Abstract
Influenza viruses are common respiratory pathogens in humans and can cause serious infection that leads to the development of pneumonia. In this study, the clinical and laboratory features of 36 patients from Turkey who are hospitalized in intensive care unit due to pandemic influenza A (H1N1) associated pneumonia and respiratory failure were retrospectively evaluated. The most common symptoms were cough and fever. Consolidation (36.1 %) and interstitial changes (30.6 %) were the most frequently identified findings on chest radiographs at the time of admission. Six of the patients (16.7 %) died. Mortality occurred in 3 of 13 patients (23.1 %) with underlying disease, whilst it occurred in only 3 of 23 patients (13 %) who were previously healthy. Mortality was found to be significantly associated only with an elevated lactate dehydrogenase level. A significant relationship was determined only between the presence of lymphopenia and acute respiratory distress syndrome and the need for intensive care treatment. The average time elapsed from the onset of the symptoms until admission was 8.67 ± 2.87 days for the patients died, and 6.0 ± 3.8 days for the patients survived.
Electronic supplementary material
The online version of this article (doi:10.1007/s13337-012-0122-z) contains supplementary material, which is available to authorized users.
Keywords: Pneumonia, Respiratory failure, Swine influenza virus, H1N1
Influenza is one of the most important human infectious diseases [15]. The first isolation of a swine influenza virus from a human occurred in 1974 [12]. Swine influenza virus infections in humans have been reported in the United States, Canada, Europe, and Asia. In 2009, a novel H1N1 virus, which seems to have originated from one or more swine influenza viruses, emerged in human populations [3, 13]. Influenza is an acute respiratory disease that is characterized by the sudden onset of high fever, chills, coughing, sore throat, muscle pain, severe headache, prostration, malaise, and inflammation of the upper respiratory tract and trachea, with general discomfort, but it rarely induces severe inflammatory lung diseases, including pneumonic involvement [7, 14]. There are no unique clinical features that distinguish swine influenza in humans from typical influenza. Although a number of the patients had predisposing immunocompromising conditions, healthy persons are also clearly at risk for illness and death from swine influenza [11]. In this study, we present clinical and laboratory features of 36 hospitalized patients due to pandemic influenza-related pneumonia and respiratory failure in Turkey.
This study included patients with pneumonia admitted to Van Education and Research Hospital, Turkey with presumed H1N1 during the period from November 2009 to January 2010. In this period, a total of 71 patients were hospitalized. Clinical, laboratory and epidemiological data were collected retrospectively. Nasopharyngeal specimens were sent to Virology Laboratory of Refik Saydam National Public Health Agency. The analyses were done using real-time polymerase chain reaction (RT-PCR). Consequently, 36 patients positive for H1N1 were included in the study. The data were assessed with SPSS for Windows 11.0 pack software. The mean values were shown as “arithmetic mean ± SD”. For between-group comparisons, Chi square test and Mann–Whitney U test were used. P < 0.05 was accepted as significant.
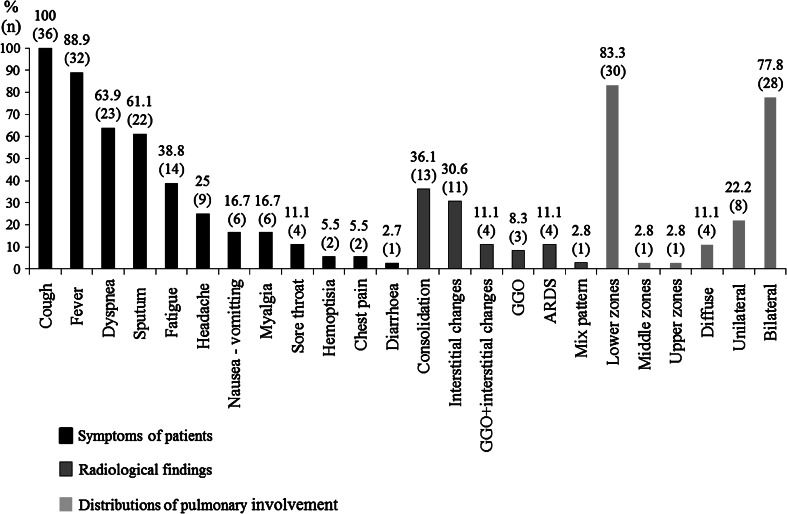
The ages of patients ranged from 14 to 71 years with a mean of 35.27 ± 14.35 years. Of the patients, 17 (47.2 %) were male and 19 (52.8 %) were female. A total of 13 (36.1 %) patients had an underlying chronic disease or pregnancy. Five of these patients had chronic obstructive pulmonary disease (COPD) (additionally, one patient had asthma, one had a history of steroid use because of pemphigus, and one patient had a history of lobectomy); one patient had diabetes mellitus type 2; one patient had cardiovascular disease; one patient had Down syndrome and epilepsy; one patient had asthma; one patient had a history of azathioprine and steroid use because of pemphigus, one patient had chronic lymphocytic leukemia; one patient had obesity; and one was pregnant. The average time elapsed between the onset of the symptoms and admission was 6.4 ± 3.8 days (range, 2–21 days). The most common symptoms were cough and fever; the symptoms are shown in Fig. 1. On physical examination, breath sounds were decreased in three patients (at the left in one patient, and on basal areas in the remaining two patients). There was wheezing in four patients. In addition to the findings of lower respiratory system infection, seven patients had pharyngeal hyperemia and one patient had postnasal drainage. On laboratory examination, the average leukocyte count of the patients was 5,353 ± 2,911/mm3, ANC was 3,927 ± 2,547/mm3, ALC 980 ± 518/mm3, absolute monocyte count was 393 ± 321/mm3, the mean hemoglobin value was 12.8 ± 2.2 g/dL, the average thrombocyte count was 164 ± 72 × 103/mm3. Anemia occurred in, 33.3 %, thrombocytopenia in 36.1 %, neutropenia in 2.8 % and lymphopenia in 58.3 % of patients. With regard to biochemical parameters, average C-reactive protein (CRP) value was 8.8 ± 6.9 mg/dL, sedimentation was 42.5 ± 23.6 mm/h, AST was 55.7 ± 45.3 U/L, ALT was 35.4 ± 26.2 U/L, LDH was 701.7 ± 282.1 U/L, and CK 516.2 ± 631.8 U/L. CRP was high in 91.7 % of the patients, sedimentation was high in 83.3 %, AST was elevated in 52.8 %, ALT was increased in 44.4 %, LDH was elevated in 75 % of the patients and CK was high in 47.2 % of the patients. Radiologically, consolidation and interstitial changes were the most frequently identified findings on chest radiographs at the time of admission. These changes involved particularly lower lobes bilaterally. The distribution of chest radiographic findings was shown in Fig. 1. All patients were administered oseltamivir on the day of hospitalization. An oseltamivir dose of 75 mg was given twice a day for at least 5 days to patients with a mild clinical picture and a dose of 150 mg, twice a day, for at least 10 days to patients who needed intensive care treatment and developed symptoms of acute respiratory distress syndrome (ARDS) during following period. All patients were administered ceftriaxone + clarithromycin or ampicillin-sulbactam + ciprofloxacin for bacterial co-infection. Afterwards, antibiotic treatment was modified on the basis of clinical picture and culture results. Blood cultures of three patients yielded Acinetobacter baumannii, one patient yielded Streptococcus pneumonia. Growth of non-albicans candida was detected in the blood culture of one patient and candida growth on sputum culture of another patient. Additionally, intravenous immunoglobulin was given to two patients due to sepsis. Six patients (16.7 %) were administered steroids (methylprednisolone 1 mg/kg/day) with ARDS and/or sepsis. Two of the six patients that were given steroids died, whilst 4 of the 30 patients who were not given steroids died. The difference was not statistically significant (P > 0.05). The average duration of hospitalization was 10.4 ± 7.0 days, ranging from 2 to 26 days. Ten patients (27.8 %) needed intensive care treatment. Nine of these patients required mechanic ventilation. Average duration of mechanic ventilation was 9.5 ± 8.2 days, it ranged from 1 to 25 days. Multiple organ failures were encountered in two patients (5.6 %), that these were two of four patients with sepsis. Only one had a neurological complication (2.8 %) as generalized tonic–clonic seizure. Six patients died (16.7 %). The mean dead age was 37.1 ± 12.4 years (range, 26–53 years). The characteristics of the patients who died are shown in Table 1 in Supplementary material. There was a significant relationship between the presence of lymphopenia and ARDS (P = 0.011). Also, the relationship between presence of lymphopenia and the need for ICU was significant (P = 0.042). But, there was no relationship among ARDS, the need for intensive care treatment and other laboratory parameters. Given the association between the time elapsed from the onset of the symptoms to admission and mortality, the average time elapsed until hospital admission was 8.67 ± 2.87 days for the patients died, and 6.0 ± 3.8 days for the patients survived. The difference was statistically significant (P = 0.02). Given the association between mortality and the presence of underlying disease, mortality occurred in 3 (23.1 %) of 13 patients with underlying disease, whilst it occurred in only 3 (13 %) of 23 patients who were previously healthy. However, the difference was not statistically significant (P > 0.05). With regard to the association between mortality and laboratory parameters, mortality was found to be significantly associated only with an elevated LDH level (P = 0.001), whilst there was no statistically significant relationship between mortality and other laboratory parameters (Table 1).
Fig. 1.
The symptoms and chest X-ray findings in patients with H1N1 related pneumonia and respiratory failure. The most frequent symptoms were cough and fever. Approximately two-thirds of the patients had dyspnea and sputum. Consolidation and interstitial changes were detected in one-thirds of the patients. Fewer patients had a ground-glass appearance. The lesions were often bilateral. Usually, it was influenced by the lower zone of the lungs. Additionally, one patient had pleural effusion, and one patient had atelectasis, and another patient had pneumothorax. GGO ground-glass opacities, ARDS ARDS
Table 1.
The relationship between laboratory and mortality in patients with H1N1 related pneumonia and respiratory failure
| Laboratory findings | Survived (n, %) | Died (n, %) | p value |
|---|---|---|---|
| Presence of leucopenia | 16–76.2 | 5–23.8 | >0.05 |
| Absence of leucopenia | 14–93.3 | 1–6.7 | |
| Presence of neutropenia | 1–100 | – | >0.05 |
| Absence of neutropenia | 29–82.9 | 6–17.1 | |
| Presence of lymphopenia | 14–73.7 | 5–26.3 | >0.05 |
| Absence of lymphopenia | 16–94.1 | 1–5.9 | |
| Presence of anemia | 9–75 | 3–25 | >0.05 |
| Absence of anemia | 21–87.5 | 3–12.5 | |
| Presence of thrombocytopenia | 10–76.9 | 3–23.1 | >0.05 |
| Absence of thrombocytopenia | 20–87 | 3–13 | |
| CRP | |||
| Normal | 3–100 | – | >0.05 |
| Elevated | 27–81.8 | 6–18.2 | |
| ESR | |||
| Normal | 6–100 | – | >0.05 |
| Elevated | 24–80 | 6–20 | |
| LDH (U/L) | |||
| <500 | 9–100 | – | |
| 500–1,000 | 19–90.5 | 2–9.5 | |
| >1,000 | 2–33.3 | 4–66.7 | 0.001a |
| CK | |||
| Normal | 16–84.2 | 3–15.8 | >0.05 |
| Elevated | 14–82.4 | 3–17.6 | |
| AST | |||
| Normal | 16–94.1 | 1–5.9 | >0.05 |
| Elevated | 14–73.7 | 5–26.3 | |
| ALT | |||
| Normal | 17–85 | 3–15 | >0.05 |
| Elevated | 13–81.3 | 3–18.8 |
CRP C-reactive protein, ESR erythrocyte sedimentation rate, LDH lactate dehydrogenase, CK creatine kinase, AST aspartate aminotransferase, ALT alanine aminotransferase
aCompared with survived patients and dead patients, LDH levels were significantly higher in patients who died
Pandemic influenza infections usually have a mild course. In a majority of the cases, it was determined that the symptoms of viral respiratory tract infection were indistinguishable from those of normal flu with mild course. However, more severe cases may develop particularly in the patients at high risk. As in our study, it has been reported that pandemic influenza A (H1N1) is also able to cause severe cases such as pneumonia and ARDS [1, 6, 8, 9]. Clinically, there may be fever, dry cough, headache, sore throat, cold feeling, fatigue, malaise, muscle and joint pain, vomiting, nasal drainage, and diarrhea [4]. In our study, all symptoms, most frequently cough, fever and shortness of breath, were observed. Clinical symptoms tend to be more severe in high-risk groups. Children under 5 years old, elderly people older than 65 years, pregnant women, people who have chronic disorders (asthma, COPD, diabetes, cardiovascular diseases, malignancy, hemoglobinopathies, immunosuppression, metabolic disorders, chronic renal failure), obesity and those living in long-term care facilities are defined as high-risk groups [4]. It was reported that 73 % of the patients hospitalized because of pandemic H1N1 influenza A 2009 in United States of America (USA) was in high-risk group [6]; in another study from Mexico, 44.4 % of the patients was reported to be in risk group [9]. Of the patients in our study, 36.1 % was in high-risk group. In an article from USA, it was reported that 68 % of the patients died had an underlying disease; however, it was also highlighted that deaths among healthy young people should not be underestimated [6]. As in our study, a study from Mexico has been reported that did not effect on mortality by whether or not the underlying disease [9]. Jain et al. [6] reported that 20 % had leukopenia, 37 % had anemia, 14 % had thrombocytopenia, 45 % had elevated ALT and 44 % had elevated AST of the hospitalized patients in a study from USA. Of 18 hospitalized patients with pneumonia and respiratory failure in Mexico, 88.9 % had high LDH levels exceeding 1,000 U/L in the majority of them; it was found that CK was elevated in 88.9 % of the patients and high aminotransferases in 61.1 % of the patients. In the same study, lymphopenia was reported in 61 % of the patients. In that study, high LDH and CK levels and lymphopenia were reported to be associated with mortality [9]. In our study, 33.3 % of the patients had anemia, 36.1 % had thrombocytopenia, 58.3 % had leukopenia, 2.8 % had neutropenia and 52.8 % had lymphopenia. AST was elevated in 52.8 % of the patients, ALT was elevated in 44.4 % of the patients, 75 % had high LDH level and 47.2 % had high CK level. In our study, only elevated LDH was found to be associated with mortality, the presence of lymphopenia was found to be correlated with development of ARDS and the need for intensive care treatment. Radiographically, there are signs of viral pneumonia such as interstitial changes, ground glass opacities, centrilobular nodules and consolidation. These signs vary and may manifest together. Symptoms of ARDS may develop in the patients, but pleural effusion is rare [2]. In a study, major radiological signs were reported as interstitial changes, ground glass opacities singly or together with interstitial changes, and consolidation; it was indicated that the involvement occurred particularly bilateral and in lower lobes. In the same study, ARDS signs were present in 7.5 % of the patients whilst pleural effusion attended in 10 % of the patients [2]. Similarly, the most commonly encountered signs in our study were interstitial changes, consolidation and ground glass opacities. ARDS signs were present in 11.1 % of the patients. Pleural effusion attended to the event in 8.3 % of the patients. The treatment involves antiviral treatment, antibiotic therapy for bacterial co-infections and supportive therapy. Antiviral treatment must be established as soon as possible. Therefore, the treatment is recommended for all patients with confirmed, probable or presumed pandemic H1N1 influenza A 2009 infection, who are hospitalized. The most widely used agent for this purpose is oseltamivir in a dose of 75 mg, twice a day for adolescents and adults weighing over 40 kg. The duration of the treatment is at least 5 days. Another drug is zanamivir, it is used in a dose of 10 mg, twice a day by oral inhalation, for 5 days [4, 5]. In our study, oseltamivir was administered to all patients as soon as they were hospitalized in a dose of 75 mg, twice a day; the dose was augmented to 150 mg, twice a day for patients who required intensive care treatment. In USA, severe bacterial co-infection was recognized in 29 % of 77 cases died due to pandemic influenza A and performed lung autopsy. Hence, additive antibiotic treatment is recommended for the patients with severe pulmonary disease and infiltrates. In adults, treatment involves third generation cephalosporins or ampicillin-sulbactam plus any drug from macrolide or quinolone group [4, 5]. So, we started to the patients particularly ceftriaxone + clarithromycin or ampicillin-sulbactam + ciprofloxacin for bacterial co-infection at the time of hospitalization. Although the treatment does not involve steroids, there are opinions supporting their use in case of the presence signs of septic shock and severe ARDS. In a study [9], it was reported that 27.7 % of the patients were given steroids; in another study [6], it was reported that 36 % of the patients were given steroids. Quispe-Laime et al. [10] reported that ARDS and multiple organ dysfunction significantly improved in the patients given steroids. In our study, we gave steroids to six (16.7 %) patients who had ARDS and/or sepsis. However, we did not find any positive or negative effect of steroid treatment on mortality. There are reports about that delayed antiviral treatment increases mortality. It was denoted that establishment of the antiviral treatment especially within 48 h after the onset of the symptoms considerably reduced mortality [4–6, 9]. In a study reported from USA, the time of admission to the hospital of the patents died was an average of 8 days after the onset of the symptoms. In that study, it was highlighted that no patients who were started antiviral treatment within 48 h died [6]. In a study from Mexico, mortality rate was found to be 38.8 % among the patients hospitalized because of pneumonia and respiratory failure and it was reported that the patients died were young and previously healthy persons [9]; in another study from USA, it was reported that the mean age of the patients died was 26 years [6]. In our study, the mortality rate was 16.7 %. Similarly, we found that the deaths intensified among young adult (mean age is 37.1 years) patients, and that there was no difference with regard to mortality between the patients who had underlying diseases and the persons who were previously healthy.
In conclusion, we found the mortality rate to be 16.7 % among 36 patients hospitalized with the clinical picture of pneumonia and respiratory failure related to pandemic influenza A 2009. We found that the deaths intensified in young adult ages, occurred also in previously healthy persons and that the presence of lymphopenia was associated with ARDS development and the need for intensive care treatment, whilst elevated LDH level was related to mortality.
Electronic supplementary material
(DOC 51 kb)
References
- 1.Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009;193:1488–1493. doi: 10.2214/AJR.09.3599. [DOI] [PubMed] [Google Scholar]
- 2.Busi Rizzi E, Schininà V, Ferraro F, Rovighi L, Cristoforo M, Chiappetta D, Lisena F, Lauria F, Bibbolino C. Radiological findings of pneumonia in patients with swine-origin influenza A virus (H1N1) Radiol Med. 2010;115:507–515. doi: 10.1007/s11547-010-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacımustafaoğlu M. Pandemic H1N1 influenza infections in 2009. Turk Arch Ped. 2010;45:31–36. [Google Scholar]
- 5.Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, McGeer AJ, Neuzil KM, Pavia AT, Tapper ML, Uyeki TM, Zimmerman RK, Expert Panel of the Infectious Diseases Society of America Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/598513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 7.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26(Suppl 4):59–66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollura DJ, Asnis DS, Crupi RS, Conetta R, Feigin DS, Bray M, Taubenberger JK, Bluemke DA. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1) AJR Am J Roentgenol. 2009;193:1500–1503. doi: 10.2214/AJR.09.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 10.Quispe-Laime AM, Bracco JD, Barberio PA, Campagne CG, Rolfo VE, Umberger R, Meduri GU. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36:33–41. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez A, Capuano AW, Wellman DA, Lesher KA, Setterquist SF, Gray GC. Preventing zoonotic influenza virus infection. Emerg Infect Dis. 2006;12:996–1000. doi: 10.3201/eid1206.051576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith TF, Burgert EO, Jr, Dowdle WR, Noble GR, Campbell RJ, Van Scoy RE. Isolation of swine influenza virus from autopsy lung tissue of man. N Engl J Med. 1976;294:708–710. doi: 10.1056/NEJM197603252941308. [DOI] [PubMed] [Google Scholar]
- 13.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 14.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson WW, Moore MR, Weintraub E, Cheng PY, Jin X, Bridges CB, Bresee JS, Shay DK. Estimating influenza-associated deaths in the United States. Am J Public Health. 2009;99(Suppl 2):225–230. doi: 10.2105/AJPH.2008.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 51 kb)