | OR=1.01 (0.39–2.61) | n/a | |
| 151 | 47 | TP53 A→T transversion | OR=23.18 (1.38–388.15) | |||||||
| 148 | 38 | AL-DNA adducts plus TP53 A→T transversion | OR=17.77 (1.06–297.80) | |||||||
| 8 | Jelakovic et al., 2012 (26) (Case control study) | Croatia, Bosnia, and Serbia | 43–89 | Aristolochic acid | Upper tract urothelial carcinoma | 72 | 47 | AL-DNA adducts | OR=48.66 (2.72–870.27) | n/a |
| 63 | 16 | TP53 A→T transversion | OR=3.82 (0.2–72.92) |
The techniques used to detect, identify, and quantify AL-DNA adducts include 32P-postlabeling and mass spectroscopy analyses. Nortier et al. (4) used the nuclease P1 enrichment version of the 32P-postlabeling method to detect and quantify DNA adducts formed by AA, while Jelakovic et al. (26) applied liquid chromatography electrospray /multistage mass spectrometry (LC-ESI/MS/MS3) to identify the predominant DNA adduct, 7-(deoxyadenosin-N6-yl) AL-I (dA-AL-I), in the renal cortex. Similarly, in addition to quantify the level of DNA-AL adducts using 32P-postlabeling assay, Grollman et al. (8) and Chen et al. (25) employed the same method to identify dA-AL adducts. One recent study (27) compared the two methodologies, and concluded that both Ultra-Performance Liquid Chromatography/Ion-Trap Mass Spectrometry (UPLC-ESI/MS3) and the 32P-postlabeling methods are both highly sensitive for the detection of dA-AL, but that UPLC-ESI/MS3 is superior under certain circumstances; for example, measuring trace levels of AL-DNA adducts.
It is worth noting that Chen et al. (25) investigated the TP53 mutation spectra in UUC cases and controls as a biomarker of effect in addition to the AL-DNA adduct. The TP53 mutational signature is dominated by rare A:T to T:A transversion in patients with UUC. AL-DNA adducts were present in the renal cortex of 83% of patients with A:T to T:A mutations in TP53, FGFR3 or HRAS oncogenes. With the data provided in the paper, we estimated several odds ratios for AA-related UUC. Using the signature TP53 A→T transversion as a biomarker of exposure, and applying a continuity correction ε=0.5 (because the number of exposed controls was zero), the odds ratio of AA-related UUC is 23.18 (1.38–388.15). ORs were also estimated using, as measures of exposure, the presence of AL-DNA adducts and the presence of both TP53 A→T transversions and AL-DNA adducts. The corresponding ORs are 1.01 (0.39–2.61) and 17.77 (1.06–297.80), respectively.
Benchmark dose modeling
Two studies (28, 29) provided data on disease prevalence corresponding to at least four different cumulative doses of AA-containing herbal medicines. The Lai et al. (29) study was used for the purposes of benchmark dose modeling, as this study provided a conversion factor from doses of the medicine MuTong into aristolochic acid, the substance of interest. Muniz Martinez et al. (28) measured disease progression as a function of doses of Aristolochia fangchi, but this was not translated to doses of AA.
Lai et al. (29) conducted a case control study in Taiwan to investigate the relationship between kidney failure and cumulative consumption of herbal medicines MuTong or Fangchi. The authors drew ESRD cases and random samples from the national health insurance reimbursement database from 1997–2002. Adjusted ORs obtained for ESRD associated with MuTong were 1.47 (1.01–2.14) for 61–100 g cumulative dose, and 5.82 (3.89–8.71) for >200 g cumulative dose; and for ESRD associated with Fangchi were 1.60 (1.20–2.14) for 61–100 g cumulative dose and 1.94 (1.29–2.92) for >200 g cumulative dose. Hence, total consumption of >60 g MuTong or Fangchi from herbal supplements was associated with a statistically significant increased risk of ESRD. However, when we fitted statistical BMD models on the more extensive MuTong and Fangchi dose ranges, all the P-values were zero for the Fangchi data, which meant that no statistical models in the software fit the data well.
For MuTong-related ESRD, six dose ranges and their associated numbers of ESRD cases and controls were provided. These data were entered into the benchmark dose software, and the corresponding dose-response model is shown in Figure 2. For the MuTong dose-response data, the Weibull distribution was the most appropriate fit, resulting in a BMDL value of 162.8 g cumulative exposure to MuTong (equivalent to 0.42 g AA, according to the conversion provided in the paper) resulting in a 10% increased risk to the population of ESRD. This specific conversion factor of amount of AA in MuTong was derived by the study authors themselves for this particular study (29), and is not necessarily the same conversion factor that should be used to estimate amount of AA in a particular Chinese herbal medicine. Indeed, Lai et al. (29) note that their conversion factor was slightly different from those derived in other Dutch and Belgian studies.
Figure 2.
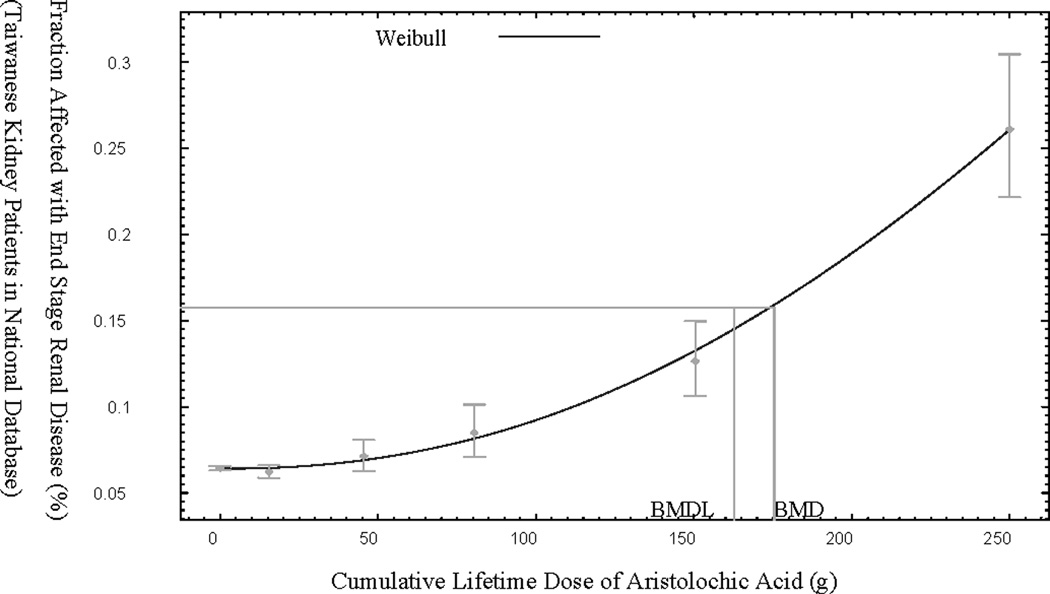
Benchmark dose model for Lai et al. (29) on end stage renal disease (ESRD) prevalence as a function of cumulative MuTong dose in Taiwan.
Meta-analysis
A forest plot with estimates from a random effects model and the contribution of each epidemiological study to the meta-analysis is shown in Figure 3. The I-V (Inverse-Variance method) pooled OR denotes fixed-effects estimates, and the D+L (DerSimonian and Laird method) pooled OR denotes random-effects estimates. Heterogeneity in the study pool was significant (p-value from Cochran’s test <0.001; I2=88.7%). Therefore, in this study, the random-effects estimates were reported as the primary analysis, while fixed-effects estimates were provided for comparison. AA exposure is significantly associated with UUC, with a pooled OR of 5.97 (2.78–12.84). The Egger’s test of asymmetry suggests no presence of bias (intercept=1.84; p=0.446).
Figure 3.
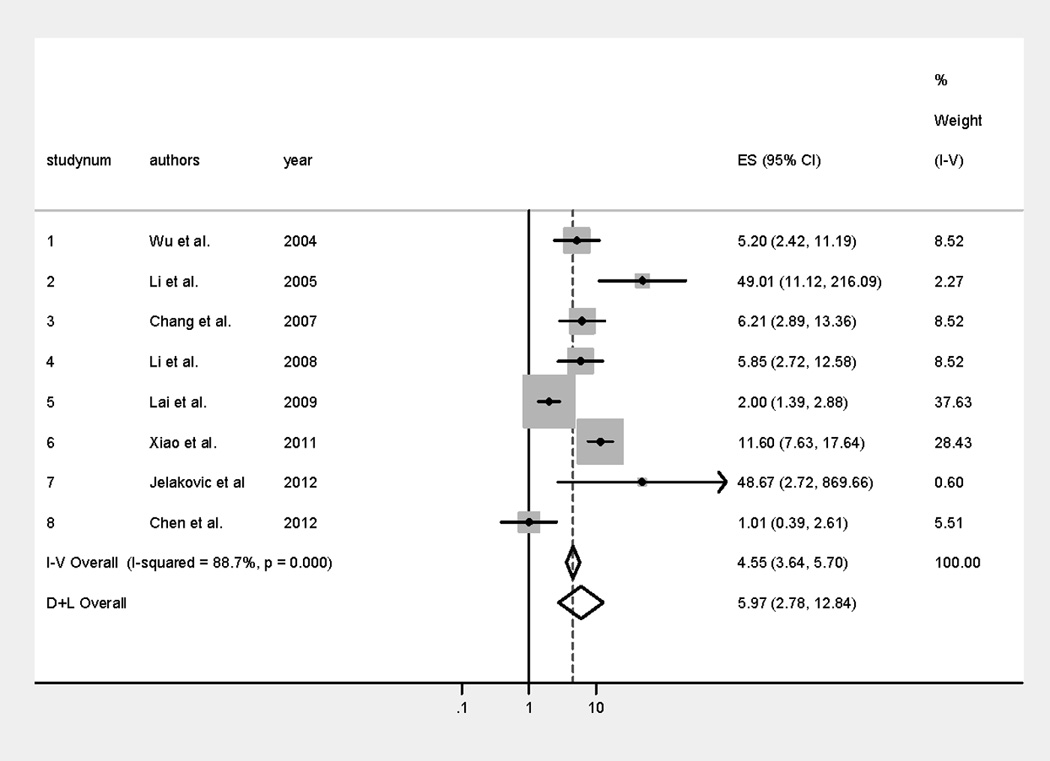
Forest plot of risk ratios (ES) of aristolochic acid-related urothelial cancer with the contribution of each study to the meta-analysis. I–V (Inverse Variance method) pooled OR denotes the fixed-effects estimates, and the D+L (DerSimonian and Laird method) pooled OR denotes the random-effects estimates.
Exposure to aristolochic acid worldwide
There are at least two major routes of human exposure to AA. The more well-characterized exposure, potentially affecting millions worldwide (25), is consumption of Aristolochia plants in certain herbal medicines. The less-understood exposure route concerns the inadvertent presence of some part of Aristolochia plants commingled into the diets of farming populations in the Balkans (and possibly other parts of the world). It is estimated that over 100,000 individuals may be at risk for AA-related BEN in the Balkans alone (30).
At national levels, regulatory efforts are being made to reduce risk of human exposure to herbal medicines containing AA. All Aristolochia-based medicines were prohibited for supply, sale or use in therapeutic goods in Australia (17, 31). In 2001, the United Kingdom Committee on Safety of Medicines issued a statutory instrument to prohibit the sale, supply and importation of any medicinal products containing Aristolochia (17, 32). Herbal products containing AA were prohibited in 2003 in Taiwan (29). In addition, advisories concerning the use and marketing of botanical products that may contain AA have been issued by the European Agency for the Evaluation of Medicinal Products (33), Health Canada, and the Food and Drug Administration of the USA (17). In these nations, it is likely that AA exposure has decreased over the last decade and will continue to decrease, as fewer herbal medicines available in the market will contain AA. However, unreported sales of these herbal medicines may continue for some time. Moreover, many who have already been exposed to AA in the past may develop AA-related cancer or other AA-related diseases in the years to come, despite recent regulatory efforts to curb exposure. Given the benchmark doses estimated above, probably many individuals who have regularly consumed these herbal medicines for years have reached cumulative doses that could result in disease.
Inadvertent exposures to AA are more difficult to characterize and control. It has been speculated that, because Aristolochia weeds have been seen to grow in cereal fields, Aristolochia seeds may commingle with cereal grains in the field and during harvest. Aristolactam-DNA adducts have been found in the kidneys of BEN patients (8), which indicates AA exposure in these individuals. Moreover, AA was detected in the seeds of Aristolochia clematitis growing in the endemic regions (9). Although it is highly likely that AA has caused BEN in this population, the toxin has not actually been detected in the food consumed in this region; in part because of lack of efforts in the past to detect it, and the difficulty of chemical analysis if AA binds to cereal components. It may not be possible at this point to find such food samples, as relevant exposures may have occurred from food consumed decades ago. However, it is known that Aristolochia clematitis has grown continuously in the cultivated fields in Balkan endemic regions for at least five decades (34).
DISCUSSION
The review of the epidemiological literature indicates that aristolochic acid exposure is causally related to urothelial carcinoma and end-stage renal disease in humans, in a dose-dependent manner. Thus, the more herbal medicines containing AA are consumed over the course of a lifetime, or the longer that inadvertent exposures to AA continue, the higher the risk that one or both of these diseases will develop.
From a risk assessment standpoint, it is useful to understand the cumulative dose of AA that is associated with an increased risk of human disease. The benchmark dose has been used in recent governmental risk assessments as a point of departure by which to establish tolerable human intakes of certain substances. In this study, we found a benchmark dose of cumulative aristolochic acid exposure of 0.42 g, associated with increased risk of end-stage renal disease in humans, based on a Taiwanese study that analyzed extensive data on Chinese herbal medicine intake and disease incidence (29). This value may be useful in providing risk analysts with information about establishing a cumulative dose of AA exposure, below which there may not be increased risk of ESRD in humans.
Our meta-analysis found a pooled odds ratio of AA-related upper tract urothelial carcinoma of 5.97 (2.78–12.84). As UUC is a relatively rare condition, the odds ratio can be used to estimate relative risk. Hence, the risk of developing UUC is about six times higher for those exposed to aristolochic acid than for those who have not been exposed to AA. One limitation of our approach is the differences in how exposure to AA was measured amongst the studies in the meta-analysis. Several studies were based upon medical or prescription records, with or without an accompanying questionnaire; others were based on detection of the AL-DNA adduct. In particular, one weakness of questionnaire-based studies is the possibility of recall bias. Although in the case of these studies, it is likely that the participants would have remembered whether they had consumed a particular Chinese herbal medicine, the amount consumed would be more difficult to remember or estimate.
Additionally, there are differences in how much AA is present in different preparations of the Aristolochia-containing Chinese herbal medicines, as well as differences in methods for quantifying the AA in these medicines. Several classic analytical methods, including thin layer chromatography (TLC), liquid chromatography UV-vis (LC/UV), and liquid chromatography mass spectroscopy (LC/MS), were employed to determine AAs in Chinese herbs and dietary supplements (35, 36). An enhanced method of LC/MS, liquid chromatography- tandem mass spectrometry (LC/MS/MS) with superior sensitivity, selectivity and specificity, was also developed (35). Simple and rapid techniques, such as capillary zone electrophoresis (CZE) and capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) (37, 38), were also proposed as alternatives for analyzing AAs.
Finally, there are limitations associated with extrapolating from the results of the benchmark dose modeling linking AA to ESRD. Even if AA exposure was fairly well-characterized in (29) because the conversion factor from the MuTong to AA was directly provided by the authors for their study, this conversion factor cannot be used for other herbal medicine exposure studies worldwide; because there is a wide variability in the amount of AA in different medicinal preparations. Thus, in future studies of this nature, new conversion factors must be found for the relevant herbal medicine preparations, which themselves may not be consistent over the lifetime of an individual’s use of these medicines. In the future, measuring AA exposure through the AL-DNA biomarker may be a much more accurate way to assess individuals’ cumulative exposures.
Two unique routes of human exposure to AA exist. The route for which there is the greatest evidence for causality of adverse health effects is through consumption of traditional Chinese herbal medicines labeled Fangchi or MuTong. Even within populations that consume these herbal medicines, however, some may take the medicine continually, while others may take it for several days or weeks in a lifetime (e.g., in response to a bout of illness). Hence, lifetime exposures to AA can vary substantially among these herbal medicine-consuming populations. Another, less defined, route of human exposure concerns inadvertent presence in foods due to presence of Aristolochia plants growing alongside food crops, thus incorporated unintentionally in human diets. This route of exposure has been postulated in certain Balkan populations, in which AA exposure has been associated with Balkan Endemic Nephropathy. While AA has not been detected in food, it has been detected in the seeds of plants growing in those regions, and AA-specific DNA adducts have been found in the kidneys of those with the disease.
Possible genetic factors associated with chronic kidney diseases, and UUC specifically, were investigated in several studies. In a recent meta-analysis focusing on genome-wide linkage scans for renal function traits (39), no chromosomal region reached genome-wide statistical significance, and subgroup analyses by status of chronic kidney diseases did not yield additional information. On the other hand, two other very recent studies (40, 41) identified a particular genotype – a polymorphism located at the T allele of rs9642880 on chromosome 8q24 - that appears to confer susceptibility to urothelial carcinomas of the upper urinary tract. Thus, the genetic link to the diseases associated with aristolochic acid should be studied further, particularly in identifying vulnerable subpopulations in terms of both genetics and AA exposure.
Because the association between AA exposure and severe renal disease is strong, efforts must be made to prevent exposures in at-risk human populations. Both herbal medicine producers and the populations that consume these medicines must be informed of these risks; and, where possible, regulations should be enforced to prevent the presence of Aristolochia plants in medicines. Fortunately, many regulatory bodies worldwide have in the last decade taken measures to reduce the sales of herbal medicines containing Aristolochia. In communities in which inadvertent exposure to AA is suspected through commingling of Aristolochia seeds with cereal grains, particularly in the Balkans, farmers should be alerted to the possible presence of harmful weeds in their fields and encouraged to adopt primary prevention methods: removing the weeds, or carefully sorting weed plants or seeds from the grains following harvest. Primary prevention is key to preventing AA-related diseases in human populations in the future.
Acknowledgment
We thank Dr. Arthur Grollman for his helpful comments.
Grant support:
This work was funded by the National Cancer Institute (NCI) of the National Institutes of Health (NIH), Grant No. 5R01CA153073-2. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI or NIH.
Footnotes
Disclosure
The authors declare no competing interests.
REFERENCES
- 1.Grollman AP, Scarborough J, Jelakovic B. Aristolochic acid nephropathy: An environmental and iatrogenic disease. In: Fischbein JC, editor. Advances in Molecular Toxicology. Vol. 3. Oxford, UK: Elsevier; 2009. [Google Scholar]
- 2.Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 3.Cosyns JP, Jadoul M, Squifflet JP, van Cangh PJ, van Ypersele de Strihou C. Urothelial malignancy in nephropathy due to Chinese herbs. Lancet. 1994;344:188. doi: 10.1016/s0140-6736(94)92786-3. [DOI] [PubMed] [Google Scholar]
- 4.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 5.Olivier M, Hollstein M, Schmeiser HH, Straif K, Wild CP. Upper urinary tract urothelial cancers: where it is A:T. Nature Rev Cancer. 2012;12:503–504. doi: 10.1038/nrc3311. [DOI] [PubMed] [Google Scholar]
- 6.IARC (International Agency for Research on Cancer) A review of human carcinogens. Part A: pharmaceuticals. [accessed November 2011];Lyon, France: IARC Monogr Eval Carcinog Risks Hum. 2008 100:367–383. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100A/mono100A-23.pdf. [Google Scholar]
- 7.Batuman V. Fifty years of Balkan endemic nephropathy: daunting questions, elusive answers. Kidney Int. 2006;69:644–646. doi: 10.1038/sj.ki.5000231. [DOI] [PubMed] [Google Scholar]
- 8.Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, et al. Aristolochic acid and the etiology of (Balkan) endemic nephropathy. PNAS. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hranjec T, Kovac A, Kos J, Mao W, Chen JJ, Grollman AP, et al. Endemic nephropathy: the case for chronic poisoning by aristolochia. Croat Med J. 2005;46:116–125. [PubMed] [Google Scholar]
- 10.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Wu F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ Health Perspect. 2010;118(6):818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Chang CC, Marsh GM, Wu F. Population Attributable Risk of Aflatoxin-Related Liver Cancer: Systematic Review and Meta-Analysis. Eur J Cancer. 2012;48(14):2125–2136. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York, USA: Springer-Verlag New York, Inc; 2003. [Google Scholar]
- 15.Hartung J, Knapp G, Sinha BK. Statistical Meta-Analysis with Applications. 1st ed. Hoboken, New Jersey: John Wiley & Sons, Inc; 2008. [Google Scholar]
- 16.Ngo AD, Taylor R, Roberts CL, Nguyen TV. Association between Agent Orange and birth defects: systematic review and meta-analysis. Int J Epidemiol. 2006;35(5):1220–1230. doi: 10.1093/ije/dyl038. [DOI] [PubMed] [Google Scholar]
- 17.IARC (International Agency for Research on Cancer) Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. [accessed November 2011];Lyon, France: IARC Monogr Eval Carcinog Risks Hum. 2002 82:69–128. Available from: http://monographs.iarc.fr/ENG/Monographs/vol82/mono82-6B.pdf. [PMC free article] [PubMed] [Google Scholar]
- 18.NTP (National Toxicology Program) Report on Carcinogens Background Document for Aristolochic Acids. [accessed February 2012];NTP. 2008 Available from: http://ntp.niehs.nih.gov/NTP/roc/twelfth/2010/FinalBDs/AristolochicAcids20101014.pdf. [PubMed]
- 19.Wu MJ, Lian JD, Yang CR, Cheng CH, Chen CH, Lee WC, et al. High Cumulative Incidence of Urinary Tract Transitional Cell Carcinoma After Kidney Transplantation in Taiwan. Am J Kidney Dis. 2004;43(6):1091–1097. doi: 10.1053/j.ajkd.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Li WH, Yang L, Su T, Song Y, Li XM. Influence of taking aristolochic acid –containing Chinese drugs on occurrence of urinary transitional cell cancer in uremic patients undergoing dialysis. Zhonghua Yi Xue Za Zhi. 2005;85(35):2487–2491. Chinese. [PubMed] [Google Scholar]
- 21.Chang CH, Yang CM, Yang AH. Renal Diagnosis of Chronic Hemodialysis Patients With Urinary Tract Transitional Cell Carcinoma in Taiwan. Cancer. 2007;109(8):1487–1492. doi: 10.1002/cncr.22557. [DOI] [PubMed] [Google Scholar]
- 22.Li XB, Xing NZ, Wang Y, Hu XP, Yin H, Zhang XD. Transitional cell carcinoma in renal transplant recipients: A single center experience. Int J Urol. 2008;15(1):53–57. doi: 10.1111/j.1442-2042.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 23.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-Based Case-Control Study of Chinese Herbal Products Containing Aristolochic Acid and Urinary Tract Cancer Risk. JNCI. 2009;102(3):179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J, Zhu X, Hao GY, Zhu YC, Hou HJ, Zhang J, et al. Association Between Urothelial Carcinoma After Kidney Transplantation and Aristolochic Acid Exposure: The Potential Role of Aristolochic Acid in HRas and TP53 Gene Mutations. Transplant Proc. 2011;43(10):3751–3754. doi: 10.1016/j.transproceed.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Dickman KG, Moriya M, Zavadil J, Sidorenko VS, Edwards KL, Gnatenko DV, et al. Aristolochic acid-associated urothelial carcinoma in Taiwan. PNAS. 2012;109(21):8241–8246. doi: 10.1073/pnas.1119920109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelaković B, Karanović S, Vuković-Lela I, Miller F, Edwards KL, Nikolić J, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012;81(6):559–567. doi: 10.1038/ki.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun BH, Rosenquist TA, Sidorenko V, Iden CR, Chen CH, Pu YS, et al. Biomonitoring of Aristolactam-DNA Adducts in Human Tissues Using Ultra-Performance Liquid Chromatography/Ion-Trap Mass Spectrometry. Chem Res Toxicol. 2012;25(5):1119–1131. doi: 10.1021/tx3000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muniz Martinez MC, Nortier J, Vereerstraeten P, Vanherweghem JL. Progression rate of Chinese herb nephropathy: impact of Aristolochia fangchi ingested dose. Nephrol Dial Transplant. 2002;17(3):408–412. doi: 10.1093/ndt/17.3.408. [DOI] [PubMed] [Google Scholar]
- 29.Lai MN, Lai JN, Chen PC, Hsieh SC, Hu FC, Wang JD. Risks of Kidney Failure Associated with Consumption of Herbal Products Containing Mu Tong or Fangchi: A Population-Based Case-Control Study. Am J Kidney Dis. 2010;55(3):507–518. doi: 10.1053/j.ajkd.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 30.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 31.Therapeutic Goods Administration (TGA) Aristolochia Fact Sheet – 25 May 2001. [accessed February 2012];TGA. 2001 Available from: http://www.tga.gov.au/safety/alerts-medicine-aristolochia-010524.htm.
- 32.UK Committee on Safety of Medicines (MHRA) The Medicines (Aristolochiaand Mu Tong etc.) (Prohibition) Order 2001 (Statutory Instrument 2001 No. 1841). London. [accessed February 2012];The Stationary Office. 2001 Available from: http://www.legislation.gov.uk/uksi/2001/1841/contents/made.
- 33.European Agency for the Evaluation of Medicinal Products (EMEA) Working Party on Herbal Medicinal Products: Position paper on the risks associated with the use of herbal products containing Aristolochia species (EMEA/HMPWP/23/00). London. [accessed February 2012];EMEA. 2000 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Position_statement/2009/11/WC500015537.pdf. [Google Scholar]
- 34.Grollman AP, Jelakovic B. Role of Environmental Toxins in Endemic (Balkan) Nephropathy. J Am Soc Nephrol. 2007;18:2817–2823. doi: 10.1681/ASN.2007050537. [DOI] [PubMed] [Google Scholar]
- 35.Huang CY, Tseng MC, Lin JH. Analyzing Aristolochic Acids in Chinese Herbal Preparations Using LC/MS/MS. J Food Drug Anal. 2005;13:125–131. [Google Scholar]
- 36.Chan W, Hui KM, Poon WT, Lee KC, Cai Z. Differentiation of herbs linked to “Chinese herb nephropathy” from the liquid chromatographic determination of aristolochic acids. Analytica Chimica Acta. 2006;576:112–116. doi: 10.1016/j.aca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Kvasnička F, Ševčík R, Voldřich M, Krátká J. Determination of aristolochic acid by capillary zone electrophoresis. Cent Eur J Chem. 2004;2(3):417–424. [Google Scholar]
- 38.Hsieh SC, Huang MF, Lin BS, Chang HT. Determination of aristolochic acid in Chinese herbal medicine by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr A. 2006;1105:127–134. doi: 10.1016/j.chroma.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 39.Rao M, Mottl AK, Cole SA, Umans JG, Freedman BI, Bowden DW, et al. Meta-analysis of genome-wide linkage scans for renal function traits. Nephrol Dial Transplant. 2012;27:647–656. doi: 10.1093/ndt/gfr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouprêt M, Drouin SJ, Cancel-Tassin G, Comperat E, Larré S, Cussenot O. Genetic variability in 8q24 confers susceptibility to urothelial carcinoma of the upper urinary tract and is linked with patterns of disease aggressiveness at diagnosis. J Urol. 2012;187(2):424–428. doi: 10.1016/j.juro.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 41.Yates DR, Rouprêt M, Drouin SJ, Audouin M, Cancel-Tassin G, Comperat E, et al. Genetic polymorphisms on 8q24.1 and 4p16.3 are not linked with urothelial carcinoma of the bladder in contrast to their association with aggressive upper urinary tract tumours. World J Urol. 2013;31(1):53–59. doi: 10.1007/s00345-012-0954-6. [DOI] [PubMed] [Google Scholar]


