Abstract
The spread of tumor in the peritoneum can be understood, although it is a complex organ. A study of its embryology, anatomy and function is of clear benefit. It is formed from a network of folds, reflections, and potential spaces produced by the visceral and parietal peritoneum. These folds and reflections begin as a dorsal and ventral mesentery, supporting the primitive gut in early embryologic development. The dorsal mesentery connects the stomach and other organs to the posterior abdominal wall, while the ventral mesentery connects the stomach to the ventral abdominal wall. As the embryo develops, there is further organ growth, elongation, cavitation and rotation. The dorsal and ventral mesentery also develops along with the viscera, forming ligaments, mesenteries, omenta and potential spaces from the resulting reflections and folds. These ligaments, mesenteries, and omenta, support and nurture the organs of the peritoneum, providing a highway for arteries, veins, nerves and lymphatics. The potential spaces created from these folds and reflections of the visceral and parietal peritoneum are also important to realize. For example, the transverse mesocolon divides the peritoneal cavity into a supramesocolic and inframesocolic space in the abdomen and paravesicular spaces within the pelvis. The falciform ligament is well known in the supramesocolic space, dividing it further into a left and right compartment. Knowledge of the peritoneal vascular anatomy is beneficial in locating the spaces and ligaments about the peritoneum. For example, identifying the left gastric artery or vein will lead to the gastrohepatic ligament, which is part of the supramesocolic space. Besides serving a life sustaining role, the multiple compartments, ligaments, mesenteries and omenta within the peritoneum can also facilitate the spread of disease. Tumors can spread directly from one organ to another, seed metastatic deposits in the peritoneal cavity, and travel through the lymphatic or hematogenous route to invade other organs in the peritoneum.
Keywords: Peritoneal, Abdomen, Pelvis
INTRODUCTION
The peritoneum is indeed complex. To discern the spread of tumor in the peritoneum, one must understand its embryology, anatomy and function. It is a formed from a complex network of parietal peritoneum lining the abdominal wall and the visceral peritoneum covering the abdominal and pelvic organs. It reflects and folds as it covers the visceral organs, forming potential spaces between its lining along the abdominal and pelvic walls. Additionally, the ligaments, mesenteries and omenta are also formed from its reflections over the abdominal and pelvic viscera.
The network of reflections, folds, boundaries and potential spaces serves a physiologic and life sustaining role[1]. It also bridges the retroperitoneum to the peritoneum. However, it can act as a potential pathway for disease spread within the peritoneum and between the peritoneum and retroperitoneum via the subperitoneal space.
Primary tumors of the peritoneum are rarely encountered[2]. More commonly there will be metastases from cancers such as the stomach, colon, ovary, pancreas and lymphoma. With advancement in cross sectional imaging such as ultrasound, computed tomography (CT), magnetic resonance imaging and positron emission tomography/CT, the peritoneal spread of tumor can now be evaluated; improving screening, staging and detection for tumor recurrence.
ANATOMY
Embryology
The peritoneum arises from a single layer of mesothelial cells. There is then a submesothelial layer of connective tissue containing blood vessels, lymphatics, collagen, elastic fibers, and fibroblast-like cells[3]. The subperitoneal space is formed from the submesothelial layer of connective tissues. It is a space that serves as a contiguous highway between the ligaments, mesenteries, omenta, abdominal wall and retroperitoneum.
The embryologic development of the peritoneum is complex and a detailed account of its formation is not the purpose of this article[3-7]. In short, the primitive gut is supported and suspended by a dorsal and ventral mesentery, which divides the peritoneum into a left and right cavity. The dorsal mesentery connects the stomach and the remainder of the primitive gut to the posterior abdominal wall. On the other hand, the ventral mesentery is discontinuous, only connecting the stomach to the ventral abdominal wall. Communication between the left and right peritoneum is possible because the ventral mesentery is absent from the lower abdomen.
The dorsal mesentery contains the pancreas and spleen and allows for their development. The dorsal mesentery associated with the spleen and pancreas will later develop into the gastrosplenic and splenorenal ligament. The liver is supported and grows along the ventral mesentery, conversely. The ventral mesentery anterior to the liver and attaching it to the abdominal wall will later become the falciform ligament. The gastrohepatic and hepatoduodenal ligament will develop from the ventral mesentery that is between the liver and stomach.
Further organ development, growth, elongation, cavitation, and rotation bring about the adult peritoneum. (Figures 1 and 2). The formation of the lesser and greater sac and their communication via the Foramen of Winslow is also a result of the maturing of the peritoneum.
Figure 1.
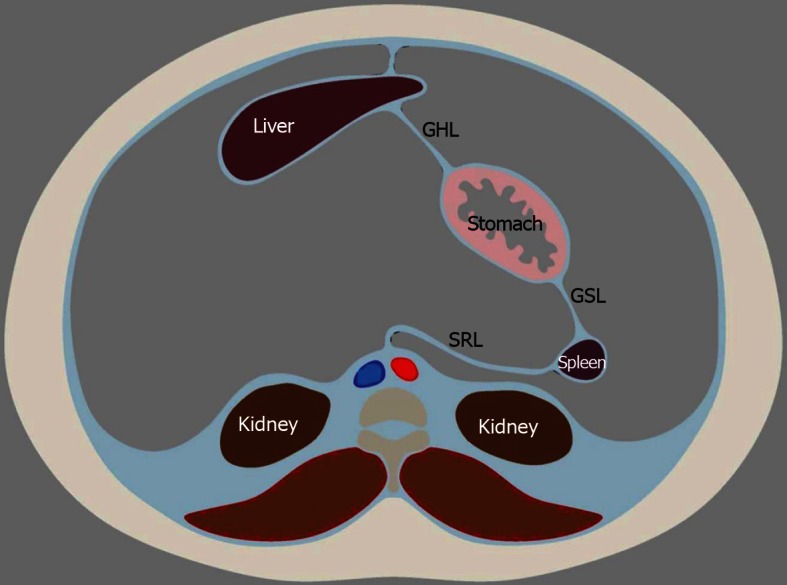
Embryologic development and rotation of the peritoneal structures to the eventual adult position. The liver fills and moves to the right side of the peritoneal cavity as it grows. The spleen moves likewise to the left. GHL: Gastrohepatic ligament; GSL: Gastrosplenic ligament; SRL: Splenorenal ligament.
Figure 2.
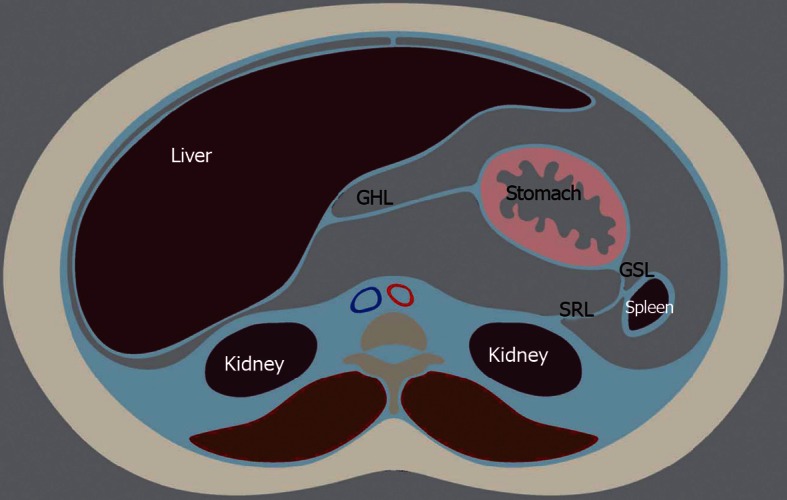
Peritoneal structures in the adult position after growth and rotation. GHL: Gastrohepatic ligament; GSL: Gastrosplenic ligament; SRL: Splenorenal ligament.
Visceral and parietal peritoneum
The parietal peritoneum is defined as that part of the lining along the anterior abdominal wall, the anterior surface of the retroperitoneum, and undersurface of the hemidiaphragms[8]. The visceral peritoneum is continuous with the parietal peritoneum, but covers the visceral organs and forms the mesenteries and omenta.
The greater peritoneal cavity is a closed continuous space in males, but is discontinuous in females at the ostia of the oviducts. The ostia provides a communication between the peritoneum and extraperitoneum[3].
The organs in the peritoneum are supported by ligaments, mesenteries and omenta. These structures are formed from double folds of the peritoneum (Figures 3 and 4). The small bowel, for example, is suspended in place in the abdominal cavity by the mesentery. The ligaments, mesentery, and omenta also serve as conduits and support for arteries, vein, nerves and lymphatics. Boundaries and interconnecting compartments are also arranged by these structures.
Figure 3.
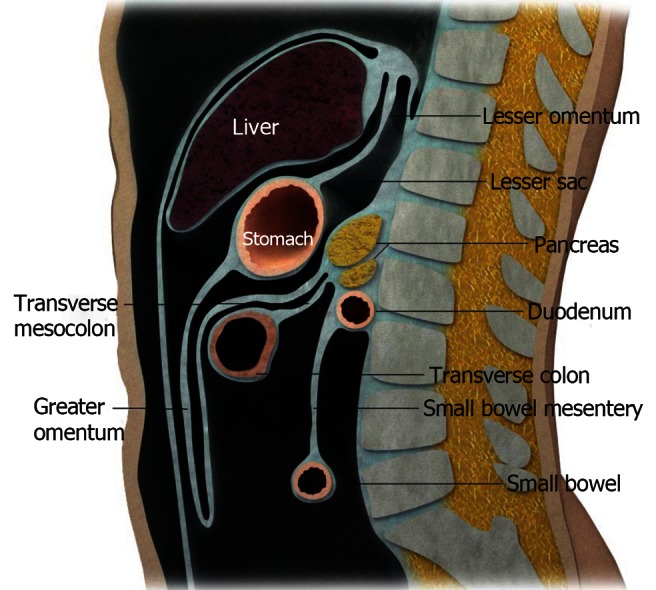
Diagrammatic representation of the peritoneal structures in sagittal view.
Figure 4.
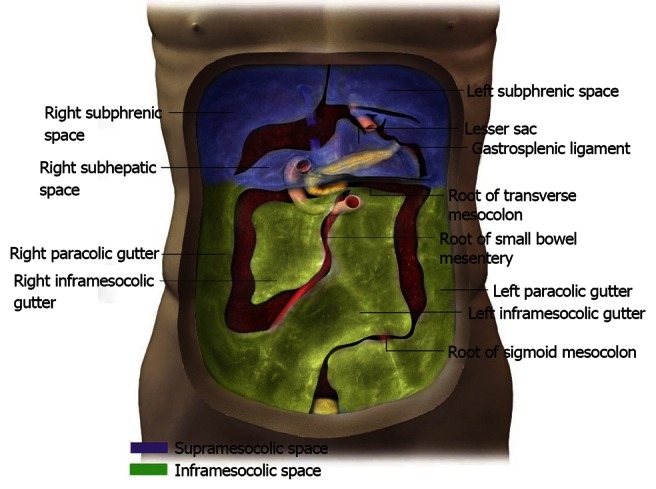
Coronal view of the peritoneal spaces and attachments within the supramesocolic and inframesocolic spaces.
Supramesocolic and inframesocolic space
The transverse mesocolon divides the peritoneum into the supramesocolic and inframesocolic space[4] (Figure 4). The supramesocolic space is further divided into the left and right supramesocolic space by the falciform ligament. Furthermore, the left supramesocolic space is further subdivided into the anterior and posterior perihepatic space, while the right supramesocolic space divides into the anterior perihepatic space and a posterior perihepatic space known as the lesser sac. The Foramen of Winslow allows communication between the right and left supramesocolic space and between the lesser sac and remainder of the peritoneal cavity or greater sac.
Inferior to the transverse mesocolon is the inframesocolic space. It is divided into the left and right inframesocolic space by an obliquely oriented small bowel mesentery.
Prominent supporting ligaments in the supramesocolic space include the hepatoduodenal ligament, gastrohepatic ligament, gastrosplenic ligament and splenorenal ligament. The root of the mesentery, jejunal mesentery, ileal mesentery, ascending mesocolon, descending mesocolon, sigmoid mesentery, and the pelvic floor and peritoneal folds are the corresponding ligaments in the inframesocolic space.
The location and relationships of the ligaments, mesentery, and omenta can be identified by certain landmarks, mostly vasculature (Tables 1 and 2). For example, the gastrohepatic ligament contains the left gastric artery. It, therefore, can be identified by locating the said artery.
Table 1.
Supramesocolic ligaments, their organ association and identifying vasculature
| Left hepatic lobe to the stomach’s lesser curvature | Left gastric artery and vein | Landmark vasculature |
| Gastrohepatic ligament | ||
| Hepatoduodenal ligament | Hepatic hilum to the lesser curvature of the stomach | Extrahepatic bile ducts, hepatic artery, and portal vein |
| Gastrocolic ligament | Greature curvature of the stomach to the transverse colon | Right and left gastroepiploic arteries |
| Greater omentum | From transverse colon covering the small bowel like an apron | Epliploic and branches of the gastroepiploic arteries |
| Gastrosplenic ligament | From fundus and proximal body of stomach to splenic hilum | Short gastric and left gastroepliploic arteries |
| Splenorenal ligament | Connects spleen to tail of pancreas | Distal splenic artery or proximal splenic vein |
Table 2.
Inframesocolic compartment, organ association and their landmark vasculature
| Ligament | Organ association | Landmark vasculature |
| Root of mesentery | Transverse duodenum to right iliac fossa | Superior mesenteric and ileocolic artery and veins |
| Jejunal mesentery | Root of mesentery to jejunal bowel | Jejunal artery and vein |
| Ileal mesentery | Root of mesentery to ileal bowel | Ileal artery and veins |
| Ascending mesocolon | Root of mesentery to ascending colon | Right colic and cecal artery and vein |
| Descending mesocolon | Base of transverse mesocolon to descending colon | Left colic artery and vein |
| Sigmoid mesocolon | Root of sigmoid mesocolon | Sigmoid and superior hemorrhoidal artery and vein |
Paravesicular spaces
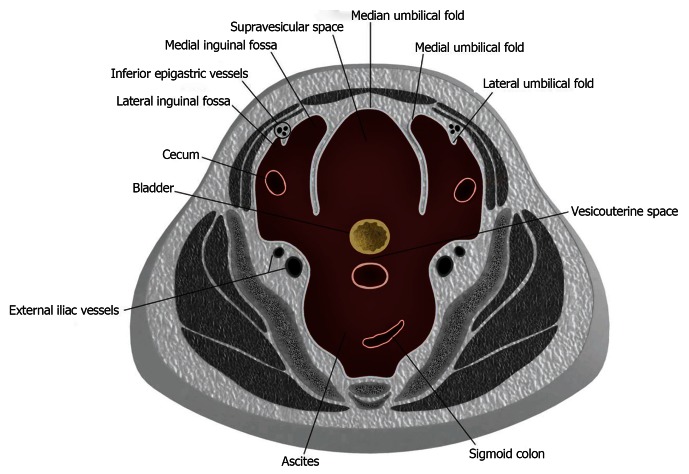
The peritoneum continues its coverings in the pelvis, forming additional boundaries and compartments with reflections and folds[9-11]. Indentations made along the parietal peritoneum of the anterior pelvic wall by the urachus, obliterated umbilical arteries, inferior epigastric vessels and bladder divides the pelvic peritoneum into the anterior and posterior paravesicular spaces (Figure 5).
Figure 5.
The paravesicular spaces in schematic cross section. The anterior paravesicular space is divided into the supravesicular space, medial inguinal fossa and lateral inguinal fossa. The supravesicular space lies in between the medial umbilical fold. The lateral umbilical fold divides the medial and lateral inguinal fossa.
The anterior paravesicular space is further subdivided into the lateral inguinal fossa, medial inguinal fossa and supravesicular space, each of which is demarcated by the lateral, medial, and median umbilical folds. These umbilical folds can be located by the inferior epigastric artery, obliterated umbilical artery and urachus respectively. The internal inguinal ring lies within the lateral inguinal fossa and the femoral ring lies within the medial inguinal fossa.
In males, the posterior paravesicular space is subdivided into the rectovesicular and pararectal space by the peritoneal coverings of the rectum and bladder. In females, peritoneal coverings of the uterus divide the rectovesicular space into the vesicouterine and rectouterine space (cul-de-sac) (Figure 6).
Figure 6.
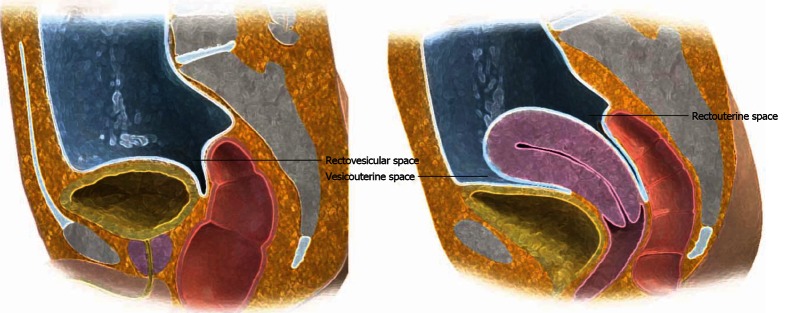
Posterior paravesicular spaces. Sagittal view of the male (left) and female (right) pelvis demonstrates the peritoneal covering of the superior bladder, uterus and upper one-third of the rectum.
These potential spaces in the pelvis formed by extension of the peritoneal coverings can be easily delineated with the accumulation of ascites and other disease process within the pelvis (Figure 7).
Figure 7.
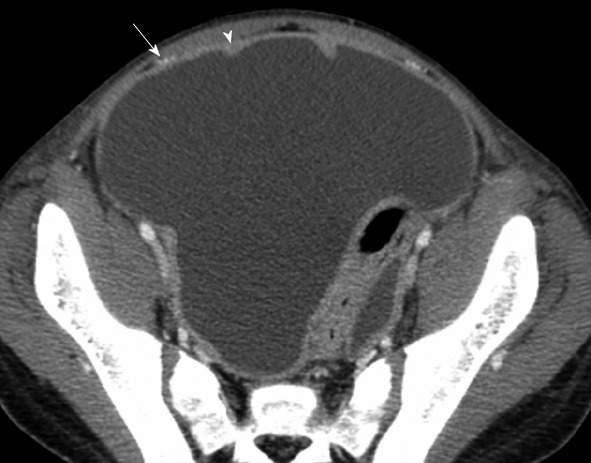
Computed tomography with intravenous contrast. Thirteen years old with rhabdomyosarcoma. The anterior paravesicular spaces are well defined with ascites. The lateral umbilical fold (containing the inferior epigastric vessels, arrow) divides the lateral and medial inguinal fossa. In between the medial umbilical fold (containing the obliterated umbilical vein, arrowhead) is the supravesicular fossa.
SPREAD OF DISEASE
The multiple potential spaces, ligaments, mesenteries and omenta support and nourish the peritoneal organs. However, this complex network of spaces and supporting structures also can serve as conduits for the spread of disease, including tumor. There are four pathways of tumor spread in the peritoneum[1,12]. First, contiguous spread of tumor from one organ to another can occur directly via the serosa. Tumor can also spread directly from one noncontiguous organ to another via the subperitoneal space along the ligaments, mesenteries and omenta. Second, tumor can spread by intraperitoneal seeding along the flow of ascitic fluid. Tumor spread along the lymphatics is the third route. Finally, tumor can embolize via the hematogenous route.
Direct spread
Tumors of the stomach, colon and pancreas commonly spread directly to contiguous and noncontiguous organs by means of the direct route[13]. For example, gastric cancer can spread to the left lobe of the liver via the gastrohepatic ligament. Evaluation of the left gastric artery, which is in the gastrohepatic ligament, is necessary as disease can be expected to spread directly via this pathway, especially when tumor involves the lesser curvature of the stomach (Figure 8).
Figure 8.
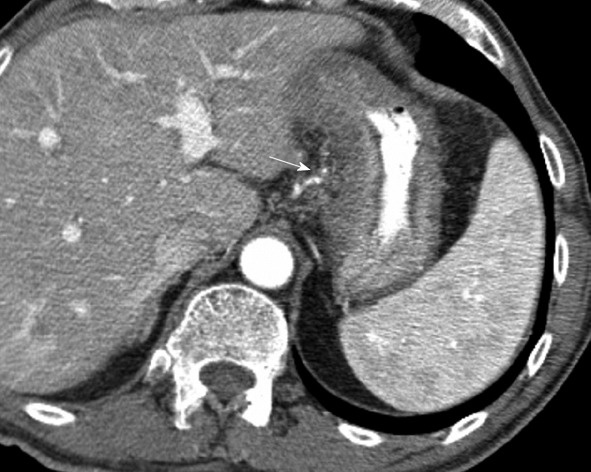
Computed tomography with intravenous contrast. Metastatic gastric cancer involves a 66-year-old man. Tumor spreads along the gastrohepatic ligament from the stomach. This is evidenced by soft tissue infiltration of tumor along the left gastric artery, with loss of a normal fat plane (arrow).
“Omental caking” occurs when direct tumor spread involves the gastrocolic ligament, greater omentum or transverse mesocolon[14] (Figure 9). Again, carcinoma of the stomach, pancreas and colon can present in such a fashion.
Figure 9.
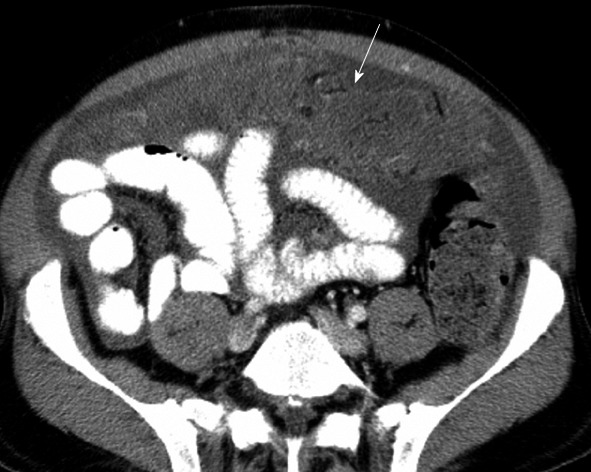
Computed tomography with intravenous contrast. There is “omental caking” (arrow) in a patient with spread of colon cancer. Ascites is also present.
The hepatoduodenal ligament, which contains the portal triad of bile duct, hepatic artery and portal vein, is also a roadway for the direct spread of tumor. It should be evaluated for the spread of pancreatic cancer to the liver (Figure 10).
Figure 10.
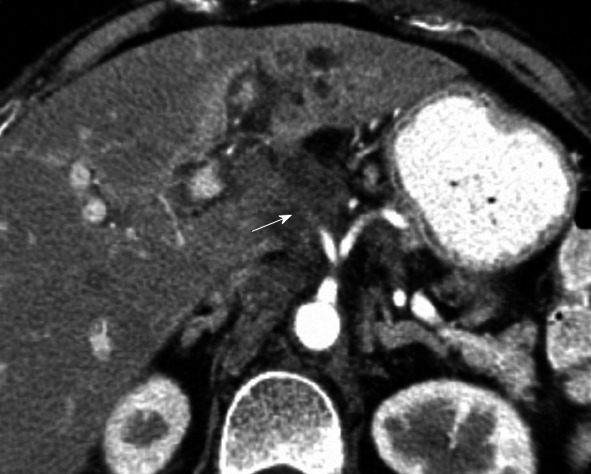
Computed tomography with intravenous contrast. Intrahepatic cholangiocarcinoma spreads along the hepatoduodenal ligament in a 63-year-old female (arrow).
Furthermore, the root of the mesentery enables delivery of tumor from lymphoma, carcinoid, or pancreatic cancer. The root of the mesentery extends from the horizontal portion of the duodenum and fans out to the iliac fossa. Soft tissue thickening, tethering of bowel and perivascular encasement can be anticipated along the root of the mesentery as seen on imaging (Figure 11)[1].
Figure 11.
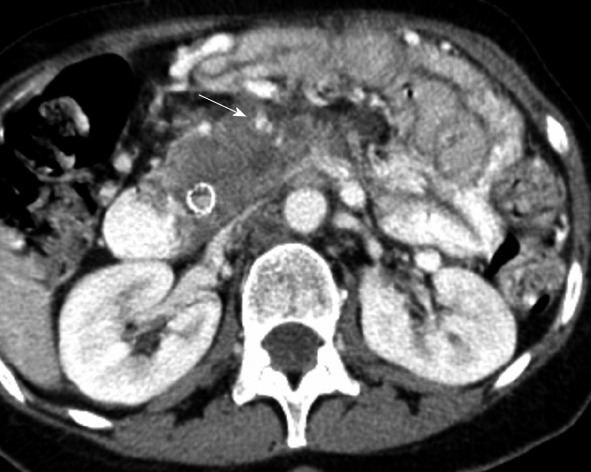
Computed tomography with intravenous contrast. Metastatic disease spreads along the mesenteric root in a 62-year-old with pancreatic cancer. This is seen as soft tissue infiltration along the SMA (arrow). The SMV is occluded and not seen.
Intraperitoneal seeding
The location of intraperitoneal spread of disease is influenced by gravity and the negative subdiaphragmatic pressure caused by respiratory motion[1]. The circulation and pooling of ascites in dependent compartments and recesses of the peritoneum will also spread and deposit tumor.
The pouch of Douglass/rectovesicular space, right lower quadrant at the inferior junction of the small bowel mesentery, right paracolic gutter, and superior aspect of the sigmoid mesocolon are common sites for intraperitoneal deposition of tumor.
Intraperitoneal metastases can manifest as soft tissue nodules or mass-like plaques on imaging such as CT. Parietal peritoneal involvement can be seen as smooth or nodular thickening with enhancement on CT imaging with intravenous contrast. Associated ascites are often present.
Tumors such as serous cystandenocarcinoma, carcinoid and less commonly gastric and colon cancer can produce calcified peritoneal metastatic deposits[15]. Mucin may occasionally also calcify. Mucin associated with pseudomyxoma peritonei from rupture of mucinous cystadenoma/cystadenocarcinoma of the ovary or appendix exerts pressure upon the visceral organs. This can be seen as scalloping defects along the surface of organs such as the liver, spleen or bowel surface[16] (Figure 12). Unlike ascites, the pressure from mucin will prevent bowel from floating upwards.
Figure 12.
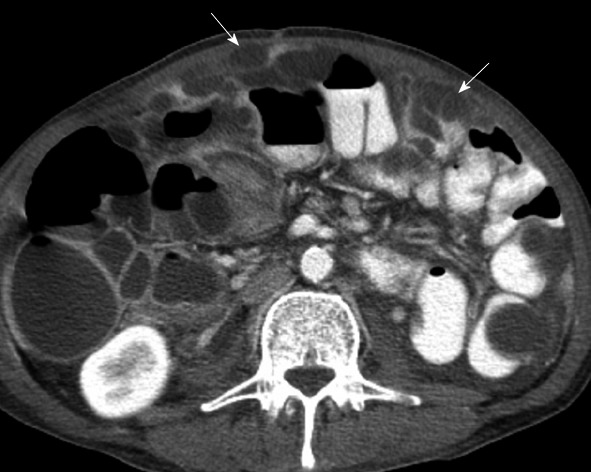
Computed tomography with intravenous contrast. Pseudomyxoma peritonei from appendiceal carcinoma in a 66-year-old male seen as hypodense collections scalloping and exerting pressure on the surface of the bowel (arrows).
Lymphatic spread
Lymphatic channels are ubiquitous along the ligaments and mesenteries of the peritoneum and can serve as routes for spread of disease[17] (Figure 13). However, tumor spread in the peritoneum via the lymphatics plays a minor role. It is, nevertheless, commonly seen with lymphoma, especially non-Hodgkin’s lymphoma. Lymphomatous involvement and spread along the ligaments and mesentery are seen as round or oval mass, often with displacement of the adjacent bowel. The mesenteric and retroperitoneal lymphadenopathy may be separated by a fat plane of the anterior pararenal space, the so called “sandwich sign”[18] (Figure 14).
Figure 13.
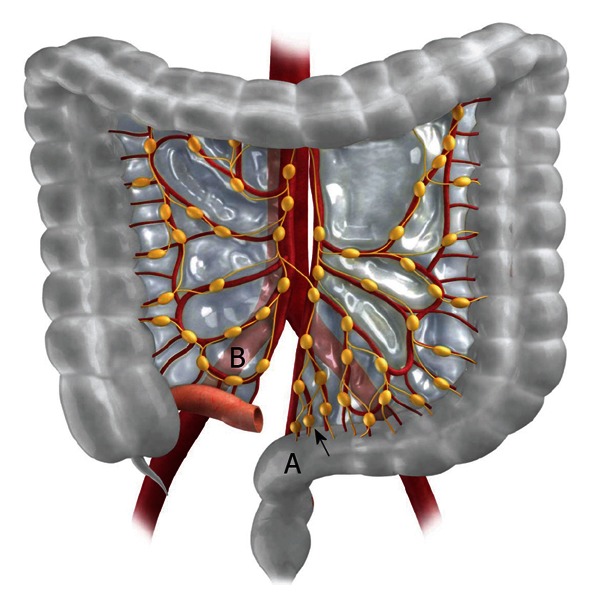
Schematic example of the ubiquitous lymphatic channels and nodes along the mesentery. A mass in the rectosigmoid colon (labeled A) can spread superiorly along the lymphatic highway of the sigmoid mesocolon (arrow). Likewise, a mass located in the cecum can spread along the lymphatic channels of the ileocolic mesentery (labeled B).
Figure 14.
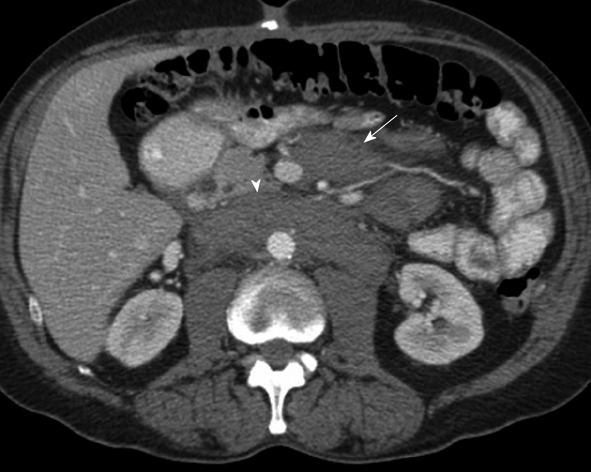
Computed tomography with intravenous contrast in a 66-year-old with lymphoma. Enlarged lymph nodes along the mesentery represent lymphatic spread of disease (arrow). Retroperitoneal lymphadenopathy is also present (arrowhead).
Hematogenous spread
Finally, intra-abdominal and extra-abdominal tumors can spread to the peritoneum via the hematogenous route. This is commonly seen in melanoma, breast and lung cancer (Figure 15). The embolic metastatic focus can begin as small nodule with eventual growth. Ulceration or thickening can been seen when there is bowel wall involvement. Abdominal pain may ensue as a result of intussusception.
Figure 15.
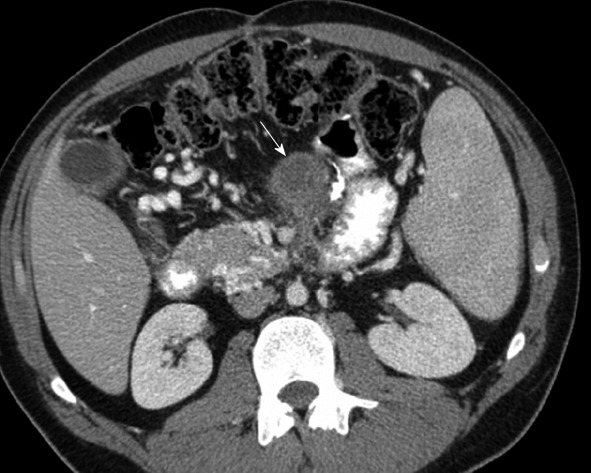
Computed tomography with intravenous contrast. There is hematogenous spread of disease to the root of the mesentery in a 21-year-old with melanoma (arrow).
CONCLUSION
The peritoneum is indeed a complex organ. An adequate understanding of its embryology and anatomy enables evaluation of tumor spread within the peritoneum. The peritoneal coverings, reflections and cavities form potential spaces and pathways for the spread of tumor and other disease processes. Methods of tumor spread within the peritoneum involves direct spread, intraperitoneal seeding, utilizing the lymphatic route or hematogenously.
Footnotes
P- Reviewer Chapel A S- Editor Cheng JX L- Editor A E- Editor Zheng XM
References
- 1.Meyers MA, Oliphant M, Berne AS, Feldberg MA. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593–604. doi: 10.1148/radiology.163.3.3575702. [DOI] [PubMed] [Google Scholar]
- 2.Elsayes KM, Staveteig PT, Narra VR, Leyendecker JR, Lewis JS, Brown JJ. MRI of the peritoneum: spectrum of abnormalities. AJR Am J Roentgenol. 2006;186:1368–1379. doi: 10.2214/AJR.04.1522. [DOI] [PubMed] [Google Scholar]
- 3.Levy AD, Arnáiz J, Shaw JC, Sobin LH. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008;28:583–607; quiz 621-622. doi: 10.1148/rg.282075175. [DOI] [PubMed] [Google Scholar]
- 4.Healy JC, Reznek RH. The peritoneum, mesenteries and omenta: normal anatomy and pathological processes. Eur Radiol. 1998;8:886–900. doi: 10.1007/s003300050485. [DOI] [PubMed] [Google Scholar]
- 5.Sompayrac SW, Mindelzun RE, Silverman PM, Sze R. The greater omentum. AJR Am J Roentgenol. 1997;168:683–687. doi: 10.2214/ajr.168.3.9057515. [DOI] [PubMed] [Google Scholar]
- 6.Silverman PM. The subperitoneal space: mechanisms of tumour spread in the peritoneal cavity, mesentery, and omentum. Cancer Imaging. 2004;4:25–29. doi: 10.1102/1470-7330.2003.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong YJ, Kim S, Kwak SW, Lee NK, Lee JW, Kim KI, Choi KU, Jeon TY. Neoplastic and nonneoplastic conditions of serosal membrane origin: CT findings. Radiographics. 2008;28:801–817; discussion 817-818; quiz 912. doi: 10.1148/rg.283075082. [DOI] [PubMed] [Google Scholar]
- 8.Hamrick-Turner JE, Chiechi MV, Abbitt PL, Ros PR. Neoplastic and inflammatory processes of the peritoneum, omentum, and mesentery: diagnosis with CT. Radiographics. 1992;12:1051–1068. doi: 10.1148/radiographics.12.6.1439011. [DOI] [PubMed] [Google Scholar]
- 9.Auh YH, Rubenstein WA, Markisz JA, Zirinsky K, Whalen JP, Kazam E. Intraperitoneal paravesical spaces: CT delineation with US correlation. Radiology. 1986;159:311–317. doi: 10.1148/radiology.159.2.3515415. [DOI] [PubMed] [Google Scholar]
- 10.Gambino J, Cohen AJ, Friedenberg RM. The direction of bladder displacement by adnexal masses. Clin Imaging. 1993;17:8–11. doi: 10.1016/0899-7071(93)90003-6. [DOI] [PubMed] [Google Scholar]
- 11.Auh YH, Rubenstein WA, Schneider M, Reckler JM, Whalen JP, Kazam E. Extraperitoneal paravesical spaces: CT delineation with US correlation. Radiology. 1986;159:319–328. doi: 10.1148/radiology.159.2.2938210. [DOI] [PubMed] [Google Scholar]
- 12.Sheth S, Horton KM, Garland MR, Fishman EK. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003;23:457–473; quiz 535-536. doi: 10.1148/rg.232025081. [DOI] [PubMed] [Google Scholar]
- 13.Oliphant M, Berne AS. Computed tomography of the subperitoneal space: demonstration of direct spread of intraabdominal disease. J Comput Assist Tomogr. 1982;6:1127–1137. doi: 10.1097/00004728-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Woodward PJ, Hosseinzadeh K, Saenger JS. From the archives of the AFIP: radiologic staging of ovarian carcinoma with pathologic correlation. Radiographics. 2004;24:225–246. doi: 10.1148/rg.241035178. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Yeh BM, Breiman RS, Qayyum A, Coakley FV. Peritoneal calcification: causes and distinguishing features on CT. AJR Am J Roentgenol. 2004;182:441–445. doi: 10.2214/ajr.182.2.1820441. [DOI] [PubMed] [Google Scholar]
- 16.Sulkin TV, O’Neill H, Amin AI, Moran B. CT in pseudomyxoma peritonei: a review of 17 cases. Clin Radiol. 2002;57:608–613. doi: 10.1053/crad.2002.0942. [DOI] [PubMed] [Google Scholar]
- 17.Karaosmanoglu D, Karcaaltincaba M, Oguz B, Akata D, Ozmen M, Akhan O. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. Eur J Radiol. 2009;71:313–317. doi: 10.1016/j.ejrad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Hokama A, Nakamoto M, Kinjo F, Fujita J. ‘The sandwich sign’ of mesenteric lymphoma. Eur J Haematol. 2006;77:363–364. doi: 10.1111/j.1600-0609.2006.00710.x. [DOI] [PubMed] [Google Scholar]