Abstract
Significant advances in ultrasound technology have created new opportunities for its use in oncologic imaging. The advent of new transducers with focal beam technology and higher frequency has solidified the role of intraoperative sonography (IOUS) as an invaluable imaging modality in oncologic surgery of the liver, kidneys and pancreas. The ability to detect and characterize small lesions and the precise intraoperative localization of such tumors is essential for adequate surgical planning in segmental or lobar hepatic resections, metastasectomy, nephron-sparing surgery, and partial pancreatectomy. Also, diagnostic characterization of small equivocal lesions deemed indeterminate by conventional preoperative imaging such as multidetector computed tomography or magnetic resonance imaging, has become an important application of IOUS. This article will review the current applications of IOUS in the liver, kidneys and pancreas.
Keywords: Abdominal ultrasound, Oncologic imaging
INTRODUCTION
Ultrasound was first used intraoperatively in abdominal imaging in 1979 for the evaluation of biliary calculi[1]. Acceptance of this new modality by the surgical community was slow despite clear reports of its potential benefits and applications. Advances in imaging technology in ultrasound have resulted in a higher rate of utilization of this procedure in various intra-abdominal surgical procedures and interventions[2,3]. The ability to provide high resolution real-time imaging of the organ of interest, along with accurate lesion detection and characterization, has established the role of intraoperative sonography (IOUS) in a number of surgical procedures such as lobar and segmental hepatic resections, hepatic metastasectomy, single or multiple nephron-sparing surgery for renal cell carcinoma (RCC), and partial pancreatectomy or pancreatic enucleation[4-11]. The indications for IOUS are broad and include intraoperative guidance and localization of lesions, assistance in determining lesion resectability and surgical planning, intraoperative characterization of indeterminate lesions, and metastatic survey[4,5,7-9]. The purpose of the article is to provide a review of the applications of state-of-the-art IOUS in the abdomen based on the authors’ experience in our institution, focusing on the contributions of this important imaging modality to oncologic surgery in the liver, kidneys and pancreas.
LIVER
The liver is the intra-abdominal organ most commonly involved in metastatic disease[12]. Colorectal cancer is the most frequent malignancy metastasizing to the liver, followed by pancreatic, esophageal, gastric, and gallbladder cancer[12]. Recent advances in oncologic surgery have placed additional demands on radiologists to precisely define the number and location of liver metastases. This information is essential to define resectability and plan the correct surgical approach. The applications for intraoperative ultrasound of the liver are vast and include tumor staging, metastatic survey, documentation of vessel patency or involvement, assessment of the biliary tree, and surveillance for metastatic disease[4,5,8,13,14]. IOUS has proven accuracy for the detection of liver metastases, with reported sensitivity of 93.8%, specificity of 94.4%, positive predictive value of 92.0% and negative predictive value of 95.7%[15]. In a recent study involving 561 malignant lesions, including primary and metastatic lesions, the sensitivity of IOUS for liver lesion detection was 95.1%[16]. Performing IOUS requires a sound knowledge of the hepatic segmental anatomy, familiarity with the intraoperative transducers and technique, and range of normal and abnormal findings in the liver[8,17]. Lastly, the radiologist should be familiar with the artifacts that may be encountered in the setting of open hepatic surgery.
Technique
Various intraoperative transducers are available for use in intraabdominal surgery and a detailed discussion on the technical capabilities and applications of each transducer is beyond the scope of this article. It suffices to know that linear array, curvilinear array, and phased array IOUS transducers are made available by different vendors. Size of the probe does matter, and it should pass comfortably through the surgical incision and be easily manipulated in a narrow operative field. Higher frequency transducers will provide greater resolution images when compared with lower frequency transducers. However, high frequency ultrasound waves have limited tissue penetration due to more rapid attenuation as the ultrasound beam travels through the tissues. For this reason, the selection of a specific ultrasound transducer for intraoperative use must take into consideration the size of the target organ to be evaluated or region of interest to be covered, in order to insure adequate penetration of the deeper tissues with appropriate spacial resolution to allow proper lesion detection and characterization. In our institution, Aloka multifrequency 5-10 MHz T-shaped or I-shaped convex array transducers with color Doppler and pulsed Doppler capabilities are preferred. These probes are small and fit comfortably between the index and middle fingers, which allows the target organ to be palpated and scanned at the same time. Excellent near-field resolution is essential for proper lesion detection and characterization. The entire examination is performed with strict observation of standard sterile technique, and the radiologist scrubs and gowns for the exam. There are different options for transducer sterilization[1,5,8]. Ethylene oxide gas with high-temperature aeration of both transducer probe and cord. The turn-around time is approximately 24 h, limiting its use to a single procedure per day. Most vendors do not recommend it due to potential damage to the equipment. Hydrogen peroxide gas plasma sterilization techniques use low temperature, and are considered safe for use with heat sensitive equipment[1,8]. The turn-around time is about 2 h, enabling use of the probe for more procedures per day. Use of glutaraldehyde immersion is not accepted in most institutions due to concerns of surface residue from this agent causing inflammatory reaction on visceral contact. The most widely accepted and commonly used sterilization technique involves the use of specially designed sterile condom sheaths for the transducer probe and electrical cord. The transducer is sheathed by a sterile cover and should fit comfortably between the fingers. The palm of the hand is moved over the liver surface to scan and it is important that radiologist obtain a good grip on the transducer in order to allow the establishment of an acoustic window to image the dome and right lateral segments of the liver[8]. The surgeon also resects the falciform ligament and pulls the liver down so that the radiologist can easily scan the entire liver.
Metastatic survey
One of the more common indications for IOUS of the liver is the search for metastatic disease in liver sectors which will remain following lobar or segmental hepatic resection for primary or metastatic hepatic disease. Small indeterminate hepatic lesions are commonly identified on routine preoperative imaging obtained with multidetector computed tomography (MDCT) or even magnetic resonance imaging (MRI)[4,17]. IOUS evaluation of such lesions, with or without us-guided biopsy, is often required for a definitive diagnosis and further characterization of such lesions (Figure 1). High frequency intraoperative transducers with focus-beam technology allow scanning directly over the surface of the liver, giving IOUS unmatched spatial resolution and a significant advantage over other preoperative cross-sectional imaging modalities. Not infrequently, new lesions are discovered in segments where they were not suspected, significantly impacting surgical planning[17]. In addition, some equivocal or indeterminate lesions are further characterized as definite benign or malignant lesions (Figures 2 and 3). Those lesions which remain indeterminate following intraoperative ultrasound can undergo ultrasound-guided fine needle aspiration or core biopsy intraoperatively, and a definitive diagnosis established in a timely fashion. Lastly, precise lesion localization is essential for the adequate surgical planning of wedge resections, radiofrequency ablation (RFA) procedures, or segmental hepatic resections. In our institution, IOUS is an indispensable asset for successful surgical planning of complex hepatic resections and is frequently used in partial hepatectomy, hepatic segmentectomy, and complex multi-staged hepatic resections involving partial hepatectomies associated with contralateral wedge resections or RFA procedures in the remaining liver.
Figure 1.
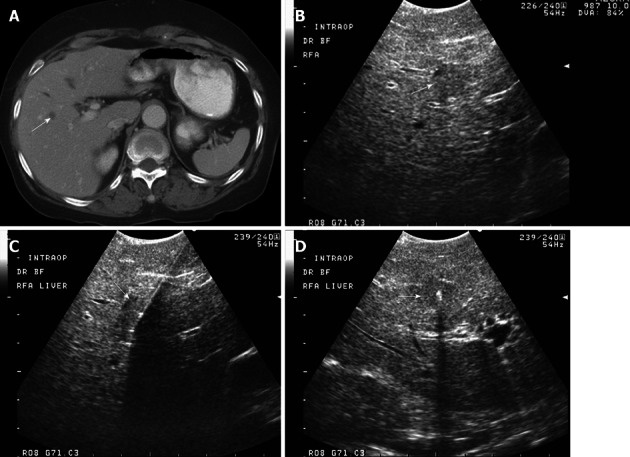
A 79-year-old male with metastatic colon with rising carcinoembryonic antigen and new hypodense lesion in the right liver on computed tomography examination. A: Axial contrast-enhanced computed tomography shows a small hypodense lesion at the junction of segments VIII and V, concerning for metastasis; B: Intraoperative transverse sonogram identifies a solid slightly hypoechoic lesion in the right liver, at the junction of segments VIII-V, compatible with a metastasis; C, D: Longitudinal and transverse intraoperative ultrasound image shows the position of a radiofrequency ablation needle, with its in appropriate location within the center of the lesion. Intraoperative ultrasound is extremely useful to precise localize hepatic lesions and guide therapeutic interventions.
Figure 2.
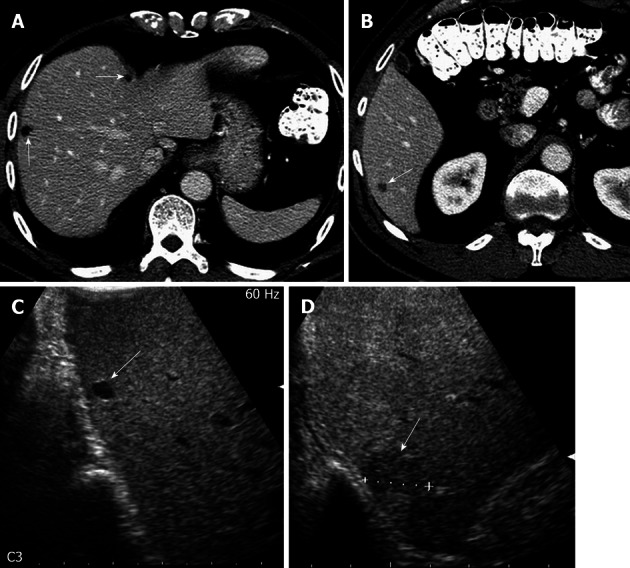
A 73-year-old male with colorectal cancer and indeterminate hepatic lesion on computed tomography, suspicious for metastasis. A: Axial contrast-enhanced computed tomography (CT) image shows small hypodense lesions in the right liver, possibly representing cysts; B: Axial contrast-enhanced CT image more inferiorly in the same patient reveals a subcentimeter lesion, deemed too small to be accurately characterized. Metastasis was not excluded; C: Intraoperative ultrasound image in the longitudinal plane shows a homogeneously hypoechoic lesion in the dome of the liver consistent with a cyst; D: Longitudinal Intraoperative ultrasound image more inferiorly shows a solid hypoechoic lesion in the inferior right liver, consistent with a metastasis.
Figure 3.
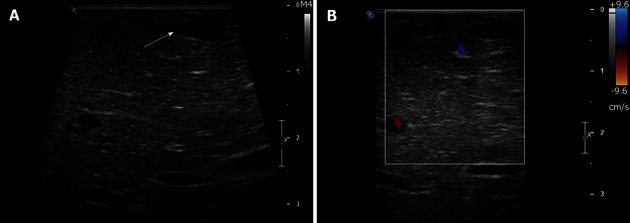
A 60-year-old male with potentially resectable pancreatic cancer and indeterminate liver lesions on preoperative imaging. A: Intraoperative ultrasound image shows a homogeneously hypoechoic subcentimeter lesion in the left liver consistent with a cyst; B: Intraoperative ultrasound image with Doppler interrogation shows lack of internal vascularity, confirming the benign nature of this cyst.
Tumor staging
IOUS can be a valuable tool for local staging of primary hepatic malignancies in cases which preoperative cross-sectional imaging yielded equivocal results[17]. Intravascular growth of hepatocellular carcinoma in the ipsilateral or contralateral portal vein, involvement of the hepatic veins, or extension into the ipsilateral or contralateral bile duct can be easily diagnosed with IOUS[17]. Radial tumor growth of cholangiocarcinoma with encasement, occlusion of the ipsilateral or contralateral portal vein, or occlusion of the hepatic veins can also be diagnosed with IOUS and provide critical information for adequate surgical planning.
KIDNEYS
Significant advances in urologic surgery in recent years have resulted in a wide variety of nephron-sparing surgical techniques as open or laparoscopic partial nephrectomy (PN), robotic-assisted PN, enucleation, RFA, and cryoablation procedures[7,10,18-21]. Nephron-sparing procedures have become the treatment of choice for T1 renal tumors and recent data suggests that PN may be as effective as radical nephrectomy for larger renal tumors[22]. These nephron-sparing procedures rely on radiology to precisely define size and location of the renal tumors, as well as extension or involvement of the renal sinus fat, and vessels and the hilum of the kidneys[19,23]. Besides state-of-the-art preoperative MDCT or MRI, IOUS is routinely utilized in our institution in the vast majority of nephron-sparing surgeries. IOUS plays an important role in lesion localization and characterization, precisely defining their margins and extent into the renal sinus fat, invasion into the collecting system or renal vein[24]. Lastly, IOUS is useful for surveying the remaining renal parenchyma for unsuspected additional lesions. It also helps identify additional lesions and may change surgical planning from partial to a complete nephrectomy.
Technique
As previously mentioned, a variety of intraoperative transducers and vendors are available. In our institution, we utilize the Philips iu22 C5-8 MHz broadband curved array transducer for the majority of cases. It provides excellent near-field resolution, steerable pulsed wave and color Doppler imaging, color Power angio, and panoramic imaging capabilities. This probe allows easily visualization of the entire kidney and is better suited to assess larger, partly exophytic lesions that may become challenging when a compact linear array transducer is used. For smaller intrarenal lesions, we prefer the Philips iu22 L15-5 MHz broadband compact liner array “hockey stick” transducer. This probe has a narrow 23 mm effective aperture length, but provides superb near-field resolution, steerable pulsed Doppler imaging, Color Doppler, and panoramic imaging. It is excellent for high-resolution intraoperative imaging applications. The radiologist is called to the operating room approximately 10 min prior to scanning, and scrubs for the procedure. Through the small flank incision the kidney is visible, and free for mobilization. A small amount of saline is poured over the kidney at the time of scanning, and excellent acoustic coupling is usually achieved. The entire kidney is scanned and the surgeon is sometimes asked to mobilize the kidney out of the renal fossa, in order to ensure thorough surveillance of the entire renal parenchyma. Images of the entire kidney are documented.
Imaging findings
The ability to place the transducer directly over the kidney surface produces exquisite images of the renal anatomy[7,20,21]. The renal cortex echogenicity contrasts sharply with the hypoechoic pyramids, which can be clearly delineated on IOUS. The calyces and infundibula are readily identified, outlined by the anechoic urine. The renal sinus fat is markedly hyperechoic and can be easily differentiated from the adjacent renal parenchyma[7,21]. The sonographic appearance of RCC varies with size and histology. Most RCCs are mildly hypoechoic or isoechoic in relation to the renal cortex and generally form a discrete, well marginated mass, often distinguishable by displacement of adjacent blood vessels. About 30% of RCCs are markedly hyperechoic and virtually indistinguishable from small angiomyolipomas. Most are hypervascular in relation to the renal parenchyma, but chromophobe RCCs are typically hypovascular[13,21,23]. Cystic RCCs usually show discrete solid mural nodules with internal vascularity or thickened irregular septations. Poorly marginated, infiltrative tumors may present a challenge in terms of clearly delineating their margins, but color Doppler shows encircling vascularity.
Lesion localization
Intraoperative ultrasound is of great value to accurately identify small completely intrarenal tumors, ensuring that the lesion is in the center of the resected specimen, and that a safe margin free of tumor is obtained on all sides[7,23]. This requires careful scanning and the surgeon often marks the renal surface with electrocautery at the location indicated by the radiologist.
Intraoperative ultrasound can reliably delineate small tumors and define if the lesions are encapsulated or infiltrative in nature (Figure 4). Lesion size and morphology is also very important for the surgeon since infiltrative and poorly marginated lesions often require a more extensive resection in order to achieve a margin free of tumor. Extension of the lesion into the sinus fat or involvement of the sinus structures is important information for the surgeon, and IOUS is able to provide this.
Figure 4.
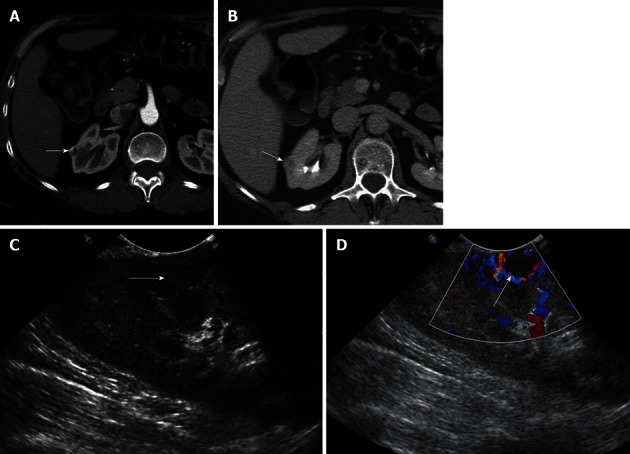
A 51-year-old female with incidentally detected small solid mass in the right kidney, compatible with a renal cell carcinoma. A, B: Axial contrast-enhanced images of the right kidney show a 1.2 cm hypervascular mass in the midportion of the right kidney, concerning for a small renal cell carcinoma; C: Longitudinal Intraoperative ultrasound image localizes the small solid mass in the midportion of the right kidney anteriorly; D: Intraoperative ultrasound with Doppler interrogation shows prominent vessels at the margin of the lesion. Intraoperative ultrasound is an invaluable resource to localize small solid renal lesions during partial nephrectomy, ensuring that negative margins are achieved, while preserving the normal renal parenchyma.
Local staging
IOUS is extremely helpful to define the size and extent of the tumor into adjacent structures, and has become a valuable tool in the decision process for partial versus radical nephrectomy for centrally located renal masses (Figure 5). Its sensitivity, and specificity for detection of renal sinus invasion and venous invasion is very high, approaching 100%[25]. Centrally located renal masses abutting the renal hilar vessels, collecting system or renal pelvis may preclude a nephron-sparing procedure, since a negative margin may not be feasible[7,19,20,23]. The pyramids, calyces, infundibula, and the renal sinus fat are readily identified on intraoperative ultrasound and tumor involvement of these structures must be commented upon and relayed to the surgeon at the time the examination is being performed. The overall diagnostic accuracy of IOUS for detection of sinus invasion is 98%, and is superior to MRI, which ranged from 70% to 72% depending on the observer[25]. In addition, small new solid intrarenal tumors may be detected on IOUS, which have not been visualized on preoperative imaging, impacting patient management and the surgical approach (Figure 6). In our experience, renal IOUS will reveal additional foci of cancer in approximately 5% of cases. In a 10-year study by Choyke et al[7], 68 nephron sparing surgeries were performed on patients where all but one had a hereditary renal cancer (i.e., von Hippel-Lindau syndrome). In 25% of these surgeries, IOUS localized renal cancers undetected by the surgeon[7]. Lastly, intravascular invasion can be reliably detected on ultrasound and represents important information for adequate surgical planning. The diagnostic accuracy of IOUS for detection of venous invasion is around 100%[25].
Figure 5.
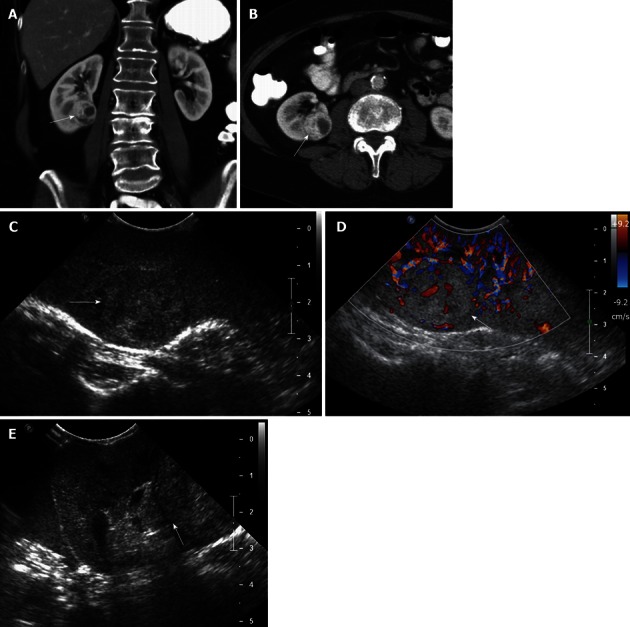
A 62-year-old female with renal cell carcinoma. A, B: Coronal (A) and axial (B) contrast-enhanced computed tomography images show a solid, hypervascular, 2.8 cm mass in the midportion - lower pole of the right kidney, consistent with a renal cell carcinoma; C, D: Intraoperative grayscale (C) and color Doppler ultrasound (D) identify a solid echogenic mass in the midportion - lower pole of the right kidney, with evidence of internal vascularity, consistent with a renal cell carcinoma; E: Intraoperative ultrasound image in a oblique transverse plane shows that the lesion abuts the hyperechoic renal sinus fat. This important information to guide the surgeon, in order that tumor-free margins are properly obtained at the time of resection.
Figure 6.
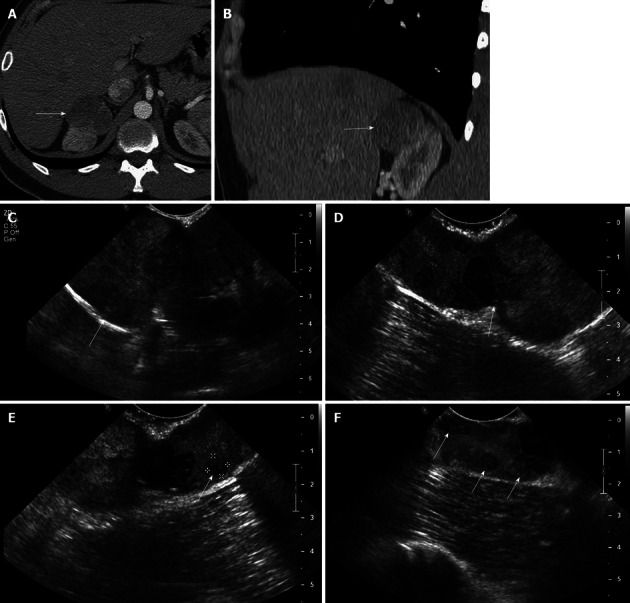
A 29-year-old male with renal cell carcinoma of the right kidney. A, B: Axial (A) and coronal (B) contrast-enhanced computed tomography image show a large solid mass in the superior pole of the right kidney, consistent with a renal cell carcinoma; C: Intraoperative longitudinal sonogram shows an exophytic large solid mass in the superior pole of the right kidney; D: Intraoperative ultrasound image shows the point of attachment of the solid mass to the superior pole of the right kidney; E, F: High resolution intraoperative ultrasound image detects additional subcentimeter solid renal masses in the superior pole of the right kidney, near the site of origin of the large exophytic right renal mass. These findings alerted the surgeon that a deeper resection needed to be performed in order to secure tumor-free resection margin.
PANCREAS
IOUS of the pancreas provides excellent spatial and contrast resolution and its real-time imaging capabilities are invaluable for a variety of surgical procedures[2,6,9,13,21]. Among its most common indications are localization of small tumors, local staging, identification of multifocal neuroendocrine tumors and regional metastatic survey[6,9]. Various studies have shown a mean detection rate of 86% to 92% for IOUS detection of pancreatic neuroendocrine tumors[26,27]. In 2009 a consensus statement revealed a mean sensitivity and detection rate of pancreatic neuroendocrine tumors to be 63%-82% on CT[28,29]. Other studies evaluating pancreatic neuroendocrine tumors on MDCT have shown CT sensitivities of 84% and 94%[28,30].
Technique
In our institution, the Philips iu22 15 MHz broadband compact linear array “hockey stick” transducer with color and pulsed Doppler capabilities is the transducer of choice for scanning the pancreas. The surgeon is asked to indicate the location of the gland in the operative field[6,9]. A small amount of saline is then bathed over the pancreas to aid the acoustic coupling. The high resolution transducer is covered with a sterile cover, placed directly over the gland, and high spatial resolution images of the gland are obtained in the transverse plane. The entire gland is carefully scanned from the head and uncinate process to the tail.
Imaging findings
The pancreatic parenchyma is homogeneously hyperechoic in relation to the liver or spleen. The main pancreatic duct is clearly visualized as a thin hypoechoic tubular structure coursing through the center of the gland[9]. Cystic or solid intrapancreatic lesions can be easily identified (Figure 7). Communication with the main pancreatic duct or lack thereof can be readily documented intraoperatively. Tumor relation to the adjacent vessels such as celiac axis and superior mesenteric artery can also be ascertained during surgery[9]. A regional metastatic survey can be performed during surgery. This usually includes search for peripancreatic or retroperitoneal lymphadenopathy, which can be easily detected with IOUS. One of the most common indications for IOUS of the pancreas in our institution is the localization of multifocal neuroendocrine tumors, specifically in the setting of multiple endocrine neoplasia syndrome. Small neuroendocrine tumors can easily elude detection with preoperative MDCT or MRI. IOUS is extremely helpful to precisely localize these tumors and search for unsuspected lesions, which can certainly impact surgical planning and alter the procedure to be performed (Figure 8). Not uncommonly, additional lesions are discovered during IOUS, which were not identified on the preoperative imaging, altering the initial surgical and patient management.
Figure 7.
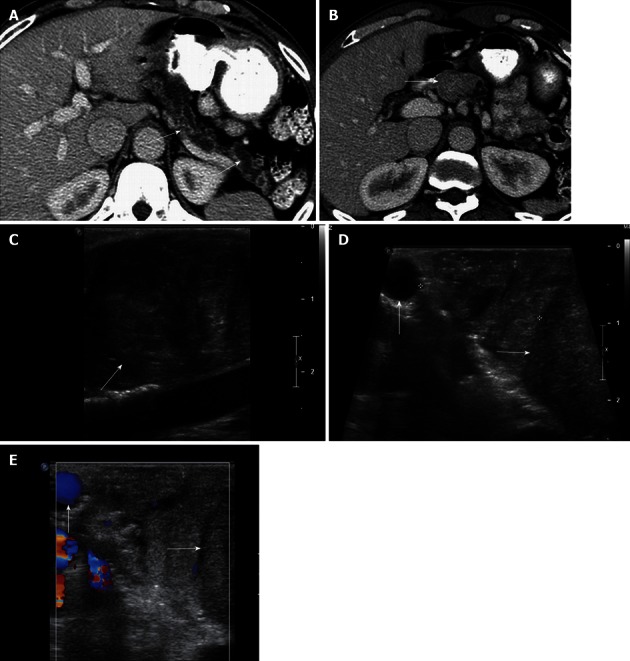
A 60-year-old male with pancreatic duct dilatation and suspected mass pancreatic head mass. A: Axial contrast-enhanced computed tomography (CT) images show marked pancreatic ductal dilatation in the tail and body of the pancreas, with atrophy of the pancreatic parenchyma; B: Axial contrast-enhanced CT shows fullness in the region of the head of the pancreas, concerning for an isodense pancreatic head mass; C: Intraoperative ultrasound image clearly defines a solid mass in the head of the pancreas, consistent with a pancreatic ductal adenocarcinoma; D, E: Grayscale (D) and color Doppler (E) intraoperative ultrasound images show to better advantage the margins of the mass (arrow) in the cephalad head of the pancreas. The lesion is confined to the pancreas, and does not involve the gastroduodenal artery (vertical arrow). Intraoperative ultrasound is useful to assess size, margins of pancreatic mass, and their relationship with the adjacent vessels. This is particularly useful in cases of isodense pancreatic masses, which can be very difficult to evaluate with CT.
Figure 8.
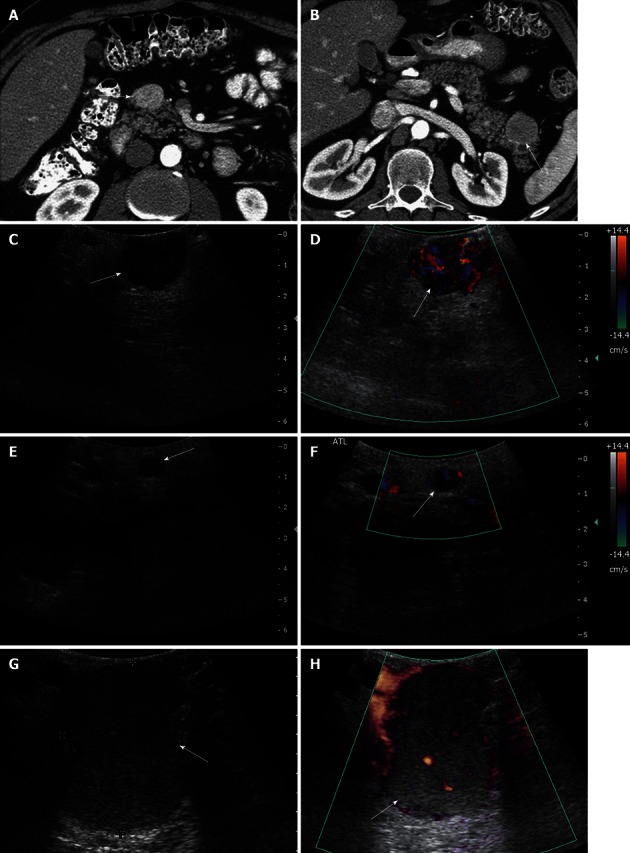
A 46-year-old male with multiple endocrine neoplasia type I syndrome and pancreatic neuroendocrine tumor. A, B: Axial contrast-enhanced computed tomography (CT) images show hypervascular masses in the head and tail of the pancreas, consistent with neuroendocrine tumors; C: Intraoperative ultrasound image shows a well-defined solid mass in the head of the pancreas, consistent with the neuroendocrine tumor on CT; D: Doppler interrogation reveals increased vascularity within this mass; E, F: Intraoperative grayscale and color Doppler ultrasound images detect a 5 mm solid mass with internal vascularity in the head of the pancreas, consistent with a small neuroendocrine tumor. This lesion was not identified on the CT examination; G, H: Intraoperative grayscale and color Doppler ultrasound reveals the large dominant mass in the tail of the pancreas, consistent with a neuroendocrine tumor.
CONCLUSION
Despite tremendous advancements in preoperative oncologic imaging of the liver, kidneys and pancreas with MDCT and MRI, IOUS provides essential diagnostic information during surgery, capable of changing surgical planning and patient management. Precise lesion localization, characterization, local staging, metastatic survey, clarifying indeterminate findings and searching for multifocal lesions not suspected on preoperative imaging are among the most common indications for IOUS in oncology. The high spatial and contrast resolution allied with its real-time imaging capabilities make IOUS an invaluable asset during oncologic surgery. Radiologists should become familiar with the technique, indications, and intraoperative imaging findings since increasing future demand for IOUS is expected in most institutions involved with oncologic surgery of the liver, kidney and pancreas.
Footnotes
P- Reviewers Yoshida H, Yazdi HR S- Editor Cheng JX L- Editor A E- Editor Zheng XM
References
- 1.Machi J, Oishi AJ, Furumoto NL, Oishi RH. Intraoperative ultrasound. Surg Clin North Am. 2004;84:1085–1111, vi-i. doi: 10.1016/j.suc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Plainfossé MC, Merran S. Work in progress: intraoperative abdominal ultrasound. Radiology. 1983;147:829–832. doi: 10.1148/radiology.147.3.6844622. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M, Torzilli G, Machi J. History of intraoperative ultrasound. Ultrasound Med Biol. 1998;24:1229–1242. doi: 10.1016/s0301-5629(98)00112-4. [DOI] [PubMed] [Google Scholar]
- 4.Stone MD, Kane R, Bothe A, Jessup JM, Cady B, Steele GD. Intraoperative ultrasound imaging of the liver at the time of colorectal cancer resection. Arch Surg. 1994;129:431–435; discussion 435-436. doi: 10.1001/archsurg.1994.01420280109014. [DOI] [PubMed] [Google Scholar]
- 5.Kruskal JB, Kane RA. Intraoperative ultrasonography of the liver. Crit Rev Diagn Imaging. 1995;36:175–226. [PubMed] [Google Scholar]
- 6.Kruskal JB, Kane RA. Intraoperative ultrasonography of the pancreas: techniques and clinical applications. Surg Technol Int. 1997;6:49–57. [PubMed] [Google Scholar]
- 7.Choyke PL, Pavlovich CP, Daryanani KD, Hewitt SM, Linehan WM, Walther MM. Intraoperative ultrasound during renal parenchymal sparing surgery for hereditary renal cancers: a 10-year experience. J Urol. 2001;165:397–400. doi: 10.1097/00005392-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Kruskal JB, Kane RA. Intraoperative US of the liver: techniques and clinical applications. Radiographics. 2006;26:1067–1084. doi: 10.1148/rg.264055120. [DOI] [PubMed] [Google Scholar]
- 9.Sun MR, Brennan DD, Kruskal JB, Kane RA. Intraoperative ultrasonography of the pancreas. Radiographics. 2010;30:1935–1953. doi: 10.1148/rg.307105051. [DOI] [PubMed] [Google Scholar]
- 10.Hellenthal NJ, Mansour AM, Hayn MH, Schwaab T. Is there a role for partial nephrectomy in patients with metastatic renal cell carcinoma. Urol Oncol. 2013;31:36–41. doi: 10.1016/j.urolonc.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Pautler SE, Choyke PL, Pavlovich CP, Daryanani K, Walther MM. Intraoperative ultrasound aids in dissection during laparoscopic partial adrenalectomy. J Urol. 2002;168:1352–1355. doi: 10.1097/01.ju.0000031272.56889.28. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society. Cancer facts and Figures; 2010. Available from: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-and-figures-2010.
- 13.Ganguli S, Kruskal JB, Brennan DD, Kane RA. Intraoperative laparoscopic ultrasound. Radiol Clin North Am. 2006;44:925–935. doi: 10.1016/j.rcl.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Santambrogio R, Costa M, Strada D, Bertolini E, Zuin M, Barabino M, Opocher E. Intraoperative ultrasound score to predict recurrent hepatocellular carcinoma after radical treatments. Ultrasound Med Biol. 2011;37:7–15. doi: 10.1016/j.ultrasmedbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Machi J, Sigel B. Operative ultrasound in general surgery. Am J Surg. 1996;172:15–20. doi: 10.1016/S0002-9610(96)00052-9. [DOI] [PubMed] [Google Scholar]
- 16.Wagnetz U, Atri M, Massey C, Wei AC, Metser U. Intraoperative ultrasound of the liver in primary and secondary hepatic malignancies: comparison with preoperative 1.5-T MRI and 64-MDCT. AJR Am J Roentgenol. 2011;196:562–568. doi: 10.2214/AJR.10.4729. [DOI] [PubMed] [Google Scholar]
- 17.Conlon R, Jacobs M, Dasgupta D, Lodge JP. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003;16:211–216. doi: 10.1016/s0929-8266(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 18.Permpongkosol S, Bagga HS, Romero FR, Sroka M, Jarrett TW, Kavoussi LR. Laparoscopic versus open partial nephrectomy for the treatment of pathological T1N0M0 renal cell carcinoma: a 5-year survival rate. J Urol. 2006;176:1984–1988; discussion 1988-1989. doi: 10.1016/j.juro.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Capitanio U, Zini L, Perrotte P, Shariat SF, Jeldres C, Arjane P, Pharand D, Widmer H, Péloquin F, Montorsi F, et al. Cytoreductive partial nephrectomy does not undermine cancer control in metastatic renal cell carcinoma: a population-based study. Urology. 2008;72:1090–1095. doi: 10.1016/j.urology.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 20.Breda A, Stepanian SV, Liao J, Lam JS, Guazzoni G, Stifelman M, Perry K, Celia A, Breda G, Fornara P, et al. Positive margins in laparoscopic partial nephrectomy in 855 cases: a multi-institutional survey from the United States and Europe. J Urol. 2007;178:47–50; discussion 50. doi: 10.1016/j.juro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 21.Silas AM, Kruskal JB, Kane RA. Intraoperative ultrasound. Radiol Clin North Am. 2001;39:429–448. doi: 10.1016/s0033-8389(05)70290-6. [DOI] [PubMed] [Google Scholar]
- 22.Breau RH, Crispen PL, Jimenez RE, Lohse CM, Blute ML, Leibovich BC. Outcome of stage T2 or greater renal cell cancer treated with partial nephrectomy. J Urol. 2010;183:903–908. doi: 10.1016/j.juro.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Vikram R, Ng CS, Tamboli P, Tannir NM, Jonasch E, Matin SF, Wood CG, Sandler CM. Papillary renal cell carcinoma: radiologic-pathologic correlation and spectrum of disease. Radiographics. 2009;29:741–754; discussion 755-757. doi: 10.1148/rg.293085190. [DOI] [PubMed] [Google Scholar]
- 24.Zini L, Patard JJ, Capitanio U, Mejean A, Villers A, de La Taille A, Ficarra V, Crepel M, Bertini R, Salomon L, et al. The use of partial nephrectomy in European tertiary care centers. Eur J Surg Oncol. 2009;35:636–642. doi: 10.1016/j.ejso.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Secil M, Elibol C, Aslan G, Kefi A, Obuz F, Tuna B, Yorukoglu K. Role of intraoperative US in the decision for radical or partial nephrectomy. Radiology. 2011;258:283–290. doi: 10.1148/radiol.10100859. [DOI] [PubMed] [Google Scholar]
- 26.Gorman B, Charboneau JW, James EM, Reading CC, Galiber AK, Grant CS, van Heerden JA, Telander RL, Service FJ. Benign pancreatic insulinoma: preoperative and intraoperative sonographic localization. AJR Am J Roentgenol. 1986;147:929–934. doi: 10.2214/ajr.147.5.929. [DOI] [PubMed] [Google Scholar]
- 27.Grover AC, Skarulis M, Alexander HR, Pingpank JF, Javor ED, Chang R, Shawker T, Gorden P, Cochran C, Libutti SK. A prospective evaluation of laparoscopic exploration with intraoperative ultrasound as a technique for localizing sporadic insulinomas. Surgery. 2005;138:1003–1008; discussion 1008. doi: 10.1016/j.surg.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Rappeport ED, Hansen CP, Kjaer A, Knigge U. Multidetector computed tomography and neuroendocrine pancreaticoduodenal tumors. Acta Radiol. 2006;47:248–256. doi: 10.1080/02841850600550716. [DOI] [PubMed] [Google Scholar]
- 29.Sundin A, Vullierme MP, Kaltsas G, Plöckinger U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009;90:167–183. doi: 10.1159/000184855. [DOI] [PubMed] [Google Scholar]
- 30.Gouya H, Vignaux O, Augui J, Dousset B, Palazzo L, Louvel A, Chaussade S, Legmann P. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. AJR Am J Roentgenol. 2003;181:987–992. doi: 10.2214/ajr.181.4.1810987. [DOI] [PubMed] [Google Scholar]


