Abstract
Intestinal microbiota metabolism of choline/phosphatidylcholine produces trimethylamine (TMA), which is further metabolized to a proatherogenic species, trimethylamine-N-oxide (TMAO). Herein we demonstrate that intestinal microbiota metabolism of dietary L-carnitine, a trimethylamine abundant in red meat, also produces TMAO and accelerates atherosclerosis. Omnivorous subjects are shown to produce significantly more TMAO than vegans/vegetarians following ingestion of L-carnitine through a microbiota-dependent mechanism. Specific bacterial taxa in human feces are shown to associate with both plasma TMAO and dietary status. Plasma L-carnitine levels in subjects undergoing cardiac evaluation (n = 2,595) predict increased risks for both prevalent cardiovascular disease (CVD) and incident major adverse cardiac events (MI, stroke or death), but only among subjects with concurrently high TMAO levels. Chronic dietary L-carnitine supplementation in mice significantly altered cecal microbial composition, markedly enhanced synthesis of TMA/TMAO, and increased atherosclerosis, but not following suppression of intestinal microbiota. Dietary supplementation of TMAO, or either carnitine or choline in mice with intact intestinal microbiota, significantly reduced reverse cholesterol transport in vivo. Intestinal microbiota may thus participate in the well-established link between increased red meat consumption and CVD risk.
INTRODUCTION
The high consumption of meat in the developed world is linked to cardiovascular disease (CVD) risk, presumably due to the large content of saturated fats and cholesterol found in meat1,2. However, recent meta-analysis of prospective cohort studies showed no association between dietary saturated fat intake and CVD, prompting the suggestion that other environmental exposures linked to increased meat consumption are responsible3. In fact, the suspicion that the cholesterol and saturated fat content of red meat may not be sufficiently high to account for observed risks has stimulated investigation of alternative disease-promoting exposures that accompany dietary meat ingestion, such as high salt content, or heterocyclic compounds generated during cooking4,5. To date no studies have explored the participation of our commensal intestinal microbiota in modifying the diet-host interaction during consumption of red meat.
Our microbiota has been linked to intestinal health, immune function, bioactivation of nutrients and vitamins, and more recently, complex disease phenotypes such as obesity and insulin resistance6-8. We recently reported a novel pathway in both humans and mice linking microbiota metabolism of dietary choline/phosphatidylcholine to CVD pathogenesis9. Choline, a trimethylamine and part of the head group of phosphatidylcholine, is metabolized by gut microbiota to produce an intermediate compound known as trimethylamine (TMA) (Fig. 1a). TMA is rapidly further oxidized by hepatic flavin monooxygenases to form trimethylamine-N-oxide (TMAO). TMAO was subsequently shown to be both proatherogenic and associated with cardiovascular risks.9 These findings raise the possibility that other dietary nutrients possessing a trimethylamine structure may also generate TMAO from gut microbiota and promote accelerated atherosclerosis. How TMAO is mechanistically linked to development of accelerated atherosclerosis and which specific microbial species contribute to TMAO formation remains unknown. L-carnitine is an abundant nutrient in red meat and contains a trimethylamine structure similar to choline (Fig. 1a). While dietary ingestion of L-carnitine is a major source of the compound in omnivores, the amino acid is also endogenously produced in mammals from lysine, and serves an essential function in transport of fatty acids into the mitochondrial compartment10,11. L-carnitine ingestion and supplementation in industrialized societies have significantly increased. Whether there is a potential health risk for recent pervasive and rapidly growing carnitine supplement practices has not been evaluated.
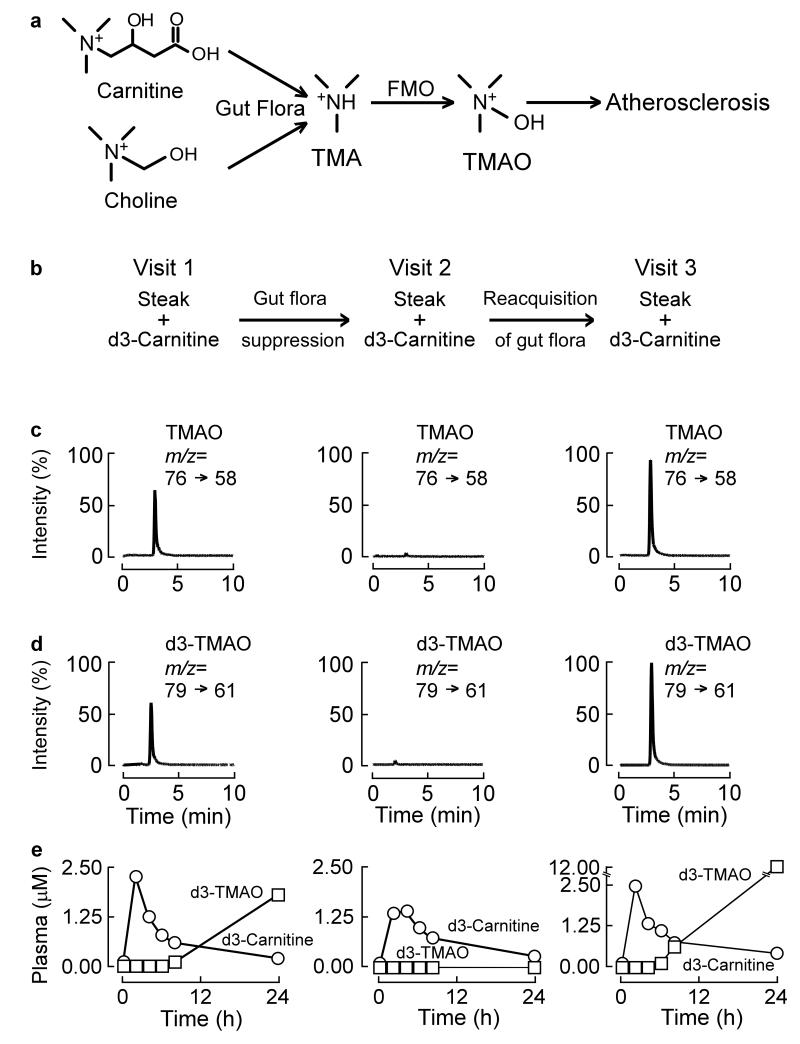
Figure 1. TMAO production from carnitine is a microbiota dependent process in humans.
(a) Structure of carnitine and scheme of carnitine and choline metabolism to TMAO. L-Carnitine and choline (are both dietary trimethylamines that can be metabolized by microbiota to TMA. TMA is then further oxidized to TMAO by flavin monooxygenases (FMOs). (b) Scheme of human carnitine challenge test. After a 12 hour overnight fast, subjects received a capsule of d3-carnitine (250 mg) alone, or in some cases (as in data for subject shown) also an 8 ounce steak (estimated 180 mg L-carnitine), whereupon serial plasma and 24h urine collection was obtained for TMA and TMAO analyses. After a weeklong regimen of oral broad spectrum antibiotics to suppress the intestinal microbiota, the challenge was repeated (Visit 2), and then again a final third time after a ≥ three week period to permit repopulation of intestinal microbiota (Visit 3). Data shown in (panels c-e) are from a representative female omnivorous subject who underwent carnitine challenge. Data is organized to vertically correspond with the indicated visit schedule above (Visit 1, 2 or 3). (c,d) LC/MS/MS chromatograms of plasma TMAO or d3-TMAO in omnivorous subject using specific precursor → product ion transitions indicated at T = 8 hour time point for each respective visit. (e) Stable isotope dilution LC/MS/MS time course measurements stable isotope (d3) labeled TMAO and carnitine, in plasma collected from sequential venous blood draws at noted times.
Herein we examine the gut microbiota-dependent metabolism of L-carnitine to produce TMAO in both rodents and humans (omnivores and vegans). Using isotope tracer studies, clinical studies, and animal models employing germ-free mice, we demonstrate a role for gut microbiota metabolism of L-carnitine in atherosclerosis pathogenesis. In addition to the upregulation of macrophage scavenger receptors potentially contributing to enhanced “forward cholesterol transport”10, we show that TMAO, and its dietary precursors choline and carnitine, suppress reverse cholesterol transport through gut microbiota-dependent mechanisms in vivo. Finally, we define microbial taxa in humans associated with both TMAO production and dietary carnitine ingestion. We also show in mice microbial compositional changes associated with chronic carnitine ingestion, and consequent marked enhancement in TMAO synthetic capacity in vivo.
RESULTS
Metabolomic studies link L-carnitine with CVD
Given the similarity in structure between L-carnitine and choline (Fig. 1a) we hypothesized that dietary L-carnitine in humans, like choline and phosphatidylcholine, might both produce TMA and TMAO in a gut microbiota-dependent fashion and be associated with atherosclerosis risk. To test this we initially examined data from our recently published unbiased small molecule metabolomics analyses of plasma analytes and CVD risks9.
An analyte with identical molecular weight and retention time to L-carnitine was not in the top tier of analytes that met the stringent P value cutoff for association with CVD. However, a hypothesis-driven examination of the data using less stringent criteria (no adjustment for multiple testing) revealed an analyte with appropriate molecular weight and retention time that was associated with cardiovascular event risk (P = 0.04)(Supplementary Table 1). In further studies we were able to confirm the identity of the plasma analyte as L-carnitine and develop a quantitative stable isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) method for measuring endogenous L-carnitine in all subsequent investigations (Supplementary Figs. 1, 2, 3).
Human gut microbiota is required to form TMAO from L-carnitine
The participation of gut microbiota in TMAO production from dietary L-carnitine in humans has not yet been shown. In initial subjects (omnivores), an “L-carnitine challenge test” was developed that incorporated a major source of dietary L-carnitine (8 ounce sirloin steak, corresponding to an estimated 180 mg L-carnitine)12,13,14 and a capsule containing 250 mg of a heavy isotope labeled L-carnitine (synthetic d3-(methyl)-L-carnitine). At baseline (Visit 1), post-prandial increases in d3-TMAO and d3-L-carnitine in plasma were readily detected, and 24 hour urine collections also revealed d3-TMAO (Fig. 1b-e; Supplementary Fig. 4, 5). Data shown in Fig. 1 and Supplementary Fig. 4 are tracings from a representative omnivorous subject, of n=5 studied with complete serial blood draws post carnitine challenge. In most subjects examined, despite clear increases in plasma d3-carnitine and d3-TMAO over time (Fig. 1e), post-prandial changes in endogenous (non-labeled) carnitine and TMAO were modest (Supplementary Fig. 5), consistent with a total body pool of carnitine and TMAO that are relatively vast in relation to the amount of carnitine ingested and TMAO produced from the carnitine challenge.
To examine the potential contribution of gut microbiota to TMAO formation from dietary L-carnitine, volunteers were placed on oral poorly absorbed broad spectrum antibiotics to suppress intestinal microbiota for a week, and then repeat L-carnitine challenge performed (Visit 2). Remarkably, near complete suppression of endogenous TMAO in both plasma and urine were noted after a weeklong treatment of the antibiotics (Visit 2) (Fig. 1b-e; Supplementary Fig. 5). Moreover, virtually no detectable formation of either native or d3-labeled TMAO was observed in post-prandial plasma or 24 hour urine samples with carnitine challenge, consistent with an obligatory role for gut microbiota in TMAO formation from L-carnitine (Fig. 1b-e; Supplementary Fig. 4). In contrast, both d3-L-carnitine and unlabeled L-carnitine were readily detected following carnitine challenge, and showed little change in the overall time course before (Visit 1) versus after antibiotic treatment (Visit 2; Fig. 1e, Supplementary Fig. 5). After discontinuation of antibiotics, subjects were re-challenged several weeks later. Baseline and post L-carnitine challenge plasma and urine samples again showed TMAO and d3-TMAO formation, consistent with intestinal re-colonization (Fig. 1b-e; Supplementary Fig. 4,5). Collectively, these data show that TMAO production from dietary L-carnitine in humans is dependent on intestinal microbiota.
Vegans and vegetarians produce less TMAO from L-carnitine
The capacity to produce TMAO (native and d3-labeled) following L-carnitine ingestion was variable among individuals. A post-hoc nutritional survey performed amongst the volunteers suggested antecedent dietary habits (red meat consumption) may influence the capacity to generate TMAO from L-carnitine. To test this prospectively, we examined TMAO and d3-TMAO production following the same L-carnitine challenge, first in a long term (>5 years) vegan who consented to the carnitine challenge (including both steak and d3-(methyl)-carnitine consumption). Figure 2a illustrates results from carnitine challenge in this vegan volunteer. Also shown for comparison are data from a single representative omnivore with reported common (near daily) dietary consumption of red meat. Post-prandially the omnivore showed both an increase in TMAO and d3-TMAO levels in sequential plasma measurements (Fig. 2a), and in a 24 hour urine collection sample (Fig. 2b). In contrast, the vegan showed nominal plasma and urine TMAO levels at baseline, and virtually no capacity to generate TMAO or d3-TMAO in plasma after the carnitine challenge (Fig. 2a,b). The vegan subject also had lower fasting plasma levels of L-carnitine compared to the omnivorous subject (Supplementary Fig. 6).
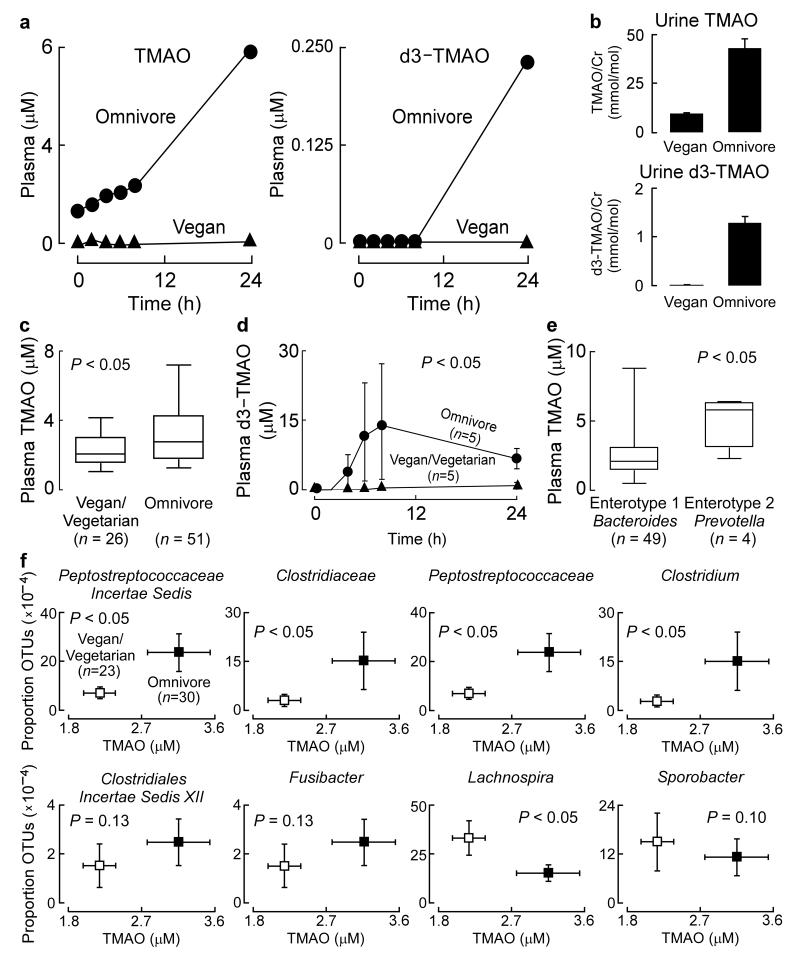
Figure 2. The formation of TMAO from ingested L-carnitine is negligible in vegans, and fecal microbiota composition associates with plasma TMAO concentrations.
(a-b) Data from a male vegan subject in the carnitine challenge consisting of co-administration of both 250 mg d3-carnitine and an 8 ounce sirloin steak, and for comparison, a representative female omnivore with frequent red meat consumption. (a) Plasma TMAO and d3-TMAO were quantified post carnitine challenge, and in a (b) 24 hour urine collection. (c) Baseline fasting plasma concentrations of from male and female (n = 26) vegans and vegetarians and (n = 51) omnivores. Boxes represent the 25th, 50th, and 75th percentile and whiskers represent the 5th and 95th percentile. (d) Plasma d3-TMAO levels in male and female (n = 5) vegan and vegetarian versus (n = 5) omnivores participating in a d3-carnitine (250 mg) challenge without concomitant steak consumption. P value shown is for comparison between area under the curve (AUC) of groups using Wilcoxon non-parametric test. (e) Baseline plasma concentrations of TMAO associates with Enterotype 2 (Prevotella) in male and female subjects with a characterized gut microbiome enterotype. (f) Plasma TMAO concentrations (plotted on x axes) and the proportion of taxonomic operational units (OTUs, plotted on Y axes) were determined as described in Supplementary Methods. Subjects were grouped by dietary status as either vegan and vegetarian (n = 23) or omnivore (n = 30). P value shown is for comparisons between dietary groups using a robust Hotelling T2 test.
To confirm and extend these findings we examined additional vegans/vegetarians (n=23) and omnivorous subjects (n=51). Fasting baseline TMAO levels were significantly lower among vegan/vegetarian subjects compared to omnivores (Fig. 2c). In a subset of these individuals an oral d3(methyl)-carnitine challenge (but with no steak) was performed, and confirmed that long term (all > 1 year) vegan/vegetarians have markedly reduced synthetic capacity to produce TMAO from oral carnitine (Fig. 2c,d). Interestingly, vegan/vegetarians challenged with d3-carnitine also had significantly more post-challenge plasma d3-carnitine compared to omnivorous subjects (Supplementary Fig. 7), a result that may reflect decreased intestinal microbial metabolism of carnitine prior to absorption.
TMAO levels associate with human gut microbial taxa
Dietary habits (e.g. vegan/vegetarian versus omnivore/carnivore) are associated with significant alterations in intestinal microbiota composition15-17. We therefore analyzed fecal samples from both vegans/vegetarians (n=23) and omnivores (n=30) for the gene encoding for bacterial 16S ribosomal RNA. In parallel, plasma TMAO, carnitine and choline levels were quantified by stable isotope dilution LC/MS/MS. Global analysis of taxa proportions (Supplemental Methods) revealed significant associations with plasma TMAO levels (P=0.03), but not plasma carnitine (P= 0.77) or choline (P =0.74) levels.
Several bacterial taxa remained significantly associated with plasma TMAO levels after false discovery rate (FDR) adjustment for multiple comparisons (Supplementary Fig. 8). When subjects were classified into previously reported enterotypes18 based upon fecal microbial composition, individuals with an enterotype characterized by enriched proportions of the genus Prevotella (n=4) demonstrated higher (p<0.05) plasma TMAO levels than subjects with an enterotype notable for enrichment of Bacteroides (n=49) genus (Fig. 2e). Examination of the proportion of specific bacterial genera and subject TMAO levels revealed several taxa (genus level) that simultaneously were significantly associated with both vegan/vegetarian versus omnivore status, and plasma TMAO levels (Fig. 2f).
TMAO production from dietary L-carnitine is inducible
Analyses of data from vegan/vegetarians versus omnivores suggested that preceding dietary habits may modulate both intestinal microbiota composition and synthetic capacity to produce TMA/TMAO from dietary L-carnitine. We therefore next investigated the ability of chronic dietary L-carnitine to induce gut microbiota-dependent production of TMA and TMAO in the murine model. Initial LC/MS/MS studies in germ-free mice showed no detectable plasma d3-(methyl)TMA or d3-(methyl)TMAO following oral (gastric gavage) d3-(methyl)carnitine challenge, but acquisition of capacity to produce both d3-(methyl)TMA and d3-(methyl)TMAO following oral d3-(methyl)carnitine after a several week period in conventional cages to allow for microbial colonization (i.e. “conventionalization”) (Supplementary Fig. 9). Parallel studies with conventional C57BL/6J, Apoe−/− mice that were placed on a cocktail of oral relatively non-absorbable broad spectrum antibiotics previously shown to suppress intestinal microbiota9,19 showed similar results (i.e., complete suppression of both TMA and TMAO formation; Supplementary Fig. 10). Collectively, these studies confirm in mice an obligatory role for gut microbiota in TMA/TMAO production from dietary L-carnitine.
To examine the impact of dietary L-carnitine on inducibility of TMA/TMAO production from intestinal microbiota, we compared the pre- and post-prandial plasma profile of C57BL/6J, Apoe−/− mice on normal chow diet versus a diet supplemented in L-carnitine for 15 weeks. The production of both d3-(methyl)TMA and d3-(methyl)TMAO following gastric gavage of d3-(methyl)carnitine was induced by approximately ten-fold in mice on the L-carnitine supplemented diet (Fig. 3a). Further, plasma post-prandial d3-(methyl)carnitine levels in mice in the carnitine supplemented diet arm were substantially lower than that observed in mice on the carnitine free diet (normal chow), consistent with enhanced microbiota-dependent catabolism prior to absorption in the carnitine supplemented mice.
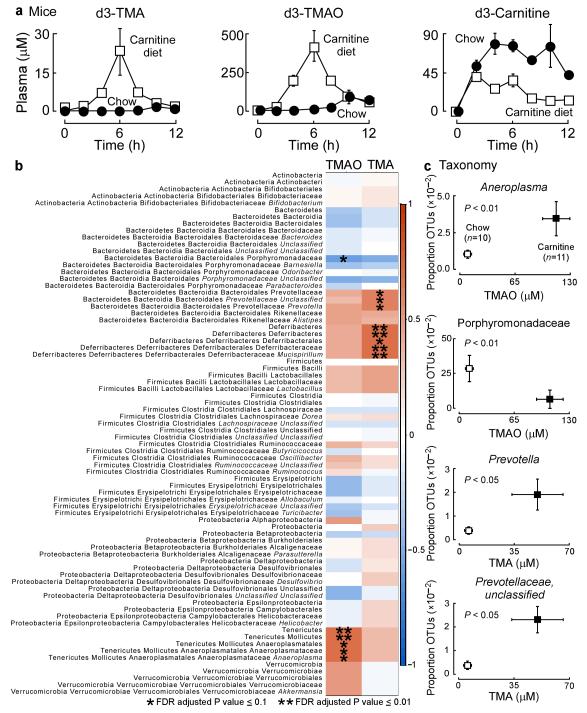
Figure 3. The metabolism of carnitine to TMAO is an inducible trait and associates with microbiota composition.
(a) d3-Carnitine challenge of mice on either a carnitine supplemented diet (1.3%) at 10 weeks and age versus age-matched normal chow controls. Plasma d3-TMA and d3-TMAO were measured at the indicated times following d3-carnitine administration by oral gavage using stable isotope dilution LC/MS/MS. Data points represents mean ± SE of 4 replicates per group. (b) Correlation heat map demonstrating the association between the indicated microbiota taxonomic genera and TMA and TMAO levels (all reported as mean ±SEM in μM) of mice grouped by dietary status (chow, n = 10 (TMA,1.3±0.4; TMAO, 17±1.9); and carnitine, n = 11 (TMA, 50±16; TMAO, 114±16). Red denotes a positive association, blue a negative association, and white no association. A single asterisk indicates a significant false discovery rate adjusted (FDR) association of P ≤ 0.1 and a double asterisk indicates a significant FDR adjusted association of P ≤ 0.01. (c) Plasma TMAO and TMA concentrations were determined by stable isotope dilution LC/MS/MS (plotted on x axes) and the proportion of taxonomic operational units (OTUs, plotted on Y axes) were determined. Statistical and laboratory analyses were performed as described in Supplementary Methods.
Plasma TMA/TMAO associate with mouse gut microbial taxa
The marked effect of dietary carnitine on enhanced TMA and TMAO production from a carnitine challenge (d3-(methyl)carnitine by gavage) suggested that chronic carnitine supplementation may significantly alter intestinal microbial composition with enrichment of taxa better suited for TMA production from carnitine. To test this we first identified the cecum as the segment of the entire intestinal tract of mice showing the highest synthetic capacity to form TMA from carnitine (data not shown). We then sequenced 16S rRNA gene amplicons from cecum of mice on either normal chow (n=10) or carnitine supplemented diet (n=11), and in parallel quantified plasma levels of TMA and TMAO (Fig. 3b). Global analyses of individual taxa proportions reveals that in general, microbial genera that show increased proportions coincident with increased plasma levels of TMA also tend to show increased proportions coincident with plasma TMAO levels. Several bacterial taxa remained significantly associated with plasma TMA and/or TMAO levels after false discovery rate (FDR) adjustment for multiple comparisons (Fig. 3b).
Further analyses examining the proportion of specific bacterial genera and mouse plasma TMA and TMAO levels revealed several taxa that significantly segregate with both mouse dietary groups and are associated with plasma TMA or TMA levels (P < 0.05) (Fig. 3c; Supplementary Fig. 11). Interestingly, a direct comparison of genera identified in humans versus mice that associated with plasma TMAO levels failed to identify common genera. These results are consistent with prior reports that microbes identified from the distal gut of the mouse represent genera that are typically not detected in humans15,20.
Plasma levels of L-carnitine are associated with CVD
We next investigated the relationship of fasting plasma levels of L-carnitine with CVD risks in an independent large cohort of stable subjects (n = 2,595) undergoing elective cardiac evaluation. Patient demographics, laboratory values, and clinical characteristics are provided in Supplementary Table 2. A significant dose – dependent association between L-carnitine levels and risk of prevalent coronary artery disease (CAD), peripheral artery disease (PAD), and overall CVD was noted (P < 0.05) (Fig. 4a-c). Moreover, these associations remained significant following adjustments for traditional CVD risk factors (P < 0.05) (Fig. 4a-c). In further analyses, plasma levels of L-carnitine were observed to be increased in subjects with significant (≥ 50% stenosis) angiographic evidence of CAD, regardless of the extent (e.g. single versus multi-vessel) of CAD, as revealed by diagnostic cardiac catheterization (P < 0.001) (Fig. 4d).
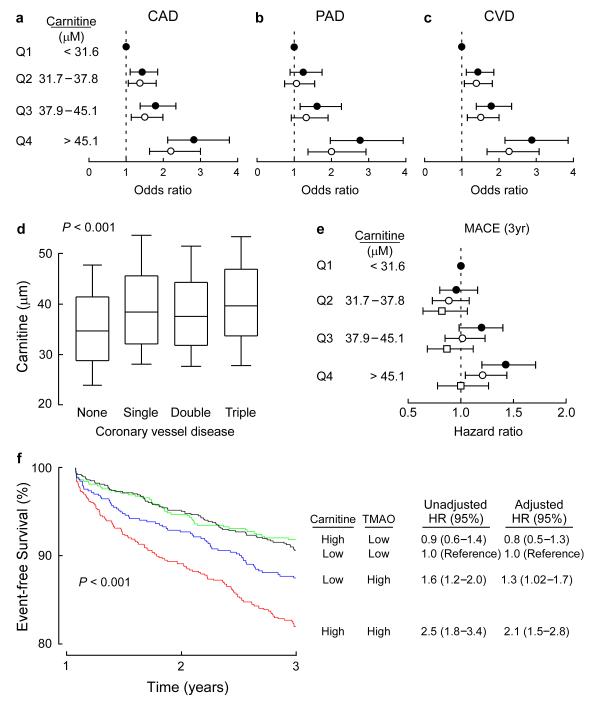
Figure 4. Relation between plasma carnitine and CVD risks.
(a-c) Forrest plots of odds ratio of CAD, PAD, and CVD and quartiles of carnitine before (closed circles) and after (open circles) logistic regression adjustments with traditional cardiovascular risk factors including age, sex, history of diabetes mellitus, smoking, systolic blood pressure, low density lipoprotein cholesterol, and high density lipoprotein cholesterol. Bars represent 95% confidence intervals. (d) Relationship of fasting plasma carnitine levels and angiographic evidence of CAD. Boxes represent the 25th, 50th, and 75th percentile of plasma carnitine and the whiskers represent the 10th and 90th percentile. The Kruskal-Wallis test was used to assess the degree of coronary vessel disease on L-carnitine levels. (e) Forrest plot of hazard ratio of MACE (death, non fatal-MI, stroke, and revascularization) and quartiles of carnitine unadjusted (closed circles), and after adjusting for traditional cardiovascular risk factors (open circles), or traditional cardiac risk factors plus creatinine clearance, history of MI, history of CAD, burden of CAD (one, two, or three vessel disease), left ventricular ejection fraction, baseline medications (ACE inhibitors, statins, β-blockers, and aspirin) and TMAO levels (open squares). Bars represent 95% confidence intervals. (f) Kaplan Meier plot (graph) and hazard ratios with 95% confidence intervals for unadjusted model, or following adjustments for traditional risk factors as in panel e. Median levels of carnitine (46.8 μM) and TMAO (4.6 μM) within the cohort were used to stratify subjects as ‘high’ (≥ median) or ‘low’ (< median) concentrations.
The relationship between fasting plasma levels of carnitine and incident (3 year) risk for major adverse cardiac events (MACE = composite of death, MI, stroke, and revascularization) was also examined. Elevated carnitine (4th quartile) levels were an independent predictor of MACE, including following adjustments for traditional CVD risk factors (Fig. 4e). After further adjustment for both TMAO and a larger number of comorbidities that might be known at time of presentation (e.g. extent of CAD, ejection fraction, medications, and estimated renal function), the significant relationship between carnitine and MACE risk was completely attenuated (Model 2) (Fig. 4e). Notably, a significant association between carnitine and incident cardiovascular event risks was observed in Cox regression models after multivariate adjustment, but only among those subjects with concurrent high plasma TMAO levels (P < 0.001) (Fig. 4f). Thus, while plasma levels of carnitine appear to be associated with prevalent and incident cardiovascular risks, the present results are consistent with TMAO, and not the dietary precursor carnitine, serving as the primary driver of the association with cardiovascular risks.
Dietary L-carnitine promotes microbiota dependent atherosclerosis
We therefore next sought to investigate whether dietary L-carnitine had any impact on the extent of atherosclerosis in the presence vs. absence of TMAO formation in animal models. C57BL/6J, Apoe−/− mice were fed normal chow diet versus the same diet supplemented with L-carnitine from time of weaning. Aortic root atherosclerotic plaque quantification revealed approximately a doubling in disease burden compared to normal chow fed animals (Fig. 5a, b). Importantly, parallel studies in mice placed on oral antibiotic cocktail to suppress intestinal microbiota showed marked reductions in plasma TMA and TMAO levels (Fig. 5c), and complete inhibition in dietary L-carnitine-dependent increase in atherosclerosis (Fig. 5b). Of note, the increase in atherosclerotic plaque burden with dietary L-carnitine occurs in the absence of pro-atherogenic changes in plasma lipids, lipoproteins, glucose, or insulin levels; moreover, both biochemical and histological analyses of livers in the mice failed to demonstrate steatosis (Supplementary Tables 3, 4; Supplementary Fig. 12).
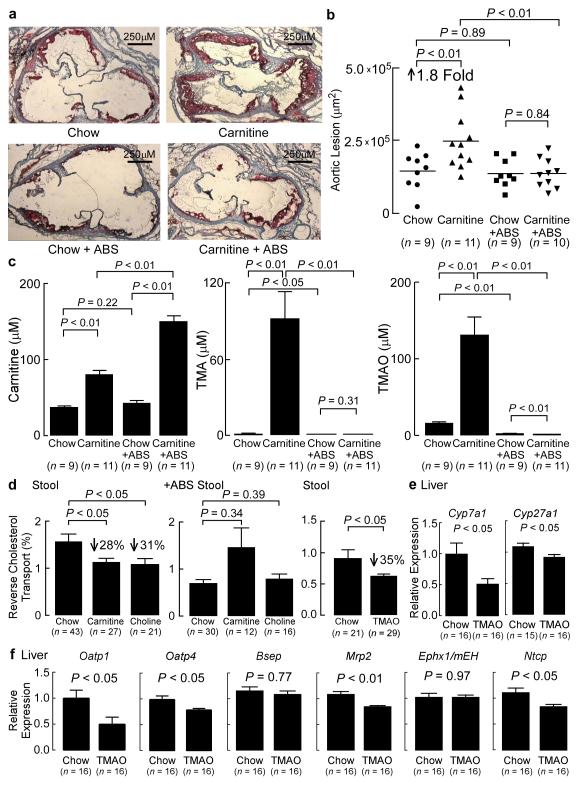
Figure 5. Dietary carnitine accelerates atherosclerosis and inhibits reverse cholesterol transport in a microbiota dependent fashion.
(a) Representative Oil-red-O stained (counterstained with hematoxylin) aortic roots of 19 week old C57BL/6J, Apoe−/− female mice on the indicated diets in the presence versus absence of antibiotics (ABS) as described under Methods. (b) Quantification of mouse aortic root plaque lesion area. 19 week-old C57BL/6J, Apoe−/− female mice were started on the indicated diets at the time of weaning (4 weeks of age) before sacrifice, and lesion area was quantified as described under Methods. (c) Carnitine, TMA, and TMAO were determined using stable isotope dilution LC/MS/MS analysis of plasma recovered from mice at time of sacrifice. (d) Reverse cholesterol transport (RCT) (72 hour stool collection) in adult female (> 8 weeks of age) C57BL/6J, Apoe−/− mice on normal chow versus diet supplemented with either carnitine or choline, as well as following suppression of microbiota using cocktail of antibiotics (+ ABS). Also shown are RCT (72 hour stool collection) results in adult female (> 8 weeks of age) C57BL/6J, Apoe−/− mice on normal chow versus diet supplemented with TMAO. (e,f) Relative mRNA levels (to β-actin) of mouse liver candidate genes involved in bile acid synthesis or transport. Ephx1, epoxide hydrolase 1, microsomal.
Quantification of plasma levels of L-carnitine in the mice revealed a significant increase in the L-carnitine fed animals versus the normal chow fed controls (P < 0.05) (Fig. 5c). Interestingly, an even higher increase in plasma L-carnitine levels were noted in mice supplemented with L-carnitine on the antibiotic arm of the study (presumably as a result of the reduced capacity of the microbiota to catabolize carnitine), which failed to show enhanced atherosclerosis. These results are consistent with not L-carnitine itself, but a down-stream microbiota-dependent metabolite that promotes the observed increased atherosclerosis burden (Fig. 5c).
TMAO inhibits reverse cholesterol transport
In an effort to identify additional mechanisms by which TMAO may promote atherosclerosis, we first noted that TMAO and its trimethylamine nutrient precursors are all cationic quaternary amines, and may have the potential to compete with arginine to limit its bioavailability and thus reduce nitric oxide synthesis. However, direct testing of this hypothesis in bovine aortic endothelial cells through competition studies using [14C]arginine and TMAO demonstrated no decrease in [14C]arginine transport (Supplementary Fig 13). In recent studies we showed that TMAO can promote macrophage cholesterol accumulation in a microbiota dependent manner by increasing cell surface expression of two proatherogenic scavenger receptors, cluster of differentiation 36 (CD36) and scavenger receptor A (SRA)9,21,22. Taking a “black box” approach, one can in general envision three non-exclusive mechanisms through which cholesterol can accumulate within cells of the artery wall: (i) enhanced rate of flux in (as noted above); (ii) enhanced synthesis; or (iii) diminished rate of flux out. To test whether TMAO might alter the canonical regulation of cholesterol biosynthesis genes23, macrophages were loaded with cholesterol in the presence vs. absence of physiologically relevant TMAO levels. However, TMAO failed to alter mRNA levels of the LDL receptor or cholesterol synthesis genes (Supplementary Fig. 14). Parallel studies examining desmosterol levels and macrophage inflammatory gene expression24, also failed to show any effect of TMAO within tissue culture media (Supplemental Figs. 14, 15).
Turning our attention next to potential mechanisms of cholesterol removal from peripheral macrophages, we sought to test the hypothesis that dietary sources of TMAO inhibit reverse cholesterol transport (RCT) in vivo using an adaptation of the model system first described by Rader and colleagues25. Remarkably, mice on either the choline or carnitine supplemented diets showed a significant (~30%, P < 0.05) decrease in RCT compared to normal chow controls (Fig. 5d, left panel). Furthermore, suppression of intestinal microbiota (and plasma TMAO levels) with oral broad spectrum antibiotics completely blocked the diet-dependent (for both choline and carnitine) suppression of RCT (Fig. 5d, middle panel), suggesting that a microbiota-generated product (e.g. TMAO) inhibits RCT (Supplementary Fig. 16). To test this hypothesis, mice were placed on a TMAO containing diet. They showed a significant 35% decrease in RCT relative to normal chow (P < 0.05) (Fig. 5d, right panel). Further examination of plasma, liver and bile compartments in the normal chow versus TMAO supplemented mice demonstrated significant reductions in [14C]cholesterol recovered within plasma of the TMAO fed mice (16%, P < 0.05), but no changes in counts recovered within the liver or bile (Supplementary Fig. 17).
TMAO alters sterol metabolism in in vivo compartments
To better understand potential molecular mechanism(s) through which TMAO reduces RCT, we examined candidate genes and biological processes in multiple compartments (i.e. macrophage, plasma, liver, intestine) known to participate in cholesterol and sterol metabolism and RCT. Mouse peritoneal macrophages recovered from C57BL/6J mice were exposed to TMAO in vitro and mRNA levels of cholesterol transporters ATP-binding cassette, sub-family A (ABC1), member 1 (Abca1), scavenger receptor class B, member 1(Srb1) and ATP-binding cassette, sub-family G, member 1 (Abcg1) were examined. Modest but statistically significant increases in expression of Abca1 and Abcg1, and corresponding cholesterol efflux were noted, indicating changes (reduction) in these transporters does not account for the observed global reductions in RCT induced by TMAO (P < 0.05) (Supplementary Fig. 18,19). Parallel examination of plasma recovered from mice involved in the RCT experiments showed no differences in total cholesterol and HDL cholesterol concentrations (Supplementary Table 5).
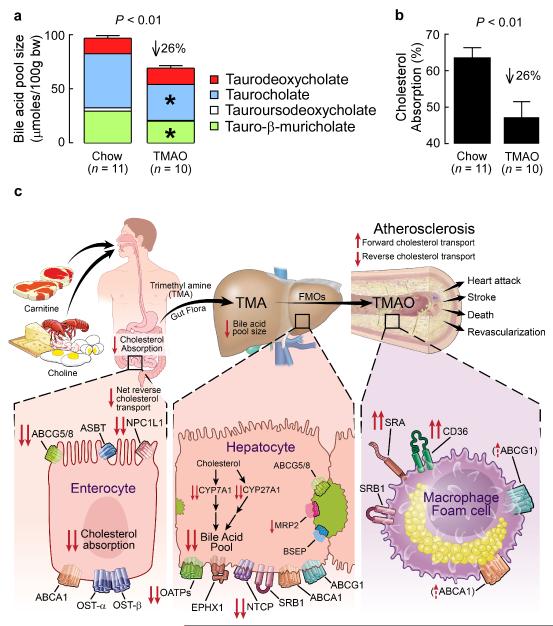
In parallel studies, we examined the expression levels of known cholesterol transporters (i.e. Srb1, Abca1, Abcg1, ATP-binding cassette, sub-family G, member 5 (Abcg5), ATP-binding cassette, sub-family G, member 8 (Abcg8), and nuclear receptor subfamily 0, group B, member 2 (Shp)) within mouse liver between the normal chow vs. TMAO dietary groups. No differences were noted (Supplementary Fig. 20, 21). In contrast, liver expression of the key bile acid synthetic enzymes cytochrome P450, family 7, subfamily a, polypeptide 1 (Cyp7a1) and cytochrome P450, family 27, subfamily a, polypeptide 1 (Cyp27a1) showed significant reductions in mice supplemented with dietary TMAO, but no change in expression of the up-stream regulator, Shp (P < 0.05 each; Fig. 5e Supplementary Fig. 20). Bile acid transporters in the liver (e.g. solute carrier organic anion transporter family, member 1a1 (Oatp1), solute carrier organic anion transporter family, member 1b2 (Oatp4), ATP-binding cassette, sub-family C (CFTR/MRP), member 2 (Mrp2), and solute carrier family 10 (sodium and bile acid cotransporter family), member 1 (Ntcp)) also showed significant dietary TMAO-induced decreases in expression (P < 0.05 each; Fig. 5f). Despite these TMAO-induced changes in mouse liver, no differences in bile acid transporter expression in the gut were noted between dietary groups (Supplementary Fig. 22). Taken together, these data show that gut-microbiota dependent metabolite TMAO fosters alterations in a major pathway for cholesterol elimination from the body, the bile acid synthetic pathway. To confirm these findings, the total bile acid pool size was examined. Mice supplemented with TMAO showed significant decreases in the total bile acid pool size (P < 0.01) (Fig. 6a). Dietary supplementation with TMAO also markedly reduced expression of both intestinal cholesterol transporters Niemann-Pick C1-like1 (Npc1L1), which transports cholesterol into enterocyte from the gut lumen26, and Abcg5/8, which transports cholesterol out of enterocytes into the gut lumen26, (Supplementary Figure 23). Previous studies with either Cyp7a1 or Cyp27a1 null mice have demonstrated a reduction in cholesterol absorption27,28. In separate studies, dietary TMAO supplementation similarly promoted a reduction (26%, P < 0.01) in total cholesterol absorption (Fig. 6b).
Figure 6. Effect of TMAO on cholesterol and sterol metabolism.
Measurement of (a) total bile acid pool size and composition, as well as (b) cholesterol absorption in adult female (> 8 weeks of age) C57BL/6J, Apoe−/− mice on normal chow diet versus diet supplemented with TMAO for 4 weeks. (c) Summary scheme outlining pathway for microbiota participation in atherosclerosis via metabolism of dietary carnitine and choline forming TMA and TMAO, as well as the impact of TMAO on cholesterol and sterol metabolism in macrophages, liver and intestines. FMOs, flavin monooxygenases; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; OST-α, solute carrier family 51, alpha subunit; ASBT, solute carrier family 10, member 2.
DISCUSSION
L-carnitine has been studied for over a century 29. Although eukaryototes can endogenously produce L-carnitine, only prokaryotic organisms can catabolize L-carnitine11. A role for intestinal microbiota in TMAO production from dietary carnitine was first suggested by studies in rats; moreover, while TMAO production from alternative dietary trimethylamines has been suggested in humans, a role for microbiota in production of TMAO from dietary L-carnitine in humans had not yet been demonstrated30-32. The present studies reveal an obligatory role of gut microbiota in the production of TMAO from ingested L-carnitine in humans (Fig. 6c). They also suggest a new nutritional pathway in CVD pathogenesis that involves dietary L-carnitine, the intestinal microbial community, and production of the pro-atherosclerotic metabolite TMAO. Finally, they show that TMAO modulates cholesterol and sterol metabolism at multiple sites in vivo with a net effect of increase in atherosclerosis (Fig. 6c).
The present studies also suggest a novel mechanism for the observed relationship between dietary red meat ingestion and accelerated atherosclerosis. Consuming foods rich in L-carnitine (predominantly red meat) can significantly increase fasting human L-carnitine plasma levels33. Meats and full fat dairy products are abundant components of the Western diet and are commonly implicated in CVD. Together, L-carnitine and choline containing lipids can constitute up to 2%13,14,34 of a Western diet. Numerous studies have suggested a decrease in atherosclerotic disease risk in vegan and vegetarian individuals when compared to omnivores with an inferred mechanism being reduction in dietary cholesterol and saturated fat35,36. Interestingly, a recent 4.8 year randomized dietary study showed a 30% reduction in cardiovascular events in subjects consuming a Mediterranean diet (with specific avoidance of red meat) compared to subjects consuming a control diet 37. The present studies suggest reduction in carnitine and total choline ingestion with attendant reductions in TMAO levels may contribute to the cardiovascular health benefits observed in vegan/vegetarians. Conversely, an increased synthetic capacity for microbiota-dependent production of TMAO from carnitine may contribute to atherosclerosis, particularly in omnivores where carnitine consumption is elevated.
One pro-atherosclerotic mechanism observed for TMAO in the current studies is reduction of RCT (Fig. 6c). Dietary carnitine and choline each reduced RCT (P < 0.05), but only in the presence of intact intestinal microbiota and ability to form TMA/TMAO. Importantly, suppression of intestinal microbiota completely eliminated choline and carnitine dependent reductions in RCT. Moreover, dietary supplementation with TMAO directly promoted a similar reduction in RCT. These results are consistent with a gut microbiota dependent mechanism whereby generation of TMAO impairs RCT, potentially contributing to the observed pro-atherosclerotic phenotype of these interventions. Another pro-atherosclerotic mechanism of TMAO is increases in macrophage SRA and CD36 surface expression and foam cell formation9 (Fig. 6c). Within the macrophage, TMAO does not appear to alter cholesterol biosynthetic and uptake pathways initially reported by Brown and Goldstein23,38, or the more recently described regulatory role of desmosterol in integrating macrophage lipid metabolism and inflammatory gene responses24. In the liver, TMAO decreased the bile acid pool size and reduced key bile acid synthesis and transport proteins (Fig. 6c). However, it is unclear whether these changes contribute to the reductions in RCT. Of note, TMAO promoted reduction in expression of Cyp7a1, the major bile acid synthetic enzyme and rate limiting step in the catabolism of cholesterol. The effect of TMAO is thus consistent with reports of human genetic variants in the Cyp7a1 gene that are associated with reduced expression and bile acid synthesis, decreased bile acid secretion, and enhanced atherosclerosis39-41. Further, up- (as opposed to down-) regulation of Cyp7a1 is reported to lead to an expansion of the bile acid pool size, increased RCT, and reduced atherosclerotic plaque in susceptible mice42-44. Within the intestines, TMAO was again associated with changes in cholesterol metabolism (Fig. 6c), but the significant reductions in cholesterol absorption observed (P < 0.01), while consistent with the reduction in intestinal Npc1L145 (and hepatic Cyp7a1 and Cyp27a127,28), cannot explain the reduction in RCT. Thus, the molecular mechanisms through which the microbiota → TMAO pathway inhibits RCT are not entirely clear. Finally, it is not known whether TMAO interacts with a specific receptor(s) directly, or whether it acts to alter signaling pathways indirectly by altering protein conformation (i.e., via allosteric effects). A small quaternary amine with aliphatic character, TMAO is reportedly capable of directly inducing conformational changes in proteins, including both stabilization of protein folding, and functioning as a small molecule protein chaperone46,47. TMA too has been reported to influence signal transduction by direct interaction with a family of G couple protein receptors48,49. It is thus conceivable that TMAO may alter a multitude of signaling pathways without directly acting at a “TMAO receptor”.
A remarkable finding of the present studies is the magnitude with which long term dietary habits impact TMAO synthetic capacity in both humans (i.e. vegan and vegetarian vs. omnivore) and mice (normal chow vs. chronic carnitine supplementation). Microbial composition analyses from humans and mice revealed specific taxa that segregated with both dietary status and plasma TMAO levels. Recent studies have shown that significant changes in gut microbial composition, or “enterotype”, are associated with long-term dietary changes18. We observed that plasma TMAO levels varied significantly according to prior reported enterotypes (P < 0.05). An obligatory role for gut microbiota in TMAO formation from dietary carnitine was shown in mice and humans. The differences observed in TMAO production following an “L-carnitine challenge” within omnivore versus vegan subjects is striking, consistent with the observed differences in microbial community composition. Recent reports have shown significant differences in microbial communities among vegetarians/vegans versus omnivores 50. Of note, we observed a significant increase in baseline plasma TMAO concentrations in what historically was called enterotype 2 (Prevotella) (P < 0.05), a relatively rare enterotype that previously in one study was associated with low animal fat and protein consumption18. Notably, in our study, 3 of the 4 individuals classified into enterotype 2 are self-identified omnivores suggesting more complexity in the human gut microbiome than anticipated. Indeed, other studies have demonstrated variable results in associating human bacterial genera, including Bacteroides and Prevotella, to omnivorous and vegetarian eating habits17,51. This complexity is no doubt in part attributed to the fact that there are many species within any genus and distinct species within the same genus may have different capacity to use carnitine as a fuel and form TMA. Indeed, prior studies have suggested that multiple bacterial strains can metabolize carnitine in culture52, and by analogy, comparison of distinct species within the genus Clostridium reveals some that are capable and others not of using choline as the sole source of carbon and nitrogen in culture53. The present studies suggest that multiple “proatherogenic” (i.e. TMA and TMAO producing) species, likely exist. Consistent with this supposition, others have reported that several bacterial phylotypes are associated with a history of atherosclerosis, and that the human microbiota biodiversity may in part be influenced by carnivorous eating habits15,18,54.
The association between plasma carnitine levels and cardiovascular risks further supports the potential physiological importance of the carnitine → gut microbiota → TMA/TMAO → atherosclerosis pathway (Fig. 6c). The association between plasma carnitine levels and CVD risks disappeared following addition of TMAO levels to the statistical model. These observations are consistent with a proposed mechanism whereby oral carnitine ingestion contributes to atherosclerotic CVD risks via the microbiota metabolite TMAO. There are only a few reports of specific intestinal anaerobic and aerobic bacterial species that can utilize L-carnitine as a carbon nitrogen source10,11,55.
L-carnitine is essential in the import of activated long chain fatty acids from the cytoplasm into mitochondria for β-oxidation and supplementation with L-carnitine has been widely studied. There are case reports of carnitine supplementation showing benefit for individuals with inherited primary and acquired secondary L-carnitine deficiency syndromes12. Review of multiple supplementation studies with carnitine reveals conflicting results, which may in part reflect variations in route (i.e. bypassing gut) of administration 56,57. Interestingly, oral L-carnitine supplementation in hemodialysis subjects raises plasma L-carnitine to normal levels, but substantially increases TMAO levels. A broader potential therapeutic scope for L-carnitine and two related metabolites, acetyl-L-carnitine and propionyl-L-carnitine, has also been explored in treating acute ischemic events and cardio-metabolic disorders (reviewed in ref 57). Here too, results are conflicting. One potential explanation for the discrepant findings of various carnitine intervention studies may be variations in the route of administration and the duration of dosing. Many studies have provided one of the L-carnitines over short intervals, or via parenteral route, bypassing gut microbiota (and hence TMAO formation).
Discovery of a link between carnitine ingestion, gut microbiota metabolism, and CVD risks has broad health related implications. Our studies reveal a new potential pathway linking dietary red meat ingestion with atherosclerosis pathogenesis. The role of gut microbiota in this pathway suggests new potential therapeutic targets for preventing CVD. Furthermore, our studies have public health relevance as carnitine is a common over-the-counter dietary supplement and additive. Our results suggest the safety of chronic L-carnitine supplementation should be examined, because high amounts of orally ingested carnitine may under some conditions prime our gut microbiota with enhanced capacity to produce TMAO, and potentially promote atherosclerosis.
Online Methods
Materials and general procedures
Breeders of all conventional mice were obtained from Jackson Laboratories. All animal studies were performed under approval of the Animal Research Committee of the Cleveland Clinic. Liver cholesterol was quantified by GC/MS and liver triglyceride was measured using GPO reagent as described in Supplementary Methods. Mouse plasma lipids and glucose, and human fasting lipid profile, CRP, and glucose were measured using the Abbott ARCHITECT platform, model ci8200 (Abbott Diagnostics). Mouse HDL was isolated using density ultracentrifugation and insulin levels were quantified by enzyme linked enzyme-linked immunosorbent assay as described in Supplementary Methods. Human plasma MPO was measured using the FDA-cleared CardioMPO assay (Cleveland Heart Lab).
Research subjects
All research subjects gave written informed consent. All protocols were approved by the Cleveland Clinic Institutional Review Board. Two cohorts of subjects were used in the present studies. The first group of volunteers (n = 30 omnivores and n = 23 vegetarians/vegans) had extensive dietary questioning, and stool, plasma and urine collection. A subset of subjects with stool collected also underwent oral carnitine challenge testing (n = 5 omnivores and n = 5 vegans), consisting of d3(methyl)carnitine (250 mg within a veggie capsule). Where indicated, additional omnivores, and an individual vegan, also underwent carnitine challenge testing with combined ingestion of the synthetic d3-carnitine capsule (250 mg) and an 8 ounce steak (consumed within 10 minutes). Male and female volunteers were at least 18 years of age. Volunteers participating in the carnitine challenge tests were excluded if they were pregnant, had chronic illness (including a known history of heart failure, renal failure, pulmonary disease, gastrointestinal disorders, or hematologic diseases), an active infection, received antibiotics within 2 months of study enrollment, used any over the counter or prescriptive probiotic or bowel cleansing preparation within the past 2 months, ingested yogurt within the past 7 days, or had undergone bariatric or other intestinal (e.g. gall bladder removal, bowel resection) surgery. All other research subjects were derived from GeneBank, a large longitudinal tissue repository with connecting clinical database from sequential consenting stable subjects undergoing elective cardiac evaluation. Further description of the GeneBank cohort can be found in Supplementary Methods.
Human L-carnitine challenge test
Consented adult men and women fasted overnight (12 hours) before performing the “L-carnitine challenge test”, which involved baseline blood and spot urine collection, and then oral ingestion (T = 0 at time of initial ingestion) of a veggie caps capsules containing 250 mg of a stable isotope labeled d3-L-carnitne (under Investigational New Drug exemption). Where indicated, for a subset of subjects, the carnitine challenge also included a natural source of L-carnitine (an 8 ounce sirloin steak cooked medium on a George Forman Grill) in a 10 minute period concurrent with taking the capsule containing the d3-carnitine. After combined ingestion of the steak and d3-L-carnitine, sequential venous serial blood draws were performed at respective time points, and a 24 hour urine collection was performed. An ensuing 1 week treatment period of oral antibiotics (Metronidazole 500 mg bid, Ciprofloxacin 500 mg bid) was given to suppress intestinal microbiota that use carnitine to form TMA and TMAO before repeating the L-carnitine challenge. After at least 3 weeks off of all antibiotics to allow reacquisition of intestinal microbiota, a third and final L-carnitine challenge test was performed. Dietary habits (vegan vs. ominivore) were determined using a questionnaire assessment of dietary L-carnitine intake, similar to that conducted by the Atherosclerotic Risk in Community (ARIC) study58. d3-Carnitine was prepared by taking sodium L-norcarnitine dissolved in methanol and reaction with d3-methyl iodide (Cambridge Isotope) in the presence of potassium hydrogen carbonate to give d3-L-carnitine. Further details regarding d3-L-carnitine synthesis, purification and characterization are described in Supplementary Methods.
Metabolomics study
We previously reported results from a metabolomics study where small molecule analytes were sought that associated with cardiovascular risks9. The metabolomics study had a two stage screening strategy. In the first phase, totally unbiased metabolomics studies were performed on randomly selected plasma samples from a Learning Cohort generated from Genebank subjects that experienced a major adverse cardiovascular event (defined as non-fatal myocardial infarction, stroke, or death) (n = 50) in the 3 year period following enrollment versus age- and gender-matched controls (n = 50) that did not experience an event. A second phase (Validation Cohort) of unbiased metabolomics analyses was then performed on a non-overlapping second cohort of cases (n = 25) and age- and gender-matched controls (n = 25) using identical inclusion / exclusion criteria. Further details regarding the unbiased metabolomic approach can be found in Supplementary Methods.
Identification of L-carnitine and quantification of TMAO, TMA, and L-carnitine
Matching CID spectra of an unknown plasma metabolite with identical retention time and mass-to-charge ratio (m/z) as authentic L-carnitine (m/z = 162) were obtained as described in Supplementary Methods. Concentrations of carnitine, TMA and TMAO isotopologues in mouse and human plasma samples were determined by stable isotope dilution LC/MS/MS in positive MRM mode using deuterated internal standards on an AB Sciex API 5000 triple quadrupole mass spectrometer (Applied Biosystems) as described in Supplementary Methods. In studies quantifying endogenous carnitine and ingested d3-carnitine, d9-carnitine was used as internal standard. D9-carnitine was prepared by dissolving 3-hydroxy-4-aminobutyric acid (Chem-Impex Intl.) in methanol and exhaustive reaction with d3-methyl iodide (Cambridge Isotope Labs) in the presence of potassium hydrogen carbonate. Further details regarding synthesis, purification and characterization of d9-carnitine can be found in Supplementary Methods.
Human microbiota analyses
Stool samples were stored at −80 °C and DNA for the gene encoding 16SrRNA was isolated using the MoBio PowerSoil kit according to the manufacturer’s instructions. DNA samples were amplified using V1-V2 region primers targeting bacterial 16S genes and sequenced using 454/Roche Titanium technology. Sequence reads from this study are available from the Sequence Read Archive (CaFE: SRX037803, SRX021237, SRX021236, SRX020772, SRX020771, SRX020588, SRX020587, SRX020379, SRX020378 (metagenomic). COMBO: SRX020773, SRX020770). Overall association between TMAO measurements and microbiome compositions was assessed using PermanovaG59 by combining both the weighted and unweighted UniFrac distances. Associations between TMAO measurements and individual taxa proportions were assessed by Spearman’s rank correlation test. False discovery rate (FDR) control based on the Benjamini–Hochberg procedure was used to account for multiple comparisons when evaluating these associations. Each of the samples was assigned to an enterotype category based on their microbiome distances (Jensen-Shannon distance) to the medoids of the enterotype clusters as defined in the COMBO data18. Association between enterotypes and TMAO level was assessed by Wilcoxon rank sum test. Student’s t-test was used to test the difference in means of TMAO level between omnivores and vegans. A robust Hotelling T2 test was used to examine the association between both the proportion of specific bacterial taxa and TMAO levels in groups using R software version 2.1560.
Mouse microbiota analysis
Microbial community composition was assessed by pyrosequencing 16S rRNA genes derived from mouse cecums from either a normal chow diet (n = 11) and L-carnitine diet (n = 13). DNA was isolated using the MoBio PowerSoil DNA Isolation Kit. The V4 region of the 16S ribosomal DNA gene was amplified using bar-coded fusion primers (F515/R806) with the 454 A Titanium sequencing adapter as further described in Supplementary Methods. The relative abundances of bacteria at each taxonomic level were computed for each mouse, a single representative sequence for each OTU was aligned using PyNAST, and a phylogenetic tree was built using FastTree as further described in Supplementary Methods. Spearman correlations were calculated to assess correlations between relative abundance of gut microbiota and mouse plasma TMA and TMAO levels. False discovery rates (FDR) of the multiple comparisons were estimated for each taxon based on the P-values resulted from correlation estimates, as further described in Supplementary Methods. A robust Hotelling T2 test was used to examine the association between both the proportion of specific bacterial taxa and mouse plasma TMA/TMAO levels in groups using R software version 2.1560.
Aortic root lesion quantification
Apolipoprotein E knockout mice on C57BL/6J background (C57BL/6J, Apoe−/−) were weaned at 28 days of age and placed on a standard chow control diet (Teklad 2018). L-carnitine was introduced into the diet by supplementing mouse drinking water with 1.3% L-carnitine (Chem-Impex Intl.), 1.3% L-carnitine and antibiotics, or antibiotics respectively. The antibiotic cocktail dissolved in mouse drinking water has previously been shown to suppress commensal gut microbiota, and included 0.1% Ampicillin sodium salt (Fisher Scientific), 0.1% Metronidazole, 0.05% Vancomycin (Chem Impex Intl.), and 0.1% Neomycin sulfate (Gibco)19. Mice were anaesthetized with Ketamine/Xylazine before terminal bleeding by cardiac puncture to collect blood. Mouse hearts were fixed and stored in 10% neutral buffered formalin before being frozen in OCT for sectioning. Aortic root slides were stained with Oil-red-O and counterstained with Haematoxylin. The aortic root atherosclerotic lesion area was quantified as the mean of sequential sections of 6 microns approximately 100 microns apart9.
Germ-free mice and conventionalization studies
10-week-old female Swiss Webster germ-free mice (SWGF) were obtained from the University of North Carolina Gnotobiotics Core Facility. Germ-free mice underwent gastric gavage with the indicated isotopologues of L-carnitine (see below for details of L-carnitine challenge) immediately following removal from the germ-free microisolator shipper. After performing the L-carnitine challenge, germ-free mice were conventionalized by being housed in cages with non-sterile C57BL/6J female mice. Approximately 4 weeks later, the L-carnitine challenge was repeated. Quantification of natural abundance and isotope labeled carnitine, TMA and TMAO in mouse plasma was performed using stable isotope dilution LC/MS/MS as described above.
Mouse L-carnitine challenge studies
C57BL/6J female or C57BL/6J, Apoe−/− female mice were provided via gastric gavage synthetic d3-L-carnitine (150 μl of 150 mM stock) dissolved in water using a 1.5-inch 20-gauge intubation needle. Plasma was collected from the saphenous vein at baseline and at the indicated time points. C57BL/6J, Apoe−/− female mice were used in the study examining the inducibility of microbiota to generate TMA and TMAO following carnitine feeding. For these studies, animals were placed on an L-carnitine supplemented diet (1.3% L-carnitine in mouse drinking water) for 10 weeks. Quantification of natural abundance and isotope-labeled forms of carnitine, TMA and TMAO in mouse plasma was performed using stable isotope dilution LC/MS/MS as described above.
Mouse reverse cholesterol transport, cholesterol absorption, and bile acid pool size studies
Adult female (> 8 weeks of age) C57BL/6J, Apoe−/− mice were place on either a chow diet, or a L-carnitine, a choline, or a TMAO supplemented diet for 4 weeks prior to performance of reverse cholesterol transport, cholesterol absorption, or bile acid pool size/composition studies as described in Supplementary Methods. In some reverse cholesterol transport experiments, mice were treated with a cocktail of oral antibiotics (as in atherosclerosis studies described above) for 4 weeks prior to enrollment. Reverse cholesterol transport studies were performed using subcutaneous (in the back) injection of [14C]cholesterol labeled bone marrow-derived macrophages, as further detailed in Supplementary Methods. Feces were collected and analyzed as described in Supplementary Methods. For cholesterol absorption experiments, animals were fasted 4 hours before gavage with olive oil supplemented with [14C]cholesterol/ [3H]β-sitostanol. Feces were collected over a 24 hour period and analyzed as described in Supplementary Methods. Total bile acid pool size and composition were determined in female C57BL/6J, Apoe−/− analyzing the combined small intestine, gallbladder, and liver, which were extracted together in ethanol with nor-Deoxycholate (Steraloids) added as an internal standard. The extracts were filtered (Whatman paper #2), dried and resuspended in water. The samples were then passed through a C18 column (Sigma) and eluted with methanol. The eluted samples were again dried down and resuspended in methanol. A portion of this was subjected to HPLC using Waters Symmetry C18 column (4.6 × 250 mm No. WAT054275, Waters Corp.) and a mobile phase consisting of methanol: acetonitrile: water (53:23:24) with 30 mM ammonium acetate, pH 4.91, at a flow rate of 0.7 ml min−1. Bile acids were detected by evaporative light spray detector (Alltech ELSD 800, nitrogen at 3 bar, drift tube temperature 40°C) and identified by comparing their respective retention times to those of valid standards (Taurocholate and Tauro-β-muricholate from Steraloids; Taurodeoxycholate and Taurochenodeoxycholate from Sigma; Tauroursodeoxycholate from Calbiochem). For quantitation, peak areas were integrated using Chromperfect Spirit (Justice laboratory software) and bile acid pool size was expressed as μmol/100 g body weight after correcting for procedural losses with nor-deoxycholate.
Effect of TMAO on macrophage cholesterol biosynthesis, cholesterol efflux, inflammatory genes, and desmosterol levels
The effect of cholesterol loading on macrophage cholesterol biosynthetic and inflammation genes, LDL receptor expression levels, and desmosterol levels, were performed as previously described24. Thioglycollate elicited mouse peritoneal macrophages (MPMs) were harvested and cultured in RPMI 1640 supplemented with 10% FCS and penicillin/streptomycin. MPMs were then lipoprotein starved further in culture for 18 hours in the absence versus presence of increasing concentrations of cholesterol, acetylated LDL or vehicle with (+) or without (−) 300 μM TMAO dehydrate (Sigma). Desmosterol in the cholesterol loading studies was quantified by stable isotope dilution GC/MS analysis. Further details of these studies and cholesterol efflux studies are described in Supplementary Methods.
RNA preparation and real time PCR analysis
RNA was first purified from tissue (macrophage, liver, or gut) using the animal tissue protocol from the Qiagen rneasy mini kit. Small bowel used for RNA purification was sectioned sequentially in 5 equal segments from the duodenum to illeum before RNA preparation. Purified total RNA and random primers were used to synthesize first strand cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) reverse transcription protocol. Quantitative real-time PCR was performed using Taqman qRT-PCR probes (Applied Biosystems, Foster City, CA) and normalized to tissue β-Actin by the ΔΔCT method using StepOne Software v2.1 (Applied Biosystems, Foster City, CA).
General Statistics
Student’s t-test or a Wilcoxon non parametric test were used to compare group means as deemed appropriate. The analysis of variance (ANOVA, if normally distributed) or Kruskal-Wallis test (if not normally distributed) was used for multiple group comparisons of continuous variables and a Chi-square test was used for categorical variables. Odds ratios for various cardiac phenotypes (CAD, PAD, and CVD) and corresponding 95% confidence intervals were calculated using logistic regression models. Kaplan–Meier analysis with Cox proportional hazards regression was used for time-to-event analysis to determine Hazard ratio (HR) and 95% confidence intervals (95%CI) for adverse cardiac events (death, MI, stroke, and revascularization). Adjustments were made for individual traditional cardiac risk factors (age, gender, diabetes mellitus, systolic blood pressure, former or current cigarette smoking, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol), extent of CAD, left ventricular ejection fraction, history of MI, baseline medications (aspirin, statins, β-blockers, and ACE inhibitors), and renal function by estimated creatinine clearance. Kruskal-Wallis test was used to assess the effect of the degree of coronary vessel disease on L-carnitine levels. A robust Hotelling T2 test was used to examine the difference in the proportion of specific bacterial genera along with subject TMAO levels between the different dietary groups60. All data was analyzed using R software version 2.15 and Prism (Graphpad Software).
Supplementary Material
Acknowledgements
We thank L. Kerchenski and C. Stevenson for assistance in performing the clinical studies; A. Pratt, S. Neale, M. Pepoy, and B. Sullivan for technical assistance with human specimen processing and routine clinical diagnostic testing; E. Klipfell, F. McNally, and M. Berk for technical assistance, and the subjects who consented to participate in these studies. Mass Spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility with partial support through a Center of Innovation by AB SCIEX. Germ free animals used were obtained from the University of North Carolina Gnotobiotic Facility, which is supported by P30-DK034987-25-28 and P40-RR018603-06-08.
This research was supported by National Institutes of Health grants R01 HL103866 (S.L.H.), P20 HL113452 (S.L.H. and W.H.W.T.), PO1 HL30568 (A.J.L.), PO1 H28481 (A.J.L.), R00 HL096166 (J.M.B.), UH3-DK083981 (J.D.L.), 1RC1DK086472 (R.M.K.), and the Leducq Foundation (S.L.H.). The clinical study GeneBank was supported in part by P01 HL076491, P01 HL098055, R01 HL103931 and the Cleveland Clinic Foundation General Clinical Research Center of the Cleveland Clinic/Case Western Reserve University CTSA (1UL1RR024989). S.L.H. is also partially supported by a gift from the Leonard Krieger Fund. Z.W. was partially supported by a Scientist Development Grant from the American Heart Association. E.O. was supported by MOBILITAS Postdoctoral Research Grant (MJD252).
Footnotes
Author Contributions: R.A.K. participated in laboratory, animal and human studies, assisted in statistical analyses, helped design the experiments, and drafted the manuscript. Z.W. performed the initial metabolomics study, assisted with animal and mass spectrometry analyses. B.S.L. synthesized d3- and d9-carnitine for studies, assisted with mass spectrometry analyses, and helped draft the manuscript. E.B.B and X.F. assisted in performance of mass spectrometry analyses of the large human clinical cohort study. Y.W. performed the statistical analysis and critical review of the manuscript. J.D.S. helped with aortic root atherosclerosis analyses and critical review of the manuscript. J.A.D. assisted in experimental design. J.A.B. and B.T.S. assisted in laboratory and animal experiments. E.O. and A.J.L. performed and helped interpret mouse cecal microbiota analyses. J.C., H.L., G.D.W., J.D.L., and R.M.K. provided samples, performed human microbiota analyses, and helped interpret human microbiota data. M.W. and J.M.B. assisted with measurement of bile acid pool size and helped with critical review of the manuscript. W.H.W.T helped with human studies and critical review of the manuscript. S.L.H. conceived the idea, helped design the experiments, provided the funding for the study, and helped draft and critically revised the manuscript.
Competing Financial Interest Disclosure
Drs. Wang and Levison are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Drs. Wang and Levison report that they have the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Liposciences, Inc. Dr. Tang received research grant support from Abbott Laboratories, and served as consultants for Medtronic Inc and St. Jude Medical. Drs. Hazen and Smith are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports he has been paid as a consultant or speaker by the following companies: Cleveland Heart Lab, Inc., Esperion, Liposciences Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports he has received research funds from Abbott, Cleveland Heart Lab, Esperion and Liposciences, Inc. Dr. Hazen has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Abbott Laboratories, Cleveland Heart Lab, Inc., Frantz Biomarkers, Liposciences, Inc., and Siemens.
REFERENCES
- 1.Bernstein AM, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutrit. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, et al. Projected effect of dietary salt reductions on future cardiovascular disease. The N. Engl. J. Med. 2010;362:590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen ES. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/2. Shared risk factors for cancer and atherosclerosis--a review of the epidemiological evidence. Mutat. Res. 1990;239:163–179. doi: 10.1016/0165-1110(90)90004-u. [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Goodman AL, Gordon JI. Our unindicted coconspirators: human metabolism from a microbial perspective. Cell Metab. 2010;12:111–116. doi: 10.1016/j.cmet.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremer J. Carnitine--metabolism and functions. Physiol. Rev. Suppl. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 11.Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 1998;18:39–61. doi: 10.1146/annurev.nutr.18.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Stanley CA. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 2004;1033:42–51. doi: 10.1196/annals.1320.004. [DOI] [PubMed] [Google Scholar]
- 13.Demarquoy J, et al. Radioisotopic determination of L-carnitine content in foods commonly eaten in western countries. Food Chem. 2004;86:137–142. [Google Scholar]
- 14.Rigault C, Mazue F, Bernard A, Demarquoy J, Le Borgne F. Changes in l-carnitine content of fish and meat during domestic cooking. Meat Sci. 2008;78:331–335. doi: 10.1016/j.meatsci.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Ley RE, et al. Evolution of mammals and their gut microbes. Science (New York, N.Y.) 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muegge BD, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmer J, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012;66:53–60. doi: 10.1038/ejcn.2011.141. [DOI] [PubMed] [Google Scholar]
- 18.Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science (New York, N.Y.) 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Ley RE, et al. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Febbraio M, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 23.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 24.Spann NJ, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am. J. Cardiol. 2003;92:42J–49J. doi: 10.1016/s0002-9149(03)00615-5. [DOI] [PubMed] [Google Scholar]
- 26.Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011;73:239–259. doi: 10.1146/annurev-physiol-012110-142233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 28.Repa JJ, et al. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J. Biol .Chem. 2000;275:39685–39692. doi: 10.1074/jbc.M007653200. [DOI] [PubMed] [Google Scholar]
- 29.Gulewitsch W, Krimberg R. Zur Kenntnis der Extrakivstoffe der Muskein, II. Mitteilung. Uber das Carnitin. Hoppe-Seyler’s Z. Physiol. Chem. 1905:326–330. [Google Scholar]
- 30.Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. J. Nutr. 1991;121:539–546. doi: 10.1093/jn/121.4.539. [DOI] [PubMed] [Google Scholar]
- 31.Rebouche CJ, Mack DL, Edmonson PF. L-Carnitine dissimilation in the gastrointestinal tract of the rat. Biochemistry. 1984;23:6422–6426. doi: 10.1021/bi00321a022. [DOI] [PubMed] [Google Scholar]
- 32.Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study. Food Chem. Toxicol. 1999;37:515–520. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 33.Delany JP, snook JT, Vivian VM, Cashmere K. Metabolic effects of a carnitine-free diet fed to college students. Fed. Proc. 1986;45:815. [Google Scholar]
- 34.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 35.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009;89:1607S–1612S. doi: 10.3945/ajcn.2009.26736K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Key TJ, et al. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 1999;70:516S–524S. doi: 10.1093/ajcn/70.3.516s. [DOI] [PubMed] [Google Scholar]
- 37.Estruch R, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013 doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 38.Brown MS, Goldstein JL. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974;185:61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- 39.Charach G, Rabinovich A, Argov O, Weintraub M, Rabinovich P. The role of bile Acid excretion in atherosclerotic coronary artery disease. Int. J. Vasc. Med. 2012;2012949672 doi: 10.1155/2012/949672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charach G, et al. Decreased fecal bile acid output in patients with coronary atherosclerosis. J. Med. 1998;29:125–136. [PubMed] [Google Scholar]
- 41.Lu Y, Feskens EJ, Boer JM, Muller M. The potential influence of genetic variants in genes along bile acid and bile metabolic pathway on blood cholesterol levels in the population. Atherosclerosis. 2010;210:14–27. doi: 10.1016/j.atherosclerosis.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Miyake JH, et al. Transgenic expression of cholesterol-7-alpha-hydroxylase prevents atherosclerosis in C57BL/6J mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:121–126. doi: 10.1161/hq0102.102588. [DOI] [PubMed] [Google Scholar]
- 43.Post SM, de Crom R, van Haperen R, van Tol A, Princen HM. Increased fecal bile acid excretion in transgenic mice with elevated expression of human phospholipid transfer protein. Arterioscler. Thromb. Vasc. Biol. 2003;23:892–897. doi: 10.1161/01.ATV.0000067702.22390.20. [DOI] [PubMed] [Google Scholar]
- 44.Zong C, et al. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp. Biol. Med. (Maywood) 2012;237:194–200. doi: 10.1258/ebm.2011.011275. [DOI] [PubMed] [Google Scholar]
- 45.Altmann SW, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 46.Bai C, Biwersi J, Verkman AS, Matthay MA. A mouse model to test the in vivo efficacy of chemical chaperones. J. Pharmacol. Toxicol. Methods. 1998;40:39–45. doi: 10.1016/s1056-8719(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 47.Mello CC, Barrick D. Measuring the stability of partly folded proteins using TMAO. Protein Sci. 2003;12:1522–1529. doi: 10.1110/ps.0372903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 49.Suska A, Ibanez AB, Lundstrom I, Berghard A. G protein-coupled receptor mediated trimethylamine sensing. Biosensors & bioelectronics. 2009;25:715. doi: 10.1016/j.bios.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Cordain L, et al. Origins and evolution of the Western diet: health implications for the 21st century. The Am. J. Clin. Nutrit. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 51.Liszt K, et al. Characterization of bacteria, clostridia and Bacteroides in faeces of vegetarians using qPCR and PCR-DGGE fingerprinting. Ann. Nutr. Metab. 2009;54:253–257. doi: 10.1159/000229505. [DOI] [PubMed] [Google Scholar]
- 52.Elssner T, Preusser A, Wagner U, Kleber HP. Metabolism of L(−)-carnitine by Enterobacteriaceae under aerobic conditions. FEMS Microbiol. Lett. 1999;174:295–301. doi: 10.1111/j.1574-6968.1999.tb13582.x. [DOI] [PubMed] [Google Scholar]
- 53.Moller B, Hippe H, Gottschalk G. Degradation of various amine compounds by mesophilic clostridia. Arch. Microbiol. 1986;145:85–90. doi: 10.1007/BF00413032. [DOI] [PubMed] [Google Scholar]
- 54.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science (New York, N.Y.) 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleber HP. Bacterial carnitine metabolism. FEMS Microbiol Lett. 1997;147:1–9. doi: 10.1111/j.1574-6968.1997.tb10212.x. [DOI] [PubMed] [Google Scholar]
- 56.Hedayati SS. Dialysis-related carnitine disorder. Semin. Dial. 2006;19:323–328. doi: 10.1111/j.1525-139X.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 57.Mingorance C, Rodriguez-Rodriguez R, Justo ML, Alvarez de Sotomayor M, Herrera MD. Critical update for the clinical use of L-carnitine analogs in cardiometabolic disorders. Vasc. Health Risk Manag. 2011;7:169–176. doi: 10.2147/VHRM.S14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am. J. Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 59.Chen J. Powerful statistical analysis for associating microbiomes to enviromental covariates using generalized Unifrac distances. Bioinformatics. 2012;28:2106–2113. doi: 10.1093/bioinformatics/bts342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willems G. A robust hotelling test. Metrika. 2002;55:125–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.