Abstract
Background
Allergic transfusion reactions (ATRs) are among the most common complications of transfusion. Storage in platelet additive solution (PAS) has been shown to reduce ATRs from apheresis platelets (APs). This study evaluated the cost-effectiveness of using PAS storage as an alternative method to reduce ATRs.
Study Design and Methods
A Markov-based decision tree was constructed to compare ATR rates and associated costs expected from current practice and from alternative strategies of using APs stored in PAS. The potential use of premedication was also incorporated. Using a hospital perspective and including direct medical expenses only (US$2012), Monte Carlo microsimulations were run to evaluate outcomes under a base-case analysis. One-way and probabilistic sensitivity analyses were used to assess outcome uncertainty.
Results
Under base-case parameters, using APs stored in PAS for all patients as an initial transfusion protocol is expected to avert ATRs and associated costs, as compared to current practice. Using PAS for all patients along with premedication would be cost-saving only when the additional cost of PAS is below $9.14. If PAS storage could eliminate premedication use, it is expected to result in cost savings when the additional unit cost of PAS is under $11.90. At a PAS cost of $15, averting 1 ATR would cost $701.95. Using PAS storage only in response to recurring mild ATRs is associated with cost savings under all costs of PAS evaluated.
Conclusions
Using PAS storage for all AP transfusions to prevent ATRs may be financially and clinically beneficial, as compared to current practice.
Keywords: allergic transfusion reaction (ATR), platelet additive solution (PAS), platelet, plasma, urticaria, hives, anaphylaxis, hypersensitivity, Markov model, decision-tree
Introduction
Allergic transfusion reactions (ATRs) are the most common complications of blood transfusion, with estimates of ATR rates among platelet transfusions ranging from 1 to 3 percent.1–3 ATRs range from relatively minor urticaria with or without pruritis to severe anaphylactic reactions that produce systemic symptoms of dyspnea, wheezing, hypotension, tachycardia, loss of consciousness, shock, and in rare cases, death.3
In addition to morbidity and mortality from potential adverse clinical effects, ATRs lead to increased health care expenditure by prolonging the time required by physicians, nursing staff, and technologists for the transfusion procedure and the evaluation of ATRs, as well as by requiring additional resources to address the ATRs directly.4 Mild ATRs typically require treatment with antihistamines, and severe ATRs can lead to costly hospitalization for outpatients or prolonged hospital stays for inpatients. Methods to prevent ATRs are still in development. Although premedication is often administered in an attempt to prevent ATRs,5 observational studies and clinical trials have shown that current use of diphenhydramine premedication is not effective.6 Clinical experience has suggested, however, that ATRs may be avoided by reducing the volume of plasma in transfused platelet products; it has recently been demonstrated that concentrating platelets, washing platelets, and washing red blood cells (RBCs) reduces the risk of ATRs.7 Unfortunately, the manipulation of platelets by concentrating or washing is time-consuming for medical technologists, increasing labor costs associated with each unit transfused. Previous studies have also demonstrated that platelet manipulation may reduce the corrected count increment.8 Storing platelets in platelet additive solution (PAS) has been proposed as an alternative to concentrating or washing platelets, and has been shown to have a similar impact on reducing ATRs as these manipulation methods.9–12
A cost-effectiveness analysis of using PAS storage for apheresis platelets (APs) to reduce ATRs has not been conducted, but would be useful in facilitating efficient transfusion-related policies.
Materials and Methods
A Markov-based13,14 decision tree model was constructed using TreeAge Pro Suite 2012 (TreeAge Pro, Williamstown, MA) to compare expected health and financial outcomes between potential strategies of preventing mild and severe ATRs caused by AP transfusions. Markov-based models have been used frequently for cost-effectiveness evaluations involving health-related processes that evolve over time. Monte Carlo microsimulations were run to follow a cohort of hypothetical patients as they experienced AP transfusion therapy and faced risks from potential ATRs. All reactions were assumed to be clinically diagnosed and classified by severity in a manner consistent with the AABB’s Technical Manual and hemovigilance guidelines from the National Healthcare Safety Network.3,15 Mild reactions were defined by urticaria or hives, while severe reactions were defined by presentation with systemic symptoms of dyspnea, wheezing, hypotension, tachycardia, loss of consciousness, shock, or death. 3,15 For each patient microsimulation, transfusion procedures, ATRs, and resulting AP manipulation were tracked, along with the costs associated with these transfusion-related events. A hospital perspective was adopted, including direct medical costs only and adjusting all costs to $US2012, using the medical care component of the Consumer Price Index.
Model structure
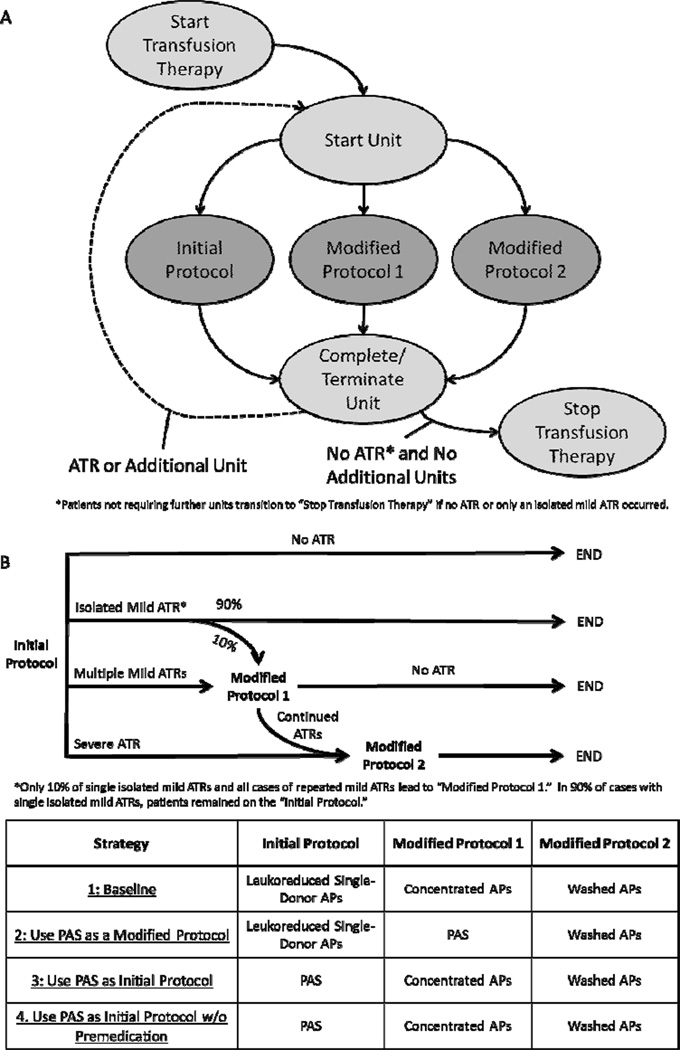
Seven Markov states, shown in Figure 1A, were used to model stages of a 1-year period of transfusion therapy: “Start Transfusion Therapy,” “Start Unit,” “Initial Protocol,” “Modified Protocol 1,” “Modified Protocol 2,” and “Complete/Terminate Unit,” and “Stop Transfusion Therapy.” During each microsimulation, simulated patients traversed the Markov process, facing strategy-dependent risks from ATRs, and accumulating costs associated with the transfusion, potential ATRs, and transfusion protocol modifications. All patients began the simulation in the “Start Transfusion Therapy” state, and transitioned to the “Start Unit” state, corresponding to beginning transfusion of a new AP unit. Units were transfused using one of three protocols: “Initial Protocol,” “Modified Protocol 1,” or “Modified Protocol 2.” All patients were assumed to have no history of prior ATRs, and began transfusion therapy under the “Initial Protocol,” but could undergo modified protocols after developing ATRs. After passing through any of the three protocol states, patients transitioned to the “Complete/Terminate Unit” state. If further transfusions were required, the patient returned to the “Start Unit” state for an additional unit. Otherwise, the patient transitioned to the “Stop Transfusion Therapy.”
Figure 1. Markov Bubble Diagram and Strategy Definitions.
(A) The transfusion procedure was modeled as a Markov process, where all patients began in the “Start Transfusion Therapy” state and transitioned in the next cycle to the “ Start Unit” state to correspond to beginning a new AP unit. Three additional Markov states defined the specific protocol used for transfusing the unit, and all patients proceeded to the “Complete/Terminate Unit” state. If an ATR occurred or another unit was needed, the patient transitioned to the “Start Unit” state, and if no other units were required and no ATRs occurred, the patient transitioned to the “Stop Transfusion Therapy” terminal state. ATR history was tracked, such that patients previously experiencing ATRs were placed immediately on the appropriate modified protocol when being transfused with a new unit. (B)Four strategies, differing by the protocols used initially and in response to allergic transfusion reactions (ATRs), were evaluated. Under each strategy, if multiple mild ATRs occurred in response to the “Initial Protocol,” patients were placed on “Modified Protocol 1.” In 10% of cases with isolated mild ATRs, patients were also placed on “Modified Protocol 1.” If, instead, a severe ATR occurred in response to the “Initial Protocol,” patients were placed on “Modified Protocol 2.” No changes in the transfusion procedure occurred if there was no ATR. If ATRs continued after being placed on “Modified Protocol 1,” patients were placed on “Modified Protocol 2.” Patients who were placed on modified protocols were maintained on those protocols for further AP transfusions.
Transition probabilities between states were defined by ATR incidence rates1 and efficacy of AP manipulation7 or PAS storage9 to reduce ATRs. The three Markov states describing possible transfusion protocols were associated with costs, including expenses associated with the transfusion procedure and any procedure modifications (washing, concentrating, or using PAS storage). Transitions between Markov states were also associated with costs to account for expenses incurred as a result of ATRs.
Each transfused unit could result in one of four outcomes: no ATR, an isolated mild ATR, a repeat mild ATR (occurring among patients who have previously experienced one or more mild ATRs to prior units), or a severe ATR (Fig. 1B). Those patients who experienced mild or severe ATRs would receive drug treatment for the ATRs. Mild ATRs were treated with 25 mg diphenhydramine, 20 mg famotidine, 50 mg hydroxyzine, or 125 mg methylprednisolone, where patients had an equal chance of receiving each medication. Severe reactions were treated with epinephrine (0.3 mg IM). After this treatment, these patients would be placed on modified transfusion protocols for further units. However, because many physicians may not implement transfusion protocol modifications in response to isolated mild ATRs, 90% of those patients who experienced an isolated mild ATR but had no prior ATR history would continue to be transfused with the initial unit under the same “Initial Protocol,” once the ATR had been treated. In the remaining 10% of such patients, the unit would be terminated and after treating the ATR, transfusion would commence with a new unit under “Modified Protocol 1.” In the event that a patient experienced a repeat mild ATR in response to a unit transfused under the “Initial Protocol,” he or she would also be placed on “Modified Protocol 1” after treatment of the ATR. Finally, patients who experienced a severe ATR from the initial transfusion protocol would receive a more rigorous “Modified Protocol 2,” after treatment of the ATR. Patients who had been placed on “Modified Protocol 1,” but continued to experience ATRs would also be placed on “Modified Protocol 2.” Patients transfused using modified protocols would continue therapy using these modified methods for additional AP units received.
Transfusion Protocols Evaluated
Four strategies of addressing ATRs to AP transfusions were evaluated (Figure 1B), with each strategy characterizing different specifications of each protocol (“Initial Protocol,” “Modified Protocol 1,” and “Modified Protocol 2”). Strategy 1 was defined as the baseline, and characterized current practice at major US hospitals. Under this strategy, the “Initial Protocol” involved leukoreduced APs, with 67% of patients also receiving 25 mg diphenhydramine as premedication.5,7,16,17 “Modified Protocol 1” (for use among 10% of patients who experienced an isolated mild ATR or multiple mild ATRs in response to the “Initial Protocol”) involved transfusion with another unit of APs which had been concentrated using a standard cell washer to remove approximately 67% of plasma, while retaining at least 100 mL for resuspension. “Modified Protocol 2” involved transfusion with APs that had been washed with 1 L normal saline.
Strategy 2 describes a similar approach, but APs stored in PAS were used instead of concentrated APs as “Modified Protocol 1”. Other components of the strategy, including the portion of patients being placed on a modified protocol after experiencing an isolated mild ATR, would be the same as under Strategy 1. Under Strategy 3, all patients would be initially placed on leukoreduced APs stored in PAS (“Initial Protocol”). Subsequent ATRs would be addressed using the same modifications 7 as under Strategy 1. Strategy 4 characterized an identical procedure as Strategy 3, but since premedication has been shown to be ineffective,6 it assumed the elimination of premedication use prior to transfusion of APs.
Under all strategies, ATR history of patients was tracked to inform future transfusion procedures. Patients who experienced repeated mild ATRs from the “Initial Protocol” but were transfused without ATRs after being placed on “Modified Protocol 1” would remain on “Modified Protocol 1” for additional AP transfusions. Those patients who continued to experience ATRs after being placed on “Modified Protocol 1” and were subsequently placed on “Modified Protocol 2” would remain on “Modified Protocol 2” for future AP transfusions. Similarly, those patients who had been placed on “Modified Protocol 2” after experiencing severe ATRs remained on this modified method for further transfusions.
By comparing outcomes under Strategies 2 or 3 to outcomes under Strategy 1, the impact of incorporating PAS storage as a modified or initial protocol was assessed. Comparing outcomes between strategies 3 and 4 provided an estimate of the incremental impact of eliminating premedication usage.
Input Parameters
Each of the transfusion strategies evaluated was associated with different transition probabilities. Input parameters, with base-case values and ranges used in probabilistic sensitivity analysis, are provided in Table 1. Costs associated with each leukoreduced AP unit transfused included blood product acquisition cost, based on reimbursement data from the Centers for Medicare and Medicaid Services,18 non-product costs of labor in the blood bank and during patient care,17 and product and labor costs of premedication. Costs associated with AP manipulation were estimated from existing literature, and product loss expected from AP manipulation by concentrating or washing was incorporated by inflating the manipulated AP unit cost by 20%.8 AP product wastage associated with units that were terminated early due to an ATR was also incorporated, assuming that 10% of transfusions resulting in an ATR would be associated with product wastage, where 50% of the product would be unused. Because consistent costs have not been established for the additional cost of storage in PAS, a base-case value of $15 was assumed and the model was reanalyzed using a range of plausible values ($5, $10, $15, $25, and $50).
Table 1.
Model Input Parameters: Base-Case Values and Ranges used for Sensitivity Base
| Input Parameter | Base-Case Value (Range) | Source |
|---|---|---|
| Transfusion Products and Services | ||
| Product Costs per Unit Leukoreduced Single Donor Apheresis Platelets (APs) | 538.74 (404.–673.43) | 18 |
| Non-product Costsa | 84.13 (63.–105.16) | 4 |
| Manipulation Costs-Washing | 111.69 (83.–139.61) | 29 |
| Manipulation Costs-Concentrating | 111.69 (83.–139.61) | 29 |
| Additional Cost of PAS | 15 (5–50) | Assumed |
| Prophylactic Premedication Costb | 4.11 (3.–5.13) | 17,19 |
| Number of AP Units Transfused per Patient | 10 (1–20) | Assumed |
| Portion of Transfusions using Premedication(%) | 67 (58–72) | 5 |
| Portion of Platelets Lost due to Washing or Concentrating (%) | 20 (15–25) | 8 |
| Portion of Transfusions Resulting in Loss from Early Unit Termination (%) | 10 (5–15) | Assumed |
| Portion of Platelets Lost due to Early Termination (%) | 50 | Assumed |
| Allergic Transfusion Reactions | ||
| Mild ATR Risk from APs (%)c | 1.57 –3) | 1,21 |
| Mild ATR-Non-product costsd | 200.78 (150.–250.98) | 4 |
| Mild ATR-Cost of Antihistamine Medicationse | 2.77(208–3.46) | 19 |
| Portion of Single Isolated Mild ATRs Resulting in Modified Protocol (%) | 10 (0–20) | Assumed |
| Severe ATR Risk from APs (%)c | 0.13 (0.–0.18) | 1 |
| Severe ATR - Cost of Hospital Care | 5515.94 (4136.–6894.92) | 20 |
| Severe ATR-Cost of Epinephrine | 64.44 (48.33–80.55) | 19 |
| AP Manipulation Efficacy to Reduce ATRs | ||
| Efficacy of Concentrating APs (% Reduction) | 73 (65–79) | 7 |
| Efficacy of Washing APs (% Reduction) | 95 (91–97) | 7 |
| Efficacy PAS (% Reduction) | 56(30–80) | 9 |
Non-product costs expenses incurred by labor during patient care or by the blood bank.
Costs of prophylactic premedication included both cost of diphenhydramine (25 mg IV) and the cost of labor associated with premedication administration.
Mild ATR risk calculated as the product of the probability of any ATR1,21 and the probability that an ATR is not severe7. Severe ATR risk calculated as the product of the probability of any ATR and the probability that an ATR is severe.
Cost of labor assumes 1 hour of physician time (combining time of the clinical and transfusion medicine departments), 1 hour of technician time, and 0.67 hours of nurse time.
Cost of antihistamine medications used to treat mild ATRs was calculated as the average cost of diphenhydramine (25 mg IV), famotidine (20 mg IV), hydroxyzine (50 mg PO), and methylprednisolone(125 mg IV).
ATR cost parameters incorporated the published average wholesale price for treatment medication19 and the non-product costs of ATR-specific medical care. The costs of care associated with severe ATRs were assumed to be similar to the direct costs associated with inpatient treatment for food allergies and food-induced anaphylaxis.20 The non-product costs associated with a mild ATR were drawn from previous literature.4
Parameters describing the risk of mild or severe ATRs and the efficacy of AP manipulation in reducing these risks were drawn from previously reported estimates from studies of patients in major US hospitals.2,7,21 Efficacy estimates for the reduction in ATR risk from storage of APs in PAS were drawn from previous published literature.9–11
Analysis
Under the base-case scenario, strategies were analyzed under each potential cost value for PAS storage, using 100,000 individual trials for each simulation run. Expected ATRs and costs were reported under each strategy per unit of APs transfused, assuming that the number of total units per patient was characterized by a triangular distribution (mode= 10, min= 1, max= 20). Although the underlying distribution characterizing this parameter was unknown, a triangular distribution, which allowed for the specification of a minimum, maximum, and most common value, was used as a simplifying assumption. by a triangular distribution (mode= 10, min= 1, max= 20). Although the underlying distribution characterizing this parameter was unknown, a triangular distribution, which allowed for the specification of a minimum, maximum, and most common value, was used as a simplifying assumption. Under strategies which involved the use of PAS storage (Strategies 2–4), the additional cost per averted ATR was also calculated when the strategy was not cost-saving compared to the current practice defined by Strategy 1. One-way sensitivity analysis was used to evaluate the impact of variation in the cost of PAS. Probabilistic sensitivity analysis, using 10,000 samples of 10,000 trials each was conducted to evaluate the impact of uncertainty in sample-level input parameters. Costs were varied by 25% in either direction using an adjustment factor sampled from a triangular distribution (mode=1; min=0.75, max=1.25). Efficacy estimates and incidence rates were drawn from beta distributions, using the 95% CI reported by the original data source.
Results
Under the base-case scenario, for the entire range of PAS storage costs evaluated ($5–50), use of APs stored in PAS as a modified protocol in response to mild ATRs (Strategy 2) was cost-saving compared to the current practice of using concentrated APs after a mild ATR (Strategy 1), as shown in Table 2. Net cost per AP unit transfused, including costs from the transfusion and any costs associated with ATRs, would be $1.65 to $2.03 lower under Strategy 2 than Strategy 1, depending on the additional cost of PAS storage. Strategies 3 and 4, characterized by the use of APs stored in PAS as an initial transfusion protocol for all patients, were also cost-saving compared to Strategy 1 when the additional cost of PAS storage was low. Under a scenario where the cost of PAS storage was $5, total costs per AP unit transfused were $3.57 less under Strategy 3 than Strategy 1. At this cost of PAS, Strategy 4, characterized by the use of PAS as an initial protocol for all patients and the elimination of premedication use, was associated with an additional cost savings of $2.73, equivalent to a total cost savings of $6.30 per unit transfused compared to Strategy 1. Compared to Strategy 1, Strategy 2 was not associated with reduced ATRs, while Strategies 3 and 4 were associated with a 55.42% reduction in ATR risk. Because premedication has not been shown to reduce ATR risk, the clinical benefits of Strategies 3 and 4 were identical, and thus Strategy 4 was dominant to Strategy 3.
Table 2.
Base-Case Analysis: Cost and ATR Risk per AP Unit Transfused under Evaluated Strategies
| Cost to Prevent 1 ATR ($) | ||||||
|---|---|---|---|---|---|---|
| Cost of PAS ($) | Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 | Strategy 3f | Strategy 4g |
| 5 | ||||||
| Total Cost ($)a | 641.16 | 639.13 | 637.59 | 634.86 | --- | --- |
| Cost of Transfusion ($)b | 631.64 | 629.57 | 633.25 | 630.52 | ||
| Cost of ATRs ($)c | 9.52 | 9.56 | 4.34 | 4.34 | ||
| Change in Total Cost ($)d | 0.00 | −2.03 | −3.57 | −6.30 | ||
| 10 | ||||||
| Total Cost ($)a | 641.52 | 639.51 | 642.37 | 639.63 | 98.38 | --- |
| Cost of Transfusion ($)b | 631.76 | 629.74 | 638.14 | 635.40 | ||
| Cost of ATRs ($)c | 9.76 | 9.77 | 4.23 | 4.23 | ||
| Change in Total Cost ($) d | 0.00 | −2.01 | 0.85 | −1.89 | ||
| 15 | ||||||
| Total Cost ($) a | 641.44 | 639.44 | 647.49 | 644.76 | 701.95 | 384.86 |
| Cost of Transfusion ($)b | 631.75 | 629.72 | 643.21 | 640.47 | ||
| Cost of ATRs ($)c | 9.69 | 9.72 | 4.28 | 4.28 | ||
| Change in Total Cost ($)d | 0.00 | −2.00 | 6.05 | 3.32 | ||
| 25 | ||||||
| Total Cost ($)a | 641.10 | 639.21 | 657.13 | 654.39 | 1882.45 | 1561.17 |
| Cost of Transfusion ($)b | 631.67 | 629.77 | 653.00 | 650.26 | ||
| Cost of ATRs ($)c | 9.43 | 9.44 | 4.13 | 4.13 | ||
| Change in Total Cost ($)d | 0.00 | −1.89 | 16.03 | 13.30 | ||
| 50 | ||||||
| Total Cost ($)a | 641.52 | 639.88 | 682.23 | 679.49 | 4747.36 | 4428.24 |
| Cost of Transfusion ($)b | 631.83 | 630.17 | 677.91 | 675.17 | ||
| Cost of ATRs ($)c | 9.70 | 9.71 | 4.32 | 4.32 | ||
| Change in Total Cost ($)d | 0.00 | −1.65 | 40.70 | 37.97 | ||
| Average Across PAS Costs Evaluated | ||||||
| ATR Risk (%)e | 1.54 | 1.55 | 0.69 | 0.69 | ||
| Change in ATR Risk (%) | 0.20 | −55.42 | −55.42 | |||
Total cost per AP unit transfused includes direct medical expenses associated with the transfusion, any ATRs, and any AP manipulations.
Cost of Transfusion includes any direct medical expenses associated with the initial procedure, any AP manipulation, and any modified transfusion procedure.
Cost of ATRs includes any direct medical expenses associated with diagnosis, treatment, and monitoring of a mild or severe ATR.
Change in total cost calculated using Strategy 1 total cost as the baseline. Negative values represent cost savings.
ATR Risk calculated as an average across evaluated costs of PAS ($5, 10, 15, 25, 50)
Cost to avert 1 ATR calculated by comparing the costs and rate of ATRs between Strategy 1 and Strategy 3. Strategy 3 is cost-saving compared to Strategy 1 when PAS costs $5.
Cost to avert 1 ATR calculated by comparing the costs and rate of ATRs between Strategy 1 and Strategy 4. Strategy 4 is cost-saving compared to Strategy 1 when PAS costs $5 or $10.
Cost savings associated with Strategy 2 were attributable to decreased expenses from transfusion, as the additional cost of AP storage in PAS was less than the additional cost of AP concentration. However, because the efficacy of PAS storage9 in decreasing the risk of ATRs was assumed to be slightly less than the efficacy of concentrated APs7, the estimated prevalence of ATRs was slightly greater under Strategy 2 than Strategy 1. Since the majority of patients experiencing an isolated mild ATR were not placed on a modified transfusion protocol, any increase in transfusion costs associated with increased ATRs under Strategy 2 was minor.
The total cost (including transfusion-related and ATR-related expenses) to prevent one ATR using PAS storage increased from $701.95 when the cost of PAS storage was $15 to $4747.36 when the cost of PAS storage was $50 (Table 2).
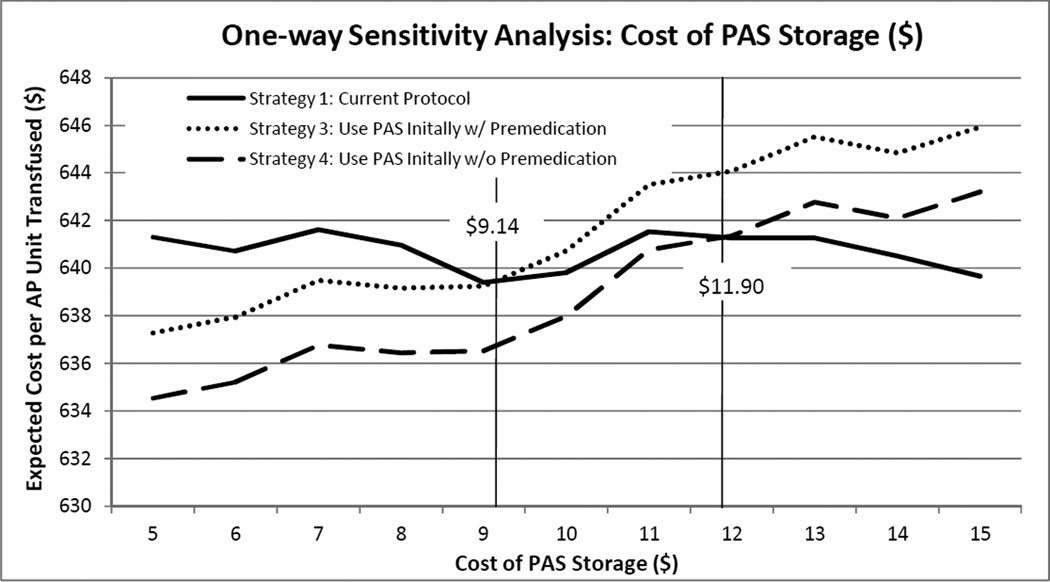
Cost savings associated with Strategies 3 and 4 (PAS storage as the initial protocol, with or without premedication) were driven by reduction in ATRs and resulting costs. As the parameter describing the cost of PAS increased, however, the increased costs associated with transfusion under Strategies 3 and 4 overwhelmed savings associated with reduced ATRs, resulting in these alternatives no longer being cost saving compared to Strategy 1. As shown in Figure 2, one-way sensitivity analysis varying the cost of PAS storage while holding all other input parameters constant at their base-case values demonstrated that Strategy 3 would be cost-saving compared to Strategy 1 while the cost of PAS storage was less than $9.14, and that Strategy 4 would be cost-saving compared to Strategy 1 while the cost of PAS storage was less than $11.90. The difference in these cost threshold values was attributable to the cost of premedication associated with Strategy 3.
Figure 2. One-way Sensitivity Analysis Varying the Cost of PAS Storage.
Strategy 1, defining current practice at major US hospitals, is compared to Strategies 3 and 4, defining the use of APs stored in PAS as an initial protocol for transfusion patients. Strategy 4 is distinguished from Strategy 3 in that premedication use is eliminated. Using one-way sensitivity analysis, all other input variables were held constant at their base-case values while the cost of PAS storage was varied. When PAS storage costs less than $11.90, Strategy 4 is cost-saving compared to Strategy 1. This threshold cost for Strategy 3 is $9.14.
The total cost (including transfusion-related and ATR-related expenses) to prevent one ATR using PAS storage increased from $701.95 when the cost of PAS storage was $15 to $4747.36 when the cost of PAS storage was $50. Probabilistic sensitivity analysis results, shown in Table 3, suggest that cost savings associated with fewer ATRs when using APs stored in PAS persist after considering uncertainty in input parameters.
Table 3.
Probabilistic Sensitivity Analysis: and ATR Risk per AP Unit Transfused under Evaluated Strategies
| Cost to Prevent 1 ATR ($) | ||||||
|---|---|---|---|---|---|---|
| Cost of PAS($) | Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 | Strategy 3f | Strategy 4g |
| 5 | ||||||
| Total Cost($)a | 642.20 | 640.12 | 638.30 | 635.57 | --- | --- |
| 95% CI | (523.03, 766.57) | (522.57, 764.02) | (520.24, 762.88) | (517.14, 760.17) | ||
| Cost of Transfusion($)b | 632.74 | 630.63 | 634.12 | 631.39 | ||
| Cost of ATRs($)c | 9.46 | 9.49 | 4.18 | 4.18 | ||
| Change in Total Cost($)d | 0.00 | −2.08 | −3.90 | −6.63 | ||
| 10 | ||||||
| Total Cost($)a | 640.39 | 638.31 | 641.40 | 638.68 | 118.97 | --- |
| 95% CI | (518.24, 761.73) | (517.11, 759.58) | (522.85, 762.6) | (520.06, 759.35) | ||
| Cost of Transfusion($)b | 630.86 | 628.76 | 637.15 | 634.43 | ||
| Cost of ATRs($)c | 9.53 | 9.55 | 4.25 | 4.25 | ||
| Change in Total Cost($)d | 0.00 | −2.08 | 1.02 | −1.71 | ||
| 15 | ||||||
| Total Cost($)a | 637.13 | 635.09 | 643.03 | 640.28 | 678.19 | 362.50 |
| 95% CI | (510., 766.21) | (509.13, 763.2) | (520.63, 770.11) | (518.05, 767.09) | ||
| Cost of Transfusion($)b | 627.35 | 625.29 | 638.66 | 635.91 | ||
| Cost of ATRs($)c | 9.78 | 9.81 | 4.37 | 4.37 | ||
| Change in Total Cost($)d | 0.00 | −2.04 | 5.90 | 3.15 | ||
| 25 | ||||||
| Total Cost($)a | 641.80 | 639.84 | 657.82 | 655.08 | 1896.08 | 1572.36 |
| 95% CI | (524.53, 763.06) | (522.83, 760.29) | (541.15, 778.28) | (538.82, 775.64) | ||
| Cost of Transfusion($)b | 632.44 | 630.46 | 653.67 | 650.94 | ||
| Cost of ATRs($)c | 9.36 | 9.38 | 4.15 | 4.15 | ||
| Change in Total Cost($)d | 0.00 | −1.96 | 16.02 | 13.29 | ||
| 50 | ||||||
| Total Cost($)a | 643.73 | 641.98 | 684.27 | 681.53 | 4681.67 | 4365.12 |
| 95% CI | (519.77, 764.5) | (518.97, 762.05) | (561.08, 804.04) | (558.45, 801.3) | ||
| Cost of Transfusion($)b | 633.99 | 632.22 | 679.90 | 677.16 | ||
| Cost of ATRs($)c | 9.74 | 9.77 | 4.37 | 4.37 | ||
| Change in Total Cost($)d | 0.00 | −1.74 | 40.54 | 37.80 | ||
| Average Across PAS Costs Evaluated | ||||||
| ATR Risk (%)e | 1.55 | 1.55 | 0.69 | 0.69 | ||
| Change in ATR Risk (%) | 0.27 | −55.37 | −55.37 | |||
Total cost per AP unit transfused includes direct medical expenses associated with the transfusion, any ATRs, and any AP manipulations.
Cost of Transfusion includes any direct medical expenses associated with the initial procedure, any AP manipulation, and any modified transfusion procedure.
Cost of ATRs includes any direct medical expenses associated with diagnosis, treatment, and monitoring of a mild or severe ATR.
Change in total cost calculated using Strategy 1 total cost as the baseline. Negative values represent cost-savings.
ATR Risk calculated as an average across evaluated costs of PAS ($5, 10, 15, 25, 50)
Cost to avert 1 ATR calculated by comparing the costs and rate of ATRs between Strategy 1 and Strategy 3. Strategy 3 is cost-saving compared to Strategy 1 when PAS costs $5.
Cost to avert 1 ATR calculated by comparing the costs and rate of ATRs between Strategy 1 and Strategy 4. Strategy 4 is cost-saving compared to Strategy 1
Discussion
ATRs are among the most common problems faced by many transfusion services. Despite premedication, many patients continue to experience severe urticarial reactions.6 It has been shown that similar to concentrating and washing APs, storage of platelets in PAS can reduce ATRs.9,11,22
This model assessed the financial and health implications of transfusing patients with APs stored in PAS to prevent ATRs. Direct medical expenses and ATR rates expected from a strategy defined by current practice of using AP manipulation (concentrating or washing) to respond to ATRs were compared to outcomes expected from alternative strategies of using PAS storage as an initial or modified transfusion protocol. The three strategies incorporating PAS storage included one using APs stored in PAS as a modified protocol in response to repeated mild ATRs (Strategy 2), another using PAS storage as an initial protocol for all AP transfusion patients (Strategy 3), and a third using PAS storage along with the elimination of premedication use as an initial protocol (Strategy 4). Results suggest that using APs stored in PAS instead of APs stored in plasma for all transfusion patients as an initial protocol would result in fewer ATRs. Using PAS as an initial protocol along with premedication is cost-saving when the additional cost of PAS storage is less than $9.14, and using PAS storage without premedication is expected to be cost-saving while the additional cost of PAS storage is less than $11.90. Despite its use in many major US hospitals, there does not appear to be any clinical or financial benefit to using premedication.6 A strategy of using APs stored in PAS instead of concentrated APs as a modified protocol in response to recurrent mild ATRs was cost -saving under all evaluated values of the cost of PAS storage.
This study has several limitations and likely underestimates the true cost savings associated with PAS storage. The precise efficacy of PAS storage to prevent ATRs is unknown, but may be similar to the preventative efficacy of concentrating APs (73%).7 This analysis incorporated a conservative estimate of PAS storage efficacy, using a previously published estimate of 56%.9 Cost parameters incorporated direct medical expenses only, ignoring direct non-medical costs, patient or caregiver time, and lost productivity, which may cause additional financial burden associated with ATRs.20 Thus, potential savings from averted ATRs may actually be greater than suggested by these results. Furthermore, storage of APs in PAS will result in additional available plasma for use in other transfusions or for fractionation, as well as cost savings associated with this additional blood resource.
Although this model focused on the reduction of ATRs associated with PAS stored APs, other advantages of using PAS may exist, including reduced risk of transfusion related acute lung injury (TRALI), and reduced risk of ABO mismatched hemolysis.10 Any cost savings associated with these additional advantages were not incorporated in this analysis.
AP manipulation through concentration or washing substantially reduces the number of ATRs,7 but preliminary studies indicate that this manipulation decreases the number of platelets and the platelets may not be as functional.8,23,24 Although expected product loss from washing or concentrating APs was accounted for in this model, decreased corrected count increment (CCI) with washed APs, resulting in more frequent transfusions to maintain platelet counts, was not incorporated. The impact of PAS of CCI is not well documented. Thus, these influences were not included in the model.
The costs of transfusion incorporated in this model did not include expected expenses associated with HLA matching, irradiation, or other AP product testing or manipulation, since these are not likely to affect ATR risk16 and would not be affected by washing, concentrating, or PAS storage. These additional costs would likely be consistent across strategies evaluated and thus would not affect outcomes. It is also important to note that differences in cost are considered in universal terms and are not apportioned among costs for product acquisition, blood bank services, or clinical services. Such apportionment may be critical for incentivizing changes in transfusion practices.
While this analysis suggests that strategies 3 and 4 are expected to yield cost savings under threshold values of the cost of PAS and result in decreased ATRs, these strategies may require additional expenditure from hospitals for AP product acquisition. If those individuals at higher risk of experiencing an ATR could be identified before transfusion, an alternative strategy of only using PAS storage among higher-risk patients could be implemented. This approach would avoid additional unnecessary costs for those patients who are at low-risk of having an ATR and would not benefit from the PAS. However, it is often difficult to predict which patients would be at increased ATR risk.
Because the safety of blood transfusions is highly prioritized, transfusion-related interventions that are not cost-saving may still be widely encouraged. Although cost-saving programs which also provide clinical benefit are clearly advantageous, programs associated with a wide range of additional costs are still considered reasonable if they provide sufficient clinical benefit. For example, substantial risk reduction in TRALI and hemolytic transfusion reactions is obtained using patient barcoding, online databases, and exclusion of high-risk donors, at an additional cost of just $14–28 per unit.25 A more expensive intervention, HIV nucleic acid amplification testing, has been shown to have a marginal cost effectiveness of $2 million per additional quality-adjusted life year gained.26 Thus, the potential to reduce ATRs using PAS storage may be acceptable even if the additional cost of PAS is higher than the threshold values reported.
Although the pathophysiology of ATRs has not been fully elucidated, it is clear that the plasma component of APs and RBCs play an essential role in etiology. Biogenic amines, eosinophil and neutrophil chemotactic factors, enzymes, leukotrienes, prostaglandins, platelet activating factor and numerous cytokines have all been found in the plasma and been implicated in ATRs.27,28 Understanding how to prevent ATRs by limiting plasma exposure could provide the basis of new preventative techniques. This analysis suggests that using APs stored in PAS as an initial transfusion product for AP transfusion patients may be a financially and clinically appealing strategy to prevent ATRs.
Acknowledgements
A.A.R.T. and S.K. were supported by the Doris Duke Charitable Foundation Clinician Scientist Development Award (#22006.02), and A.A.R.T. was also supported by the NIH 1K23AI093152-01A1. W.J.S. was supported by an American Society of Hematology Scholar award and NIH R21HL107828-01A1.
This study was supported in part by an unrestricted gift from Fenwal Inc. to Johns Hopkins University. J.M. serves on the scientific advisory board of Fenwal Inc.
Footnotes
Conflicts of Interest:
S.K, W.J.S., K.D.F, P.M.N, K.E.K, and A.A.R.T declare no conflicts of interest.
References
- 1.Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–320. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 2.Enright H, Davis K, Gernsheimer T, McCullough JJ, Woodson R, Slichter SJ. Factors influencing moderate to severe reactions to PLT transfusions: experience of the TRAP multicenter clinical trial. Transfusion. 2003;43:1545–1552. doi: 10.1046/j.1537-2995.2003.00529.x. [DOI] [PubMed] [Google Scholar]
- 3.Roback JDCM, Grossman MR, Hillyer CD. Technical Manual. 16th ed. Bethesda: American Association of Blood Banks; 2008. [Google Scholar]
- 4.Riley W, Smalley B, Pulkrabek S, Clay ME, McCullough J. Using lean techniques to define the platelet (PLT) transfusion process and cost-effectiveness to evaluate PLT dose transfusion strategies. Transfusion Published online. 2012 Feb 10; doi: 10.1111/j.1537-2995.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanders RP, Maddirala SD, Geiger TL, Pounds S, Sandlund JT, Ribeiro RC, Pui CH, Howard SC. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130:781–787. doi: 10.1111/j.1365-2141.2005.05670.x. [DOI] [PubMed] [Google Scholar]
- 6.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–1096. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 7.Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51:1676–1683. doi: 10.1111/j.1537-2995.2010.03008.x. [DOI] [PubMed] [Google Scholar]
- 8.Karafin M, Fuller AK, Savage WJ, King KE, Ness PM, Tobian AA. The impact of apheresis platelet manipulation on corrected count increment. Transfusion. 2012;52:1221–1227. doi: 10.1111/j.1537-2995.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerkhoffs JL, Eikenboom JC, Schipperus MS, van Wordragen-Vlaswinkel RJ, Brand R, Harvey MS, de Vries RR, Barge R, van Rhenen DJ, Brand A. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108:3210–3215. doi: 10.1182/blood-2006-04-020131. [DOI] [PubMed] [Google Scholar]
- 10.Alhumaidan H, Sweeney J. Current status of additive solutions for platelets. J Clin Apher. 2012;27:93–98. doi: 10.1002/jca.21207. [DOI] [PubMed] [Google Scholar]
- 11.de Wildt-Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: a prospective, randomized study. Transfusion. 2000;40:398–403. doi: 10.1046/j.1537-2995.2000.40040398.x. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney J. Additive solutions for platelets: is it time for North America to go with the flow? Transfusion. 2009;49:199–201. doi: 10.1111/j.1537-2995.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 14.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Manual: Biovigilance Component. http://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-Protocol-1-3-1-June-2011.pdf.
- 16.Savage WJ, Tobian AA, Savage JH, Wood RA, Schroeder JT, Ness PM. Scratching the Surface of Allergic Transfusion Reactions. Transfusion. doi: 10.1111/j.1537-2995.2012.03892.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions. Management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128:991–995. doi: 10.5858/2004-128-991-FNTR. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services. Hospital Outpatient Prospective Payment System: Anndendum A Update. 2012 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates-Items/2012-July-Addendum-Ahtml.
- 19.Red Book 2010. Pharmacy's Fundamental Reference. 114. Thomson, Montvale: New Jersey: PDR Network; [Google Scholar]
- 20.Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. Journal of Allergy and Clinical Immunology. 2011;128 doi: 10.1016/j.jaci.2011.03.013. 110-U87. [DOI] [PubMed] [Google Scholar]
- 21.Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic transfusion reactions to platelets are associated more with recipient and donor factors than with product attributes. Transfusion. 2011;51:1716–1722. doi: 10.1111/j.1537-2995.2010.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azuma H, Hirayama J, Akino M, Miura R, Kiyama Y, Imai K, Kasai M, Koizumi K, Kakinoki Y, Makiguchi Y, Kubo K, Atsuta Y, Fujihara M, Homma C, Yamamoto S, Kato T, Ikeda H. Reduction in adverse reactions to platelets by the removal of plasma supernatant and resuspension in a new additive solution (M-sol) Transfusion. 2009;49:214–218. doi: 10.1111/j.1537-2995.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 23.Moroff G, Friedman A, Robkin-Kline L, Gautier G, Luban NL. Reduction of the volume of stored platelet concentrates for use in neonatal patients. Transfusion. 1984;24:144–146. doi: 10.1046/j.1537-2995.1984.24284173346.x. [DOI] [PubMed] [Google Scholar]
- 24.Tanz WS, Ansari-Lari M, King KE, Ness PM. Efficacy of washed platelets for the treatment of severe allergic transfusion reactions. Blood. 2001;98:827a. [Google Scholar]
- 25.Klein HG, Anderson D, Bernardi MJ, Cable R, Carey W, Hoch JS, Robitaille N, Sivilotti ML, Smaill F. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion. 2007;47:2338–2347. doi: 10.1111/j.1537-2995.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 26.van Hulst M, de Wolf JT, Staginnus U, Ruitenberg EJ, Postma MJ. Pharmaco-economics of blood transfusion safety: review of the available evidence. Vox Sang. 2002;83:146–155. doi: 10.1046/j.1423-0410.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- 27.Vamvakas EC, Pineda AA. Allergic and Anaphylactic Reactions. In: Popovsky MA, editor. Transfusion reactions. Bethesda: AABB Press; 2001. pp. 83–127. [Google Scholar]
- 28.Savage WJ, Tobian AA, Savage JH, Wood RA, Schroeder JT, Ness PM. Scratching the surface of allergic transfusion reactions. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta P, LeRoy SC, Luikart SD, Bateman A, Morrison VA. Long-term blood product transfusion support for patients with myelodysplastic syndromes (MDS): cost analysis and complications. Leuk Res. 1999;23:953–959. doi: 10.1016/s0145-2126(99)00113-7. [DOI] [PubMed] [Google Scholar]