Abstract
Aims and Objectives
To compare the efficacy, safety, and rate of response of intravenous iron sucrose and intramuscular iron sorbitol therapy for anemia during pregnancy.
Material and Methods
100 antenatal cases of gestational age 14–32 weeks were included in this prospective study. Cases were randomly divided into two groups. Group A, having 50 cases received intravenous iron sucrose, and 50 cases in Group B received intramuscular iron sorbitol. Response to therapy in both groups was studied and compared.
Results
The mean pretherapy hemoglobin in group A was 6.49 gm/dl and in group B was 6.48 gm/dl. The rise in hemoglobin after 4 weeks of starting therapy was 3.52 gm/dl in group A and 2.33 gm/dl in group B. The difference was statistically significant (P < 0.01). The mean time taken to achieve target hemoglobin (≥11 gm/dl) was 6.37 weeks in group A and 9.04 weeks in group B. In group A, 8 % (four) cases had grade I adverse effects. In group B, 24 % (12) cases had grade I adverse effects. The difference was statistically significant (P = 0.027). In both the groups, no case discontinued the therapy.
Conclusion
Intravenous iron sucrose is safe, convenient, more effective, and faster acting therapy than intramuscular iron sorbitol therapy for treating moderate to severe anemia during pregnancy.
Keywords: Iron sucrose, Pregnancy, Iron deficiency anemia, Iron sorbitol
Introduction
Anemia is estimated to affect nearly two-third of the pregnant women in developing countries and iron deficiency anemia accounts for 95 % cases. It is the most common nutritional pathology in pregnant women [1]. Among them, about 45 % of pregnant women suffer from moderate to severe anemia. Anemia is associated with high perinatal morbidity and mortality.
Over the past years, various routine methods like oral iron therapy, intramuscular iron therapy, and blood transfusion were used to treat anemia during pregnancy [2]. These methods are not without deficiencies, and also there are conditions in which these conventional iron therapies are not helpful, like inadequate gastrointestinal absorption, late pregnancy, intolerance to required oral iron, requirement of emergency supplement, and severe anemia with contraindications to blood transfusion [3]. So, to treat these conditions, we require a relatively new mode of iron therapy with better efficacy, less side effects, fast action, and better compliance. Intravenous iron sucrose therapy seems to be a safe, convenient, and more effective treatment for anemia during pregnancy.
Materials and Methods
Total 100 antenatal women between 14 and 32 weeks of gestation with hemoglobin level of 8 gm/dl or less, serum iron less than 60 μg/dl, and total iron-binding capacity more than 400 μg/dl attending the out patient department and antenatal wards were included in the study. The cases having hemoglobin level >8 gm/dl, gestational age <14 or >32 weeks, normal serum iron levels, history of allergic reaction to previous iron therapy, and anemia due to causes other than iron deficiency were excluded from the study. All the 100 cases enrolled in the study were assigned into two groups. Group A including 50 cases received intravenous iron sucrose and Group B including 50 cases received intramuscular iron sorbitol therapy. Iron was given after proper sensitivity testing in both the groups. All the selected cases were subjected to a thorough history taking, general, systemic, and obstetrical examination. The dose of iron required in both the groups was calculated by the formula
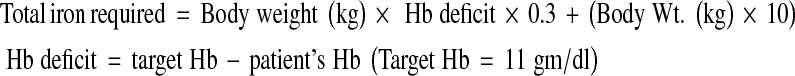 |
In group A, iron sucrose was given as 150 mg (3 ampules, each of 2.5 ml) in 100 ml of 0.9 % normal saline infusion over 1 h every third day up to the total calculated dose. In group B, iron sorbitol complex was given as daily intramuscular injection of 1.5 ml till total calculated dose by means of ‘Z’ technique. All the cases were monitored for adverse effects. Adverse effects were graded as grade I and grade II. Grade I reactions were mild to moderate and settled with an antiallergic drug but not requiring discontinuation of drug. Grade II reaction was severe in nature threatening the life of patient and requiring discontinuation of therapy.
Results
Both the groups were comparable for age, parity, socioeconomic status, and period of gestation (Table 1). The mean pretherapy hemoglobin level in group A was 6.49 gm/dl and in group B was 6.48 gm/dl (Table 2). In group A, the mean hemoglobin level after 2 weeks of starting therapy was 8.79 gm/dl with a rise of 2.3 gm/dl. In group B, the mean hemoglobin level after 2 weeks of starting therapy was 7.74 gm/dl with a rise of 1.26 gm/dl (Table 3). The difference was statistically significant(P < 0.01).
Table 1.
Demographic distribution of cases
| Group A (n = 50) | Group B (n = 50) | |
|---|---|---|
| Mean age (years) | 26.46 | 26.62 |
| Mean period of gestation (weeks) | 24.48 | 23.94 |
| Parity ≥2 (% of cases) | 68 | 56 |
| Soecioeconomic status class IV or lower (% of cases) | 76 | 80 |
Table 2.
Hemoglobin level before starting therapy
| Hemoglobin level (gm/dl) | Group A | Group B | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| ≤4 | 6 | 12 | 3 | 6 |
| 4.1–6 | 8 | 16 | 12 | 24 |
| 6.1–8 | 36 | 72 | 35 | 70 |
| Mean | 6.49 | 6.48 | ||
| P value | >0.05 | |||
Table 3.
Hemoglobin level 2 and 4 weeks after starting therapy
| Hemoglobin level (gm/dl) | After 2 weeks of therapy | After 4 weeks of therapy | ||||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |||||
| No. | % | No. | % | No. | % | No. | % | |
| 5–7 | 7 | 14 | 12 | 24 | – | – | 5 | 10 |
| 7.1–9 | 16 | 32 | 33 | 66 | 9 | 18 | 21 | 42 |
| 9.1–11 | 27 | 52 | 5 | 10 | 39 | 78 | 24 | 48 |
| >11 | – | – | – | – | 2 | 4 | – | – |
| Mean | 8.79 | 7.74 | 10.01 | 8.81 | ||||
| P value | <0.01 | <0.01 | ||||||
After 4 weeks of starting therapy, the mean hemoglobin level in group A was 10.01 gm/dl with a rise of 3.52 gm/dl and in group B mean hemoglobin was 8.81 gm/dl with a total rise of 2.33 gm/dl from the pretherapy level (Table 3). The difference was statistically significant (P < 0.01). In group A, 90 % (45) cases achieved target hemoglobin level after 8 weeks of starting therapy, while in group B only 34 % (17) cases achieved target hemoglobin levels after 8 weeks of therapy. The difference was statistically highly significant (P < 0.001).
The mean time period taken to achieve target hemoglobin level was 6.37 gm/dl in group A and 9.04 weeks in group B (Table 4). The difference was found to be statistically significant (P < 0.01). In group A, 8 % (four) cases had grade I adverse effects; while in group B, 24 % (12) cases had grade I adverse effects (Table 5). The adverse effects were minimal and managed symptomatically. On statistically analyzing the results, the difference was found significant (P = 0.027).
Table 4.
Time period taken to achieve target hemoglobin level (≥11 gm/dl)
| Time period (weeks) | Group A | Group B | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| 2–4 | 3 | 6 | – | – |
| >4–8 | 42 | 84 | 17 | 34 |
| >8–12 | 5 | 10 | 28 | 56 |
| >12 | – | – | 5 | 10 |
| Mean | 6.37 | 9.04 | ||
| P value | <0.01 | |||
Table 5.
Adverse effects in both the groups
| Adverse effects (all grade I) | Group A (n = 50) | Group B (n = 50) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Local phlebitis | 2 | 4 | – | – |
| Shivering and weakness | 1 | 2 | – | – |
| Moderate abdominal pain | 1 | 2 | – | – |
| Local pain | – | – | 6 | 12 |
| Skin staining | – | – | 5 | 10 |
| Headache | – | – | 1 | 2 |
| Total | 4 | 8 | 12 | 24 |
In group A, 60 % (30) cases were completely relieved of their clinical symptoms at 4 weeks after therapy; while in group B, only 20 % (10) cases were completely relieved of their symptoms. The difference was statistically highly significant.
Discussion
The current study was undertaken to evaluate the efficacy and safety of intravenous iron sucrose therapy for treating anemia during pregnancy and compare it with intramuscular iron sorbitol therapy. The rise in hemoglobin level in cases who were given intravenous iron sucrose therapy was 2.3 gm/dl after 2 weeks and 3.52 gm/dl after 4 weeks (Table 3). It was significantly higher when compared to rise after intramuscular iron sorbitol therapy. The rise in hemoglobin level was 2.6 gm/dl in intravenous group and 1.2 gm/dl in intramuscular group after 3 weeks of therapy in the study by Hashmi et al. [4]. In the study conducted by Wali et al. [5], the rise in hemoglobin level was 2.8 gm/dl after 3 weeks of intravenous iron sucrose therapy.
90 % (45) cases achieved the target hemoglobin level of ≥11 gm/dl after 8 weeks of intravenous iron sucrose therapy, while only 34 % (17) cases achieved target hemoglobin level after 8 weeks of intramuscular iron sorbitol therapy. The difference was statistically significant (P < 0.001). In the study by Hashmi et al. [4], 80 % cases achieved target hemoglobin in intravenous iron group and only 20 % cases achieved target hemoglobin level in intramuscular iron group after 6 weeks of therapy.
In our study, the mean time period taken to achieve the target hemoglobin level was 6.37 weeks in intravenous iron sucrose group and 9.04 weeks in intramuscular iron sorbitol group (Table 4). This difference was statistically significant (P < 0.01).In the study by Raja et al. [6], the mean time period to achieve target hemoglobin level was 5 weeks in the intravenous iron sucrose group.
Only 8 % (four) cases had adverse effects which were of grade I type with intravenous iron sucrose therapy, while 24 % (12) cases had grade I adverse effects with intramuscular iron sorbitol therapy (Table 5). The difference was statistically significant (P = 0.027). There were no grade II adverse effects in either of the groups. In the study conducted by Wali et al. [5], 12 % cases had grade I adverse effects with intravenous iron sucrose therapy, while 50 % cases had grade I adverse effects with intramuscular iron sorbitol therapy. In group A, 60 % (30) cases were completely relieved of their symptoms; while in group B, only 20 % (10) cases were completely relieved of their symptoms. The difference was statistically significant (P < 0.001). The results of our study show that the mean hemoglobin level achieved in intravenous iron sucrose group was significantly higher and the rate of increase in hemoglobin level was also higher in intravenous group. The number of cases achieving target hemoglobin was significantly higher in intravenous group and also the target hemoglobin was achieved in a shorter time period in intravenous group. The incidence of adverse effects was also significantly lower in intravenous group.
Conclusion
Intravenous iron sucrose therapy is safe, convenient, more effective, and faster acting than intramuscular iron sorbitol therapy for the treatment of moderate to severe anemia during pregnancy.
References
- 1.Allen LH. Pregnancy and iron deficiency: unresolved Issues. Nutr Rev. 1997;55:91–101. doi: 10.1111/j.1753-4887.1997.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 2.Bayoumeu F, Subiran-Buisset C, Baka NE, et al. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol. 2002;186:518–522. doi: 10.1067/mob.2002.121894. [DOI] [PubMed] [Google Scholar]
- 3.Perewusny KG, Huck R, Huck A, et al. Parenteral iron-sucrose complex. Br J Nutr. 2002;88:3–10. doi: 10.1079/BJN2002577. [DOI] [PubMed] [Google Scholar]
- 4.Hashmi Z, Bashir G, Azeem P, et al. Effectiveness of intra-venous iron sucrose complex versus intra-muscular iron sorbitol in iron deficiency anemia. Ann Pak Inst Med Sci. 2006;2:188–191. [Google Scholar]
- 5.Wali A, Mushtaq A. Comparative study—efficacy, safety and compliance of intravenous iron sucrose and intramuscular iron sorbitol in iron deficiency anemia of pregnancy. J Pak Med Assoc. 2002;52:392–395. [PubMed] [Google Scholar]
- 6.Raja KS, Janjua NB, Khokhar N. Intravenous iron sucrose complex therapy in iron deficiency anemia in the pregnant women. Rawal Med J. 2003;28:40–43. [Google Scholar]


