Abstract
Physical force environment is a major factor that influences cellular homeostasis and remodeling. It is not well understood, however, as a potential role of force intensities in the induction of cellular mechanotransduction. Using a fluorescence resonance energy transfer (FRET)-based approach, we asked whether activities of GTPase RhoA in chondrocytes are dependent on intensities of flow induced shear stress. We hypothesized that RhoA activities can be either elevated or reduced by selecting different levels of shear stress intensities. The result indicate that C28/I2 chondrocytes have increased RhoA activities in response to high shear stress (10 or 20 dyn/cm2), whereas a decrease in activity was seen with an intermediate shear stress of 5 dyn/cm2. No changes were seen under low shear stress (2 dyn/cm2). The observed 2-level switch of RhoA activities is closely linked to the shear stress-induced alterations in actin cytoskeleton and traction forces. In the presence of constitutively active RhoA (RhoA-V14), intermediate shear stress suppressed RhoA activities, while high shear stress failed to activate them. In chondrocytes, expression of various metalloproteinases is, in part, regulated by shear and normal stresses through a network of GTPases. Collectively, the data suggest that intensities of shear stress are critical in differential activation and inhibition of RhoA activities in chondrocytes.
Keywords: fluorescence resonance energy transfer (FRET), mechanical loading, cartilage, Rho GTPase, actin cytoskeleton, traction force
Introduction
Mechanical forces within a physiological range are an important regulator for insuring tissue homeostasis and remodeling (Discher et al., 2005; Giannone and Sheetz, 2006; Janmey and McCulloch, 2007). Deviations from this physiological loading condition, in contrast, often result in pathological outcomes associated with various diseases (Ingber, 2003; Jaalouk and Lammerding, 2009). Moderate mechanical loading, for instance, is reported to decrease proteolytic activities of degenerative enzymes in the articular cartilage, while excessive loading may lead to an increase in expression of matrix metalloproteinases (Yokota et al., 2011). Although substantial progress has been made toward the understanding of how cells sense mechanical forces and convert them into biochemical signals (Wang et al., 2005; Sawada et al., 2006; Vogel and Sheetz, 2006; Johnson et al., 2007; Na et al., 2008), it is not well understood how cells perceive and differentially respond to a wide spectrum of mechanical forces in terms of their intensities.
We have investigated the role of RhoA in response to fluid flow-induced shear stress in individual chondrocytes. Mechanical forces regulate RhoA activation in many types of cells including endothelial cells (Tzima et al., 2001; Wojciak-Stothard and Ridley, 2003; Shiu et al., 2004), smooth muscle cells (Putnam et al., 2003; Smith et al., 2003), cardiomyocytes (Kawamura et al., 2003), and chondrocytes (Haudenschild et al., 2008; Haudenschild et al., 2010). RhoA is a member of the family of Rho GTPases that act as a molecular switch in the early mechanotransduction responses (Olson, 2004; Guilluy et al., 2011). RhoA regulates a number of intracellular signaling cascades that elicit changes in gene expression and cellular functions including remodeling of actin cytoskeleton and exertion of traction forces (Ridley, 2000; Jaffe and Hall, 2005). When activated by mechanical force, RhoA regulates multiple downstream effectors. One of the downstream effectors, Rho-associated kinase (ROCK), promotes the assembly of actin cytoskeleton and phosphorylation of myosin light chains. By regulating intracellular tension through the cytoskeleton, this RhoA-ROCK signaling alters cell shape, and migration patterns as well as cellular differentiation (Etienne-Manneville and Hall, 2002).
The specific question we addressed was whether different magnitudes of shear stress can dissimilarly regulate RhoA activity in C28/I2 chondrocytes. Based on differential regulation of matrix metalloproteinases in response to moderate and acute loading, we hypothesized that RhoA activity can be both reduced and increased by shear stress depending on its intensity. To monitor shear stress-modulated RhoA activity with high spatiotemporal resolution, we employed a fluorescence resonance energy transfer (FRET)-based approach (Yoshizaki et al., 2003). This FRET approach allowed us to determine time-course dynamical activities of RhoA in individual live cells under shear stress at 2-20 dyn/cm2 before, during, and after fluid flow treatment. We used a FRET-based RhoA biosensor as well as constitutively active RhoA mutant.
Materials and methods
DNA plasmids
A FRET-based, cyan fluorescent protein (CFP)-yellow fluorescent protein (YFP) RhoA biosensor (Raichu-RhoA) was used, a gift of Dr. M. Matsuda (Yoshizaki et al., 2003). The probe consists of truncated RhoA, a RhoA binding domain (RBD) of an effector protein, and a pair of CFP and YFP. The intramolecular binding of active RhoA to RBD leads to the close association of CFP with YFP, resulting in an increase of FRET from CFP to YFP. Thus, the FRET activity of the RhoA biosensor was monitored to determine RhoA activity. The RhoA biosensor has been well characterized in terms of its specificity (Yoshizaki et al., 2003). As RhoA mutants, a constitutively active RhoA (RhoA-V14) and a dominant negative RhoA (RhoA-N19) were used (Li et al., 1999). An mCherry-actin probe was a gift of Dr. R. Tsien.
Cell culture and transfection
The human chondrocyte cell line, C28/I2, was used (Goldring et al., 1994). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Lonza) containing 10% FBS (Hyclone) and 1% penicillin/streptomycin (Lonza), and maintained at 37°C and 5% CO2 in a humidified incubator. The DNA plasmids were transfected into the cells using a Neon transfection system (Invitrogen). After transfection, the cells were transferred to a type I collagen-coated μ-slide cell culture chamber (Ibidi) and incubated in DMEM containing 0.5% FBS for 24-36 h before imaging experiments.
Inhibitors
Cytochalasin D (1 μg/ml; Enzo life sciences) and latrunculin A (1 μM; Enzo life sciences) were used to disrupt actin filaments. Blebbistatin (50 μM; Toronto research chemicals) was used to inhibit myosin II. ML-7 (25 μM; Biomol) was used to inhibit myosin light chain kinase. C3 transferase (1 μg/ml; Cytoskeleton) was used to inhibit Rho signaling.
Traction force microscopy
Polyacrylamide gel was used for traction measurements (Pelham and Wang, 1997). The elastic Young’s modulus of the gels used in this study was 35 kPa (0.3% bisacrylamide and 10% acrylamide, both purchased from Bio-Rad), which is close to the microscale stiffness of cartilage (Park et al., 2009). Red fluorescent submicrobeads (0.2 μm in diameter; Invitrogen) were embedded in the polyacrylamide gel to track their deformation. Images of the same region of the gel were taken at different times before or after experimental interventions. The displacement field was determined by monitoring the changes in the position of corresponding fluorescent beads between the reference (cell free) image and the image containing a cell. The traction map was calculated from the displacement field of the fluorescent beads by implementing the solution (Butler et al., 2002).
Shear stress application and microscopy
During imaging, a unidirectional flow was applied to the cells grown in the μ-slide cell culture chamber (Ibidi) without serum at 37°C. Shear stress of 2-20 dyn/cm2 was applied to the chamber by controlling the flow rate of the peristaltic pump (Cole-Parmer). A pulse dampener (Cole-Parmer) was used to minimize pulsation of the flow. All images were obtained by using an automated fluorescence microscope (Nikon) equipped with an electron-multiplying charge-coupled device (EMCCD) camera (Photometrix), a filter wheel controller (Sutter) and a Perfect Focus System (PFS; Nikon) that maintains the focus during time-lapse imaging. The following filter sets were used (Semrock): CFP excitation: 438/24 (center wavelength/bandwidth in nm); CFP emission (483/32); YFP (FRET) emission: 542/27; mCherry excitation: 562/40; mCherry emission: 641/75. Cells were illuminated with a 100 W Hg lamp through an ND64 (~1.5% transmittance) neutral density filter to minimize photobleaching. Time-lapse images were acquired at intervals of 2 min with a 63X (0.75 numerical aperture) objective. FRET images for RhoA activity was generated with NIS-Elements software (Nikon) by computing emission ratio of YFP/CFP for the individual cell.
Statistical analysis
Statistical data are presented as the mean ± standard error of the mean (SEM). One-way ANOVA followed by Dunnett’s post hoc test was used to determine the statistical differences for time-course experiments. Student’s t-test was used to compare 2 groups. Statistical analyses used Prism 5 software (GraphPad Software). P<0.05 was considered significant.
Results
Selective RhoA activity is regulated by the magnitude of shear stress
To determine whether the magnitude of shear stress is a key factor in regulating RhoA activities, we transfected a FRET-based, CFP-YFP RhoA biosensor (Yoshizaki et al., 2003) into C28/I2 cells and plated the cells on a type I collagen-coated flow chamber. Spatiotemporal changes of RhoA activities were assessed by monitoring changes in the emission ratio of YFP/CFP of the RhoA biosensor in the cell. During imaging, the cells were subjected to no flow for 2 min, flow-induced shear stress at 2, 5, 10, or 20 dyn/cm2 for 1 h, and lastly no flow for ~15 min (Fig. 1B). RhoA activity was not altered when shear stress of 2 dyn/cm2 was applied (Fig. 1C). However, in response to shear stress at 5 dyn/cm2, a rapid (< 2 min) RhoA inhibition occurred (~15 % FRET decrease; Fig. 1D, G). When shear stress was removed following the application of 5 dyn/cm2, RhoA activity increased and returned to the basal levels within 10 min (Fig. 1D, G). In marked contrast to shear stress of 5 dyn/cm2, 10 dyn/cm2 and 20 dyn/cm2 led to slow (>30 min), but strong RhoA activation (~ 20% FRET increase; Fig. 1E, F, H). As a control for shear stress-induced RhoA activity, we conducted FRET imaging experiments without applying shear stress (zero dyn/cm2) and observed that without the shear stress RhoA activity does not change (Fig. 1A). These substantially different activity patterns and time-courses for RhoA by shear stress suggest that mechanotransduction mechanisms for RhoA activities might be different depending on the magnitude of the applied shear stress.
Fig. 1.
RhoA activity is shear stress-magnitude dependent. (A) Control test. RhoA activity without shear stress. n = 8 cells. (B) Shear stress was applied for 1 h. Blue color indicates pre- and post-shear stress (no flow), and red color indicates shear stress (2-20 dyn/cm2) application. (C, D, E, F) Time course of RhoA activity in response to shear stress. The black arrow indicates the direction of shear flow applied to the cell. Color bars represent an emission ratio of YFP/CFP of the biosensor, an index of RhoA activation. Ratio images of YFP/CFP were scaled based on the color bars. Scale bars, 10 μm. Time courses of YFP/CFP emission ratio were averaged over the whole cell and were normalized to time point 0 min. (C) 2 dyn/cm2 (n = 5 cells). (D) 5 dyn/cm2 (n = 6 cells). (E) 10 dyn/cm2 (n = 7 cells). (F) 20 dyn/cm2 (n = 6 cells). (G, H) The white boxes in Fig. 1D and F are enlarged in Fig. 1G and H, respectively.
Shear stress-induced RhoA activity is correlated with actin cytoskeletal remodeling
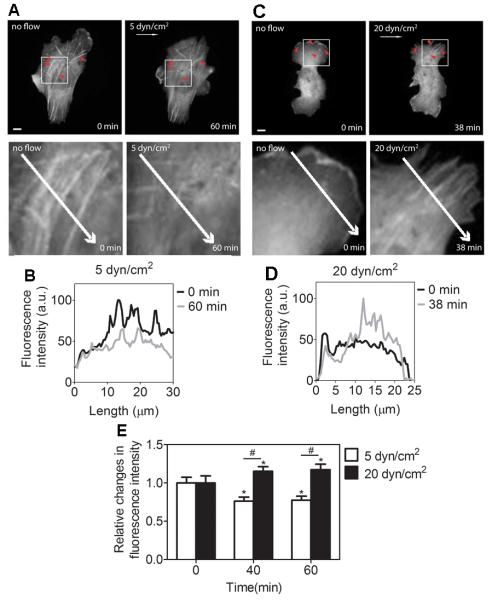
Shear stress-induced RhoA activity is associated with actin cytoskeleton organization (Tzima et al., 2001). To determine whether the selective RhoA activities by shear stress, Fig. 1, are associated with shear stress-induced changes in actin cytoskeleton organization, we transfected C28/I2 cells with mCherry-actin and visualized the actin cytoskeletal remodeling when applying shear stress to the cells. In response to shear stress at 5 dyn/cm2, actin stress fibers gradually disappeared (Fig. 2A, B). In contrast, shear stress at 20 dyn/cm2 induced an increase in actin stress fiber formation (Fig. 2C, D). Together with the statistical analysis on changes in actin stress fibers under shear stress (Fig. 2E), the data suggest that, under shear stress application, actin cytoskeletal remodeling is correlated with altered RhoA activities.
Fig. 2.
Shear stress-induced actin cytoskeleton organization is dependent on the magnitude of shear stress. (A) In response to 5 dyn/cm2, the cell displays a decrease in actin (see arrowheads). The white arrow denotes the flow direction. (B) Fluorescence intensity profile of actin along the length of the white arrows under 5 dyn/cm2 of shear stress. (C) In contrast to 5 dyn/cm2, shear stress at 20 dyn/cm2 results in an increase in actin stress fibers (see arrowheads). The white arrow denotes the flow direction. (D) Fluorescence intensity profile of actin along the length of the white arrows under 20 dyn/cm2 of shear stress. (E) Relative changes in fluorescence intensity of actin stress fibers under 5 and 20 dyn/cm2 of shear stress. The size of the region to obtain fluorescence intensity within the cell body was 4 μm × 4 μm. n = 10. * P < 0.05 relative to time zero, # P < 0.05 between groups under 5 or 20 dyn/cm2. Scale bars, 10 μm.
Actin cytoskeleton and intracellular tension are necessary for shear stress-induced RhoA activity
To further explore the potential contribution of actin cytoskeleton and intracellular tension in RhoA activity in response to shear stress, we used one of 4 different pharmacological drugs in individual experiments. First, we used cytochalasin D (CytoD) or latrunculin A (LatA) to disrupt actin filaments. Cells were pretreated with CytoD (1 μg/ml) or LatA (1 μM) and subjected to shear stress for 1 h. This treatment prevented RhoA inhibition and activation by shear stress at 5 and 20 dyn/cm2, respectively (Fig. 3 and Supplementary Fig. S1). To test the role of intracellular tension in shear stress-induced RhoA activity, we used ML-7 to inhibit myosin light chain kinase or blebbistatin (Bleb) to inhibit non-muscle myosin II. Pretreating with ML-7 (25 μM) or Bleb (50 μM) also prevented shear stress-induced RhoA activation and inhibition at corresponding shear stress levels (Fig. 3 and Supplementary Fig. S1). These results demonstrate that the myosin II-dependent, tensed actin cytoskeleton is necessary for selective RhoA regulation by shear stress regardless of the shear stress magnitude.
Fig. 3.
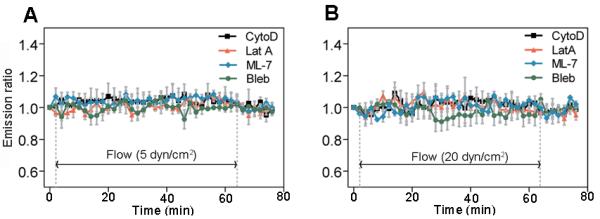
Shear stress-induced changes in RhoA activity are dependent on cytoskeleton and intracellular tension. (A) 5 dyn/cm2 (n > 5 cells). (B) 20 dyn/cm2 (n > 5 cells). The cells were transfected with RhoA biosensor and then treated with drugs. Actin filament disruption (CytoD and LatA), inhibiton of myosin light chain kinase (ML-7), or inhibition of myosin II (Bleb) prevented shear stress-induced RhoA activity.
Shear stress regulates traction forces
Because our results show that RhoA activities are regulated by shear stress (Fig. 1), and RhoA activation is known to increase traction forces (Chrzanowska-Wodnicka and Burridge, 1996), we hypothesized that the magnitude of shear stress would regulate traction force. To test this hypothesis, we quantified changes in tractions in C28/I2 cells during shear stress application using a traction force microscopy technique (Butler et al., 2002). Shear stress of 5 dyn/cm2 decreased tractions (~ 30 %) within 20 min (Fig. 4A), and shear stress of 20 dyn/cm2 significantly increased tractions (~ 70%) within 60 min (Fig. 4B). These results suggest that changes in tractions are regulated depending on the magnitude of shear stress. The significant change in tractions in Fig. 4 was preceded by rapid RhoA modulation by shear stress (Fig. 1), suggesting that shear stress magnitude-dependent RhoA activities may be required to regulate shear stress-induced traction dynamics.
Fig. 4.
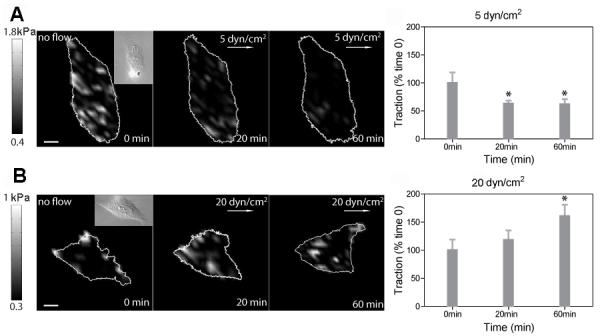
Dynamic tractions are regulated in a stress-magnitude dependent way. Dynamic traction maps and time courses of averaged tractions in response to 5 dyn/cm2 (A; n = 5 cells) and 20 dyn/cm2 (B; n = 5 cells). The color bars represent tractions. The white arrows indicate the shear stress direction. Insets are corresponding DIC images. Values are normalized by the tractions at 0 min. For statistical analysis, tractions at 20 min and 60 min were compared with tractions at 0 min (* P < 0.05). Scale bars, 10 μm.
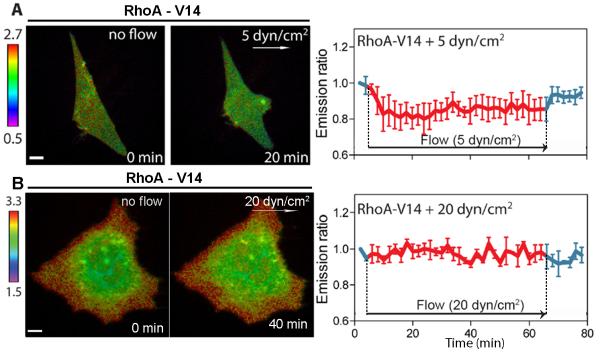
Intermediate shear stress decreases constitutively activated RhoA
To examine the specificity of RhoA in response to shear stress, we co-transfected C28/I2 cells with a RhoA biosensor and constitutively active (RhoA-V14) or dominant negative (RhoA-N19) mutant of RhoA. Unexpectedly, shear stress of 5 dyn/cm2 decreased (~15 %) RhoA activity of the cell transfected with RhoA-V14 (Fig. 5A). However, high shear stress at 20 dyn/cm2 failed to further activate RhoA in RhoA-V14-expressing cells (Fig. 5B). The inhibition of RhoA activity by a dominant negative RhoA-N19or C3 transferase (1 μg/ml) apparently blocked shear stress-driven activation of RhoA (Supplementary Fig. S2).
Fig. 5.
Shear stress of 5 dyn/cm2 downregulates RhoA activity of the cell transfected with a constitutively active RhoA. (A) RhoA activity in response to 5 dyn/cm2 (n = 3 cells). (B) RhoA activity in response to 20 dyn/cm2 (n = 3 cells). The white arrows denote the shear stress direction. Scale bars, 10 μm.
Discussion
Using a FRET-based RhoA biosensor, a novel observation is that RhoA is differentially regulated in C28/I2 chondrocytes depending on the magnitude of shear stress - 5 dyn/cm2 reduced RhoA activity, while 10 and 20 dyn/cm2 increased it. To date, most studies on mechanotransduction have focused on how mechanical loading can “activate” intracellular signaling. It has been shown, for instance, that a threshold of mechanical loading or deformation is required to activate the signaling, while the loading lower than the threshold does not directly affect the signaling (Na et al., 2008; Poh et al., 2009). However, the molecular mechanism underlying the observed two-level-switch like regulation of RhoA is apparently different from a simple on-off switch mechanism.
The results herein show that the differential RhoA activity by shear stress is correlated with the alteration in remodeling of actin cytoskeleton. The reduced RhoA activity in response to 5 dyn/cm2 corresponds to a decrease in formation of actin stress fibers, while the elevated RhoA activity by 10 or 20 dyn/cm2 an increase in their formation. This correlation of RhoA activity to actin organization has been shown in endothelial cells subjected to shear stress of 12 dyn/cm2 (Tzima et al., 2001), although the reported response (a transient decrease followed by an increase) presents a different temporal profile. Chondrocytes are in general rich in cortical actin but poor in cytosolic stress fibers. When they de-differentiate to fibroblast-like cells, they are reported to develop stress fibers (Woods et al., 2005). Our data suggest that shapes and differentiation states of chondrocytes are regulated differentially by intermediate and high shear stresses (Langelier et al., 2000; Haudenschild et al., 2009).
Previous reports using silicone rubber substrates have shown that the activation of RhoA by a soluble factor (lysophosphatidic acid; LPA) stimulates cell contractility (Chrzanowska-Wodnicka and Burridge, 1996). Although their results show the linkage of RhoA to intracellular force generation, the results were qualitative, and the time course of intracellular tension in individual cells under shear stress has been largely unknown. We found new quantitative evidence that the intracellular tension or cellular contractility is differentially regulated by the flow-induced shear stress depending on its magnitude. Using the traction force microscopy technique, dynamics of shear-driven cell contractility are clearly correlated with RhoA activity. Cell contraction plays an important role in cellular functions including the rates of cell migration as well as protein synthesis and transport. Reduced contractility in chondrocytes results in higher rates of glycosaminoglycan synthesis (Lee et al., 2003). RhoA-induced cell contractility is mediated by Rho kinase (ROCK), which regulates the appearance of actin stress fibers, phosphorylation of myosin light chains and the interaction of actin to myosin II (Amano et al., 1997). Our results with ML-7 and blebbistatin also confirm the linkage of the myosin light chain kinase and myosin II to RhoA activity under shear flow.
A potential mechanism for the observed RhoA regulation in a form of two-level switch might be achieved through interactions with integrins, stress-responsive kinases, and/or other GTPases. Activation of integrins such as αvβ3 or α5β1 by shear stress transiently downregulate RhoA activity in endothelial cells (Tzima et al., 2001). Furthermore, shear stress-induced activation of focal adhesion kinase (FAK) (Ren et al., 2000), Rac1 (Burridge and Wennerberg, 2004), or Src (Arthur et al., 2000) downregulate RhoA activity. We need to determine now whether the two-level regulation of RhoA is mediated through integrins, FAK, Rac1 or Src. We have found that intermediate shear stress at 5 dyn/cm2 downregulates RhoA activity in chondrocytes, which express constitutively active RhoA. While the exact mechanism is unclear at the current stage, it is possible that the Rho GDP dissociation inhibitor (Rho-GDI), one of the known Rho GTPase regulators, is involved in the observed regulation of RhoA activity by the phosphorylation of GDI that negatively regulates RhoA (Qiao et al., 2008). Indeed, previous reports demonstrated that Rho-GDI is responsive to shear stress and cyclic stretching (Kumar et al., 2004; Qi et al., 2008).
In summary, we have demonstrated that shear stress selectively activates and inhibits RhoA activity in a stress-magnitude-dependent manner, and that the differential activity of RhoA by shear stress is associated with the dynamical alterations in cytoskeletal remodeling and traction force. The results suggest that load-driven changes in RhoA activity affect cytoskeletal organization and dynamic force generation in chondrocytes. Future studies will address how RhoA interacts with other Rho family GTPases such as Rac1 and Cdc42 under mechanical loading, and how the load-induced GTPases are involved in metalloproteinase activities in chondrocytes.
Supplementary Material
Acknowledgements
We thank Dr. M. Goldring (Hospital for Special Surgery, NY) for providing C28/I2 chondrocytes; Dr. M. Matsuda (Kyoto University, Japan) and Dr. J. Miyazaki (Osaka University, Japan) for the gift of RhoA biosensor; Dr. R. Tsien (University of California, San Diego, CA) for the mCherry-actin; Dr. Y. Wang (University of Illinois, Urbana-Champaign, IL) for the RhoA mutants (RhoA-V14 and RhoA-N19). This work was supported by Indiana University (S.N.), and National Institutes of Health Grant AR052144 (H.Y.).
References
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–11. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Current biology: CB. 2000;10:719–22. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. American journal of physiology Cell physiology. 2002;282:C595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–23. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Birkhead JR, Suen LF, Yamin R, Mizuno S, Glowacki J, et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. The Journal of clinical investigation. 1994;94:2307–16. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nature cell biology. 2011;13:722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Chen J, Pang N, Lotz MK, D’Lima DD. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis and rheumatism. 2010;62:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Chen J, Steklov N, Lotz MK, D’Lima DD. Characterization of the chondrocyte actin cytoskeleton in living three-dimensional culture: response to anabolic and catabolic stimuli. Molecular & cellular biomechanics: MCB. 2009;6:135–44. [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Nguyen B, Chen J, D’Lima DD, Lotz MK. Rho kinase-dependent CCL20 induced by dynamic compression of human chondrocytes. Arthritis and rheumatism. 2008;58:2735–42. doi: 10.1002/art.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–77. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews Molecular cell biology. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annual review of biomedical engineering. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–6. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–7. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- Kumar A, Murphy R, Robinson P, Wei L, Boriek AM. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J. 2004;18:1524–35. doi: 10.1096/fj.04-2414com. [DOI] [PubMed] [Google Scholar]
- Langelier E, Suetterlin R, Hoemann CD, Aebi U, Buschmann MD. The chondrocyte cytoskeleton in mature articular cartilage: structure and distribution of actin, tubulin, and vimentin filaments. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2000;48:1307–20. doi: 10.1177/002215540004801002. [DOI] [PubMed] [Google Scholar]
- Lee CR, Grodzinsky AJ, Spector M. Modulation of the contractile and biosynthetic activity of chondrocytes seeded in collagen-glycosaminoglycan matrices. Tissue engineering. 2003;9:27–36. doi: 10.1089/107632703762687500. [DOI] [PubMed] [Google Scholar]
- Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, et al. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. The Journal of clinical investigation. 1999;103:1141–50. doi: 10.1172/JCI5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6626–31. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MF. Contraction reaction: mechanical regulation of Rho GTPase. Trends Cell Biol. 2004;14:111–4. doi: 10.1016/j.tcb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Park S, Costa KD, Ateshian GA, Hong KS. Mechanical properties of bovine articular cartilage under microscale indentation loading from atomic force microscopy. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 2009;223:339–47. doi: 10.1243/09544119JEIM516. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh YC, Na S, Chowdhury F, Ouyang M, Wang Y, Wang N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PloS one. 2009;4:e7886. doi: 10.1371/journal.pone.0007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam AJ, Cunningham JJ, Pillemer BB, Mooney DJ. External mechanical strain regulates membrane targeting of Rho GTPases by controlling microtubule assembly. American journal of physiology Cell physiology. 2003;284:C627–39. doi: 10.1152/ajpcell.00137.2002. [DOI] [PubMed] [Google Scholar]
- Qi YX, Qu MJ, Long DK, Liu B, Yao QP, Chien S, et al. Rho-GDP dissociation inhibitor alpha downregulated by low shear stress promotes vascular smooth muscle cell migration and apoptosis: a proteomic analysis. Cardiovascular research. 2008;80:114–22. doi: 10.1093/cvr/cvn158. [DOI] [PubMed] [Google Scholar]
- Qiao J, Holian O, Lee BS, Huang F, Zhang J, Lum H. Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA. American journal of physiology Cell physiology. 2008;295:C1161–8. doi: 10.1152/ajpcell.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–8. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Ridley A. Rho GTPases. Integrating integrin signaling. J Cell Biol. 2000;150:F107–9. doi: 10.1083/jcb.150.4.f107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu YT, Li S, Marganski WA, Usami S, Schwartz MA, Wang YL, et al. Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophysical journal. 2004;86:2558–65. doi: 10.1016/S0006-3495(04)74311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PG, Roy C, Zhang YN, Chauduri S. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2003;28:436–42. doi: 10.1165/rcmb.4754. [DOI] [PubMed] [Google Scholar]
- Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. The EMBO journal. 2001;20:4639–47. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature reviews Molecular cell biology. 2006;7:265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, et al. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–5. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–39. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–34. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- Yokota H, Leong DJ, Sun HB. Mechanical loading: bone remodeling and cartilage maintenance. Current osteoporosis reports. 2011;9:237–42. doi: 10.1007/s11914-011-0067-y. [DOI] [PubMed] [Google Scholar]
- Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–32. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.