Abstract
Aim
To report the vitreoretinal (VR) surgical case mix in the United Kingdom, the intraoperative complication rate of pars plana vitrectomy (PPV), and the incidence of post-vitrectomy cataract extraction.
Methods
Participating hospitals prospectively collected ophthalmic data using a single electronic medical record system, with automatic extraction of anonymised data to a national database. This study included the subset of 11 618 VR operations undertaken on 9619 eyes, of 8741 patients, over 8 years, from 27 sites. Surgical data included the indication for surgery, all procedure elements, and whether or not an intraoperative complication occurred. Post-vitrectomy cataract data were also analysed. The main outcome measures were a description of the indications for surgery, intraoperative PPV complication rate, and percentage of eyes undergoing post-vitrectomy cataract surgery (PVCS).
Results
The most common indications for VR intervention were retinal breaks and rhegmatogenous retinal detachment (48.5%), macular hole (9.8%), epiretinal membrane (9.6%), and diabetic eye disease (7.3%). Overall, 7.8% of PPVs had at least one intraoperative complication—the most common were iatrogenic retinal breaks (3.2%), and lens touch (1.2–1.6% of phakic eyes). PVCS occurred in 50.2, 68.7, and 74.0% of eyes at 1, 2, and 3 years, respectively.
Conclusion
VR surgery is undertaken for a wide range of conditions, but a small number of diagnoses encompass the majority of cases. Intraoperative PPV complications are not uncommon, and post-vitrectomy cataract is to be expected in most phakic eyes.
Keywords: database, vitreoretinal, surgery, complications, pars plana vitrectomy, cataract
Introduction
Robust surgical data are important for both clinicians and patients. They allow surgeons to benchmark their complication rates and, if necessary, refine their techniques; they enable patients to make an informed decision about whether or not to proceed with surgery. The complication rates reported in the literature may not be generalisable, in that they are often highly select cases, part of a clinical trial, collected retrospectively, or potentially subject to publication bias. It would be preferable to prospectively collect anonymous, pooled, data from a standard clinical setting, with open inclusion criteria, and a large number of contributing centres.
The National Ophthalmology Database (NOD) was initiated by The Royal College of Ophthalmologists to collate pseudoanonymised data collected during routine clinical care, using electronic medical records (EMRs). The aim was to facilitate national audit, research, and continuing professional development. Previous proof of concept studies, that led to the creation of the NOD, collated very large, high-quality, pragmatic, cataract data sets, with high levels of data completeness.1, 2, 3, 4, 5
This is the first in a planned series of studies using the NOD to study vitreoretinal (VR) surgery. It aimed to survey the surgical retina case mix in the United Kingdom, and to determine the intraoperative complication rate associated with pars plana vitrectomy (PPV). It also aimed to estimate the incidence of post-vitrectomy cataract surgery (PVCS).
Materials and methods
Data extraction
The data presented in this report relate to VR operations performed between December 2002 and the date of data extraction in November 2010. Data came from the NOD, which has 31 contributing National Health Service (NHS) hospitals. Of these, 27 hospitals contributed VR surgical data, as shown in the acknowledgements section. All data were captured using a single EMR system (Medisoft Ophthalmology, Medisoft Limited, Leeds, UK). In the future, the NOD will accept data from any EMR system that collects nationally agreed data sets. Data were transferred automatically, in anonymised form, to the NOD. Ethnicity data were obtained via an automatic link between the EMR and the hospital's patient administration system. At each contributing centre, the lead clinician and Caldicott Guardian (who oversees data protection) gave written approval for the extraction of anonymised data. No patient identifiable data are held on the NOD, but each patient has a unique identifier assigned by the EMR provider, such that future data extractions can be matched to existing patients. As the data were anonymised, an ethics committee advised that their approval was not required to extract and analyse data, provided the Caldicott Guardian and lead clinician at each site were satisfied with the data governance arrangements. This study was conducted in accordance with the declaration of Helsinki, and the UK's Data Protection Act.
Case mix allocation and eligibility
The case mix allocation was made using the ‘indication for surgery' field from the EMR. If the indication for surgery was not recorded (in earlier years it was not compulsory), then cases were allocated to an ‘indication unknown' category. The indication for surgery field contained a search field of all VR diagnoses. To be included in this study an eye had to have one or more operations that included a VR procedure element, as referenced to the Office of Populations and Census and Surveys Classification of Interventions and Procedures (OCPS-4.6),6 either alone or in combination with other VR or non-VR procedures.
Complications of PPV
The EMR forced surgeons to record whether or not there were any surgical complications, before they were able to save the operation note. If a complication occurred the surgeon was forced to select from a pre-populated list of well-recognised complications specific to the procedure elements that formed that operation, or select ‘other' and record the complication using free text.
Post-PPV cataract surgery
All hospitals using the EMR for VR procedures also recorded cataract surgery using the same system, and these data were also automatically submitted to the NOD. It was therefore possible to estimate the incidence of PVCS following PPV. Eyes were eligible to be included in the PVCS analysis following their first PPV. Eyes were excluded if they were recorded as having previously undergone cataract surgery, or had a cataract operation as a part of their primary PPV. Eyes with <3 weeks follow-up were also excluded. A short minimum follow-up time was selected to capture any cases of lens touch cataract.
Statistical analysis
The time to PVCS was modelled using the Kaplan–Meier7 method, with PVCS modelled as failure. Eyes were censored at the last date on which follow-up data of any type was recorded on the EMR (and hence the NOD), if they had not had cataract surgery. All analysis was conducted using STATA version 11 (StataCorp, College Station, TX, USA).
Results
Patient demographics
There were 11 618 operations that included one or more VR procedure element recorded on the NOD, within the study period. These operations were performed on 9619 eyes (4686 left eyes and 4933 right eyes) of 8741 patients (Figure 1). Of the 8741 patients, 4539 (51.9%) were male, 4190 (47.9%) were female, and the gender was not specified for 12 (0.1%) patients. The median age at the time of the patient's first recorded VR procedure was 65.1 years (inter-quartile range 54.8–74.1 years; range 0.1–100.0 years). Demographic variables were fairly balanced across gender; although on average females were slightly older than males at the time of their first recorded VR procedure (Table 1). The diabetic and ethnic background of many patients was not known.
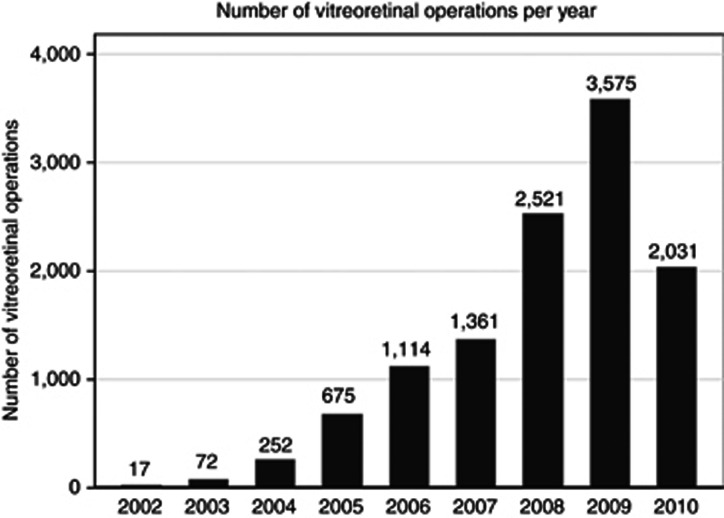
Figure 1.
The figure shows the number of vitreoretinal operations recorded on the NOD. The increasing number of operations per year reflects an increasing number of sites contributing to the NOD. The years shown are as defined by the NHS, running from 1st April to 31 March. In 2010, data were collected from the 1st of April to the 30th of November. The total number of operations is 11 618 over 8.7 years.
Table 1. Demographic details.
| Variable (column %) | Male | Female | Not specified | Total |
|---|---|---|---|---|
| n |
4539 |
4190 |
12 |
8741 |
| Diabetic status | ||||
| Diabetic | 635 (14.0) | 511 (12.2) | 2 (16.7) | 1148 (13.1) |
| Not diabetic | 1157 (25.5) | 1173 (28.0) | 2 (16.7) | 2332 (26.7) |
| Not recorded | 2747 (60.5) | 2506 (59.8) | 8 (66.7) | 5261 (60.2) |
| Ethnicity | ||||
| Caucasian | 2418 (53.3) | 2165 (51.7) | 2 (16.7) | 4585 (52.5) |
| Mixed race | 16 (0.4) | 19 (0.5) | 0 (0.0) | 35 (0.4) |
| Asian | 66 (1.5) | 40 (1.0) | 0 (0.0) | 106 (1.2) |
| Black | 50 (1.1) | 59 (1.4) | 0 (0.0) | 109 (1.2) |
| Other | 25 (0.6) | 18 (0.4) | 0 (0.0) | 43 (0.5) |
| Not recorded | 1964 (43.3) | 1889 (45.1) | 10 (83.3) | 3863 (44.2) |
| Age at first vitreoretinal operation (years) | ||||
| Median | 63.5 | 66.8 | 66.3 | 65.1 |
| Inter-quartile range | 52.0–72.9 | 57.9–75.3 | 58.6–75.5 | 54.8–74.1 |
| Range | 0.2–97.2 | 0.1–100.0 | 45.3–82.1 | 0.1–100.0 |
Of the 9619 eyes, 8224 (85.5%) eyes had one VR operation, 994 (10.3%) eyes had two VR operations, 259 (2.7%) eyes had three VR operations, 99 (1.0%) eyes four VR operations, and 43 (0.4%) eyes had five or more VR operations. The maximum number of VR operations in one eye was nine.
Surgical case mix
Of all VR procedures, 4217 (36.3%) were for rhegmatogenous retinal detachment, 1417 (12.2%) for retinal tears, 1136 (9.8%) for macular holes, 1117 (9.6%) for epiretinal membrane, and 848 (7.3%) for diabetic eye disease. All other surgery categories contained <5% of the total operations, as shown in Table 2.
Table 2. Indication for surgery.
| Indication for surgery Surgery for each group included PPV unless otherwise stated | No. of operations (column %) | No. of eyesa (column %) | No. of operations with PPV (row %) |
|---|---|---|---|
| Retinal detachment (includes cases without PPV) | 4217 (36.3) | 3549 (33.6) | 3683 (87.3) |
| Retinal tear (no PPV) | 1417 (12.2) | 1291 (12.2) | N/A |
| Macular hole | 1136 (9.8) | 1089 (10.3) | 1131 (99.6) |
| Epiretinal membrane peel | 1117 (9.6) | 1089 (10.3) | 995 (89.1) |
| Removal of tamponading agent±cataract | 489 (4.2) | 464 (4.4) | 64 (13.1) |
| Diabetic vitrectomy | 447 (3.8) | 414 (3.9) | 443 (99.1) |
| Diabetic vitrectomy & traction | 401 (3.5) | 386 (3.7) | 389 (97.0) |
| Cataract & PPV (PPV indication not known) | 309 (2.7) | 309 (2.9) | 309 (100.0) |
| Vitreoretinal examination under anaesthesia (no PPV) | 278 (2.4) | 275 (2.6) | 0 (0.0) |
| Dropped nucleus | 223 (1.9) | 216 (2.0) | 202 (90.6) |
| PRP laser | 210 (1.8) | 194 (1.8) | 7 (3.3) |
| Vitreomacular traction | 174 (1.5) | 167 (1.6) | 171 (98.3) |
| Vitreous haemorrhage (no DR) | 160 (1.4) | 155 (1.5) | 157 (98.1) |
| Vitrectomy indication unknown | 143 (1.2) | 142 (1.3) | 143 (100.0) |
| Uveitis | 123 (1.1) | 110 (1.0) | 53 (43.1) |
| AMD/retinal haemorrhage | 112 (1.0) | 105 (1.0) | 103 (92.0) |
| Trauma/intraocular foreign body removal | 106 (0.9) | 95 (0.9) | 77 (72.6) |
| Rare | 85 (0.7) | 76 (0.7) | 50 (58.8) |
| PVD/vitreous opacity | 85 (0.7) | 84 (0.8) | 82 (96.5) |
| IOL/lens/aphakic | 81 (0.7) | 80 (0.8) | 74 (91.4) |
| Endophthalmitis | 79 (0.7) | 69 (0.7) | 35 (44.3) |
| Scleral buckle removal (no PPV) | 75 (0.6) | 74 (0.7) | N/A |
| Glaucoma & VR Procedure | 72 (0.6) | 59 (0.6) | 27 (37.5) |
| Retinal vein occlusion | 48 (0.4) | 47 (0.4) | 30 (62.5) |
| Ectopia lentis | 31 (0.3) | 30 (0.3) | 30 (96.8) |
| Total | 11 618 | 10 569 | 8257 (71.1) |
Abbreviations: AMD, age-related macular degeneration; DR, diabetic retinopathy; IOL, intraocular lens; PPV, pars plana vitrectomy; PRP, panretinal photocoagulation; PPV, pars plana vitrectomy; PVD, posterior vitreous detachment; VR, vitreoretinal.
Each eye could appear in >1 group if having >1 operation over the time period.
Of all the operations, 8257 (71.1%) included a PPV, 4781 (41.2%) included internal tamponade using gas, 1090 (9.4%) included panretinal photocoagulation (PRP), and 894 (7.7%) included a scleral buckle (438 in combination with PPV).
Of the 85 operations that were classified as rare, 23 were for operations that had cryotherapy to a lesion of the retina as the only VR operative procedure, 22 were for tractional retinal detachments of uncertain cause, 11 for retinopathy of prematurity, 7 for exudative retinal detachment, 5 for choroidal folds associated with hypotony, 5 for cystoid macular oedema in patients who were either not diabetic or the diabetic status was not recorded, 4 for optic disc pit with serous detachment of the macula, 2 for suprachoroidal haemorrhages, and 1 each for anterior capsule opacification, central serous retinopathy associated with retinal detachment, hyphaema, phthisis, retinoschisis, and sickle cell retinopathy. One of the eyes assigned to the posterior vitreous detachment/vitreous opacity group had amyloidosis.
Intraoperative complications
Overall, 10 937 operations (94.1%) were recorded as having no intraoperative complication. In eyes undergoing PPV, 7617 of 8257 cases (92.2%) were recorded as having no intraoperative complication. Two intraoperative complications were reported for 56 PPVs, and three intraoperative complications for 8 PPVs. The most commonly reported intraoperative complications were iatrogenic retinal tears in 3.2%, lens touch in 0.9%, and iatrogenic retinal trauma in 0.7%. The lens status of eyes undergoing primary PPV was not known in 3183 cases, and as a result the exact rate of lens touch in phakic eyes was not known. The lens touch rate can, however, be estimated to lie between approximately 1.2 and 1.6%: 1.2% if all the eyes with unknown status were phakic, and 1.6% if all the eyes with unknown status were pseudophakic (see footnote in Table 3 for details of the calculation). Other complications occurred in 0.5% or less of cases (Table 3). If the lens-related complications such as posterior capsular rupture were excluded (in the eyes undergoing combined cataract surgery and vitrectomy), then the complication rate attributable to PPV reduced from 7.8 to 6.2%. Table 4 shows the intraoperative complication rates associated with primary PPV undertaken for the most common indications.
Table 3. Reported intraoperative complications.
| (%)=Column percentage | With a PPV (N=8257) | No PPV (N=3361) | Total (N=11 618) |
|---|---|---|---|
| Number of operations with no complications | 7617 (92.2%) | 3320 (98.8%) | 10 937 (94.1%) |
| Number of operations with complications | 640 (7.8%) | 41 (1.2%) | 681 (5.9%) |
| Reported operative complications | |||
| Iatrogenic tear | 263 (3.2) | 1 (<0.1) | 264 (2.3) |
| Lens touch | 73 (0.9)a | 1 (<0.1) | 74 (0.6) |
| Iatrogenic retinal trauma | 57 (0.7) | 1 (<0.1) | 58 (0.5) |
| Posterior capsule rupture—no vitreous lossb | 43 (0.5) | 1 (<0.1) | 44 (0.4) |
| Other | 35 (0.4) | 15 (0.4) | 50 (0.4) |
| Choroidal/suprachoroidal haemorrhage | 33 (0.4) | 1 (<0.1) | 34 (0.3) |
| Posterior capsule rupture—vitreous lossb | 34 (0.4) | 2 (<0.1) | 36 (0.3) |
| Nuclear/epinuclear fragment into vitreousb | 31 (0.4) | 0 (0.0) | 31 (0.3) |
| Conjunctival buttonhole | 22 (0.3) | 0 (0.0) | 22 (0.2) |
| Infusion cannula in subretinal/suprachoroidal space | 21 (0.3) | 0 (0.0) | 21 (0.2) |
| Retinal haemorrhage | 19 (0.2) | 1 (<0.1) | 20 (0.2) |
| Zonule dialysisb | 14 (0.2) | 1 (<0.1) | 15 (0.1) |
| Corneal epithelial abrasion | 12 (0.1) | 3 (<0.1) | 15 (0.1) |
| Subretinal haemorrhage | 11 (0.1) | 3 (<0.) | 14 (0.1) |
| Iris trauma | 8 (<0.1) | 0 (0.0) | 8 (<0.1) |
| Corneal oedema | 7 (<0.1) | 3 (<0.1) | 10 (<0.1) |
| Zonule rupture—no vitreous lossb | 5 (<0.1) | 0 (0.0) | 5 (<0.1) |
| Hyphaema | 4 (<0.1) | 1 (<0.1) | 5 (<0.1) |
| Retinal incarceration | 3 (<0.1) | 0 (0.0) | 3 (<0.1) |
| Vitreous haemorrhage | 2 (<0.1) | 2 (<0.1) | 4 (<0.1) |
| Inadvertent subretinal fluid drain | 1 (<0.1) | 6 (0.2) | 7 (<0.1) |
| Lens exchange required/other intraocular lens problemsb | 3 (<0.1) | 0 (0.0) | 3 (<0.1) |
| Subretinal heavy liquid | 1 (<0.1) | 0 (0.0) | 1 (<0.1) |
| Subretinal silicone oil | 1 (<0.1) | 0 (0.0) | 1 (<0.1) |
| Suprachoroidal silicone oil | 1 (<0.1) | 0 (0.0) | 1 (<0.1) |
| Variable burn intensity | 0 (0.0) | 1 (<0.1) | 1 (<0.1) |
| Total reported operative complicationsc | 704 | 43 | 747 |
The value shown is the percentage of all eyes undergoing pars plana vitrectomy (PPV), despite the fact that lens touch would not occur in a pseudophakic eye. Of the 6986 eyes undergoing primary PPV, 668 had previously had cataract surgery and were therefore known to be pseudophakic, 1897 had combined PPV and cataract surgery and were therefore phakic at the time of their primary PPV, and 1238 subsequently had cataract surgery and were therefore also known to be phakic during their primary PPV. In 3183 eyes, the lens status was not known and in these eyes there were 24 cases of lens touch. With this information it is possible to estimate the range of possible lens touch rate. If all of the eyes with unknown lens status were phakic then the lens touch rate in phakic eyes undergoing primary PPV would be 1.2% (73/[1897+1238+3183]). If all the eyes with unknown lens status were pseudophakic, and therefore excluded from the analysis of phakic lens touch, then there were 49 cases of lens touch remaining in the group available for analysis, and the rate of lens touch would be 1.6% (49/[1897+1238]). The highest rate would occur if there were only 24 phakic eyes in the 3183 eyes with unknown lens status and all had a lens touch. In this unlikely situation the lens touch rate would be 2.3% (73/[1,897+1,238+24]). Therefore, the rate of recorded lens touch is likely to range between 1.2 and 1.6%, but theoretically could be up to 2.3%.
Cataract surgery-related complications occurring in eyes with combined vitreoretinal and cataract surgery.
More than one intraoperative complication could be reported for each operation, and therefore the sum of the individual complication percentages exceeds the percentage of operations with a complication.
Table 4. Reported intraoperative complication rate during primary pars plana vitrectomy, by indication.
| Condition | Overall intraoperative complication rate in eyes undergoing primary pars plana vitrectomy n/N (%) |
|---|---|
| Rhegmatogenous retinal detachment | 191/2990 (6.4) |
| Macular hole22 | 134/1078 (12.4) |
| Epiretinal membrane | 81/956 (8.5) |
| Diabetic vitrectomy without traction | 36/383 (9.4) |
| Diabetic vitrectomy with traction | 67/365 (18.4) |
Post-PPV cataract surgery
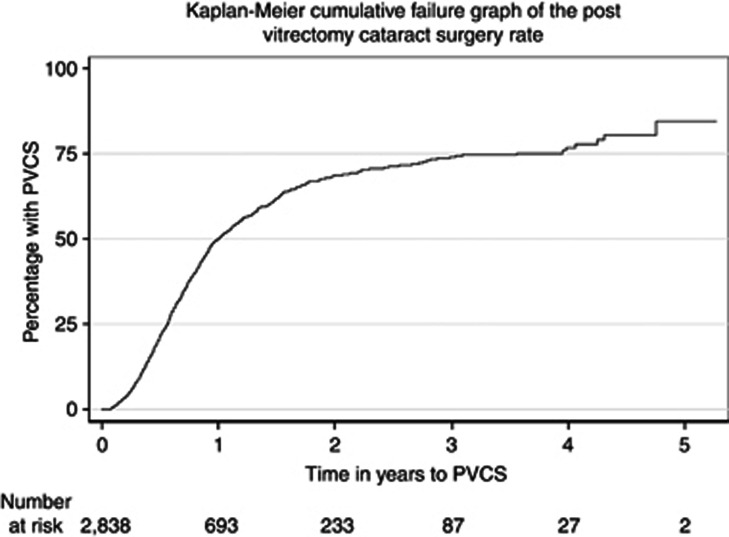
Of the 11 618 VR operations, 6883 were on eyes having their first PPV. Of these, 4045 eyes were excluded from the PVCS analysis: 659 because they had previously had cataract surgery, 1889 as the first PPV operation was combined with cataract surgery, and 1497 as they had no, or <3 weeks, follow-up data recorded on the NOD. Of the remaining 2838 eyes eligible for analysis, the median follow-up was 0.7 years (range 22 days–5.6 years), and 1197 (42.2%) were subsequently recorded as having cataract surgery. The 1-, 2-, 3-, and 5-year PVCS rates were 50.2, 68.7, 74.0, and 85.5%, respectively, with no events recorded more than 5 years after VR surgery (Figure 2).
Figure 2.
Graph shows the percentage of eyes requiring cataract surgery, in relation to the time following primary pars plana vitrectomy. The number of eyes at risk for each time point comprises the number of eyes available for assessment, in that they were still providing follow-up data to the National Ophthalmology Database.
Conclusion
An insight into the activity of typical VR operating rooms is important for resource allocation, to help clinicians, researchers and Industry set priorities, to follow trends over time, and to allow individual surgeons to compare their own case mix, and complication rate, with others'.
This study used the UK's NOD to capture VR surgical data from NHS hospitals, over an 8-year window. As such, it provides an overview of surgical case mix, an estimate of complications occurring during PPV, and an estimate of the rate of cataract surgery following PPV that is representative of the participating centres. It is important to note that practice patterns vary, and thus these numbers and rates might not be applicable in other settings or countries.
This study found that retinal detachment and retinal tears were the most common indication for VR intervention. Together they accounted for almost half of all VR procedures, and slightly more than half if subsequent silicone oil removal was included. Macular hole and ERM were the second and third most common indications, both at slightly <10%. The other large burden of disease related to diabetes—7% of operations were undertaken specifically for diabetic eye disease.
PPV was found to have an intraoperative complication rate of 7.8%, with iatrogenic retinal tears by far the most frequent complication at 3%, followed by lens touch at 1.2–1.6%. All other complications had an incidence of <1%. Several complications were attributable to cataract surgery, such as posterior capsular rupture, and occurred in eyes undergoing combined phakoemulsification and PPV. If lens-related complications were excluded, then the complication rate attributable to PPV reduced to 6.2%.
Case series have reported entry sites breaks occurring in 2–16% of cases.8, 9, 10, 11, 12 It can, however, be difficult to compare across series, as authors use different definitions of entry site break, and iatrogenic breaks. There is also a risk of publication bias, if clinicians are reluctant to report high complication rates, and the outcomes in case series of clinical interest may not be generalisable, due to their complex nature or targeted case selection. Retrospective analysis is also potentially problematic because of incomplete or selective data capture.
There are few large, prospective studies of PPV. The submacular surgery trial13 and diabetic retinopathy vitrectomy study14 were undertaken before the widespread introduction of wide-angle viewing systems and small-gauge surgery, which may alter the detection and occurrence of retinal breaks, respectively.11, 12 There are recent, large prospective studies of PPV for the treatment of retinal detachment,15, 16 and these reported that iatrogenic retinal breaks occur in 8–13% of cases,16 and entry site breaks in 0.65%.15 A recent large, prospective study of PPV for the treatment of age-related macular degeneration reported retinal tears in 2% of cases.17 Surgery involved a relatively simple intervention (epimacular brachytherapy), and therefore the slightly lower rate of retinal tears is consistent with the 3% found in the present study, which included a much wider range of indications.
There are relatively few published database studies of vitrectomy. Dogramaci et al18 studied the risk of iatrogenic retinal breaks in 2471 eyes undergoing PPV in a single teaching centre. They reported that 10.1% of eyes had an iatrogenic break. Rizzo et al19 compared 20-gauge and small-gauge vitrectomy in 2432 eyes with either macular hole or epiretinal membrane, in another single-centre study. The incidence of iatrogenic retinal breaks was not stated, but postoperative retinal detachment occurred in 1.5% of eyes. Ho et al20 studied the effect of surgeon age on the primary retinal detachment success rate, using a large national database that included 7427 eyes. A large retrospective analysis of retinal procedures undertaken on Medicare beneficiaries in the United States, from 1997 to 2007, found that the number PPVs with membrane stripping was approximately the same as the number of procedures undertaken for the treatment or prevention of retinal detachment.21 For many of the categories the diagnosis was not known. For example, vitreomacular traction, epiretinal membrane, and macular hole are likely to have all been grouped together.
A strength of the present study is that diagnoses could be linked to the procedures, and all procedure elements were detailed. In addition, the EMR forced clinicians to record if complications did, or did not, occur and consequently these data variable were available in 100% of cases. This is likely to enhance the accuracy of recording of complications, as clinicians could not omit complications unless they made a false declaration. Further, clinicians were assured that their contribution to the NOD was anonymous, so their own success rate remained private. Despite these advantages, clinicians may nonetheless have an innate reluctance to record complications. Therefore, the complication rates reported herein may underestimate the true rate. In addition, the threshold for reporting complications, and what defines a complication, may vary across individuals, institutions, and countries.
A limitation of this study is that it was not designed to assess postoperative complications, other than post-vitrectomy cataract. Cataract is by far the most common postoperative complication of PPV,16, 17 and this study was able to access the NOD cataract data, for eyes undergoing primary PPV. Figure 2 shows that, in those not lost to follow-up, half of the eyes required cataract surgery within a year of primary PPV. Beyond the first year the follow-up rates declined, and the data need to be interpreted with caution, but they suggest that two-thirds of eyes require cataract surgery by year 2, and three-quarters by year 3. This is consistent with data from large clinical trials.15, 16, 17
All the contributing centres offer cataract surgery, and are likely to treat their own post-vitrectomy cataracts, but it is possible that some eyes had cataract surgery elsewhere and were lost to follow-up. In addition, eyes that had combined cataract surgery and primary PPV were necessarily excluded from the analysis of PVCS. These eyes may have been more likely to have had pre-existing lens opacity and this would increase the likelihood of needing subsequent cataract surgery, had this not been undertaken as part of the primary intervention. Therefore, the true rate of PVCS in phakic eyes undergoing primary PPV may be higher than we report. Conversely, it is possible that eyes with developing cataract were more likely to remain under review and contribute to the NOD data set. This selection bias may tend to overestimate the true cataract rate.
In summary, this study found that VR surgical services in the United Kingdom treat a wide range of diseases, but that three-quarters of cases have retinal detachment, retinal breaks, macular hole, epiretinal membrane, or diabetic eye disease. Almost half of the eyes undergoing PPV have combined cataract surgery, and in those which do not, approximately half require cataract surgery within a year. The most common intraoperative complication is an iatrogenic retinal break, occurring in 3% of cases, with 1.2–1.6% of phakic eyes having lens touch. Other complications occur in <1% of cases.

Acknowledgments
We thank the vitreoretinal surgeons from the following sites for contributing data: Aintree Hospitals NHS Trust; Airedale NHS Foundation Trust & Bradford Teaching Hospitals NHS Foundation Trust; Barking, Havering and Redbridge University hospitals NHS Trust; Bedford Hospital NHS Trust; Cambridge University Hospitals NHS Foundation Trust; Calderdale and Huddersfield NHS Foundation Trust; Dumfries and Galloway Community Health NHS Trust; Epsom and St Helier University Hospitals NHS Trust; Gloucestershire Hospitals NHS Foundation Trust; Grampian Healthcare NHS Trust; King's College Hospital NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Mid Cheshire Hospitals NHS Foundation Trust; Mid Yorkshire Hospitals NHS Trust; Norfolk and Norwich University Hospitals NHS Foundation Trust; North Devon Healthcare NHS Trust; Peterborough and Stamford Hospitals NHS Foundation Trust; Portsmouth Hospitals NHS Trust; Royal Berkshire NHS Foundation Trust; Royal United Hospital Bath NHS Trust; South London Healthcare NHS Trust; South Warwickshire NHS Foundation Trust; The Hillingdon Hospital NHS Trust; University Hospitals Birmingham NHS Foundation Trust; University Hospitals Bristol NHS Foundation Trust; Winchester and Eastleigh Healthcare NHS Trust; Wirral University Teaching Hospital NHS Foundation Trust.
T. Jackson and P. Donachie's employer received unrestricted funding from Thrombogenics to analyse these data. Thrombogenics had no data access, nor any input in the study design, data analysis, or manuscript preparation. R. Johnston is the Medical Director of Medisoft Limited, which developed the electronic medical record from which data were extracted, for the first iteration of the National Ophthalmology Database.
References
- Johnston RL, Sparrow JM, Canning CR, Tole D, Price NC. Pilot national electronic cataract surgery survey: I. Method, descriptive, and process features. Eye. 2005;19:788–794. doi: 10.1038/sj.eye.6701644. [DOI] [PubMed] [Google Scholar]
- Javitt JC. Rule Britannia. Eye. 2005;19:727–728. doi: 10.1038/sj.eye.6701645. [DOI] [PubMed] [Google Scholar]
- Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, Galloway P, et al. The cataract national dataset electronic multi-centre audit of 55,567 operations: updating benchmark standards of care in the United Kingdom and internationally. Eye. 2009;23:38–49. doi: 10.1038/sj.eye.6703015. [DOI] [PubMed] [Google Scholar]
- Narendran N, Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, et al. The cataract national dataset electronic multicentre audit of 55 567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye. 2009;23:31–37. doi: 10.1038/sj.eye.6703049. [DOI] [PubMed] [Google Scholar]
- Sparrow JM, Taylor H, Qureshi K, Smith R, Johnston RL. The cataract national data set electronic multi-centre audit of 55 567 operations: case-mix adjusted surgeon's outcomes for posterior capsule rupture. Eye. 2011;25:1010–1015. doi: 10.1038/eye.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Connecting for Health . OPCS Classification of Interventions and Procedures Version 4.6 (Vols 1 and 2) The Stationery Office: London; 2011. [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- Misra A, Ho-Yen G, Burton RL. 23-Gauge sutureless vitrectomy and 20-gauge vitrectomy: a case series comparison. Eye. 2009;23:1187–1191. doi: 10.1038/eye.2008.175. [DOI] [PubMed] [Google Scholar]
- Ehrlich R, Goh YW, Ahmad N, Polkinghorne P. Retinal breaks in small-gauge pars plana vitrectomy. Am J Ophthalmol. 2012;153:868–872. doi: 10.1016/j.ajo.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Ramkissoon YD, Aslam SA, Shah SP, Wong SC, Sullivan PM. Risk of iatrogenic peripheral retinal breaks in 20-G pars plana vitrectomy. Ophthalmology. 2010;117:1825–1830. doi: 10.1016/j.ophtha.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Gosse E, Newsom R, Lochhead J. The incidence and distribution of iatrogenic retinal tears in 20-gauge and 23-gauge vitrectomy. Eye. 2012;26:140–143. doi: 10.1038/eye.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa SA, Connor A, Habib M, Steel DHW. Comparison of retinal breaks observed during 23 gauge transconjunctival vitrectomy versus conventional 20 gauge surgery for proliferative diabetic retinopathy. Clin Ophthalmol. 2011;5:109–114. doi: 10.2147/OPTH.S16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Submacular Surgery Trials Pilot Study Investigators Submacular surgery trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration: I. Ophthalmic outcomes. SST pilot study report no. 1. Am J Ophthalmol. 2000;130:387–407. doi: 10.1016/s0002-9394(00)00729-7. [DOI] [PubMed] [Google Scholar]
- Anon Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic retinopathy vitrectomy study (DRVS) report #1. Ophthalmology. 1985;92:492–502. doi: 10.1016/s0161-6420(85)34002-2. [DOI] [PubMed] [Google Scholar]
- Wickham L, Bunce C, Wong D, McGurn D, Charteris DG. Randomized controlled trial of combined 5-Fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachments undergoing primary vitrectomy. Ophthalmology. 2007;114:698–704. doi: 10.1016/j.ophtha.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–2154. doi: 10.1016/j.ophtha.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Dugel PU, Bebchuk JD, Nau J, Reichel E, Singer M, Barak A, et al. Epimacular brachytherapy for neovascular age-related macular degeneration: (CABERNET): a randomized, controlled trial. Ophthalmology. 2013;120:317–327. doi: 10.1016/j.ophtha.2012.07.068. [DOI] [PubMed] [Google Scholar]
- Dogramaci M, Lee EJ, Williamson TH. The incidence and the risk factors for iatrogenic retinal breaks during pars plana vitrectomy. Eye. 2012;26:718–722. doi: 10.1038/eye.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo S, Belting C, Genovesi-Ebert F, di Bartolo E. Incidence of retinal detachment after small-incision, sutureless pars plana vitrectomy compared with conventional 20-gauge vitrectomy in macular hole and epiretinal membrane surgery. Retina. 2010;30:1065–1071. doi: 10.1097/IAE.0b013e3181cd48b0. [DOI] [PubMed] [Google Scholar]
- Ho JD, Kuo NW, Tsai CY, Liou SW, Lin HC. Surgeon age and operative outcomes for primary rhegmatogenous retinal detachment: a 3-year nationwide population-based study. Eye. 2010;24:290–296. doi: 10.1038/eye.2009.99. [DOI] [PubMed] [Google Scholar]
- Ramulu PY, Do DV, Corcoran KJ, Corcoran SL, Robin AL. Use of retinal procedures in medicare beneficiaries from 1997–2007. Arch Ophthalmol. 2010;128:1335–1340. doi: 10.1001/archophthalmol.2010.224. [DOI] [PubMed] [Google Scholar]
- Jackson TL, Donechie PHJ, Sparrow JM, Johnston RL.United Kingdom national ophthalmology database study of vitreoretinal surgery: report 2, macular hole Ophthalmology 2012. e-pub ahead of print 1 December 2012; doi: 10.1016/j.ophtha.2012.09.003 [DOI] [PubMed]