Abstract
The G-protein coupled receptor, Mc1r, plays a major role in pigment production and has been reported to be important in the inflammatory response. We have investigated the effect of deficiency in Mc1r on UV inflammation. Mice on the same genetic background were used- C57BL/6-c (albino), C57BL/6 (black), C57BL/6-Mc1re/e deficient (yellow). FACS analysis of disaggregated skin showed a similar dose-dependent increase in Ly6G+ and CD11b+ cells in response to UV radiation in all groups. No differences in UV-induced edema or in DNA damage were detected between groups. The contact hypersensitivity response, neonatal immune tolerance and UV immunosuppression were all similar in C57BL/6 and C57BL/6-Mc1re/e mice. We conclude that the absence of Mc1r does not impair the inflammatory response to UV radiation or the generation of immunosuppression.
Keywords: UV, inflammation, Mc1r, immune suppression
Background
The melanocortin-1 receptor (MC1R), a member of the G-protein- coupled receptor family (GPCR), is expressed by all cutaneous cell types and is responsive to alpha-melanocyte stimulating hormone (α-MSH) is derived from the pro-opiomelanocortin (POMC) precursor protein (1,2). The MC1R regulates the amount and type of melanin production and thus human skin phototype and its sensitivity to UV-induced damage (1,2). Polymorphisms in the human MC1R are a well-established risk factor for melanoma (3–5). The MC1R has been proposed as the receptor responsible for the anti-inflammatory effects of α-MSH and related peptides (6–8) since administration of α-MSH results in reduction of the expression of pro-inflammatory cytokines (9) and adhesion molecules (7), thus affecting the humoral and cellular phases of inflammation (10–12). Further, MSH has been shown to decrease contact hypersensitivity and induce immune tolerance in mice, indicating an immunosuppressive role (13).
Mc1re/e yellow mice, however, which have a spontaneous mutation resulting in a non-functional Mc1r, have yielded conflicting findings on the role of Mc1r in inflammation (14,15). UV radiation causes inflammation (16) and stimulates production and release of MSH (17) but the role of Mc1r in these events has not been directly addressed in vivo.
Questions addressed
We have used quantitation of the influx of inflammatory cells in response to UV radiation (18) and of immunosuppression by UV radiation (19) to establish if the Mc1r is required for UV-induced inflammation and immunosuppression in the mouse.
Experimental design
We used syngeneic black pigmented (C57BL/6), yellow Mc1r deficient (C57BL/6-Mc1re/e) and albino (C57BL/6-c) mice. Routine histology and FACS analysis of disaggregated skin for Ly6G+ and CD11b+ cells were employed to assess the inflammatory response to UV radiation and DNA damage was detected by immunohistochemistry (18). Edema was assessed as increase in skin thickness. We investigated both systemic suppression by UV radiation of contact hypersensitivity to trinitrochlorobenzene (TNCB) and UV-independent neonatal tolerance. All animal work was performed in accordance with NIH guidelines for the Human Care and Use of Laboratory Animals. For detailed methods see Supplementary materials.
Results
UV irradiation (8.4 kJ/m2 from F40 sunlamps) induced similar DNA damage in the epidermis and dermis in C57BL/6-c, C57BL/6 and C57BL/6-Mc1re/e strain animals (Fig. 1a) and resulted in marked cellular infiltrate into the upper dermis and epidermis of all strains of mice (Fig. 1b). Inflammation was accompanied by significant edema with a more than two-fold increase in skin thickness with no significant differences between strains (Fig. S1). The underside of skin upon UV irradiation showed an angiogenic effect (18) which was similar in all tested groups (data not shown).
Figure 1.
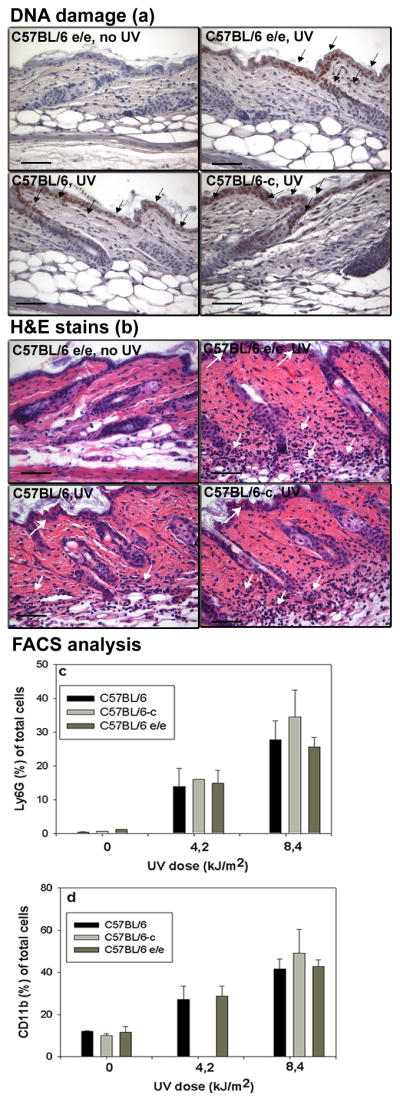
(a) DNA damage in mouse skin within 1h after irradiation with 8.4 kJ/m2 UV irradiation detected by antithymine dimer antibody conjugated with HRP. DNA damage (brown nuclear staining marked by black arrows). Bar= 50μm.
(b) H&E stain of adult mouse skin 48h after UV irradiation (8.4 kJ/m2). White arrows designated infiltrating inflammatory cells. Bar= 50μm.
Dose dependent infiltrate of Ly6G+ cells (c) and CD11b+ cells (d) in adult C57BL/6-c (albino), C57BL/6-Mc1re/e (yellow) and C57BL/6 (black) mouse skin 48h after UV irradiation. Significant difference between UV irradiated and untreated skin (t-test, p <0.001) in each group. No differences between the UV response in the 3 tested groups of mice (p=NS, ANOVA).
We previously identified in UV irradiated skin a population of CD11b+Ly6G+ infiltrating cells, almost exclusively neutrophils. Ly6G single staining is sufficient to recognize and quantify this population (18). We also used CD11b to monitor other leukocytes such as monocytes, macrophages, and lymphocytes. FACS sorting confirmed that UV irradiated adult skin of all strains 48h after treatment showed a dose-dependent neutrophil influx. However, the percentage of Ly6G + cells was similar in all tested groups (Fig. 1c). A similar effect with lack of difference between strains was found with CD11b staining (Fig. 1d)
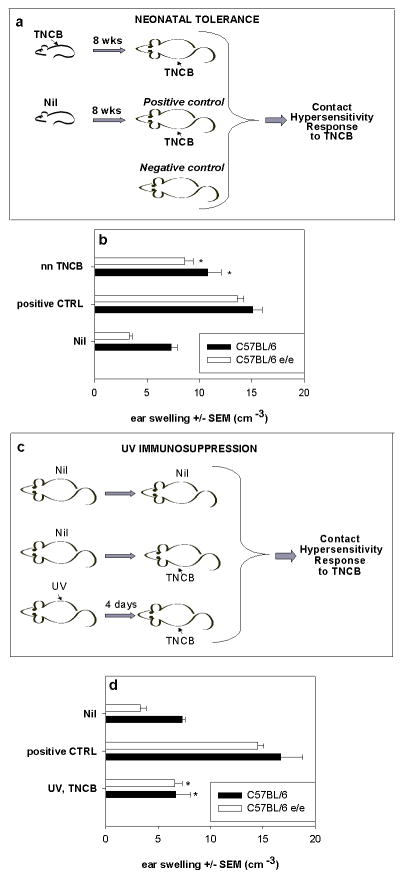
We previously demonstrated that FVB strain mice showed neonatal immune tolerance (18). The contact hypersensitivity response of adult mice was significantly reduced if the animals had previously been exposed to the same antigen as neonates. Similar levels of neonatal tolerance were found in C57BL/6-Mc1re/e and C57BL/6 strains after neonatal exposure to TNCB (Fig. 2a,b) similar to those previously described in albino FVB mice (18). Systemic immunosuppression of contact hypersensitivity by UV irradiation prior to sensitization occurs in most strains of mice (19) and is not genetically linked to black or brown pigmentation (20). UV immunosuppression in C57BL/6-Mc1re/e mice occurred at a level similar to that for C57BL/6 mice (Fig. 2c,d) (71% vs 75%). Thus, C57BL/6-Mc1re/e mice did not show differences from the parent C57BL/6 animals in the generation of immunosuppression.
Figure 2.
Neonatal tolerance (a,b) and UV immunosuppression (c,d) are not affected by loss of Mc1r. Negative control mice (Nil) were not sensitized, positive control mice (CTRL) were sensitized only as adults, test group (nnTNCB) were first sensitized with TNCB as pups and resensitized as adults according to the schema in a). In b) CHS to TNCB determined as ear swelling ± SEM, 24h after challenge in 8-week-old C57BL/6 and C57BL/6-Mc1re/e mice is shown. Ear swelling in mice which had nnTNCB was significantly different from the positive control in each group (*p<0.05, t-test). 4 mice per group.
UV immunosuppression was induced in 8 week old mice by exposure to 4.5 kJ/m2 of UV, followed by sensitization at an unirradiated site 3 days later with TNCB and challenge 4 days later as shown in schema c). Ear swelling in UV,TNCB mice (d) was significantly different from positive control within each group (*p<0.05, t-test). Each experimental group consisted of 4 to 5 animals.
Conclusions
We have demonstrated that a genetically determined lack of functional Mc1r in mice did not alter inflammatory responses to UV or affect either UV immunosuppression or UV-independent neonatal immune tolerance. These findings are in contrast to published work in which administration of α-MSH was anti-inflammatory and induced immune tolerance (11–13). Our studies do not, however, exclude an anti-inflammatory and immunomodulatory role for α-MSH, but show that Mc1r is not a necessary player. Our findings agree with studies showing that Mc3r but not Mc1r is the predominant anti-inflammatory receptor for melanocortins (14,21). Although UV upregulates α-MSH in keratinocytes and melanocytes (1,17) and POMC, the α-MSH precursor in cultured skin explants (22) there is limited evidence that UV upregulates α-MSH in human or mouse skin in vivo (1). It appears likely that α-MSH will be upregulated in skin in response to the inflammatory UV doses we have used but further studies will be required to resolve this point.
Mouse and human melanocortin 1 receptors differ in receptor number, responsiveness to MSH and ligand-independent signalling (10,23) and loss of POMC in C57BL/6 mice does not affect pigmentation (24). C57BL/6 skin also expresses Mc2r, raising the question of a role for ACTH mediated effects (25). The truncated Mc1r receptor in the recessive yellow mouse, where the ability to engage cAMP is totally absent, differs from the human polymorphisms in MC1R which encode a complete receptor, albeit one with altered function. In humans there is conflicting evidence on correlation between MC1R genotype and erythema (26,27) and between MC1R genotype and immune responses (12,28). Most studies have focussed on the anti-inflammatory properties of α-MSH from which a role for MC1R in UV sensitivity has been inferred.
Our studies in a well established mouse model thus do not support a role for Mc1r in UV induced inflammation and immunosuppression and indicate further studies are required to understand the anti-inflammatory and immunosuppressive actions of α-MSH.
Supplementary Material
Acknowledgments
We thank Dr Lynn Lamoureux for Mc1r deficient mice and Jesse Bahn for technical assistance. Funded by NIH RO1CA53765 (FPN). AWG designed the experiments, performed UV studies, immunohistochemistry and FACS analysis and wrote the paper. EDF set up UV optical sources and monitored dose and wavelength output. FPN determined neonatal tolerance and UV immunosuppression and assisted with writing the paper.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;803:979–1020. doi: 10.1152/physrev.2000.80.3.979. Review. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Malek Z, Scott MC, Suzuki I, et al. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;8:156–62. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- 3.Rana BK, Hewett-Emmett D, Jin L, et al. High polymorphism at the human melanocortin 1 receptor locus. Genetics. 1999;151:1547–1557. doi: 10.1093/genetics/151.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ. Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet. 2001;10:2397–2402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- 5.Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- 6.Manna SK, Aggarwal BB. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. JImmunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- 7.Kalden DH, Scholzen T, Brzoska T, Luger TA. Mechanisms of the antiinflammatory effects of alpha-MSH. Role of transcription factor NF-kappa B and adhesion molecule expression. Ann NY Acad Sci. 1999;885:254–261. doi: 10.1111/j.1749-6632.1999.tb08682.x. [DOI] [PubMed] [Google Scholar]
- 8.Ichiyama T, Sakai T, Catania A, Barsh GS, Furukawa S, Lipton JM. Inhibition of peripheral NF-kappaB activation by central action of alpha-melanocyte-stimulating hormone. JNeuroimmunol. 1999;99:211–217. doi: 10.1016/s0165-5728(99)00122-8. [DOI] [PubMed] [Google Scholar]
- 9.Mason MJ, Van Epps D. Modulation of IL-1, tumor necrosis factor, and C5a-mediated murine neutrophil migration by alpha-melanocyte-stimulating hormone. J Immunol. 1989;142:1646–1651. [PubMed] [Google Scholar]
- 10.Abdel-Malek ZA. Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci. 2001;58:434–441. doi: 10.1007/PL00000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loser K, Brzoska T, Oji V, Auriemma M, Voskort M, Kupas V, Klenner L, Mensing C, Hauschild A, Beissert S, Luger TA. The neuropeptide alpha-melanocyte-stimulating hormone is critically involved in the development of cytotoxic CD8+ T cells in mice and humans. PLoS One. 2010;5:e8958. doi: 10.1371/journal.pone.0008958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auriemma M, Brzoska T, Klenner L, Kupas V, Goerge T, Voskort M, Zhao Z, Sparwasser T, Luger TA, Loser K. α-MSH-Stimulated Tolerogenic Dendritic Cells Induce Functional Regulatory T Cells and Ameliorate Ongoing Skin Inflammation. J Invest Dermatol. 2012;132:1814–1824. doi: 10.1038/jid.2012.59. [DOI] [PubMed] [Google Scholar]
- 13.Grabbe S, Bhardwaj RS, Mahnke K, Simon MM, Schwarz T, Luger TA. alpha-Melanocyte-stimulating hormone induces hapten-specific tolerance in mice. J Immunol. 1996;156:473–478. [PubMed] [Google Scholar]
- 14.Getting SJ, Christian HC, Lam CW, et al. Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol. 2003;170:3323–3330. doi: 10.4049/jimmunol.170.6.3323. [DOI] [PubMed] [Google Scholar]
- 15.Maaser C, Kannengiesser K, Specht C, et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415–1422. doi: 10.1136/gut.2005.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portugal-Cohen M, Soroka Y, Frušić-Zlotkin M, et al. Skin organ culture as a model to study oxidative stress, inflammation and structural alterations associated with UVB-induced photodamage. Exp Dermatol. 2011;20:749–755. doi: 10.1111/j.1600-0625.2011.01317.x. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty AK, Funasaka Y, Slominski A, et al. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 18.Wolnicka-Glubisz A, Damsker J, Constant S, Corn S, De Fabo E, Noonan F. Deficient inflammatory response to UV radiation in neonatal mice. J Leukoc Biol. 2007;81:1352–1361. doi: 10.1189/jlb.1206729. [DOI] [PubMed] [Google Scholar]
- 19.Noonan FP, Hoffman HA. Susceptibility to immunosuppression by ultraviolet B radiation in the mouse. Immunogenetics. 1994;39:29–39. doi: 10.1007/BF00171794. [DOI] [PubMed] [Google Scholar]
- 20.Noonan FP, Hoffman HA. Control of UVB immunosuppression in the mouse by autosomal and sex-linked genes. Immunogenetics. 1994;40:247–256. doi: 10.1007/BF00189969. [DOI] [PubMed] [Google Scholar]
- 21.Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol. 2011;179:259–269. doi: 10.1016/j.ajpath.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson IJ, Budd PS, Keighren M, McKie L. Humanized MC1R transgenic mice reveal human specyfic receptor function. Hum Mol Genet. 2007;16:2341–2348. doi: 10.1093/hmg/ddm191. [DOI] [PubMed] [Google Scholar]
- 24.Slominski A, Plonka PM, Pisarchik A, et al. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146:1245–53. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ermak G, Slominski A. Production of POMC, CRH-R1, MC1, and MC2 receptor mRNA and expression of tyrosinase gene in relation to hair cycle and dexamethasone treatment in the C57BL/6 mouse skin. J Invest Dermatol. 1997;108:160–165. doi: 10.1111/1523-1747.ep12332925. [DOI] [PubMed] [Google Scholar]
- 26.Flanagan N, Ray AJ, Todd C, Birch-Machin MA, Rees JL. The relation between melanocortin 1 receptor genotype and experimentally assessed ultraviolet radiation sensitivity. J Invest Dermatol. 2001;117:1314–1317. doi: 10.1046/j.0022-202x.2001.01532.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith G, Wilkie MJ, Deeni YY, et al. Melanocortin 1 receptor (MC1R) genotype influences erythemal sensitivity to psoralen-ultraviolet A photochemotherapy. Br J Dermatol. 2007;157:1230–1234. doi: 10.1111/j.1365-2133.2007.08209.x. [DOI] [PubMed] [Google Scholar]
- 28.Cooper A, Robinson SJ, Pickard C, Jackson CL, Friedmann PS, Healy E. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. 2005;175:4806– 48013. doi: 10.4049/jimmunol.175.7.4806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.