Abstract
Background
Smoking tobacco preparations in a water pipe (hookah) is widespread in many places of the world and is perceived by many as relatively safe. We investigated biomarkers of toxicant exposure with water pipe compared to cigarette smoking.
Methods
We conducted a cross-over study to assess daily nicotine and carcinogen exposure with water pipe and cigarette smoking in 13 people who were experienced in using both products.
Results
While smoking an average of 3 water pipe sessions compared to smoking 11 cigarettes per day, water pipe use was associated with a significantly lower intake of nicotine, greater exposure to carbon monoxide and a different pattern of carcinogen exposure compared to cigarette smoking, with greater exposure to benzene and high molecular weight PAHs, but less exposure to tobacco-specific nitrosamines, 1,3-butadiene and acrolein, acrylonitrile, propylene oxide, ethylene oxide, and low molecular weight PAHs.
Conclusions
A different pattern of carcinogen exposure might result in a different cancer risk profile between cigarette and water pipe smoking. Of particular concern is the risk of leukemia related to high levels of benzene exposure with water pipe use.
Impact
Smoking tobacco in water pipes has gained popularity in the United States and around the world. Many believe that water pipe smoking is not addictive and less harmful than cigarette smoking. We provide data on toxicant exposure that will help guide regulation and public education regarding water pipe health risk.
Introduction
It is estimated that about 100 million people worldwide smoke tobacco in water pipes. Water pipe is also known as hookah (Indian subcontinent and Africa), shisha, borry, goza (Egypt, Saudi Arabia), narghile, arghile (Jordan, Lebanon, Syria, and Israel), shui yan dai (China), or hubble-bubble [1, 2]. Smoking tobacco in water pipes has gained popularity in the United States, particularly in areas with sizable Arab-American populations, and also among young non-Arab-American people, with hookah bars often being located near college campuses [3]. A typical session at a hookah bar involves smoking for 45-60 minutes, often with a group of friends [4-6]. Many believe that water pipe smoking is not addictive and is less harmful than cigarette smoking [1, 5, 7].
A water pipe consists of a head that is connected to a bowl containing water and a hose with mouthpiece. A tobacco preparation is placed in the head, and burning charcoal is placed on top of the tobacco. The smoker inhales through a mouthpiece, which draws air and hot combustion products from the burning charcoal through the tobacco preparation, creating an aerosol consisting of volatilized and pyrolized tobacco components. The smoke passes through the water in the bowl, cooling the smoke, before being carried through the hose to the smoker.
Water pipe tobacco is a moist paste-like preparation made from about 5-10% crude cut tobacco that is fermented with honey, molasses, and pulp of different fruits to add flavor. Differences in composition of the products smoked and different temperatures involved in the smoking process result in substantial difference in the composition of hookah smoke compared to cigarette smoke. Water pipe smoke is produced at about 450° C compared to about 900° C for cigarettes [8]. Furthermore, water pipe smoke also contains charcoal combustion products, including substantial amounts of carbon monoxide (CO)
Based on smoking machine data, the amount of water pipe tobacco used in a single smoking session was reported to produce 100-fold more tar, 4-fold more nicotine, 11-fold more CO, and 2 to 5-fold more polycyclic aromatic hydrocarbons than did a single cigarette [8]. Other investigators have confirmed these findings, but PAH delivery was higher for some PAHs and lower for others [9]. Shafagoj et al found that the water pipe smokers had about 2-fold higher expired CO levels and about 3-fold higher plasma nicotine levels than cigarette smokers [10]. We recently studied biomarkers of nicotine and carcinogen exposure after single water pipe sessions, and found that peak plasma nicotine concentrations were comparable and expired CO levels were much higher than those typically seen after smoking a cigarette [11]. We found that the estimated systemic dose of nicotine from one session of water pipe smoking was similar to smoking 2 to 3 cigarettes and water pipe smoking significantly increased urine excretion of tobacco specific nitrosamines and polycyclic aromatic hydrocarbons, representing two major classes of tobacco smoke carcinogens [12].
The goal of the present study was to compare toxicant exposure from water pipe smoking with exposure from cigarette smoking using biomarker measurements. We conducted a cross-over study to assess daily nicotine and carcinogen exposure with water pipe and cigarette smoking in people who were experienced users of both products.
Materials and Methods
Subjects
Thirteen healthy volunteers who smoked both cigarettes and water pipes completed the study. They included 8 men and 5 women, eight non-Hispanic whites, 1 Hispanic white, 3 Asians and 1 African American with a mean age of 24 years (range 18-33) and an average BMI of 26 (range 21-35). Subjects smoked an average of 10 cigarettes per day (cpd) (range 4-20) and had an average Fagerström Test of Nicotine Dependence score of 3 (range 0-6). Subjects reported smoking an average of 3 water pipe sessions per week (range 1-7) for an average of 4.8 years (range 1.5-7). The average saliva cotinine at screening was 72 ng/ml (range 20-150).
Participants were recruited through Internet postings (Craigslist) and word of mouth. Subjects were financially compensated for their time. The study was approved by the Committee on Human Research at University of California, San Francisco.
Study Procedures
This was a randomized, two arm, cross over study of water pipe and cigarette smoking. The arms comprised exclusive water pipe smoking and exclusive cigarette smoking, each requiring 4 inpatient days in the Clinical Research Center (CRC) at San Francisco General Hospital, with at least 1 week separating each arm. Randomization of the sequence of treatment arms was done separately for males and females. Subjects were requested to refrain from smoking from 9 PM on the night before CRC admission, which occurred at 7AM the next day. On each hospital day, subjects were required to have their first smoking session (cigarette or water pipe) at 9 AM. This was to maintain the same day- night tobacco use schedule throughout. A 24-hour urine was collected daily, with a split urine collection on day 4 as described below.
Subjects were allowed to smoke cigarettes as desired between 9AM and 10 PM (CRC policy). Subjects were required to smoke the water pipe for a minimum of twice per day (9 AM and 1 PM), but otherwise could smoke water pipe ad libitum between 9AM and 6 PM. Evening water pipe smoking was not allowed because the kitchen, where the charcoal was lighted, closes at 6 PM. The following were recorded daily, depending on the study arm: CPD number and weight of cigarettes smoked or weight of water pipe tobacco smoked, times, duration and number of sessions. Each day the water in the pipe was replaced (825 ml), and at the end of the day a water sample was retained for nicotine analysis.
Subjects were intensively studied on the 4th hospital day of each hospital stay. A blood sample was collected and expired CO recorded prior to and 2 minutes after completing the first smoking session at 9 AM and again after another smoking session at 1 PM Additional blood and expired CO samples were collected at 7, 9, 11, 13 and 24 hours from the start of the first smoking session. To examine the time course of excretion of toxicants, urine was collected at intervals of 0-4, 4-8, 8-12 and 12-24 hrs.
The U.S. Federal Trade Commission method machine-determined yields of the usual cigarette brands averaged 1.07 mg (SD 0.37) nicotine, 13.0 mg (2.9) tar and 13.1 mg (1.0) carbon monoxide. The self-selected water pipe tobacco brands and flavors smoked during the water pipe arm of the study are: Nakhla Double Apple; Nakhla Strawberry; Nakhla Mango (2 subjects); Nakhla Apple (3 subjects); Nakhla Peach (3 subjects); Al-Waha Peach; and Al-Waha 2-Apple (2 subjects).
Laboratory Analysis
Biomarkers of exposure to several toxic substances were measured (Table 1). Analyses of biofluid samples were carried out using published methods methods [13] [14] [15] or are described in Supplementary Materials Section.
Table 1.
Urinary Excretion of Toxic Substance Biomarkers*
| Toxic Substance1 | Biomarker1 | Water pipe | Cigarette | p | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Study Day 3 | Study Day 4 | Average | Study Day 3 | Study Day 4 | Average | |||
|
| ||||||||
| NNK (TSNA) | NNAL (pmol/24h) | 226 (136 -373) | 210 (137 -319) | 220 (140 -349) | 387 (206 -726) | 446 (261 -767) | 424 (242 -742) | <0.01 |
| 328 (119 -447) | 226 (110 -336) | 247 (127 -374) | 707 (151 -858) | 836 (215 -1051) | 770 (176 -946) | |||
|
| ||||||||
| Naphthalene (PAH) | 2-Naph (pmol/24h) | 3844 (2649 -5574) | 3174 (2234 -4524) | 3556 (2523 -5043) | 5696 (3764 -8642) | 5968 (4140 -8646) | 5944 (4114 -8640) | <0.01 |
| 3383 (2270 -5653) | 3513 (2094 -5607) | 3354 (2100 -5453) | 8507 (3009 -11516) | 7320 (3543 -10863) | 8015 (3158 -11173) | |||
|
| ||||||||
| Fluorene (PAH) | 1-Fluor (pmol/24h) | 96 (52 -178) | 90 (52 -158) | 94 (53 -167) | 262 (162 -426) | 293 (191 -450) | 284 (185 -437) | <0.01 |
| 235 (39 -273) | 143 (44 -187) | 194 (41 -235) | 251 (187-439) | 360 (189 -549) | 327 (180 -507) | |||
|
| ||||||||
| Fluorene (PAH) | 2-Fluor (pmol/24h) | 65 (29 -146) | 135 (59 -309) | 118 (55 -253) | 347 (220 -545) | 364 (230 -580) | 360 (230 -564) | 0.02 |
| 195 (18 -212) | 370 (36 -406) | 366 (34 -400) | 463 (211 -674) | 523 (185 -708) | 513 (222 -735) | |||
|
| ||||||||
| Fluorene (PAH) | 3-Fluor (pmol/24h) | 54 (36 -82) | 49 (35 -68) | 52 (37 -75) | 177 (102 -305) | 196 (117 -329) | 192 (115 -317) | <0.01 |
| 65 (31 -96) | 40 (33 -72.6) | 45 (32 -77) | 249 (92 -341) | 292 (113 -404) | 292 (101 -393) | |||
|
| ||||||||
| Phenanthrene (PAH) | Sum of Phen (pmol/24h) | 361 (241 -537) | 335 (242 -462) | 351 (245 -503) | 261 (224 -304) | 316 (243 -411) | 296 (249 -353) | 0.26 |
| 331 (201 -533) | 300 (203 -503) | 326 (200 -526) | 89 (215 -304) | 136 (250 -387) | 104 (239 -342) | |||
|
| ||||||||
| Pyrene (PAH) | 1-HP (pmol/24h) | 117 (85 -160) | 109 (83 -144) | 115 (87 -150) | 74 (60 -91) | 85 (64 -113) | 81 (66 -101) | 0.01 |
| 127 (80 -206) | 109 (70 -179) | 108 (87 -194) | 40 (59 -99) | 52 (61 -113) | 48 (61 -109) | |||
|
| ||||||||
| Ethylene Oxide (VOC) | HEMA (μg/24h) | 3.47 (2.45-4.91) | 5.97 (3.64 – 9.8) | <0.01 | ||||
| 2.39 (2.48 – 4.88) | 8.58 (2.97 – 11.55) | |||||||
|
|
|
|
|
|||||
| Acrylonitrile (VOC) | CNEMA (μg/24h) | 14.3 (8.3 – 24.6) | 70.9 (45.4 – 110.9) | <0.01 | ||||
| 18.7 (8.8 – 27.4) | 90.1 (43 – 133.1) | |||||||
|
|
|
|
|
|||||
| Acrolein (VOC) | 3-HPMA (μg/24h) | 418.6 (327.2 – 535.7) | 601.6 (450.8 – 802.8) | 0.01 | ||||
| 152.6 (337.6 – 490.2) | 388.6 (425.3 -814) | |||||||
|
|
|
|
|
|||||
| Propylene Oxide (VOC) | 2-HPMA (μg/24h) | 59.4 (34.9 – 101) | 94.9 (55.4 – 162.7) | 0.04 | ||||
| 80.3 (28.7 -109) | 148.1 (50.2 – 198.2) | |||||||
|
|
|
|
|
|||||
| 1,3-Butadiene (VOC) | MHBMA (μg/24h) | 0.39 (0.3 – 0.52) | 1.3 (1.02 – 1.65) | <0.01 | ||||
| 0.28 (0.27 -0.55) | 0.76 (0.96 – 1.72) | |||||||
|
|
|
|
|
|||||
| Acrylamide (VOC) | AAMA (μg/24h) | 105.8 (74.3 – 150.5) | 132.7 (99.5 – 177) | 0.20 | ||||
| 44.1 (77.7 – 121.8) | 84.4 (96.8 – 181.2) | |||||||
|
|
|
|
|
|||||
| Benzene (VOC) | PMA (μg/24h) | 1.73 (0.76 – 3.93) | 0.695 (0.39 – 1.25) | 0.03 | ||||
| 5.67 (0.49 – 6.16) | 0.75 (0.35 – 1.09) | |||||||
All values are presented in this format: Geometric mean (95% confidence interval of geometric mean) on upper line and median (interquartile interval) on lower line.
Significant differences are in bold. Mercapturic acid metabolites of volatile organic chemicals were measured on day 4 only, so there are no day 3 or average data for these analytes.
Abbreviations: NNK = 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone; TSNA = Tobacco-Specific Nitrosamine; NNAL = 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol; PAH = Polycyclic Aromatic Hydrocarbon; 2-Naph = 2-Naphthol; 1-Fluor = 1-Hydroxyfluorene; 2-Fluor = 2-Hydroxyfluorene; 3-Fluor = 3-Hydroxyfluorene; Sum of Phen = Sum of 1-, 2-, 3-, and 4-Hydroxyphenanthrenes; 1-HP = 1-Hydroxypyrene; VOC = Volatile Organic Compound; HEMA = 2-Hydroxyethylmercapturic acid; CNEMA = 2-Cyanoethylmercapturic acid; 3-HPMA = 3-hydroxypropylmercapturic acid; 2-HPMA = 2-hydroxypropylmercapturic acid; MHBMA = 2-Hydroxy-3-buten-1-yl-mercapturic acid or isomer(s); AAMA = 2-Carbamoylethylmercapturic acid; PMA = Phenylmercapturic acid.
Statistical Analysis
Area under the plasma nicotine concentration-time curve (AUC) and expired CO AUC were the primary measures of daily nicotine and CO exposure, respectively. The 24-hour excretion of various smoke toxin metabolites was used as the measure of these toxicant exposures. Based on common practice, data are presented in “ng/ml” for plasma nicotine, “ppm” for expired CO, “pmol/24 hr” for NNAL and PAH metabolites, and in “μg/24 hr” for mercapturic acids.
Differences between water pipe and cigarette smoking were analyzed using paired t-tests. Since the data were not normally distributed, log transformation of the data was performed. NNAL and PAH urine values were averaged on study days 3 and 4. Mercapturic acid metabolite data were available only on day 4. Two-tailed tests with α = 0.05 were used. Data analysis was performed using IBM SPSS 18 for Windows, 2009.
Results
Biomarkers of exposure to several toxic substances were measured. These included nicotine, carbon monoxide, NNAL, a metabolite of the lung-selective carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), biomarkers for the polycyclic aromatic hydrocarbon class of carcinogens, and mercapturic acid metabolites of several toxic volatile organic compounds (VOCs) (Table 1).
On average, subjects smoked water pipe for 2.8 (SD 0.7; range 2 -4) sessions with a total of 45.8 (SD 9.7; range 28.5 – 60) minutes of smoking and smoked 11.4 (SD 6.3; range 3.5 – 21) cigarettes per day. The average nicotine concentration in the water after smoking water pipe was 4.5 μg/ml (SD 3.7). The average total nicotine remaining in the water per water pipe session was 1.22 mg (SD 0.76); the average nicotine remaining per gram tobacco burned was 0.21 mg (SD 0.10).
Average plasma nicotine and expired CO concentrations on study day 4 are shown in Figures 1A and 1B. Average plasma nicotine concentrations throughout day 4 were substantially lower during water pipe use compared with cigarette smoking even though the mean plasma nicotine boost for the two individual smoking sessions was not significantly different for water pipe (11.4 ng/ml) compared to cigarette smoking (9.2 ng/ml). The 24-hour AUC for plasma nicotine, an integrated measure of exposure, was significantly lower for water pipe [63.9 ng/ml * hr (SD 50)] compared to cigarette smoking [127.4 ng/ml*hr (SD 81)] (P < 0.01). The average CO boost after smoking water pipe was 86 ppm compared to 5.2 ppm after cigarette smoking (p <0.001). The mean 24-hour AUC for expired CO was 903 ppm × hr (SD 712) for water pipe and 335 ppm × hr (SD 442) for cigarette smoking (p < 0.05).
Fig 1.
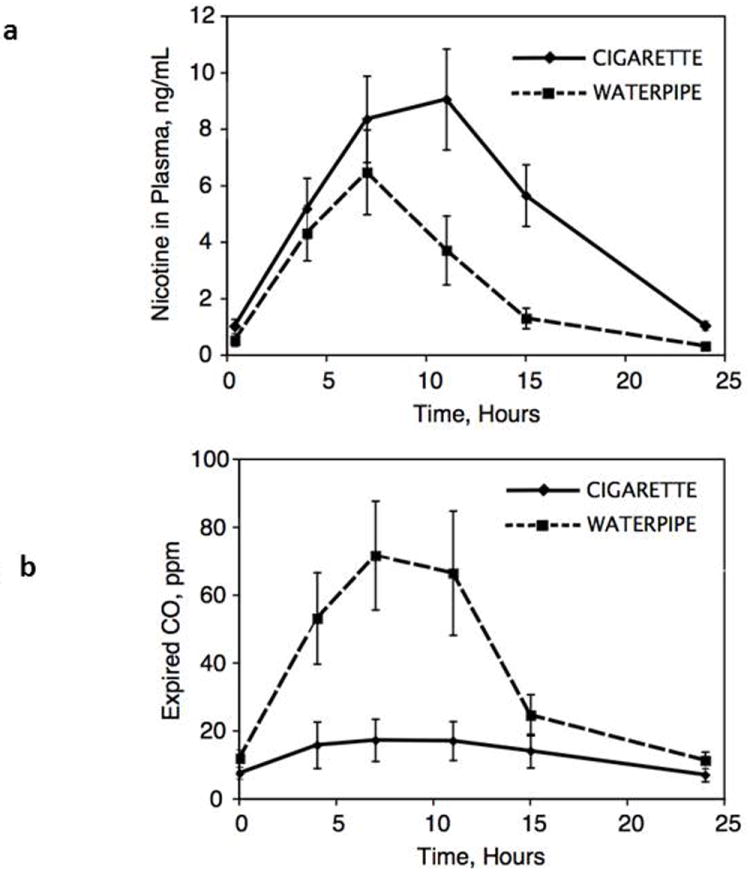
Mean plasma concentration of nicotine (Fig 1a) and expired carbon monoxide (Fig 1b) over 24 hours on day 4 of the treatment arms, comparing daily use of water pipe and cigarettes. Mean (SEM) of 13 subjects.
Urine NNAL levels were significantly lower during water pipe use compared with cigarette smoking (Figure 2A, Table 1). Relative excretion of different PAH metabolites varied according to type of tobacco. Average excretion of 2-naphthol and 1, 2 and 3-hydroxyfluorenes was significantly higher in cigarette smokers, whereas excretion of 1-hydroxypyrene was significantly higher with water pipe smoking (Table 1). The sum of hydroxyphenanthrene excretion was similar for both groups. The data are presented as a sum of metabolites, as phenanthrene is not very selective for tobacco smoke compared to environmental and dietary sources, and it was thought that this would give a better averaged measure of exposure and maximize the chance of seeing a difference between the tobacco types if one existed. In contrast, fluorene is relatively selective for tobacco smoke, and furthermore, we had previously found that the selectivity varies by metabolite in the order of 1-Fluor > 3-Fluor > 2-Fluor[16]. Circadian urine excretion data for 2-naphthol and 1-hydroxypyrene are shown in Figs 2B and 2C.
Fig 2.
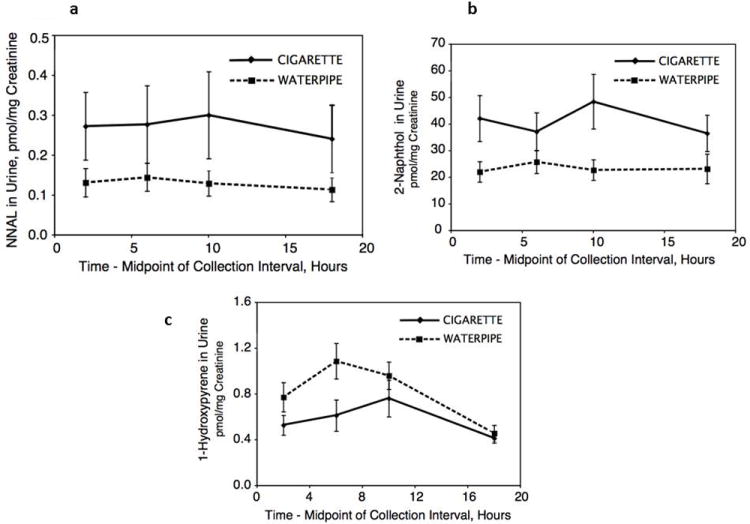
Geometric mean urine concentrations of total NNAL (Fig 2a), 2-naphthol (Fig 2b) and 1-hydroxpyrene (Fig 2c) over 24 hours on day 4 of the treatment arms, comparing daily use of water pipe and cigarettes. Geometric mean (95% CI of mean) of 13 subjects.
Relative urine excretion of different volatile organic compound (VOC) metabolites varied according to mode of smoking and type of tobacco (Table 1). Excretion of phenylmercapturic acid (metabolite of benzene) was significantly higher with water pipe use compared to cigarette smoking (Fig 3A). Excretion of 2-hydroxyethylmercapturic acid, 2-cyanoethylmercapturic acid, 3-hydroxypropylmercapturic acid, 2-hydroxypropylmercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid and isomer(s) (metabolites of ethylene or ethylene oxide, acrylonitrile, acrolein, propylene or propylene oxide and 1,3-butadiene, respectively) were significantly higher during cigarette smoking (1,3-butadiene metabolite data shown in Fig 3B). There was no significant difference in excretion of 2-carbamoylethylmercapturic acid (acrylamide metabolite)
Fig 3.
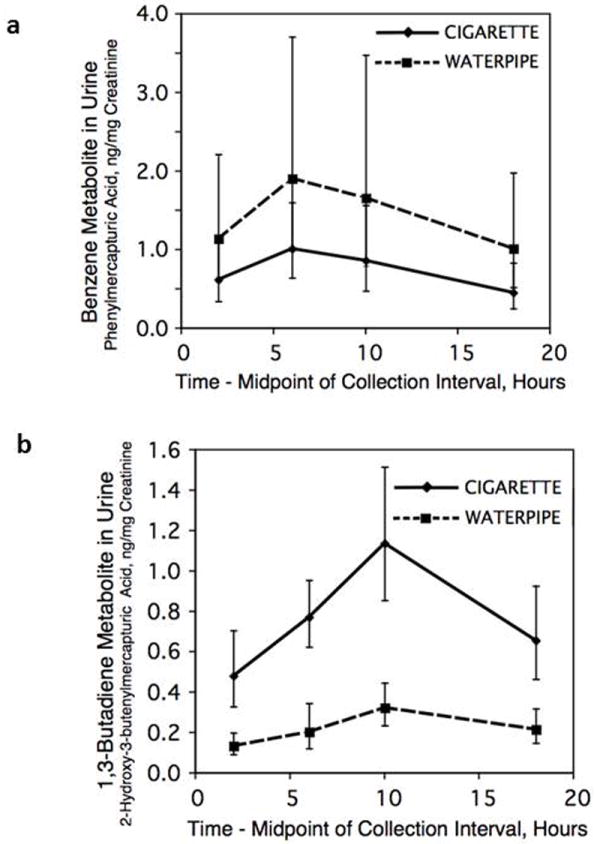
Geometric mean urine concentrations of phenylmercapturic acid (benzene metabolite, Fig 3a) and 2-hydroxy-3-butenylmercapturic acid (1,3-butadiene metabolite, Fig 3b) over 24 hours on day 4 of the treatment arms, comparing daily use of water pipe and cigarettes. Geometric mean (95% CI of mean) of 13 subjects.
A significant increase in heart rate was observed both after smoking cigarettes (11.2 bpm, p=0.011) and water pipe (11.6 bpm, p< 0.001). Systolic blood pressure increased after cigarette (9.7 mmHg, p=0.01) and water pipe smoking (8.0 mmHg, p=0.026); the changes were not significantly different comparing cigarettes vs water pipe.
Discussion
Since many people believe water pipe smoking is less harmful than cigarette smoking, and the chemistry of the two smoking processes is quite different, a study comparing the intake of toxic substances in people who customarily smoke both of these two products was warranted. To the best of our knowledge this is the first study to compare cigarette smoking to water pipe smoking using a crossover protocol. The study involved a steady state assessment of biomarkers of systemic exposure to tobacco smoke toxicants during ad libitum smoking (the exception being NNAL which has a 10 – 16 day half-life) [17] compared with ad libitum water pipe smoking. The pattern of toxicant exposure was distinctly different for water pipe smoking as compared to cigarette smoking. We made several novel and significant findings related to assessment of nicotine, carbon monoxide and three classes of carcinogens as follows.
Nicotine exposure and effects
Daily nicotine intake, estimated based on 24 hour AUC, was substantially higher while smoking cigarettes compared to water pipe. Nonetheless, the sustained levels of nicotine throughout most of the day with water pipe use are likely to cause physiologic changes in the brain that would sustain nicotine addiction [18]. Heart rate acceleration and an increase in systolic blood pressure are well-described pharmacologic effects of nicotine and were similar in our study after water pipe and cigarette smoking. Similar cardiovascular findings have been reported by Hakim et al[19].
Previously we reported that the 12.5 grams of water pipe tobacco placed in the pipe contained on average 32 mg nicotine and the average systemic intake of nicotine was 2.6 mg per water pipe session [11]. We found in the present study that only 1.2 mg nicotine on average was recovered in the water pipe water per session, representing about 4% of nicotine in 12.5 grams of water pipe tobacco. Given that nicotine is highly water soluble, the relatively low nicotine recovery in the water is likely explained by most nicotine being carried through the water in air bubbles, with little time for dissolution. This finding contrasts to beliefs of some water pipe smokers that the water removes harmful substances.
Tobacco specific nitrosamines (TSNA)
Although not at steady state, levels of the TSNA biomarker NNAL, reflecting systemic exposure to the lung carcinogen NNK, were much lower during water pipe smoking compared to cigarette smoking. Lower levels of urine NNAL have been previously reported in Egyptian water pipe compared to cigarette smokers [20]. This might be due to differences in the tobacco type or curing process used to manufacture the products or it might be due to reducing agents, such as ascorbic acid [21] in the fruit preparation inhibiting formation of TSNAs during curing or in storage.
Polycyclic aromatic hydrocarbons
Intake of naphthalene and fluorene was higher during cigarette smoking, but intake of phenanthrene and pyrene was higher during water pipe smoking. This trend suggests that there may be a continuum with higher molecular weight PAHs being more abundant in water pipe smoke than in cigarette smoke. Since higher molecular weight PAHs are generally the most carcinogenic (e.g. benzo[a]pyrene and benz[a]anthracene), this trend suggests cancer risk from PAHs might be higher in water pipe smokers than in cigarette smokers.
Volatile Organic Compounds (VOCs)
Exposure to benzene, a proven human carcinogen (leukemia and possibly lung cancer) [1] was considerably higher while smoking water pipe compared to cigarettes. This was surprising in light of the trend for PAHs of higher molecular weight being higher in water pipe smoke. It may be that the burning charcoal is a major source of benzene.[22] In contrast, intake of some other toxic VOCs – 1,3-butadiene, ethylene oxide, acrolein, acrylonitrile, and propylene oxide was higher during cigarette smoking. Both 1,3-butadiene and ethylene oxide are considered carcinogenic in humans (class 1), [1, 23]. Acrolein, an irritant and ciliotoxic chemical, is carcinogenic in animals and is thought to play a major role in tobacco-induced cardiovascular disease [24]. Acrylonitrile and propylene oxide are class 2B carcinogens [1]. Thus the profile of VOC exposure differs in water pipe and cigarettes smokers, which may have implications for different disease risks. The different pattern of VOC exposure is likely due to the different composition of the products and differences in the smoking process. The water pipe product is mostly a moist fruit preparation containing about 5-10% tobacco, and is not combusted, but rather heated to the point of charring by burning charcoal placed on top of it. Thus the temperature at which pyrolytic chemistry and aerosol formation occur is considerably lower in water pipe smoking (~450 °C) as compared to cigarette smoking (~900 °C) [8].
Carbon monoxide (CO)
As reported in previous studies [11, 25], CO intake was much higher while smoking water pipe, probably because burning charcoal is placed on top of the fruit-tobacco mixture in order to volatilize substances in the product and generate an inhalable aerosol. Carbon monoxide reduces the oxygen carrying and delivering capacity of the blood. High carbon monoxide levels are particularly hazardous in people with ischemic cardiovascular disease and chronic obstructive lung disease, where CO exposure reduces exercise capacity and increases the risk of potentially fatal cardiac arrhythmias [26, 27].
Limitations of our study warrant discussion
First, we studied dual users – that is, people who regularly smoke both cigarettes and water pipe – so that we could conduct a cross-over study. Our prior research suggested that dual users inhale water pipe more intensively and are exposed to higher levels of tobacco smoke toxicants compared to water pipe-only users [11]. Second, we studied subjects who smoked their products on a clinical research ward, by themselves. Much water pipe use is social and involves sharing of a water pipe with friends. For these reasons our estimates of exposure to tobacco smoke toxicants from water pipe are likely to be greater than that experienced by many social water pipe smokers. Third, the smoking patterns for both water pipe and cigarettes on the research ward were constrained by experimental design (first cigarette at 9 am) and by ward policy (no water pipe after 6 pm or cigarettes after 10 pm). Thus the exposures that we estimated may be less than would have occurred with ad libitum smoking in a natural environment.
In conclusion, when toxicant exposures in the same individuals were compared while smoking an average of 3 water pipe sessions versus smoking 11 cigarettes per day, differences in product composition and in the smoking processes resulted in different patterns of exposure to various tobacco toxicants. Water pipe use was associated with less nicotine intake than cigarette smoking, but with levels likely to be capable of sustaining addiction. There was a greater exposure to benzene and high molecular weight PAHs, but less exposure to 1,3-butadiene, acrolein, acrylonitrile, propylene oxide, ethylene oxide and low molecular weight PAHs. This might result in a different clinical cancer risk profile between cigarette and water pipe smoking. Epidemiological studies have reported associations between water pipe smoking and increased risks of lung cancer, respiratory illness, low birth weight and periodontal disease [28]. However these studies have limitations and reflect exposure to many different types of water pipe products. We are aware of no data on water pipe smoking and the risk of leukemia, which is of interest since benzene exposure is a risk factor in this disease. Carbon monoxide levels with regular water pipe use are extraordinarily high and could pose a risk to health in people with underlying cardiovascular or pulmonary disease. With regular daily use, water pipe smoking is not a safe alternative to cigarette smoking, nor is it likely to be an effective harm reduction strategy for cigarette smokers switching to water pipe.
Supplementary Material
Acknowledgments
The authors thank Cotys Winston and the staff of the Clinical Research Center at San Francisco General Hospital for assistance in conducting the clinical studies, Olivia Yturralde, Lita Ramos and Lawrence Chan for performing analytical chemistry, Faith Allen for data management and Marc Olmsted for editorial assistance.
Grant Support
Supported by the California Tobacco-Related Disease Research Program (15RT-0181) and the National Institutes of Health (DA012393). Clinical studies carried out at the General Clinical Research Center at San Francisco General Hospital Medical Center (NIH/NCRR UCSF CTSI UL1 RR 024131)
Footnotes
Dr. Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
References
- 1.Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 2.Maziak W, Ward KD, Soweid RAA, Eissenberg T. Tobacco smoking using a waterpipe: a re-emerging strain in a global epidemic. Tob Control. 2004;13:327–33. doi: 10.1136/tc.2004.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Primack BA, Shensa A, Kim KH, Carroll MV, Hoban MT, Leino EV, et al. Waterpipe Smoking Among U.S. University Students. Nicotine Tob Res. 2012;15:29–35. doi: 10.1093/ntr/nts076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eissenberg T, Ward KD, Smith-Simone S, Maziak W. Waterpipe tobacco smoking on a U.S. College campus: prevalence and correlates. Journal of Adolescent Health. 2008;42:526–9. doi: 10.1016/j.jadohealth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Primack BA, Sidani J, Agarwal AA, Shadel WG, Donny EC, Eissenberg TE. Prevalence of and associations with waterpipe tobacco smoking among U.S. university students. Annals of Behavioral Medicine. 2008;36:81–6. doi: 10.1007/s12160-008-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed B, Jacob P, 3rd, Allen F, Benowitz N. Attitudes and practices of hookah smokers in the San Francisco Bay Area. J Psychoactive Drugs. 2011;43:146–52. doi: 10.1080/02791072.2011.587707. [DOI] [PubMed] [Google Scholar]
- 7.Lipkus IM, Eissenberg T, Schwartz-Bloom RD, Prokhorov AV, Levy J. Affecting perceptions of harm and addiction among college waterpipe tobacco smokers. Nicotine Tob Res. 2011;13:599–610. doi: 10.1093/ntr/ntr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol. 2005;43:655–61. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Schubert J, Hahn J, Dettbarn G, Seidel A, Luch A, Schulz TG. Mainstream smoke of the waterpipe: Does this environmental matrix reveal as significant source of toxic compounds? Toxicol Lett. 2011;205:279–84. doi: 10.1016/j.toxlet.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Shafagoj YA, Mohammed FI, Hadidi KA. Hubble-bubble (water pipe) smoking: levels of nicotine and cotinine in plasma, saliva and urine. Int J Clin Pharm Th. 2002;40:249–55. doi: 10.5414/cpp40249. [DOI] [PubMed] [Google Scholar]
- 11.Jacob P, 3rd, Abu Raddaha AH, Dempsey D, Havel C, Peng M, Yu L, et al. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer Epidemiol Biomarkers Prev. 2011;20:2345–53. doi: 10.1158/1055-9965.EPI-11-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 13.Jacob P, Yu L, Wilson M, Benowitz NL. Selected Ion Monitoring Method for Determination of Nicotine, Cotinine and Deuterium-Labeled Analogs - Absence of an Isotope Effect in the Clearance of (S)-Nicotine-3’,3’-D2 in Humans. Biol Mass Spectrom. 1991;20:247–52. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 14.Jacob P, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per Milliliter Determination of the Tobacco-Specific Carcinogen Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem. 2008;80:8115–21. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob P, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–98. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 16.St Helen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P, 3rd, Benowitz NL. Exposure and kinetics of polycyclic aromatic hydrocarbons (PAHs) in cigarette smokers. Chem Res Toxicol. 2012;25:952–64. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goniewicz ML, Havel CM, Peng MW, Jacob P, Dempsey D, Yu L, et al. Elimination Kinetics of the Tobacco-Specific Biomarker and Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol. Cancer Epidem Biomar. 2009;18:3421–5. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakim F, Hellou E, Goldbart A, Katz R, Bentur Y, Bentur L. The acute effects of water-pipe smoking on the cardiorespiratory system. Chest. 2011;139:775–81. doi: 10.1378/chest.10-1833. [DOI] [PubMed] [Google Scholar]
- 20.Radwan G, Hecht SS, Carmella SG, Loffredo CA. Tobacco-Specific Nitrosamine Exposures in Smokers and Nonsmokers Exposed to Cigarette or Waterpipe Tobacco Smoke. Nicotine Tob Res. 2012;15:130–8. doi: 10.1093/ntr/nts099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porubin D, Hecht SS, Li ZZ, Gonta M, Stepanov I. Endogenous formation of N’-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J Agric Food Chem. 2007;55:7199–204. doi: 10.1021/jf0712191. [DOI] [PubMed] [Google Scholar]
- 22.Olsson M, Petersson G. Benzene emitted from glowing charcoal. Sci Total Environ. 2003;303:215–20. doi: 10.1016/S0048-9697(02)00403-5. [DOI] [PubMed] [Google Scholar]
- 23.Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of 1,3-butadiene, ethylene oxide, vinyl chloride, vinyl fluoride, and vinyl bromide. Lancet Oncol. 2007;8:679–80. doi: 10.1016/s1470-2045(07)70235-8. [DOI] [PubMed] [Google Scholar]
- 24.Bhatnagar A. Cardiovascular pathophysiology of environmental pollutants. Am J Physiol Heart Circ Physiol. 2004;286:H479–85. doi: 10.1152/ajpheart.00817.2003. [DOI] [PubMed] [Google Scholar]
- 25.Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37:518–23. doi: 10.1016/j.amepre.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, et al. Effects of carbon monoxide on myocardial ischemia. Environ Health Perspect. 1991;91:89–132. doi: 10.1289/ehp.919189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G, Rastelli G, Meschi T, Borghi L, Cervellin G. Pathophysiology, clinics, diagnosis and treatment of heart involvement in carbon monoxide poisoning. Clin Biochem. 2012;45:1278–85. doi: 10.1016/j.clinbiochem.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Warnakulasuriya S. Waterpipe smoking, oral cancer and other oral health effects. Evid Based Dent. 2011;12:44–5. doi: 10.1038/sj.ebd.6400790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


