Abstract
The mammalian host immune system has wide array of defence mechanisms against viral infections. Depending on host immunity and the extent of viral persistence, either the host immune cells might clear/restrict the viral load and disease progression or the virus might evade host immunity by down regulating host immune effector response(s). Viral antigen processing and presentation in the host cells through major histocompatibility complex (MHC) elicit subsequent anti-viral effector T cell response(s). However, modulation of such response(s) might generate one of the important viral immune evasion strategies. Viral peptides are mostly generated by proteolytic cleavage in the cytosol of the infected host cells. CD8+ T lymphocytes play critical role in the detection of viral infection by recognizing these peptides displayed at the plasma membrane by MHC-I molecules. The present review summarises the current knowledge on the regulation of mammalian host innate and adaptive immune components, which are operative in defence mechanisms against viral infections and the variety of strategies that viruses have evolved to escape host cell immunity. The understanding of viral immune evasion strategies is important for designing anti-viral immunotherapies.
Keywords: Virus, Immune regulation, Immune evasion
Introduction
Viruses are known to use host cell machinery to replicate by modulating different cellular functions. Although, the mammalian host immune system is triggered to combat viral infections, virus can still persist in the host cell due to its immune evasion mechanisms [87]. Host immunity can be downregulated with increasing viral load and subsequently the diminished immunity may allow the virus to sustain, attach itself to the adjacent healthier cells, replicate, proliferate and ultimately override the host cell immunity. On the other hand, after encountering the pathogen, the immune cells get activated and then proliferate to eliminate the pathogen or altered self cells to prevent bystander injury and maintain tissue homeostasis. There are several mechanisms, like, apoptosis, necrosis, autophagy, phagocytosis, generation of immune-regulatory cells, activation induced cell death (AICD) which are found to control the activation and maintain cellular homeostasis [6, 40, 41, 46].
The battle between viruses and host cell immunity is an evolutionary process, where successful immune evasion by viruses reduces host protective immune response. One of the major targets of immune evasion of pathogenic virus is regulation of the processing and presentation pathway of major histocompatibilty complex class I (MHC-I) [60, 62]. Moreover, there are several other important cellular components of host cell immunity which are also known to be regulated during viral infections [70, 103, 133]. However, a concise updates about the modulation of mammalian innate and adaptive immunity during viral infection is warranted. The present review focuses on the role of host innate and adaptive immune components involved in defence mechanisms against viral infections and various approaches that viruses have acquired to evade mammalian host cell immunity. The updated information of virus mediated altered cellular immune responses will help to understand those processes and design future strategies of anti-viral immunotherapies.
An Overview of Antigen Processing and Presentation
MHC is a set of cell surface molecules that are responsible for immune recognition and “antigen presentation”. Two distinct types of MHC molecules, viz., MHC class I (MHC-I) and MHC class II (MHC-II) present peptides at the cell surface to CD8+ and CD4+ T cells, respectively. The origins of these peptides are different. MHC-I molecules are expressed ubiquitously on all nucleated cells and present protein fragments of cytosolic and nuclear origin at the cell surface for further immune regulation. On the other hand, MHC-II molecules are primarily expressed by professional antigen presenting cells (APCs), such as, dendritic cells (DCs), macrophages and B cells and present exogenous peptides on the surface. In the following sections, we have discussed how pathogens have evolved various strategies to manipulate the MHC-I and II pathways [102].
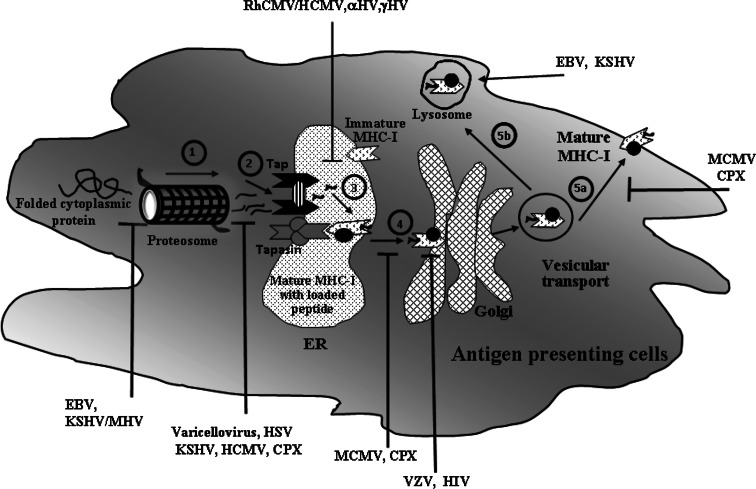
Intracellular viral antigen processing through MHC-I involves sequential steps to present peptides at the antigen presenting cell surface. At first, the proteolysis of viral antigen by the proteasome takes place in the cytosol of host APCs and the resulting peptides are translocated from cytosol to the lumen of the endoplasmic reticulum (ER) by TAP (transporter associated with antigen processing). TAP is a heterodimeric multimembrane-spanning ATP-binding cassette transporter (ABC transporter), consisting of TAP1 and TAP2 with two peptide binding sites and an ATP binding site. The peptide-loading complex of ER forms a multimeric complex consists of TAP, β2 microglobulin, calreticulin, ERp57, tapasin and MHC class I. Upon binding to the peptides on cytoplasmic site, TAP translocates them into the lumen of the ER through the secretory pathway. Ultimately, the peptide loaded MHC-I complex is transported from the ER through Golgi to reach the cell surface, where they are recognised as epitopes by cytolytic T cells (CD8+ T cells/CTLs) through their respective T cell receptors (TCR) to generate effector T cell response [31, 53, 118, 144, 146]. However, several virus-mediated immune evasion mechanisms are found to downregulate anti-viral immunity leading to defective antigen processing and presentation (Fig. 1).
Fig. 1.
Immune regulation through inhibition of MHC class I associated antigen presentation pathway during viral infection. An outline of virus mediated inhibition of various stages of MHC class-I antigen presentation pathways for recognition of CD8+ T cell has been described in this figure along with few examples of viruses that inhibit the specific step to escape immune evasion strategies. 1 The degradation of viral proteins by proteasome to generate small peptides. Epstein Barr virus (EBV), Kaposi’s sarcoma associated virus (KSHV) and Murine gamma herpes virus (MHV) escape the proteasomal degradation of viral proteins into antigenic peptides with the help of different viral proteins [27, 38, 77, 147]. 2 Translocation of proteasome generated peptides into the ER to bind TAP. Different proteins of Varicellovirus, Kaposi’s sarcoma associated virus (KSHV), Herpes Simplex virus (HSV), Cow Pox Virus (CPX) and Human Cytomegalovirus (HCMV) act as inhibitors of TAP, block the peptide and ATP binding and thereby inhibit TAP-mediated peptide transport [58, 90, 132]. 3 Formation of mature MHC class I molecule from nascent MHC class I molecule by addition of TAP, β2 microglobulin, calreticulin, ERp57 and tapasin in ER. Rhesus CMV (RhCMV)/HCMV interferes with the function of tapasin. HCMV, alpha and gamma herpes viruses restrict MHC-I molecule in ER. Adenovirus also retains MHC-I molecules in ER [36, 45, 65, 106]. The complex assembly of the mature MHC complex has been simplified and depicted with a small circle, filled in black. 4 Translocation of peptide loaded mature MHC class I to Golgi. For example, Murine cytomegalovirus (MCMV) and CPX inhibit the transport of mature MHC-I molecules out of the ER membrane. HIV-1 protein, Nef diverts MHC-I trafficking from the trans-Golgi network to the lysosomal compartment [109, 145]. Varicella zoster virus (VZV) retains mature MHC-I complexes in the cis/medial Golgi of the trans-Golgi network that regulates the vesicular trafficking of these molecules [36]. 5a Transport of peptide loaded mature MHC from Golgi to cell surface for antigen presentation to CD8+ T cell. For example MCMV gp48 and CPX can restrict MHC-I transport from Golgi and redirect it to lysosome [19, 113]. 5b Degradation of peptide through lysosomal and/or autophagy pathway [24]. EBV and KSHV enhance the endocytosis and lysosomal degradation of mature MHC-I molecule of the cell surface [95, 151]
Proteins from exogenous sources can also be processed for peptide presentation by MHC-I molecules and “cross-priming” to CD8+ T cells [14]. Similarly, the ‘cross-presentation’ is considered as a presentation process of exogenous peptides by MHC-I complex, without being synthesized by translational machinery of the respective APCs [4, 15, 129, 150]. Numerous examples have been shown to illustrate the essential role of CD8+ T cells in protective immunity against intracellular pathogens. Moreover, the peptide-loaded MHC-I molecules (pMHC-I) serve as ligands for CD8+ T cells [15]. These CD8+ T cells can recognize a large number of pMHC-I derived peptides of different viral proteins [79, 101]. However, viruses have evolved multiple strategies to overcome the host defense mechanism for their own survival. The following sections focus on how different pathways have been targeted by different viruses to disrupt the host immunity.
Altered MHC Response During Viral Infection
The highly sensitive recognition system of MHCs for antigenic peptides is one of the prime targets for immune evasion strategies of many viruses. Complex membrane trafficking events in the regulation of antigen processing and presentation of both endogenous and exogenous antigens may also be exploited by viruses to escape host immune response.
It has been reported that various autophagy processes can be involved in the presentation of endogenous viral antigens on MHC class I molecules during Herpes simplex virus-1 (HSV-1) infection. In autophagosome viral proteins undergo further processing by the proteasome for MHC presentation [37, 38]. It has been shown that altered HSV-1 gene transfer to neuronal cells leads to the induction of MHC-I and MHC-II, which drives inflammatory effects characterized by the recruitment of activated T cells and macrophages [143]. In SJL/J mice it has been demonstrated that Theiler’s murine encephalomyelitis virus (TMEV) infection promotes high expression level of MHC-I/II, inflammatory cytokines and chemokines along with proliferating cell nuclear antigen (PCNA) in the microglia [33]. However, in mouse model of Lassa virus (LASV) infection, a major role of MHC-I restricted T cell response has been demonstrated in the regulation of viral pathogenesis [47].
In contrast, downregulation of MHC-I has been reported during human immunodeficiency virus 1 (HIV-1) infection. Moreover, MHC-II expression in HIV-1 infection has been studied in primary monocytes and monocytic cell lines, which reveals that downregulation of MHC-II expression contributes to pathogenesis by allowing virus to replicate faster. At the same time, upregulation of MHC-II may also enhance infectivity mediated by high density of MHC-II on macrophages and increased number of virions in a dynamic immunoregulatory response [72]. HIV-1 infection has also been found to upregulate MHC-II expression in T and B cell lines, macrophages and peripheral blood mononuclear cells (PBMC) without significantly altering CD80/86 expression [42]. Moreover, increased expression of both MHC-I and II antigens has been observed in Maedi-visna virus (MVV) infected pulmonary alveolar macrophages, whereas sheep choroid plexus cells only demonstrated the upregulation of MHC-I [99]. These results suggest that upregulation of the expression of MHC molecules depend on specific viral infection, probably due to differential response of APCs towards host immunity. However, due to lack of activation of accessory co-stimulation of the infected cells, viruses may override host protective immunity.
DCs are important for MHC-I processing and presentation of peptide epitopes to memory CD8+ T cells and it can potentially be targeted to activate memory CD8+ T cells to a broad array of HIV-1 epitopes for anti-retroviral therapy. Gag and Nef epitopes of HIV-1 are found to prime DCs to induce an MHC-I restricted T cell response. Such HIV-1 epitope-primed DCs are found to promote polyclonal effector T cell response with cytokine and chemokine production (such as, IFN-γ, IL-2, TNF-α, MIP-1β) and expression of cytotoxic de-granulation molecule (CD107a) [67]. It suggests the potential implication of DC-based immunotherapy for HIV-1 infection. However, in the Foot-and-mouth disease virus (FMDV) infection, MHC-I molecules become unable to present viral peptides to T lymphocytes [123] suggesting a possible mechanism of virus evasion from host cell cytotoxic immune response.
Additionally, it has been shown that three isolates of Rinderpest virus (RPV) with different in vivo virulency are able to infect and productively replicate in bovine monocytic cells. They all produce infectious progenies by downregulating MHC-II expression without changing any apparent effect on MHC-I response. It has been suggested that the downregulation of MHC-II expression may offer a way by which the virus can evade immune recognition [114]. In Semliki forest virus (SFV) infection, which induces multiple sclerosis (MS) and in mouse model of experimental allergic encephalomyelitis (EAE), the expression of MHC-I and II have been found to be upregulated in microglia, adjacent macrophages and in mononuclear cell infiltrates in the central nervous system (CNS) [100]. Varicelloviruses, the largest subgroup of alpha herpes viruses, express two viral proteins, UL49.5 and US3, that are reported to diminish MHC-I cell surface expression. Pseudorabies virus (PRV)-induced downregulation of cell surface MHC-I has been found to be cell-type-dependent, with variable roles of US3 and UL49.5 [32]. Enterovirus infection of the pancreas induces the co-expression of IFN-γ and CXCL10 (CXC chemokine ligand 10) in beta-cells along with MHC-II expression. Hyperexpression of MHC-I in some islet cells of the pancreas and subsequent activation followed by attraction of autoreactive T cells and macrophages to the islets have also been reported [137]. Hence, it appears that in case of viral induced autoimmune disorders, MHC response and effector T cell function may be elevated as compared to immunosuppressive viral infection.
Porcine circovirus (PCV) infection in alveolar macrophages leads to a transient increase in MHC-I expression initially that is followed by a decrease in MHC-II expression in the late stage of this infection [94].
Arbovirus infection can cause encephalitis of the host neurons and it may cause death or dysfunction of neurons. It has been suggested that unlike neurons, microglia and perivascular macrophages, probably present the viral antigens through MHCs to activated T cells, which migrate to the brain from lymphoid organs [56].
It has been reported that Ebola virus-like particles (eVLPs) are immunogenic, in vitro, as they activate mouse bone marrow-derived dendritic cells leading to the upregulation of MHC-I/II and elevated production of IL-6, IL-10, MIP-1α, and TNF-α [140].
Dengue virus infection activates B and NK cells and also enhances the expression of MHC-I molecules on macrophages and dendritic cells in mice [134].
It is evident that most viruses target the MHC and its allied molecules to escape the host immunity for persistence. However, many other viruses disrupt the antigen processing and presentation pathway as an effective strategy to combat the host defence.
Regulation of Proteasomal Response
The proteasome is a multicatalytic protease complex which can generate peptides by proteolysis of viral proteins for MHC-I presentation. Viruses like HSV-1 and HIV target the immunoproteasome to regulate the MHC-I associated viral antigen processing and presentation. It has been suggested that HSV-1 evades host immunity by down-regulating IFN-γ-inducible low-molecular-mass polypeptide 7 (LMP7, an immunoproteasomal subunit of the host cells) in mature DCs [35]. HIV protein Tat (transcriptional activation) has been found to destabilize the balance of the three immunoproteasomal subunits: LMP2, LMP7 and MECL1 (multicatalytic endopeptidase complex-like 1) [7]. Moreover, both human and mouse cytomegaloviruses (HCMV and MCMV) have been suggested to evade peptide presentation by MHC-I to CD8+ T cells by blocking of immunoproteasome formation. Accordingly, it can be implied that the blockade of immunoproteasome assembly formation may regulate the CD8+ T cells repertoire during the effector phase of the immune response [75].
Epstein-Barr virus (EBV) nuclear antigen-1 (EBNA1) and latency-associated nuclear antigen 1 (LANA1) of Kaposi’s sarcoma-associated herpes virus (KSHV) express during viral latency. Both the proteins of these gamma herpes viruses are known to downregulate MHC-I function and thereby inhibit anti-viral CTL response. EBNA1 contains an internal repeat composed of glycines and alanines that inhibits the presentation of MHC-I restricted T cell epitopes and interferes ubiquitin/proteasome-dependent proteolysis [29]. It has been reported that LANA1 functionally mimics EBNA1 to evade host immunity through central repeat domain inhibiting protein processing for MHC-I response. Moreover, it has been proposed that deletion of these inhibitory regions of the viruses may promise for effective vaccine to upregulate MHC-I response and anti-viral CTL responses [81]. Accordingly, a detailed study is required to understand the role of proteasome in viral protein mediated host immune regulation and it will shed light on the effective strategies of viruses, which are operative for the immune evasion mechanism.
Regulation of TAP and Tapasin
As mentioned earlier TAP, an important component of peptide loading complex, involves in delivering cytosolic peptides to MHC-I molecules in ER. It has been suggested that TAP can be one of the major targets of viral proteins, which may lead to downregulate MHC-I dependent antigen presentation [93, 126].
Recently the inhibition of TAP function by varicellovirus has been reported. Precisely, the domains of UL49.5 of varicellovirus have been shown to block the translocation of peptides by TAP and target TAP for proteasomal degradation [138].
Clinical studies with nasopharyngeal carcinoma (NPC) lesions during EBV infection suggest that LMP2, TAP1, tapasin and HLA class I antigens are downregulated [105]. Unique short region glycoprotein (US3) of HCMV has been found to downregulate the surface expression of MHC-I molecules by inhibiting tapasin and also by ER retention [133].
Gamma-herpes viruses are able to regulate MHC-I expression by the viral mK3 protein mediated degradation of nascent class I molecules. The mK3 protein has been found to interact with TAP/tapasin and regulate the ubiquitination of TAP/tapasin-associated MHC-I response [90].
Integral membrane proteins in the early transcription unit 3 (E3) of Adenoviruses have been suggested to alter the host immune system by regulating the MHC-associated intracellular trafficking pathways. It has been suggested that E3 downregulates surface expression of MHC-I antigens and inhibits cellular cytolysis. E3-19K prevents the expression of newly synthesized MHC molecules. It binds to TAP and acts as a tapasin inhibitor, preventing MHC-I/TAP association which leads to inhibition of ER export. E3/10.4–14.5K, another E3 protein, is known to down-regulate infected host cell apoptosis receptors by redirecting them into lysosomes [142].
In summary, interaction between TAP, tapasin and viral proteins represent an important step towards MHC-I associated viral immune escape strategies.
Regulation of ER
ER is one of the important organelles which works for the assembly and transportation of viral peptides and can lead to the presentation of antigenic peptides through MHC-I to cytotoxic T cell. However, viruses evolve several strategies to disrupt the activities in ER as mentioned below.
During the replication cycle of HCMV, ER-resident transmembrane glycoproteins, US2, US3 and US10 can be expressed. It has been shown that US10 of HCMV downregulates HLA-G without modulating classical class I MHC molecules expression on the cell surface [107]. However, it has also been reported that the stably expressing cells with HCMV derived US2 and US3 protein can downregulate MHC-I from the cell surface [104]. In HIV infection, it has been proposed that a HIV Nef protein may affect immune evasion by inhibiting the trafficking of newly synthesized HLA-I from the ER to the plasma membrane [145]. KSHV and HIV can inhibit recognition of CD8+ T cell by retention of MHC-I in ER. HIV Vpu protein holds the nascent MHC-I chains in the ER and LANA1 of KSHV can inhibit MHC-I peptide presentation [71, 80]. Varicella-zoster virus (VZV) constitute an open reading frame 66 (ORF66) protein kinase, that can reduce the expression of MHC-I by delaying the maturation step of transport from ER through Golgi apparatus [36]. Adenoviruses are found to evade immune recognition by disrupting the pathway of MHC-I assembly in ER. Human adenovirus produces a glycoprotein, gp19K, that binds to MHC-I in the endoplasmic reticulum and prevents MHC transport to the cell surface, whereas, adenovirus 2 synthesizes an early glycoprotein, E19, that binds to nascent MHC-I antigens in the ER and restricts their transport to the cell surface [45, 106]. Furthermore, detailed knowledge about different viral proteins is required to ascertain the events in ER that contribute to viral immune evasion strategies.
Regulation of Trans-Golgi Network (TGN)
Viral invasion in host cells may subsequently override the APC function at the TGN level. Restriction of antigen loaded MHC trafficking through TGN is reported to inhibit APC function and thereby may help the viruses to evade host cell immunity. African swine fever virus (ASFV) is found to restrict TGN by altering the localization of TGN46, an organelle marker for the efferent secretory pathway. Interference of TGN by ASFV can retard membrane trafficking during viral infection. It has been reported that such interference of TGN function may have important role towards the infection of macrophages with virulent ASFV infection. Albeit ASFV apparently induces expression of MHC-I genes, the functional increase in MHC-I complex delivery to the cell membrane is restricted [103].
It has been suggested that HIV-1 Nef protein binds to the cellular protein phosphofurin acidic cluster sorting protein-1 (PACS-1) to downregulate MHC-I complexes in association with altered TGN function [109]. Moreover, recently it has been shown that HIV-1 Nef protein binds to a subpopulation of MHC-I throughout its trafficking itinerary including dispersal of TGN and results in downregulation of MHC-I by inducing both anterograde and retrograde trafficking [145].
TGN has also been found to be associated with Nef mediated downregulation of non classical MHC-I like CD1d molecule that has been demonstrated by retention of CD1d in TGN and internalization from the cell surface [25]. In another study, it has also been demonstrated that a KSHV induces internalization of MHC-I complex by endocytosis and subsequently redirects them to TGN and targets MHC-I to the lysosomal compartment. It facilitates the virus to evade immune effector recognition mediated by the viral protein K3 [95]. All these works suggest that TGN is one of the important targets for immune evasion processes during virulent virus infection.
Costimulation and APC Function During Viral Infection
Costimulatory molecules, such as, CD80 (B7.1), CD86 (B7.2) and CD40 are important to promote successful immune effector response on host APCs. However, virus mediated suppression of MHC response may reflect the downregulation of costimulatory response in APCs. HIV-1 Tat protein induces degradation of RON (recepteur d’origine nantais), a receptor tyrosine kinase, through ubiquitin–proteasome pathway to facilitate HIV replication and progression of AIDS-associated diseases [70]. It has been shown that Nef protein of HIV-1 mediates the loss of CD80 and CD86 in APCs, which may restrict the activation of T cells for the anti-viral effector T cell response [23]. VLPs of Ebola virus and Marburg virus (MARV) have been found to induce maturation of DCs by upregulating costimulatory molecules (CD40, CD80 and CD86), MHC-I/II surface antigens and the late DC maturation marker CD83 [16]. Accordingly, it has been suggested that VLPs can effectively stimulate DCs and thereby induce innate and adaptive immune responses. Many herpes viruses interfere with the MHC-I antigen-processing pathway in order to restrict CTL response.
Immune Regulation and Cellular Micro-Environment During Viral Infection
In an ongoing immune response during infection and/or cancer progression, in addition to altered MHC and costimulatory response, several other cellular consequences may occur. During viral infection especially for the persistent one, there may be alteration of host cellular microenvironment with immunoregulatory cell-derived cytokines and chemokines. Moreover, there may be emergence of different categories of regulatory immune cells including regulatory T cells (Tregs) and accessory immunoregulatory APCs. During viral infection, an inflammatory immune effector response may be generated with subsequent induction of tissue damage through innate and adaptive inflammatory cytokines [120]. Viral antigens are taken up by antigen-presenting cells and carried to local draining lymph nodes. Depending on the cytokine milieu generated in the draining lymph node, different types of T helper (Th) cell responses are induced. Primed CD8+ cytotoxic CTLs migrate to the site of infection and kill virus infected cells, thereby contributing to tissue damage. The interplay between effector immune cells, immunoregulatory suppressor cells, viral host evasion and persistence could be reflected with altered host microenvironment [8, 120, 132, 141].
In a highly enriched primary ovine microglial cell cultures from brain tissues of lambs, it has been shown that these cells can be infected with MVV and that leads to a selective upregulation of pro-inflammatory cytokines. Moreover, these cells are found to be positive for CD1, CD11a, CD11c, CD14, MHC-I, MHC-II and β-N-acetyl galactose expression [34].
Regulatory T Cells (Tregs)
As mentioned above that several type of Tregs are found to be operative during ongoing infection and cancer, the complex nature of Tregs’ ontogeny and its functional attributes are yet under investigation [6, 21, 119, 122]. In acute, self-limited virus infections, a vigorous CD8+ T cell response leads to viral clearance. However, many viruses induce persistent infections, despite continuous measurable CD8+ T cell responses [116]. Moreover, Tregs are known to regulate effector T cell function including an anti-viral CTL response [119]. All Tregs, both naturally occurring and induced, need T cell receptor (TCR) triggering, for their suppressive function. However, it has been suggested that once activated, Tregs’ suppressive activity found to be antigen-nonspecific [130]. The two most possible candidates for the maintenance of self tolerance are the Cytotoxic T lymphocyte antigen-4 (CTLA-4) dependent and IL-2-dependent mechanism in FOXP3+ Treg cells [49]. The mechanism of counteracting excessive tissue damage following virus infection is the induction, activation and expansion of several types of Treg cells, which mainly inhibit the function of other cell types. Tregs inhibit both CD4+ and CD8+ T cell activation, proliferation and effector function along with suppression of APC response. However, the detailed cellular and molecular mechanism of Treg mediated immune suppression needs further investigation [149].
In a clinical study with HIV-infected pregnant women, existence of higher frequencies of several Treg populations including CD4+CD25+FOXP3+, CD8+CD25+FOXP3+, CD4+TGFβ+ and CD4+IL10+ Treg cells has been observed during early pregnancy. Moreover, it has been suggested that Tregs have differential intensity during pregnancy in HIV-infected and uninfected women. Enhanced inflammatory cytokines and decreased Treg frequency during later stage of pregnancy in HIV-infected women may contribute to their increased incidence of maternal-fetal morbidity [115].
Tregs have been found to induce robust neuroprotection in murine models of neuroAIDS by promoting neuroprotection through the killing of virus-infected macrophages by caspase-3, granzyme and perforin pathways. Tregs transform virus-infected macrophage phenotype by modulating inducible nitric oxide synthase and arginase 1 [66]. In a xenogenic graft versus host disease model, it has been shown that HIV-1 envelope glycoprotein gp120 can activate human Tregs by binding and signaling through CD4. Upon stimulation with gp120, human Tregs enrich cyclic adenosine monophosphate (cAMP) in their cytosol [12]. In a study of neuromodulatory effects of CD4+CD25+ Tregs in HIV-1 associated neurodegeneration, it has been found that Treg mediated anti-inflammatory activities and neuroprotection are associated with upregulation of brain-derived neurotrophic factor and glial cell derived neurotrophic factor and downregulation of proinflammatory cytokines, oxidative stress and viral replication [89]. In feline immunodeficiency virus (FIV), which is similar to HIV, it has been suggested that CD4+CD25+ T cells of FIV infected cats had higher transcript numbers of FOXP3, programmed death-1 (PD-1) and PD-Ligand 1 than CD4− and CD4+CD25− T cells, indicating the immunosuppressive function of Treg cells in lentiviral infection [1].
In HSV-induced Behcet’s disease (BD)-like symptoms, such as, chronic and multisystemic inflammatory disorder, it has been found that upregulation of the CD4+CD25+ T cells facilitates the inflammatory symptoms in BD-like mice, while decreased CD25+ T cells may deteriorate the symptoms [131]. FOXP3 expression has a major role in regulating inflammatory responses and in inhibition of proinflammatory and effector immune responses. Interestingly, FOXP3 expression has been found to be elevated by the CD4+ T cell sub-population of peripheral blood mononuclear cells (PBMC) and in liver tissues in association with proinflammatory IL-23/IL-17 response in chronic hepatitis B (CHB) infected patients. This work suggests an inefficiency of FOXP3+ Tregs in regulating CHB driven proinflammatory response [139].
Hepatitis C virus in chronic hepatitis has been found to induce expression of CCL17 and CCL22 chemokines by dendritic cells in liver that attract regulatory T cells to the site of infection. Accumulation of Treg favours immune suppression, which may result in restricting liver damage and limiting the extent of liver fibrosis [26, 117]. In Hodkgin’s lymphoma, it has been found that EBV, an oncogenic herpes virus, upregulates the expression of the chemokine, CCL20, which can be mediated by the EBV nuclear antigen 1 protein. It has also been demonstrated that it mediates migration of CD4+FOXP3+ Tregs which prevent immune responses against the virus-infected tumor population [10].
Other than CD4+FoxP3+ Tregs, many more lineages of Tregs including CD8+ Tregs are found, which can suppress host protective immunity during viral infection [119]. It has been suggested that an alteration of the microenvironment by the production of TGF-β and IL-10 can induce CD8+ Tregs that may inhibit activated effector T cells (e.g., CTL). Additionally, subsets of CD8, NK and NK Treg cells are reported to express inhibitory dimeric CD94/NKG2A receptors associated to non-classical MHC-I molecules (e.g., Qa-1 in mouse and HLA-E in human) during viral infection resulting in suppression of CTL response [54, 73, 136].
Accordingly, it appears that different subsets of Tregs may be associated to viral infection and its persistence.
Natural Killer (NK) Cell Response During Viral Infection
NK cells can serve an important function as robust first line defense during infection. Moreover, it has also been observed that during viral immune evasion stage NK cell response may be reduced. Low numbers of circulating NK cells in HIV patients of rapidly progressive infection has been reported. This work indicates the importance of NK associated altered non-MHC restricted cytotoxicity for the viral disease progression [18]. Moreover, it has been suggested that the interaction between NK cells and other innate and adaptive immune cells are also altered during viral infection, partially through the release of IL-2 and IFN-γ cytokines [28]. In addition to that, the suppression of NK cell activity and T cell proliferation by freshly isolated HCMV has also been demonstrated [127].
Immunoregulatory NKT cells have been found to inhibit CD8+ T cell-mediated graft rejection of human papillomavirus E7-expressing skin through an IFN-γ-dependent mechanism. In a recent study of skin transplantation, it has been shown that in draining lymph nodes, Ag-specific CD8+ T cell proliferation, cytokine production and cytotoxic activity are impaired by NKT cells. It has been observed that the suppression of NKT cells is induced by CD11c+ dendritic cells. Moreover, it has been suggested that inhibition of CD8+ T cell function may not require FOXP3+ regulatory T cells or NKT cell-secreted IFN-γ, IL-10, or IL-17 [92]. CD1d-restricted NKT cells are known to serve as a bridge between innate and adaptive immunity to regulate an ongoing immune response. In vivo and in vitro investigation of human papilloma virus (HPV) demonstrated that in cell lines like a vaginal keratinocyte (Vag) bearing endogenous CD1d+ and a stably transfected HPV-negative cervical cancer cell line, C33A (C33A/CD1d), HPVs have been found to downregulate CD1d expression by E5 protein mediated disruption of ER traffic. Accordingly, it has been suggested that the decreased CD1d expression in the presence of HPV E5 may facilitate the virus infected cells to evade protective immunological surveillance [97].
B Cell Response During Viral Infection
Lymphomagenesis in HIV-infection has been correlated to diminished host immunity, which may lead to loss of immunoregulation of EBV infected B cells. Moreover, during HIV infection, altered immune responses including chronic B cell activation has been reported [39]. It has been suggested that combination of highly regulated latent infection of EBV in B cell and chronic reactivation of replication in lymphoid tissue and mucosal surfaces may lead to many patterns of virus-host interaction, which may reveal important strategies of EBV mediated immune evasion [85]. Burkitt’s lymphoma (BL) cells lack immunogenicity, in contrary to EBV-immortalized B cells. However, induced expression of c-MYC (a product of c-myc proto-oncogene) has been reported to recapitulate the cell surface phenotype, the pattern of proliferation and the immunological characteristics of BL cells. c-MYC is found to downregulate Nuclear factor kappa B NF-kB and IFN pathway in a dose-dependent manner [124]. In case of chronic hepatitis B virus infection, immunosuppressive regulatory B cells (Bregs) have been recently reported. Those Bregs are found to regulate anti-viral CTL response in an IL-10-dependent manner [30].
Dendritic Cell (DC) Response During Viral Infection
DCs are known to be one of the major APC. Moreover, DCs serve many functions during viral infection. Monocytes may differentiate into functional DCs, which express CD11c, CD123, BDCA-4, DC-SIGN and acquire the capacity to activate T cells [135]. In a co-culture experimental model, respiratory syncytial virus (RSV) infected airway epithelial cells lead to the differentiation of monocytes into partially mature DC. However, it does not upregulate other immune effector makers, such as, CD80, CD83, CD86 and CCR7. Moreover, those DCs are reported not to release proinflammatory cytokines upon induction of TLR signaling [135]. This work suggests an important role of DC in RSV mediated host cell inflammatory response. In another study, a similar observation is reported in case of HCMV where signal delivery required for T cell activation is impaired in infected DCs which lead to persistent viral infection in the host system. In addition, HCMV infection diminishes the expression of adhesion molecules which in turn may lead to inefficient DC migration [11].
HCV induces immunosuppression in DCs during maturation. The T cell stimulatory capacity of DCs from the bone marrow of two founder lineages of transgenic mice, harboring HCV cDNA expressing HCV structural proteins, have been found to be impaired significantly. Additionally, the surface expression of MHC class-I molecules has been found to be significantly abridged in infected DC, especially with respect to MHC class-I of H-2D complex. During DC maturation, the transportation of H-2D to the cell surface has been found to be inhibited by HCV expression [62].
Measles virus downregulates cellular immune response of monocyte/macrophage and dendritic cells by decreasing IL-12 production, which provides an important inhibitory mechanism for the host cell immunity. Cross-linking of the cellular receptor for measles virus, the complement regulatory protein CD46, is found to be sufficient to inhibit IL-12 production. It has been suggested that CD46-mediated downregulation of IL-12 represents a more general pattern of inhibitory control for APC function [74]. In a study of Friend retrovirus (FV) infection in mouse DCs, it has been shown that in virus-induced immunosuppression, cell contact between DCs and T cells revealed prolonged contacts of T cells with infected DCs compared to uninfected DCs. Additionally, it has been suggested that infected DCs may expand FOXP3+ regulatory T cells that in turn may play an immunosuppressive role [9]. Accordingly, it appears that the DCs play an important role for anti-viral immunity as well as it may be responsible for the impaired host immunity by inducing immunosuppressive Tregs.
Complement Proteins During Viral Infection
The complement system is one of the important players of innate immunity having cascades of complement proteins which can restrict invasion of all the foreign pathogens. The activation of the complement system can lead to neutralization of cell-free viruses, phagocytosis of complement protein coated viral particles, lysis of virus infected cells and induction of inflammatory and effector immune responses. However, to regulate host immune responses and succeed as pathogens, viruses have developed mechanisms to limit complement function(s) towards immune evasion strategies. Important examples of viruses which regulate host complement system are poxviruses, herpes viruses, retroviruses, paramyxoviruses and picornaviruses [13]. It has been reported that expression of C-reactive protein (CRP), which is known to be required for complement activation, found to be increased during HIV-1 disease progression [112]. Accordingly, it appears that complement contributes to the control of viral replication by various strategies, such as, complement-mediated lysis, triggering the B cell responses by trapping the virus on follicular DCs in the germinal center or enhancement of the antigen presentation and subsequently generation of virus-specific CTL response [68]. Moreover, certain viruses, such as HSV-1, HIV-I, EBV are found to evade immune system by modulating host complement pathway. Glycoprotein C (gC) of HSV-1 has been found to act as a receptor for the C3b complement component on infected cells [50]. This work suggests a novel function of gC of HSV-1 and demonstrates that HSV-1 glycoprotein can help the virus to evade the host immune response by regulating the host complement function.
EBV has been found to mediate factor I-mediated breakdown of C3b to iC3b and iC3b to C3c and C3dg complement proteins. It also acts as a cofactor for the factor I-mediated cleavage of C4b to iC4b and iC4b to C4c and C4d. In addition, EBV has also been shown to have accelerated the decay of the alternative pathway of C3 convertase [98]. Accordingly, it has been suggested that the survival potential for the virus involves regulation of complement response and associated host cell defence mechanisms. It has been shown that the host cell type can significantly regulate the susceptibility of HIV to complement-mediated virolysis in association with phosphatidylinositol (PI)-linked complement control proteins, such as, membrane inhibitor of reactive lysis (MIRL, CD59) and decay-accelerating factor (DAF, CD55) [121].
Innate Immune Response During Viral Infection
Innate immunity in mammalian systems harbours several innate immune cells (e.g., monocytes, macrophages, DCs and other lineages of immune cells) which also support adaptive immune cells to mount host immunity. Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-1) and NOD-like receptors (NLRs) represent the major receptors of innate immunity. Interestingly, TLRs have been well studied in case of tumor immunity, infection immunity and autoimmune diseases along with other inflammatory disorders [22]. Viral infections usually activate the endosomal TLRs (TLR3, TLR7, TLR8 and TLR9) that recognize viral nucleic acids and double-stranded RNA intermediates [110]. Many viruses that persist in the host system can induce innate immune cells, such as, DCs, NK cells and macrophages to produce anti-inflammatory molecules, such as, IL-10 and TGF-β. It has been reported that viral infection in mice may result in significant upregulation of IL-10 by APCs, leading to impaired T cell responses and immune suppression [17].
Human respiratory syncytial virus (HRSV) is the most common cause of severe respiratory infections in infants and young children. It has been reported that these viruses infect the B lymphocytes and upregulate the expression of activation markers, including MHC-II and CD86, but not MHC-I molecules [5]. Moreover, it has been suggested that activation of B cells during HRSV infection may require several activation pathways but may not require TLR4 [108, 116]. However, TLR dependent induction of IFNs in anti-viral immunity has been well cited [148]. It has been reported that A52R, a Vaccinia virus protein may activate p38 mitogen activated protein kinase (MAPK) and may induce TLR4 dependent IL-10 production [91]. Thus TLRs may play differential role in modulating host immunity.
In bovine RSV (BRSV) infection, an induction of immunomodulatory IL-4 and IL-10 in lung DCs and alveolar macrophages have been observed. This work demonstrates that neonatal lung DCs facilitate BRSV replication and produce type II cytokines after viral infection [44]. However, another study reveals that antigen processing and presentation are not altered by chronic HRSV infection. However, it has been suggested that macrophages are able to activate RSV-specific CD8+ T lymphocytes [57].
In cowpox virus infection, it has been observed that TLR agonist-induced cytokine secretion could be suppressed in infected DCs. Such infected DCs also do not induce mixed leukocyte reaction (MLR) and LPS-stimulated NF-κB nuclear translocation [59]. It has been demonstrated in human PBMCs that Ebola virus elicits VP35 protein mediated downregulation of both IFN-α and TNF-α due to impairment of interferon regulatory factor 7 (IRF7) activation, which is an integral part of TLR signaling [84]. A similar phenomenon is also observed in a study of Hepatitis C virus infection, where decreased TLR7 expression alters TLR7-induced IRF7-mediated cell activation that results in impaired release of IFN-α [20]. The NS3/4A protease of HCV functions as an antagonist of virus-induced IRF-3 activation and IFN expression. NS3/4A also inhibits RIG-I and TLR3 signaling by cleaving the Toll-interleukin-1 receptor domain-containing beta-interferon (TRIF) [48, 86]. NS3 is a bi-functional enzyme with N-terminal domain encodes a serine protease and carboxy-terminal domain encodes an RNA helicase. The helicase activity of NS3 has been found to be important for the control of IRF-3 activation. Moreover, it has been suggested that NS3/4A protease inhibition may restore the host anti-viral innate immunity [69]. Thus, understanding the molecular mechanisms by which HCV regulates the host cell immune response may reveal important targets for novel therapeutic strategies [52].
It is evident that host cell and viral interactions confer several complex mechanisms. Persistent viral infection is mostly associated with downregulation of host immune responses. As discussed in the earlier sections, a summary of the major components of mammalian host immunity which can be altered during viral infection is depicted in Fig. 2. Moreover, the important immune evasive strategies employed by different viruses, as elaborated in the earlier sections have been briefly summarized in Table 1. This includes broadly the regulation of host RNA interference (RNAi), MHC antigen presentation, innate and adaptive immune cell receptors and immune signaling pathways during viral infections.
Fig. 2.
Interaction of mammalian host immune cells during viral infection featuring altered innate and adaptive immunity as discussed in the text. Virus infected innate immune cells and/or APCs may be suppressed for host protective immunity with defective antigen presentation, costimulation and may represent an elevated immunosuppressive cytokine response (e.g., IL-10, TGF-β). These infected host immune cells may facilitate downregulation of effector B and T cell response. Moreover, immunosuppressive B cells and T cells (Tregs) may be induced, which may be facilitated by immunosuppressive cytokine milieu during viral infection. (Abbreviation: APC antigen presenting cells, Treg regulatory T cells; IL-10 interleukin 10, TGF-β transforming growth factor beta)
Table 1.
Viral immune evasion strategies through downregulation of innate and adaptive components of host cell immunity
| evasion strategy | Possible mechanisms | Examples | References |
|---|---|---|---|
| RNAi | Encodes suppressors for RNA silencing (SRS) to inhibit RNAi responses | Ebolavirus | [43] |
| Inhibition of TAP, Tapasin and disruption of MHC-I | Post-translational strategies inhibiting peptide transport and MHC-I biosynthesis by blocking peptide transporter (TAP) and chaperones like tapasin | HCMV | [58] |
| Viral proteins, BNLF2a and mK3 inhibit TAP associated MHC-I response by targeting host tail-anchored protein integration machinery and regulating ubiquitination of TAP/tapasin, respectively. | EBV, HSV-1, BHV-1,GHV |
[64, 90] | |
| Viral proteins like UL49.5 and US6 alter the conformation, degrade the TAP1/2 interfere with ATP binding. HSV ICP47 protein interferes with peptide binding. | PRV, HCMV HSV |
[3, 51, 61, 63, 65, 76, 78, 82] | |
| LANA1 mediated evasion | Inhibits the presentation of Major Histocompatibility complex class I (MHC-I) | KSHV, EBV | [80] |
| IRF3 production | Inhibits IFN-γ and CIITA and thus MHC-I expression. | KSHV | [125] |
| Nef proteins | Nef proteins down-regulate MHC-I presentation of viral peptides. | SIV, HIV | [83, 96, 109, 145] |
| Viral NLR homolog | Homolog inhibits the inflammasome and also block caspase-1 activation, IL-1β and IL-18 processing | KSHV | [55] |
| Impairs RIG-I-like receptor-dependent signaling inhibiting IFN and TNF-α. | HCV | [88] | |
| Encoding VP35 proteins PRR control | TLRs and RLRs are taken into virus control of PRR signaling and regulation of immune programs | EBV | [84] |
| Inactivating MDA5 | MDA5 is a cytosolic PRR that is inactivated. | Paramyxovirus | [111] |
| NF-κB suppression | Prevents NF-κB dependent gene expression by retaining p65 in cytoplasm when V protein binds to p65 (RelA) | Measles virus | [128] |
| Proteases targeting signal transduction | Block IFN induction by affecting molecules of the innate immune pathways | HAV, HCV | [110] |
| Degradation of cellular TRIF | Replication and transcription activator (RTA) degrades TRIF | KSHV | [2] |
| CD1d down regulation | Viral protein E5 targets CD1d (a sentinel molecule bridging innate and adaptive immunity) by inhibiting calnexin-related CD1d trafficking | HPV | [97] |
Concluding Remark
Co-evolution of viruses and the host immune system are operating over millions of years, where viruses invade and subsequently may try to evade the host immunity for the successful replication and maintenance of their viable genome. On the other hand, host immunity has the immune surveillance and protective elements to look for and eliminate both free viruses and infected host cells. However, in this quest viruses can override host immunity through complex and evolutionary privilege strategies and remain successful in evading host immunity, which can be associated to their degree of pathogenicity and virulency. Accordingly, in depth studies for the host-viral interactions and exploring the yet unknown pathways for those viral immune evasion strategies are highly warranted. Moreover, understating the new elements starting from the functional organization of viral genome to protein expression in the perspective of host immunity will help to understand those new directions for the future strategies of anti-viral immunotherapy.
Acknowledgments
The work was partly supported by Department of Biotechnology, Ministry of Science and Technology, Govt. of India (Project no: BT/PR13312/GBD/27/247/2009); (BT/PR13118/GBD/27/186/2009) and by Council of Scientific and Industrial Research (CSIR) (Project No. 37(1542)/12/EMR-II), Ministry of Science and Technology, Govt. of India. We are grateful to Professor Robert E. Cone, Department of Immunology, University of Connecticut Health Center, USA for his critical reading and suggestions for the manuscript.
Contributor Information
Soma Chattopadhyay, Email: sochat.ils@gmail.com.
Subhasis Chattopadhyay, Email: subho13@hotmail.com, Email: subho@niser.ac.in.
References
- 1.Achleitner A, Clark ME, Bienzle D. T-regulatory cells infected with feline immunodeficiency virus up-regulate programmed death-1 (PD-1) Vet Immunol Immunopathol. 2011;143:307–313. doi: 10.1016/j.vetimm.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad H, Gubbels R, Ehlers E, Meyer F, Waterbury T, Lin R, Zhang L. Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF) J Biol Chem. 2011;286:7865–7872. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, Peterson PA, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/S1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 4.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Angel Rico M, Trento A, Ramos M, Johnstone C, Del Val M, Melero JA, Lopez D. Human respiratory syncytial virus infects and induces activation markers in mouse B lymphocytes. Immunol Cell Biol. 2009;87:344–350. doi: 10.1038/icb.2008.109. [DOI] [PubMed] [Google Scholar]
- 6.Anukumar B, Shahir P. Immune regulation in Chandipura virus infection: characterization of CD4+ T regulatory cells from infected mice. Virol J. 2011;8:259. doi: 10.1186/1743-422X-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Areste C, Blackbourn DJ. HIV Tat-mediated transcriptional regulation of proteasome protein cleavage specificity. Biochem J. 2006;396:e13–e15. doi: 10.1042/BJ20060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayyavoo V, Muthumani K, Kudchodkar S, Zhang D, Ramanathan P, Dayes NS, Kim JJ, Sin JI, Montaner LJ, Weiner DB. HIV-1 viral protein R compromises cellular immune function in vivo. Int Immunol. 2002;14:13–22. doi: 10.1093/intimm/14.1.13. [DOI] [PubMed] [Google Scholar]
- 9.Balkow S, Krux F, Loser K, Becker JU, Grabbe S, Dittmer U. Friend retrovirus infection of myeloid dendritic cells impairs maturation, prolongs contact to naive T cells, and favors expansion of regulatory T cells. Blood. 2007;110:3949–3958. doi: 10.1182/blood-2007-05-092189. [DOI] [PubMed] [Google Scholar]
- 10.Baumforth KR, Birgersdotter A, Reynolds GM, Wei W, Kapatai G, Flavell JR, Kalk E, Piper K, Lee S, Machado L, Hadley K, Sundblad A, Sjoberg J, Bjorkholm M, Porwit AA, Yap LF, Teo S, Grundy RG, Young LS, Ernberg I, Woodman CB, Murray PG. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates Up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck K, Meyer-Konig U, Weidmann M, Nern C, Hufert FT. Human cytomegalovirus impairs dendritic cell function: a novel mechanism of human cytomegalovirus immune escape. Eur J Immunol. 2003;33:1528–1538. doi: 10.1002/eji.200323612. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, Taube C, Bopp T, Michel K, Kubach J, Reuter S, Dehzad N, Neurath MF, Reifenberg K, Schneider FJ, Schmitt E, Jonuleit H. Protection from graft-versus-host disease by HIV-1 envelope protein gp120-mediated activation of human CD4+CD25+ regulatory T cells. Blood. 2009;114:1263–1269. doi: 10.1182/blood-2009-02-206730. [DOI] [PubMed] [Google Scholar]
- 13.Bernet J, Mullick J, Singh AK, Sahu A. Viral mimicry of the complement system. J Biosci. 2003;28:249–264. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard N, Shastri N. Cross-presentation of peptides from intracellular pathogens by MHC class I molecules. Ann N Y Acad Sci. 2010;1183:237–250. doi: 10.1111/j.1749-6632.2009.05135.x. [DOI] [PubMed] [Google Scholar]
- 16.Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, Bavari S. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruunsgaard H, Pedersen C, Skinhøj P, Pedersen BK. Clinical progression of HIV infection: role of NK cells. Scand J Immunol. 1997;46:91–95. doi: 10.1046/j.1365-3083.1997.d01-98.x. [DOI] [PubMed] [Google Scholar]
- 19.Byun M, Wang X, Pak M, Hansen TH, Yokoyama WM. Cowpox virus exploits the endoplasmic reticulum retention pathway to inhibit MHC class I transport to the cell surface. Cell Host Microbe. 2007;2:306–315. doi: 10.1016/j.chom.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Chang S, Kodys K, Szabo G. Impaired expression and function of toll-like receptor 7 in hepatitis C virus infection in human hepatoma cells. Hepatology. 2010;51:35–42. doi: 10.1002/hep.23256. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay S, Chakraborty NG, Mukherji B. Regulatory T cells and tumor immunity. Cancer Immunol Immunother. 2005;54:1153–1161. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chattopadhyay S, Pal S, Pratheek BM, Meena VS, Singh S, Maiti PK. “Toll” extending its gate from Drosophila development to T cell response: Implication in innate immunity, adaptive immunity and immunotherapy. Immunother Insights. 2011;2:1–14. doi: 10.4137/INT.S7422. [DOI] [Google Scholar]
- 23.Chaudhry A, Das SR, Hussain A, Mayor S, George A, Bal V, Jameel S, Rath S. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J Immunol. 2005;175:4566–4574. doi: 10.4049/jimmunol.175.7.4566. [DOI] [PubMed] [Google Scholar]
- 24.Chemali M, Radtke K, Desjardins M, English L. Alternative pathways for MHC class I presentation: a new function for autophagy. Cell Mol Life Sci. 2011;68:1533–1541. doi: 10.1007/s00018-011-0660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, Screaton GR, Xu XN. HIV-1 down-regulates the expression of CD1d via Nef. Eur J Immunol. 2006;36:278–286. doi: 10.1002/eji.200535487. [DOI] [PubMed] [Google Scholar]
- 26.Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315–321. doi: 10.1016/j.jhep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Covarrubias S, Richner JM, Clyde K, Lee YJ, Glaunsinger BA. Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J Virol. 2009;83:9554–9566. doi: 10.1128/JVI.01051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox JH. Evaluation of natural killer cell activity. Methods Mol Med. 1999;17:383–389. doi: 10.1385/0-89603-369-4:383. [DOI] [PubMed] [Google Scholar]
- 29.Dantuma NP, Sharipo A, Masucci MG. Avoiding proteasomal processing: the case of EBNA1. Curr Top Microbiol Immunol. 2002;269:23–36. doi: 10.1007/978-3-642-59421-2_2. [DOI] [PubMed] [Google Scholar]
- 30.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del-Val M, Lopez D. Multiple proteases process viral antigens for presentation by MHC class I molecules to CD8(+) T lymphocytes. Mol Immunol. 2002;39:235–247. doi: 10.1016/S0161-5890(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 32.Deruelle MJ, Van den Broeke C, Nauwynck HJ, Mettenleiter TC, Favoreel HW. Pseudorabies virus US3- and UL49.5-dependent and -independent downregulation of MHC I cell surface expression in different cell types. Virology. 2009;395:172–181. doi: 10.1016/j.virol.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Duncan DS, Miller SD. CNS expression of B7–H1 regulates pro-inflammatory cytokine production and alters severity of Theiler’s virus-induced demyelinating disease. PLoS One. 2011;6:e18548. doi: 10.1371/journal.pone.0018548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebrahimi B, Allsopp TE, Fazakerley JK, Harkiss GD. Phenotypic characterisation and infection of ovine microglial cells with Maedi-Visna virus. J Neurovirol. 2000;6:320–328. doi: 10.3109/13550280009030758. [DOI] [PubMed] [Google Scholar]
- 35.Eisemann J, Prechtel AT, Muhl-Zurbes P, Steinkasserer A, Kummer M. Herpes simplex virus type I infection of mature dendritic cells leads to reduced LMP7-mRNA-expression levels. Immunobiology. 2009;214:861–867. doi: 10.1016/j.imbio.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.English L, Chemali M, Desjardins M. Nuclear membrane-derived autophagy, a novel process that participates in the presentation of endogenous viral antigens during HSV-1 infection. Autophagy. 2009;5:1026–1029. doi: 10.4161/auto.5.7.9163. [DOI] [PubMed] [Google Scholar]
- 38.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippe R, Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epeldegui M, Vendrame E, Martinez-Maza O. HIV-associated immune dysfunction and viral infection: role in the pathogenesis of AIDS-related lymphoma. Immunol Res. 2010;48:72–83. doi: 10.1007/s12026-010-8168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esolen LM, Park SW, Hardwick JM, Griffin DE. Apoptosis as a cause of death in measles virus-infected cells. J Virol. 1995;69:3955–3958. doi: 10.1128/jvi.69.6.3955-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, Codogno P, Biard-Piechaczyk M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esser MT, Graham DR, Coren LV, Trubey CM, Bess JW, Jr, Arthur LO, Ott DE, Lifson JD. Differential incorporation of CD45, CD80 (B7–1), CD86 (B7–2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J Virol. 2001;75:6173–6182. doi: 10.1128/JVI.75.13.6173-6182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabozzi G, Nabel CS, Dolan MA, Sullivan NJ. Ebolavirus proteins suppress the effects of small interfering RNA by direct interaction with the mammalian RNA interference pathway. J Virol. 2011;85:2512–2523. doi: 10.1128/JVI.01160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fach SJ, Meyerholz DK, Gallup JM, Ackermann MR, Lehmkuhl HD, Sacco RE. Neonatal ovine pulmonary dendritic cells support bovine respiratory syncytial virus replication with enhanced interleukin (IL)-4 And IL-10 gene transcripts. Viral Immunol. 2007;20:119–130. doi: 10.1089/vim.2006.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fejer G, Gyory I, Tufariello J, Horwitz MS. Characterization of transgenic mice containing adenovirus early region 3 genomic DNA. J Virol. 1994;68:5871–5881. doi: 10.1128/jvi.68.9.5871-5881.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 47.Flatz L, Rieger T, Merkler D, Bergthaler A, Regen T, Schedensack M, Bestmann L, Verschoor A, Kreutzfeldt M, Bruck W, Hanisch UK, Gunther S, Pinschewer DD. T cell-dependence of Lassa fever pathogenesis. PLoS Pathog. 2010;6:e1000836. doi: 10.1371/journal.ppat.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco S, Clotet B, Martinez MA. A wide range of NS3/4A protease catalytic efficiencies in HCV-infected individuals. Virus Res. 2008;131:260–270. doi: 10.1016/j.virusres.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 51.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, Peterson PA, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 52.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 53.Gannage M, Munz C. MHC presentation via autophagy and how viruses escape from it. Semin Immunopathol. 2010;32:373–381. doi: 10.1007/s00281-010-0227-7. [DOI] [PubMed] [Google Scholar]
- 54.Gong F, Song S, Lv G, Pan Y, Zhang D, Jiang H. Human leukocyte antigen E in human cytomegalovirus infection: friend or foe? Acta Biochim Biophys Sin(Shanghai) 2012;44:551–554. doi: 10.1093/abbs/gms032. [DOI] [PubMed] [Google Scholar]
- 55.Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JP, Damania B. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin DE. Arboviruses and the central nervous system. Springer Semin Immunopathol. 1995;17:121–132. doi: 10.1007/BF00196161. [DOI] [PubMed] [Google Scholar]
- 57.Guerrero-Plata A, Ortega E, Ortiz-Navarrete V, Gomez B. Antigen presentation by a macrophage-like cell line persistently infected with respiratory syncytial virus. Virus Res. 2004;99:95–100. doi: 10.1016/j.virusres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Halenius A, Hauka S, Dolken L, Stindt J, Reinhard H, Wiek C, Hanenberg H, Koszinowski UH, Momburg F, Hengel H. Human cytomegalovirus disrupts the major histocompatibility complex class I peptide-loading complex and inhibits tapasin gene transcription. J Virol. 2011;85:3473–3485. doi: 10.1128/JVI.01923-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen SJ, Rushton J, Dekonenko A, Chand HS, Olson GK, Hutt JA, Pickup D, Lyons CR, Lipscomb MF. Cowpox virus inhibits human dendritic cell immune function by nonlethal, nonproductive infection. Virology. 2011;412:411–425. doi: 10.1016/j.virol.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9:503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 61.Hengel H, Koopmann JO, Flohr T, Muranyi W, Goulmy E, Hammerling GJ, Koszinowski UH, Momburg F. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 1997;6:623–632. doi: 10.1016/S1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 62.Hiasa Y, Takahashi H, Shimizu M, Nuriya H, Tsukiyama-Kohara K, Tanaka T, Horiike N, Onji M, Kohara M. Major histocompatibility complex class-I presentation impaired in transgenic mice expressing hepatitis C virus structural proteins during dendritic cell maturation. J Med Virol. 2004;74:253–261. doi: 10.1002/jmv.20164. [DOI] [PubMed] [Google Scholar]
- 63.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 64.Hislop AD, Ressing ME, van Leeuwen D, Pudney VA, Horst D, Koppers-Lalic D, Croft NP, Neefjes JJ, Rickinson AB, Wiertz EJ. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med. 2007;204:1863–1873. doi: 10.1084/jem.20070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horst D, Ressing ME, Wiertz EJ. Exploiting human herpesvirus immune evasion for therapeutic gain: potential and pitfalls. Immunol Cell Biol. 2011;89:359–366. doi: 10.1038/icb.2010.129. [DOI] [PubMed] [Google Scholar]
- 66.Huang X, Stone DK, Yu F, Zeng Y, Gendelman HE. Functional proteomic analysis for regulatory T cell surveillance of the HIV-1-infected macrophage. J Proteome Res. 2010;9:6759–6773. doi: 10.1021/pr1009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang XL, Fan Z, Borowski L, Mailliard RB, Rolland M, Mullins JI, Day RD, Rinaldo CR. Dendritic cells reveal a broad range of MHC class I epitopes for HIV-1 in persons with suppressed viral load on antiretroviral therapy. PLoS One. 2010;5:e12936. doi: 10.1371/journal.pone.0012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huber G, Banki Z, Lengauer S, Stoiber H. Emerging role for complement in HIV infection. Curr Opin HIV AIDS. 2011;6:419–426. doi: 10.1097/COH.0b013e3283495a26. [DOI] [PubMed] [Google Scholar]
- 69.Johnson CL, Owen DM, Gale M., Jr Functional and therapeutic analysis of hepatitis C virus NS3.4A protease control of antiviral immune defense. J Biol Chem. 2007;282:10792–10803. doi: 10.1074/jbc.M610361200. [DOI] [PubMed] [Google Scholar]
- 70.Kalantari P, Harandi OF, Hankey PA, Henderson AJ. HIV-1 Tat mediates degradation of RON receptor tyrosine kinase, a regulator of inflammation. J Immunol. 2008;181:1548–1555. doi: 10.4049/jimmunol.181.2.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamp W, Berk MB, Visser CJ, Nottet HS. Mechanisms of HIV-1 to escape from the host immune surveillance. Eur J Clin Invest. 2000;30:740–746. doi: 10.1046/j.1365-2362.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 72.Kamp W, Breij EC, Nottet HS, Berk MB. Interactions between major histocompatibility complex class II surface expression and HIV: implications for pathogenesis. Eur J Clin Invest. 2001;31:984–991. doi: 10.1046/j.1365-2362.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 73.Kanto T. Virus associated innate immunity in liver. Front Biosci. 2008;13:6183–6192. doi: 10.2741/3146. [DOI] [PubMed] [Google Scholar]
- 74.Karp CL. Measles: immunosuppression, interleukin-12, and complement receptors. Immunol Rev. 1999;168:91–101. doi: 10.1111/j.1600-065X.1999.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 75.Khan S, Zimmermann A, Basler M, Groettrup M, Hengel H. A cytomegalovirus inhibitor of gamma interferon signaling controls immunoproteasome induction. J Virol. 2004;78:1831–1842. doi: 10.1128/JVI.78.4.1831-1842.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koppers-Lalic D, Reits EA, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes Rezende M, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, Mettenleiter TC, Rijsewijk FA, Tampe R, Neefjes J, Wiertz EJ. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc Natl Acad Sci USA. 2005;102:5144–5149. doi: 10.1073/pnas.0501463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koppers-Lalic D, Rijsewijk FA, Verschuren SB, van Gaans-Van den Brink JA, Neisig A, Ressing ME, Neefjes J, Wiertz EJ. The UL41-encoded virion host shutoff (vhs) protein and vhs-independent mechanisms are responsible for down-regulation of MHC class I molecules by bovine herpesvirus 1. J Gen Virol. 2001;82:2071–2081. doi: 10.1099/0022-1317-82-9-2071. [DOI] [PubMed] [Google Scholar]
- 78.Koppers-Lalic D, Verweij MC, Lipinska AD, Wang Y, Quinten E, Reits EA, Koch J, Loch S, Marcondes Rezende M, Daus F, Bienkowska-Szewczyk K, Osterrieder N, Mettenleiter TC, Heemskerk MH, Tampe R, Neefjes JJ, Chowdhury SI, Ressing ME, Rijsewijk FA, Wiertz EJ. Varicellovirus UL 49.5 proteins differentially affect the function of the transporter associated with antigen processing, TAP. PLoS Pathog. 2008;4:e1000080. doi: 10.1371/journal.ppat.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kotturi MF, Peters B, Buendia-Laysa F, Jr, Sidney J, Oseroff C, Botten J, Grey H, Buchmeier MJ, Sette A. The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. J Virol. 2007;81:4928–4940. doi: 10.1128/JVI.02632-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwun HJ, da Silva SR, Qin H, Ferris RL, Tan R, Chang Y, Moore PS. The central repeat domain 1 of Kaposi’s sarcoma-associated herpesvirus (KSHV) latency associated-nuclear antigen 1 (LANA1) prevents cis MHC class I peptide presentation. Virology. 2011;412:357–365. doi: 10.1016/j.virol.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwun HJ, da Silva SR, Shah IM, Blake N, Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mimics Epstein-Barr virus EBNA1 immune evasion through central repeat domain effects on protein processing. J Virol. 2007;81:8225–8235. doi: 10.1128/JVI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehner PJ, Karttunen JT, Wilkinson GW, Cresswell P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc Natl Acad Sci USA. 1997;94:6904–6909. doi: 10.1073/pnas.94.13.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leonard JA, Filzen T, Carter CC, Schaefer M, Collins KL. HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements. J Virol. 2011;85:6867–6881. doi: 10.1128/JVI.00229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leung LW, Park MS, Martinez O, Valmas C, Lopez CB, Basler CF. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunol Cell Biol. 2011;89:792–802. doi: 10.1038/icb.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levitsky V, Masucci MG. Manipulation of immune responses by Epstein-Barr virus. Virus Res. 2002;88:71–86. doi: 10.1016/S0168-1702(02)00121-1. [DOI] [PubMed] [Google Scholar]
- 86.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M, Jr, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu B, Woltman AM, Janssen HL, Boonstra A. Modulation of dendritic cell function by persistent viruses. J Leukoc Biol. 2009;85:205–214. doi: 10.1189/jlb.0408241. [DOI] [PubMed] [Google Scholar]
- 88.Liu HM, Gale M. Hepatitis C virus evasion from RIG-I-dependent hepatic innate immunity. Gastroenterol Res Pract. 2010;2010:548390. doi: 10.1155/2010/548390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182:3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lybarger L, Wang X, Harris MR, Virgin HW, IV, Hansen TH. Virus subversion of the MHC class I peptide-loading complex. Immunity. 2003;18:121–130. doi: 10.1016/S1074-7613(02)00509-5. [DOI] [PubMed] [Google Scholar]
- 91.Maloney G, Schroder M, Bowie AG. Vaccinia virus protein A52R activates p38 mitogen-activated protein kinase and potentiates lipopolysaccharide-induced interleukin-10. J Biol Chem. 2005;280:30838–30844. doi: 10.1074/jbc.M501917200. [DOI] [PubMed] [Google Scholar]
- 92.Mattarollo SR, Yong M, Gosmann C, Choyce A, Chan D, Leggatt GR, Frazer IH. NKT cells inhibit antigen-specific effector CD8 T cell induction to skin viral proteins. J Immunol. 2011;187:1601–1608. doi: 10.4049/jimmunol.1100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCluskey J, Rossjohn J, Purcell AW. TAP genes and immunity. Curr Opin Immunol. 2004;6:651–659. doi: 10.1016/j.coi.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 94.McNeilly F, Allan GM, Foster JC, Adair BM, McNulty MS, Pollock J. Effect of porcine circovirus infection on porcine alveolar macrophage function. Vet Immunol Immunopathol. 1996;49:295–306. doi: 10.1016/0165-2427(95)05476-6. [DOI] [PubMed] [Google Scholar]
- 95.Means RE, Ishido S, Alvarez X, Jung JU. Multiple endocytic trafficking pathways of MHC class I molecules induced by a Herpesvirus protein. EMBO J. 2002;21:1638–1649. doi: 10.1093/emboj/21.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minang JT, Trivett MT, Coren LV, Barsov EV, Piatak M, Jr, Ott DE, Ohlen C. Nef-mediated MHC class I down-regulation unmasks clonal differences in virus suppression by SIV-specific CD8(+) T cells independent of IFN-gamma and CD107a responses. Virology. 2009;391:130–139. doi: 10.1016/j.virol.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miura S, Kawana K, Schust DJ, Fujii T, Yokoyama T, Iwasawa Y, Nagamatsu T, Adachi K, Tomio A, Tomio K, Kojima S, Yasugi T, Kozuma S, Taketani Y. CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J Virol. 2010;84:11614–11623. doi: 10.1128/JVI.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mold C, Bradt BM, Nemerow GR, Cooper NR. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med. 1988;168:949–969. doi: 10.1084/jem.168.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monleon E, Pacheco MC, Lujan L, Bolea R, Luco DF, Vargas MA, Alabart JL, Badiola JJ, Amorena B. Effect of in vitro maedi-visna virus infection on adherence and phagocytosis of staphylococci by ovine cells. Vet Microbiol. 1997;57:13–28. doi: 10.1016/S0378-1135(97)00080-1. [DOI] [PubMed] [Google Scholar]
- 100.Morris MM, Dyson H, Baker D, Harbige LS, Fazakerley JK, Amor S. Characterization of the cellular and cytokine response in the central nervous system following Semliki Forest virus infection. J Neuroimmunol. 1997;74:185–197. doi: 10.1016/S0165-5728(96)00786-2. [DOI] [PubMed] [Google Scholar]
- 101.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 102.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 103.Netherton CL, McCrossan MC, Denyer M, Ponnambalam S, Armstrong J, Takamatsu HH, Wileman TE. African swine fever virus causes microtubule-dependent dispersal of the trans-golgi network and slows delivery of membrane protein to the plasma membrane. J Virol. 2006;80:11385–11392. doi: 10.1128/JVI.00439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noriega VM, Tortorella D. Human cytomegalovirus-encoded immune modulators partner to downregulate major histocompatibility complex class I molecules. J Virol. 2009;83:1359–1367. doi: 10.1128/JVI.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ogino T, Moriai S, Ishida Y, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. Association of immunoescape mechanisms with Epstein-Barr virus infection in nasopharyngeal carcinoma. Int J Cancer. 2007;120:2401–2410. doi: 10.1002/ijc.22334. [DOI] [PubMed] [Google Scholar]
- 106.Paabo S, Nilsson T, Peterson PA. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci USA. 1986;83:9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park B, Spooner E, Houser BL, Strominger JL, Ploegh HL. The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation. J Exp Med. 2010;207:2033–2041. doi: 10.1084/jem.20091793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 109.Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qu L, Lemon SM. Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses. Semin Liver Dis. 2010;30:319–332. doi: 10.1055/s-0030-1267534. [DOI] [PubMed] [Google Scholar]
- 111.Ramachandran A, Horvath CM. Dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J Virol. 2010;84:11152–11163. doi: 10.1128/JVI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Redd AD, Eaton KP, Kong X, Laeyendecker O, Lutalo T, Wawer MJ, Gray RH, Serwadda D, Quinn TC. C-reactive protein levels increase during HIV-1 disease progression in Rakai, Uganda, despite the absence of microbial translocation. J Acquir Immune Defic Syndr. 2010;54:556–559. doi: 10.1097/QAI.0b013e3181e0cdea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reusch U, Muranyi W, Lucin P, Burgert HG, Hengel H, Koszinowski UH. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18(4):1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rey Nores JE, McCullough KC. Rinderpest virus isolates of different virulence vary in their capacity to infect bovine monocytes and macrophages. J Gen Virol. 1997;78:1875–1884. doi: 10.1099/0022-1317-78-8-1875. [DOI] [PubMed] [Google Scholar]
- 115.Richardson K, Weinberg A. Dynamics of regulatory T-cells during pregnancy: effect of HIV infection and correlations with other immune parameters. PLoS One. 2011;6:e28172. doi: 10.1371/journal.pone.0028172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rico MA, Infantes S, Ramos M, Trento A, Johnstone C, Melero JA, Del Val M, Lopez D. TLR4-independent upregulation of activation markers in mouse B lymphocytes infected by HRSV. Mol Immunol. 2011;47:1802–1807. doi: 10.1016/j.molimm.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 117.Riezu-Boj JI, Larrea E, Aldabe R, Guembe L, Casares N, Galeano E, Echeverria I, Sarobe P, Herrero I, Sangro B, Prieto J, Lasarte JJ. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422–431. doi: 10.1016/j.jhep.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 118.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 120.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saifuddin M, Ghassemi M, Patki C, Parker CJ, Spear GT. Host cell components affect the sensitivity of HIV type 1 to complement-mediated virolysis. AIDS Res Hum Retroviruses. 1994;10:829–837. doi: 10.1089/aid.1994.10.829. [DOI] [PubMed] [Google Scholar]
- 122.Sakaguchi S. Control of immune responses by naturally arising CD4+ regulatory T cells that express toll-like receptors. J Exp Med. 2003;197:397–401. doi: 10.1084/jem.20030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanz-Parra A, Sobrino F, Ley V. Infection with foot-and-mouth disease virus results in a rapid reduction of MHC class I surface expression. J Gen Virol. 1998;79:433–436. doi: 10.1099/0022-1317-79-3-433. [DOI] [PubMed] [Google Scholar]
- 124.Schlee M, Schuhmacher M, Holzel M, Laux G, Bornkamm GW. c-MYC impairs immunogenicity of human B cells. Adv Cancer Res. 2007;97:167–188. doi: 10.1016/S0065-230X(06)97007-9. [DOI] [PubMed] [Google Scholar]
- 125.Schmidt K, Wies E, Neipel F. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J Virol. 2011;85:4530–4537. doi: 10.1128/JVI.02123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schölz C, Tampé R. The intracellular antigen transport machinery TAP in adaptive immunity and virus escape mechanisms. J Bioenerg Biomembr. 2005;37:509–515. doi: 10.1007/s10863-005-9500-1. [DOI] [PubMed] [Google Scholar]
- 127.Schrier RD, Rice GP, Oldstone MB. Suppression of natural killer cell activity and T cell proliferation by fresh isolates of human cytomegalovirus. J Infect Dis. 1986;153:1084–1091. doi: 10.1093/infdis/153.6.1084. [DOI] [PubMed] [Google Scholar]
- 128.Schuhmann KM, Pfaller CK, Conzelmann KK. The measles virus V protein binds to p65 (RelA) to suppress NF-kappaB activity. J Virol. 2011;85:3162–3171. doi: 10.1128/JVI.02342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 130.Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 131.Shim J, Lee ES, Park S, Bang D, Sohn S. CD4(+) CD25(+) regulatory T cells ameliorate Behcet’s disease-like symptoms in a mouse model. Cytotherapy. 2011;13:835–847. doi: 10.3109/14653249.2011.571245. [DOI] [PubMed] [Google Scholar]
- 132.Shin J, Park B, Lee S, Kim Y, Biegalke BJ, Kang S, Ahn K. A short isoform of human cytomegalovirus US3 functions as a dominant negative inhibitor of the full-length form. J Virol. 2006;80:5397–5404. doi: 10.1128/JVI.02397-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shisler J, Yang C, Walter B, Ware CF, Gooding LR. The adenovirus E3–10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J Virol. 1997;71:8299–8306. doi: 10.1128/jvi.71.11.8299-8306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shresta S, Sharar KL, Prigozhin DM, Snider HM, Beatty PR, Harris E. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J Immunol. 2005;175:3946–3954. doi: 10.4049/jimmunol.175.6.3946. [DOI] [PubMed] [Google Scholar]
- 135.Sluijs KF, Obregon C, Geiser TK, Muhlemann K, Nicod LP. Monocyte differentiation toward regulatory dendritic cells is not affected by respiratory syncytial virus-induced inflammatory mediators. Am J Respir Cell Mol Biol. 2011;44:655–664. doi: 10.1165/rcmb.2010-0136OC. [DOI] [PubMed] [Google Scholar]
- 136.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 137.Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, Suzuki M, Shimura H, Takizawa S, Takahashi M, Akiyama D, Arai-Yamashita S, Furuya F, Kawaguchi A, Kaneshige M, Katoh R, Endo T, Kobayashi T. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes. 2009;58:2285–2291. doi: 10.2337/db09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verweij MC, Lipińska AD, Koppers-Lalic D, Quinten E, Funke J, van Leeuwen HC, Bieńkowska-Szewczyk K, Koch J, Ressing ME, Wiertz EJ. Structural and functional analysis of the TAP-inhibiting UL49.5 proteins of varicelloviruses. Mol Immunol. 2011;48:2038–2051. doi: 10.1016/j.molimm.2011.06.438. [DOI] [PubMed] [Google Scholar]
- 139.Wang Q, Zheng Y, Huang Z, Tian Y, Zhou J, Mao Q, Wu Y, Ni B. Activated IL-23/IL-17 pathway closely correlates with increased Foxp3 expression in livers of chronic hepatitis B patients. BMC Immunol. 2011;12:25. doi: 10.1186/1471-2172-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci USA. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wilson EB, Brooks DG. The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol. 2011;350:39–65. doi: 10.1007/82_2010_96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Windheim M, Hilgendorf A, Burgert HG. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr Top Microbiol Immunol. 2004;273:29–85. doi: 10.1007/978-3-662-05599-1_2. [DOI] [PubMed] [Google Scholar]
- 143.Wood MJ, Byrnes AP, Pfaff DW, Rabkin SD, Charlton HM. Inflammatory effects of gene transfer into the CNS with defective HSV-1 vectors. Gene Ther. 1994;1:283–291. [PubMed] [Google Scholar]
- 144.Yang Y, Xiang Z, Ertl HC, Wilson JM. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yi L, Rosales T, Rose JJ, Chowdhury B, Knutson JR, Venkatesan S. HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking. J Biol Chem. 2010;285:30884–30905. doi: 10.1074/jbc.M110.135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.York IA, Goldberg AL, Mo XY, Rock KL. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol Rev. 1999;172:49–66. doi: 10.1111/j.1600-065X.1999.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 147.Zaldumbide A, Ossevoort M, Wiertz EJ, Hoeben RC. In cis inhibition of antigen processing by the latency-associated nuclear antigen I of Kaposi sarcoma herpes virus. Mol Immunol. 2007;44:1352–1360. doi: 10.1016/j.molimm.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 148.Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou Y. Regulatory T cells and viral infections. Front Biosci. 2008;13:1152–1170. doi: 10.2741/2752. [DOI] [PubMed] [Google Scholar]
- 150.Zinkernagel RM. On cross-priming of MHC class I-specific CTL: rule or exception? Eur J Immunol. 2002;32:2385–2392. doi: 10.1002/1521-4141(200209)32:9<2385::AID-IMMU2385>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 151.Zuo J, Currin A, Griffin BD, Shannon-Lowe C, Thomas WA, Ressing ME, Wiertz EJ, Rowe M. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 2009;5:e1000255. doi: 10.1371/journal.ppat.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]