Abstract
The main treatment available for restoration of the corneal endothelium is keratoplasty. This procedure is faced with several difficulties, including the shortage of donor tissue, post-surgical complications associated with the use of drugs to prevent immune rejection, and a significant increase in the occurrence of glaucoma. Recently, surgical procedures such as Descemet's stripping endothelial keratoplasty have focused on the transplant of corneal endothelium, yielding better visual results but still facing the need for donor tissue. The emergent strategies in the field of cell biology and tissue cultivation of corneal endothelial cells aim at the production of transplantable endothelial cell sheets. Cell therapy focuses on the culture of corneal endothelial cells retrieved from the donor, in the donor's cornea, followed by transplantation into the recipient. Recently, research has focused on overcoming the challenge of harvesting human corneal endothelial cells and the generation of new biomembranes to be used as cell scaffolds in surgical procedures. The use of corneal endothelial precursors from the peripheral cornea has also demonstrated to be effective and represents a valuable tool for reducing the risk of rejection in allogeneic transplants. Several animal model reports also support the use of adult stem cells as therapy for corneal diseases. Current results represent important progresses in the development of new strategies based on alternative sources of tissue for the treatment of corneal endotheliopathies. Different databases were used to search literature: PubMed, Google Books, MD Consult, Google Scholar, Gene Cards, and NCBI Books. The main search terms used were: ‘cornea AND embryology AND transcription factors', ‘human endothelial keratoplasty AND risk factors', ‘(cornea OR corneal) AND (endothelium OR endothelial) AND cell culture', ‘mesenchymal stem cells AND cell therapy', ‘mesenchymal stem cells AND cornea', and ‘stem cells AND (cornea OR corneal) AND (endothelial OR endothelium)'.
Keywords: corneal endothelium, tissue engineer, stems cells
Introduction
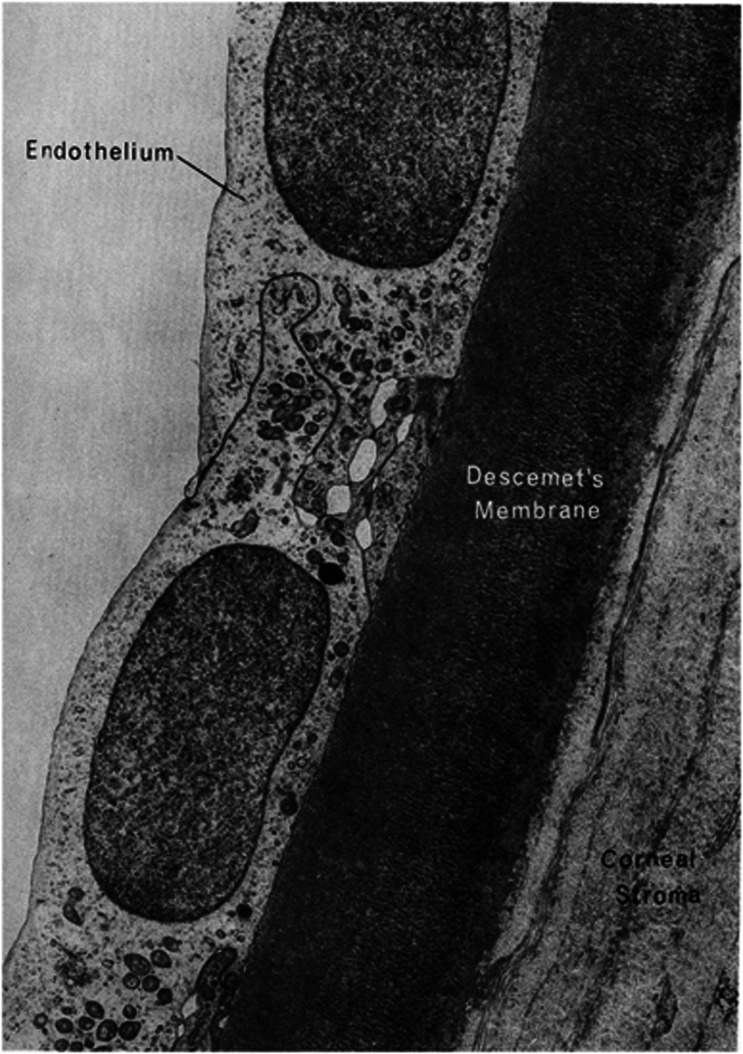
The cornea is a transparent avascular tissue that in conjunction with the sclera forms the outer portion of the eye. It is a connective tissue that acts as the primary barrier against infection and mechanical damage to the internal structures of the eye. Along with the tear film on the ocular surface, it accounts for more than two-thirds of the total refractive power of the eye. It is organized into three cell layers: epithelium, stroma, and endothelium, and two interfaces: Bowman's layer and Descemet's membrane.1 The epithelium provides a biodefense system on the anterior surface of the eye, helps to keep the corneal surface optically smooth, and provides a barrier to external biological agents and chemical damage. Bowman's layer serves as an interface between the epithelium and the stroma and consists of randomly arranged collagen fibers and proteoglycan types I and III. The stroma constitutes about 90% of the thickness of the cornea and is composed of extracellular matrix, keratinocytes, and nerve fibers. It provides structural strength, shape, stability, and transparency to the cornea. The endothelium is a thin monolayer of polygonal cells covering the posterior surface of Descemet's membrane and is in contact with the aqueous humor (Figure 1). The main function is to regulate the hydration state through an active ATP and biocarbonate-dependent pump; thereby providing transparency to the cornea, which allows the eye to perform its visual functions.2 It is also an important system for the passage of nutrients and waste removal through simple diffusion, facilitated diffusion, and active transport mechanisms.3
Figure 1.
Electron micrograph of corneal endothelium underlying Descemet's membrane of the human cornea (with permission of Cell Image Library CIL: 10944* http://creativecommons.org/licenses/by-nc-nd/3.0/legalcode).
The corneal endothelium is the cell layer with the lowest mitotic activity.4 Given the importance of its function, damage to the endothelium is potentially more serious than that to the other corneal layers and can result in cell loss and irreversible damage to the endothelial cytoskeleton, that ultimately affecting visual function.5 The main treatment for this condition is corneal transplant. Nevertheless, given the difficulty obtaining donor tissue, the development of novel strategies has focused on the use of cultured corneal endothelial cells, corneal endothelial stem cells, and stem cells of extra-ocular origin. In this article, we describe the corneal endothelium's embryology and physiology, the main conditions that affects it and the cell therapies currently under development.
Embryology
The embryological origins of the major structures of the eye are diverse. The central part of the cornea, including the endothelium, is derived from neural crest cells. The retina and the epithelial layers of the iris and ciliary body are derived from the anterior neural plate, the lens from surface ectoderm, and the corneal epithelium from epidermal ectoderm.6, 7
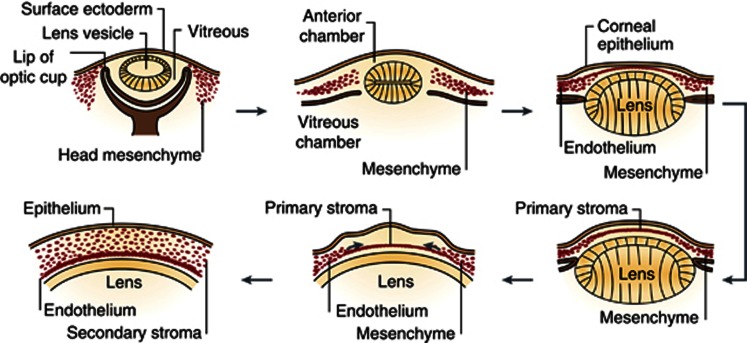
The cornea is formed as a result of the last series of major inductive events during eye development at ∼5–6 weeks of human gestation, when the surface ectoderm interacts with the lens vesicle (Figure 2). When they are completely apart, the space between them is filled with perinuclear mesenchyme cells, from the neural crest. The mesenchyme then condenses and forms several layers separated by extracellular matrix. The cells closer to the lens become the corneal endothelium and the surface ectoderm on the anterior surface, becomes the corneal epithelium.6, 7 Around the 78 mm stage, the endothelial cells become flattened and tightly connected to one another by tight junctions. Immediately anterior to this layer there is a homogeneous acellular layer, which becomes Descemet's membrane. By the 120 and 165 mm stages of development, the endothelial monolayer is of uniform thickness spans the entire posterior corneal surface and fuses with the cells of the trabecular meshwork. The endothelial cells stay arrested in the G1-phase of mitosis.8
Figure 2.
Formation of the cornea. The cornea begins to develop when the surface ectoderm closes after the formation of the lens vesicle and its detachment from the surface ectoderm. Mesenchymal cells (neural crest cells) invade the cornea and form the corneal stroma after condensation (with permission of Nature Publishing Group). Reprinted by permission from Macmillan Publishers Ltd: Academic Press.7
The mechanisms of neural crest cell migration are not fully understood. It is the coordinated action of transcription factors and inductive signals that mediate the proper development of the periocular mesenchyme. Some of the transcription factors involved in these events are: Foxc1, Foxc2, Lmx1b, Pax6, Pitx2, RARβ, RARγ, RXRα, Six3, and Smad2.9, 10, 11, 12
Pax6 is a known master gene involved in ocular development. It is required for the development of all layers of the cornea.10 In mice, it is regulated by Six3 and Lmx1b, and together with Sox2, acts on the surface ectoderm to regulate the expression of crystalline genes.13, 14 Molecules like FGF2, TGFβ1, TGFβ2, and Bmp7 are also involved in regulating expression of Pax6. FGF2 participates in the process of invagination of the optic vesicle; together with Bmp7, it regulates the optimal level of Pax6 expression.13, 15 Bmp7 indirectly controls the Sox2 signal in the lens ectoderm, which either upregulates or downregulates Pax6 expression.16 TGFβ1 and TGFβ2 have been shown to increase the expression of Foxc1 and Pitx2,17, 18 which are required for the development of the ocular anterior segment.19, 20, 21 It has been proposed that the TGF-β subfamily members initiate a supporting mechanism to regulate Pax6 function and transcription.22 In the corneal endothelium, TGFβ1 and TGFβ2 modulate cell proliferation, cell morphology, and collagen expression.23, 24
In the mouse head mesenchyme, retinoic acid receptor heterodimers RXRα/RARβ and RXRα/RARγ regulate the expression of Foxc1 and Pitx2, and control the extent of cell death during remodeling of periocular mesenchyme.25 Together, Pax6, Lmx1b, and Pitx2 have a key role in the maintenance of corneal endothelium integrity.13
Although there has been significant progress in the understanding of human eye development during embryogenesis, further research is needed to clarify the mechanisms by which expression of these (and other) transcription factors lead to the proper development of corneal endothelium and other structures of the eye's anterior segment.
Corneal endothelium physiology
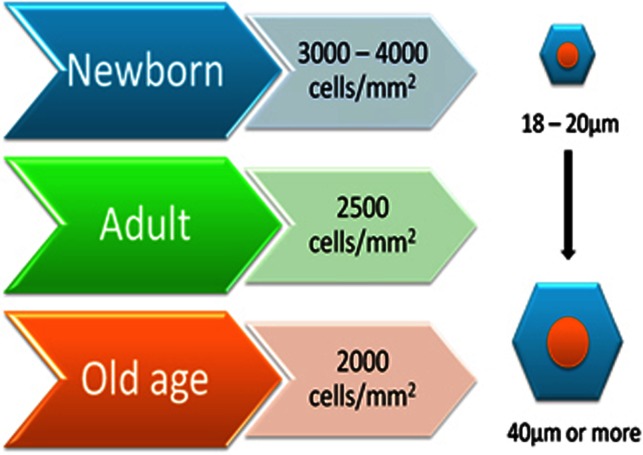
The corneal endothelium consists of a 4-μm thick monolayer of polygonal, mostly hexagonal cells. In the adult, the average cell density is ∼3000 cells/mm2 and the percentage of hexagonal cells is about 75%.26, 27 The density of corneal endothelial cells and their surface changes throughout life are noted in Figure 3. From the second to the eighth decade, the cell density declines to about 2600 cells/mm2 and the percentage of hexagonal cells decreases to ∼60%. The central endothelial cell density decreases at an average rate of 0.6% per year.28, 29 To preserve ocular transparency, endothelial cell density must remain above a critical level, usually between 400 and 500 cells/mm2.30 Adjacent cells communicate through gap junctions and tight junctions, whereas the basal surface is adhered to Descemet's membrane by hemidesmosomes.31, 32 Tight junctions (ZO-1) are supramolecular assemblies, that form intercellular junctions, and are found close to the apical domains of the endothelial cells. This allows the endothelium to function as a barrier, forming resistance to the permeability of solutes and fluid through paracellular transport routes.30, 33
Figure 3.
Changes in corneal endothelial cell density and size throughout life.
There are integral proteins in the cellular membrane, the aquaporins (AQP), which with the Na+/K+ ATPase pump participate in the fluid movement across the endothelium and function as water selective channels. The isoform AQP-1 is expressed by the corneal endothelial cells and the lens epithelium.34, 35
Corneal endothelial cells secrete collagen type VIII,36 an important component of Descemet's membrane that is actively produced during cellular differentiation and proliferation, as well as during postnatal development and in vitro cell culture.37 Collagen VIII is suggested to be partially responsible for the correct assembly of Descemet's membrane to ensure corneal stability.38
Endothelial cells contain numerous mitochondria and the Golgi complex, indicating that they are metabolically active and secretory.39 This is related to the Na+/K+-ATP pump and Descemet's membrane secretion, respectively. The former is a major function of the corneal endothelium and is driven by ionic gradients located in the basolateral side of the membrane.3 An osmotic gradient of sodium is present between the aqueous humor and the stroma and results in the influx of sodium ions from the aqueous humor and in an efflux of potassium ions in the opposite direction. Carbon dioxide also diffuses into the cytoplasm of the endothelial cells and in combination with water, bicarbonate ions are produced in a reaction catalyzed by carbonic anhydrase. The bicarbonate ions then diffuse or are transported into the aqueous humor. Coupled with the movement of bicarbonate ions there is a efflux of water across the endothelial cells into the aqueous humor.2 As a result of this activity, the stroma maintains a water content of ∼78%.40
A characteristic of the neural crest origin of the corneal endothelial cells is the expression of neuron-specific enolase (NSE).41 Although NSE is found in several tissues like smooth muscle cells, heart, and kidney, it can be used as a diagnostic tool for the identification of this cell type. A new monoclonal antibody has been generated (9.3.E) as a specific marker for human corneal endothelial cells (HCECs) that recognizes a protein mainly accumulated in the cell membrane and is useful for differentiating corneal endothelial cells from other cell types, especially corneal keratocytes.42
HCECs do not have a significant capacity for in vivo regeneration, thus making them unable to replace dead or damaged cells.30, 43 This occurs because HCECs are arrested in the G1-phase of the cell cycle. Three mechanisms have been identified that contribute to this: (1) cell–cell contact-dependent inhibition, (2) lack of effective growth factor stimulation, and (3) TGF-β2 suppression of S-phase.
To maintain proper structure and function, endothelial cells respond to minor damage with stretching and centripetal migration into the injured area;44 however, an increase in cell size (polymegathism) and variation in cell shape (pleomorphism) correlates to the reduced ability of the cells to hydrate the cornea.45, 46 Endothelial cell density can be significantly decreased as a result of trauma, refractive surgery, previous penetrating, or endothelial keratoplasty or stress caused by disorders such as diabetes, glaucoma, or endothelial dystrophies.47, 48, 49 When endothelial cell density decreases significantly, from the average of 3000 to nearly 1000 cells/mm2 (as in Fuchs' dystrophy) their function is compromised, corneal transparency is lost and surgery is required.50
There are promising therapies for corneal endothelium repair and wound healing, including the arrest of cell loss, endothelial cell transplantation, and stimulation of fluid secretion by the remaining endothelial cells.3, 51 Nevertheless, keratoplasty remains the main treatment to repair this layer.48, 50 The complications associated with this make the treatment of endotheliopathies a challenge for current and future research.
Clinical conditions of corneal endothelium and current treatments
Corneal blindness represents the fourth leading cause of blindness worldwide (5.1%) and is a major cause of visual impairment after cataracts, glaucoma, and age-related macular degeneration.52 Ocular trauma and corneal ulceration are also major causes of corneal blindness53, 54 and may result in 1.5–2.0 million new cases of monocular blindness every year.55 Given the difficulty of treating corneal blindness, public health prevention programs are the most effective options in terms of cost to reduce the number of cases worldwide. In fact, the main current treatment is keratoplasty; however, the access to this surgery is very difficult owing to lack of donors.56, 57
Corneal endothelial diseases that require corneal transplant include: Fuchs' dystrophy, bullous pseudophakic keratopathy, posterior polymorphous dystrophy, congenital hereditary endothelial dystrophy, iridocorneal endothelial syndrome, and some intermediate forms. The use of contact lenses and the effects of surgical procedures can also affect the endothelial tissue to a lesser extent.48, 58, 59, 60 Fuchs' dystrophy affects ∼4% of the population over 40 years. It is the major indication for penetrating keratoplasty in the United States, which accounts for 10–25% of all corneal transplants of different types. This is a significant number considering that the annual number of corneal transplants in the United States is more than 32 000.61 This disease is characterized by the presence of deposits on and thickening of Descemet's membrane, as well as changes in the shape, and size of the endothelial cells. The cornea progressively and slowly becomes opaque, causing blurred vision.62
Although penetrating keratoplasty has been the standard procedure for most diseases of the cornea, the outcomes are not usually expected due to several factors, including the risk of immune-mediated graft rejection, and a significant increase in the prevalence of glaucoma following transplantation, as concluded by Allouch et al63 and Valdez-García et al.64 Therefore, an ideal strategy would be to replace only the damaged layer.
In the last several years there have been breakthroughs in the field of corneal endothelial transplantation, such as the development of endothelial keratoplasty and Descemet's stripping endothelial keratoplasty (DSAEK) techniques. In 2005, only 4.5% of the donor corneas were used for endothelial keratoplasty. By 2007, this number increased to 50%.51 Nevertheless, the main problem with endothelial keratoplasty is a postoperative cell loss comparable to or higher than that observed with penetrating keratoplasty.65 It has been documented that endothelial cell density decreases ∼49% 24 months post-surgery and that cell loss can be higher in patients with previous glaucoma surgery. Neither donor age nor initial cell density proved to have significant influence on endothelial cell loss. The most promising strategy by which postoperative cell loss can be reduced effectively is the strict and adequate lowering of intraocular pressure.66 Techniques such as Descemet's membrane endothelial keratoplasty and Descemet's membrane automated endothelial keratoplasty were developed, offering better visual results, with less scarring and less optical stromal aberrations. These procedures opened the possibility to replace the corneal endothelium with endothelium reconstructed by bioengineering.67 However, clinically available procedures with artificial corneas have limitations such as inflammation of the retroprosthetic membrane and development of glaucoma, and are reserved for high-risk patients.68, 69
The limited availability of donor corneas and the current issues in surgical procedures require the development of new methods in the field of tissue engineering in order to improve corneal endothelial cell survival and increase corneal endothelial cell density. The emergent strategies in the field of cell biology and tissue cultivation of corneal endothelial cells aim at the production of transplantable endothelial cell sheets.
Cell therapy
Currently, cell therapy is aimed at reducing the problem of the lack of donor tissue. To repair the corneal endothelium, cell therapy focuses on the culture of corneal endothelial cells retrieved from the donor, in the donor's cornea, followed by transplantation into the recipient. Recent reports have demonstrated that corneal endothelial cells possess the ability to undergo mitosis in culture using these methods. HCEC ex vivo models are able to overcome the G1-phase and complete the cell cycle; this occurs after the release of cell–cell junctions and in the presence of appropriate growth factors. In fact, it has been proven that endothelial cells from both central and peripheral areas of the cornea proliferate in vitro70 and that they can be cultured from young and adult donors, obtaining similar numbers of cells when specific growth factors are used.71
To successfully engineer human corneal endothelium from a small number of cells, the processes of isolation, preservation and expansion are critical. Recently, research has focused on overcoming the challenge of harvesting HCECs. It is known that the main factors that influence the mitotic capacity of HCECs in vitro are: the method of culture, the nature of the growth factors contained in the medium, and the viability of the donor cornea. The latter is influenced by age, cell density, donor death-to-preservation time, preservation period, overall health of the donor, and the specific cause of death.72 The common methodology to isolate endothelial cells involves: (1) the retrieval of the corneal endothelium, (2) dissociation of cell junctions in paired Descemet's membrane/endothelial layer, and (3) culture in proper media. Based on the method used to dissociate the cell junctions, the procedures have been classified as enzymatic and non-enzymatic. The first is based on the use of enzymes such as collagenase, trypsin, or dispase.73, 74 This technique has the tendency of leading to cellular degradation due to the incubation time required to detach cells from the matrix, and because it also allows for the dissociation of the collagen matrix in which the keratocytes are located. Additionally, it often results in contamination from the stromal cells. To overcome this problem, magnetic cell separation improves HCECs yield, allowing for a high separation efficacy.75 The non-enzymatic method is based on the use of ethylenediamine tetraacetic acid (EDTA) to release cell–cell junctions at the same time as it promotes cell division upon exposure to mitogens.30, 70, 72, 76 In this process, EDTA can also cause cell damage and decrease cell yield.77 There is a combined method that uses collagenase II to generate preservable HCEC aggregates and a brief treatment with trypsin/EDTA leading to a high proliferation rate with less cell damage.78
The addition of several different growth factors in the culture media has been used to promote HCEC expansion. The use of insulin and basic fibroblastic growth factor (bFGF) have been shown to promote mitosis in cells from peripheral cornea but not in the central zone.79 In another study, nerve growth factor (NGF) along with bovine pituitary extract and epidermal growth factor (EGF) supported the expansion of cells from both central and peripheral areas of the cornea.72 In addition, a recent study concluded that a culture media that combines EGF, insulin, transferrin, bFGF, NGF, and pituitary extract promotes proliferative capacity up to the third passage.73
In order to replicate the monolayer of the corneal endothelium, there have been developments to maintain cell morphology, density, and function. Materials like collagen, amniotic membrane, and biodegradable polymers have successfully been used in culture media and animal models.80, 81, 82 Porcine corneal matrix and Descemet's membrane have also been used as scaffolds for transplanted cells allowing good transparency in cat and rat corneas, and maintaining cell properties after the transplant.60, 83 Human decellularized stromas have been used to culture HCECs, allowing them to retain the expression of Na+/K+-ATPase and ZO-1 markers for greater than 14 days.84 Hydrogel lens have also proved to be useful as a carrier device to transplant corneal endothelial cells in rabbits, maintaining graft clarity without signs of rejection and inflammation.85 An additional method consists of the use of superparamagnetic microspheres incorporated into HCECs before transplantation and their alienation after the transplant toward a magnetic field source. This method has allowed cell attachment to corneal stroma without affecting cell viability or light transmittance in an ex vivo model.86 Major advances have been reached in this field; however, efforts in developing new scaffolds are still focused on achieving full clarity of the cornea after transplantation, optimizing the number of steps needed for their production, and the generation of new biomembranes to be used in surgical procedures.
As an alternative to cultured HCECs, the use of corneal endothelial precursors from the peripheral cornea has demonstrated to be effective. Corneal endothelial stem cells are found as a sequestered niche at the junctional region between corneal endothelium and the trabecular meshwork, and are suggested to be a cell supply activated during wound healing.87 For their isolation, a sphere-forming assay method is developed, in which the cells obtained from the corneal endothelium-Descemet's membrane complex are cultured under floating conditions and generate proliferating spheres, that produce neuronal and mesenchymal cell proteins.88, 89 These corneal precursor spheres have been tested in an animal model of bullous keratopathy, without the use of a biocompatible carrier; they are injected directly into the anterior chamber and by placing the eye in downgaze enables their attachment to Descemet's membrane and corneal clarity can be achieved.90, 91
The production of corneal endothelial grafts from the same patient by culturing HCECs, or their precursors represents a valuable tool for reducing the risk of rejection in allogeneic transplants. However, the therapeutic use of stem cells in the cornea still requires an intact corneal stem cell compartment, which contradicts the main indication for the transplant. Furthermore, the amount of stem cells found in these compartments is very low and may require prolonged ex vivo culture to generate enough cells for a successful transplant.92 Therefore, further research is needed to find an alternative tissue source for corneal endothelial reconstruction, such as cells of extra-ocular origin.
Potential of using adult stem cells in the regeneration of corneal endothelium
Adult stem cells, especially those found in adipose tissue, bone marrow, and those obtained from umbilical cord blood have been widely studied for the development of new therapies for degenerative diseases.93 They have self-renewal and plasticity characteristics, plus they have an advantage over embryonic and fetal stem cells by being easy to obtain and culture. In addition, they do not face ethical problems, because it is feasible to obtain them from the same patient.94
Stem cells obtained from bone marrow and adipose tissue can differentiate into different cell types including chondrocytes, osteocytes, myocytes, and neural cells; the latter have been successfully obtained and used in an animal model in our laboratory.95
Both types of adult stem cells express very similar surface markers and genes;96 however, the procedure required to obtain bone marrow stem cells (BMSCs) can be uncomfortable for the patient. The amount of BMSCs collected is often low and the proportion of stem cells compared with the total number of nucleated cells is very low (0.001–0.01%). Subcutaneous adipose tissue is more abundant and often waste product in liposuction cosmetic and therapeutic type.97, 98 The ability of adult stem cells to secrete different types of molecules with anti-apoptotic, immunomodulatory, angiogenic, chemoattractant, and anti-scarring properties provides the basis for their use in regenerative medicine.99 Investigative therapies have been used in clinical trials with some designed to treat autoimmune diseases, including multiple sclerosis, lupus erythematosus, and Crohn's disease, as well as diabetes mellitus, myocardial infarction, different types of cancer, neurological disorders, and ocular surface diseases.93
Several animal model reports support the use of adult stem cells as therapy for corneal diseases. In a study made using Lum−/− mice (homozygous for disruptions in the lumican gene that produces aberrant collagen fibrils in the cornea), the intrastromal corneal injection of stem cells from umbilical cord blood was followed for 3 months post treatment. The corneal transparency returned after 12 weeks with an increase of about 10% in stromal thickness, which was recorded after 8 weeks with no immune rejection.100 In another study, a rabbit model for limbal stem cell deficiency was used to transplant bone marrow stem cells suspended in fibrin gel.101 After 28 days, the corneas were completely epithelialized and transplanted stem cells expressed cytokeratin 3, a protein expressed by corneal epithelial cells. They had secondary reactions like neovascularization, corneal opacification, and inflammation. However, these are short-term results and overall, the study provides evidence that bone marrow stem cells can be used for the treatment of corneal disorders. As an alternative, differentiated adult stem cells into corneal endothelial cells have demonstrated efficacy in the corneal clarity restoration in animal models. In our experience, differentiated cells are more likely to promote healing processes as compared with the effect of only adult stem cells (unpublished data). In 2010, Du et al102 demonstrated that stem cells isolated from human adipose tissue can differentiate into corneal keratocytes. After 3 weeks in pellet culture with enriched medium, stem cells adopted the keratocyte phenotype and expressed keratocan and keratan sulfate. In this study, the addition of bovine corneal extract to the culture medium did not enhance the levels of expressed keratocan, which suggest that differentiation relies more on the three dimensional culture environments than on molecular supplementation. A different study, demonstrated the ability of endothelial progenitor cells from bone marrow to differentiate into corneal endothelial cells.60 In this experiment, the co-culture of corneal endothelial cells with endothelial bone marrow precursors for 10 days produced endothelial-like cells and the expression of AQP-1, tight junctions, and NSE. Moreover, differentiated cells were transplanted using porcine corneal acellular matrix as carrier into cat's corneas with stripped endothelium, returning corneal transparency after 28 days with little edema.
Recently, we reported a preliminary over-representation analysis of the main difference in the gene expression pattern between adipose mesenchymal stem cells and corneal endothelial cells to set a baseline for in vitro differentiation assays.103 In this study, we identified 195 highly different expressed genes and their related pathways, protein interactions, growth factors, and biological processes. These data provide a valuable tool for designing a more appropriate induction media.
Among the animal models used for adult stem cell transplantation into corneal endothelium, White New Zealand rabbit has demonstrated to be advantageous given its resemblance with human corneal endothelium. In a previous study, we demonstrated that rabbits older than 6 months possess limited replicative ability to restore corneal endothelium, making it a suitable model of the human cornea in endothelial wound healing studies.104
In order to be used as therapy for the regeneration of the corneal endothelium, bioengineered tissue needs to overcome the limits of obtaining functional cells in enough quantities for transplantation, development of better techniques of tissue engineering to grow an ex vivo endothelial cell sheet, and improve the mechanisms to slow the loss of endothelial cells following transplantation. Current results represent important progresses in the development of new strategies based on alternative sources of tissue for the treatment of corneal endotheliopathies.
Conclusion
The lack of donor tissue for corneal transplants makes endothelium regeneration a challenge for researchers. The recently discovered ability of corneal endothelial cells to proliferate in vitro has opened the possibility of regenerating the corneal endothelium through bioengineering.
Currently, research is aimed at identifying optimal conditions for the isolation and culture of corneal endothelial cells and the optimal biomaterials use as scaffolds in transplantation. Differentiation assays should be supported by generic expression studies in order to ensure specific cell functionality. The advances of animal models show promising results, allowing for recovery of cornea transparency almost entirely.
Moreover, mesenchymal stem cells obtained from umbilical cord blood and bone marrow have shown an ability to regenerate the endothelium in animal models. Recently, it was demonstrated that in adipose tissue stem cells are found in quantities greater than those found in bone marrow, providing a more accessible source of cells. Furthermore, the discovery of progenitor cells in the periphery of the cornea has also shown potential for their use in healing the endothelium. Thus, bioengineered corneal endothelium using cells from the same patient represent a potential new treatment to restore visual acuity in patients with critical reduced endothelial corneal density. This new treatment would eliminate the main problems of corneal transplantation: lack of donors, the possibility of immune reaction after the surgery, and post-surgical complications such as infection and development of glaucoma.
The authors declare no conflict of interest.
References
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37 (3:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Krachmer H, Manis J, Holland J.Cornea: Fundamentals, Diagnosis and Management2nd edn.Elsevier Mosby: Beijing, China; 2005 [Google Scholar]
- Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res. 2003;22 (1:69–94. doi: 10.1016/s1350-9462(02)00059-9. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002;43 (7:2152–2159. [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86 (1:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Tuft SJ, Coster DJ. The corneal endothelium. Eye. 1990;4 (Pt 3:389–424. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- Graw J.Eye developmentIn: Koopman Peter (ed).Current Topics in Developmental Biology Vol. 90Academic Press: San Diego, CA, USA; 2010343–386. [DOI] [PubMed] [Google Scholar]
- Fargo A, McDermott L, Soong K.Corneal anatomy, physiology, and wound healingIn: Yanoff M, Jay Duker (eds).Ophthalmology3rd edn.Mosby Elsevier: China; 2004203 [Google Scholar]
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40 (12:2996–3005. [PubMed] [Google Scholar]
- Collinson JM, Quinn JC, Hill RE, West JD. The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev Biol. 2003;255 (2:303–312. doi: 10.1016/s0012-1606(02)00095-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Wong-Chong J, SundarRaj N. FGF-2- and TGF-ß1-induced downregulation of lumican and keratocan in activated corneal keratocytes by JNK signaling pathway. Invest Ophthalmol Vis Sci. 2011;52 (12:8957–8964. doi: 10.1167/iovs.11-8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Kay EP. NF-KB is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Invest Ophthalmol Vis Sci. 2012;53 (3:1530–1538. doi: 10.1167/iovs.11-9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48 (8-9:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 2006;25 (22:5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N, Laks S, Nakashima M, Lax I, Schlessinger J. FRS2 family docking proteins with overlapping roles in activation of MAP kinase have distinct spatial-temporal patterns of expression of their transcripts. FEBS Lett. 2004;564 (1-2:14–18. doi: 10.1016/S0014-5793(04)00287-X. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley A, Robertson E, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207 (1:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, et al. Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol. 2005;4 (3:1. doi: 10.1186/jbiol29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms B, Labosky P. Transcriptional Control of Neural Crest Development. Morgan & Claypool Life Sciences: San Rafael, CA, USA; 2010. [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26 (4:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupé V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320 (1:140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Zacharias AL. Signaling "cross-talk" is integrated by transcription factors in the development of the anterior segment in the eye. Dev Dyn. 2009;238 (9:2149–2162. doi: 10.1002/dvdy.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott T, Johnson S, Bailey AP. Neural crest cells organize the eye via TGF-ß and canonical Wnt signalling. Nat Commun. 2011;2:265. doi: 10.1038/ncomms1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91 (3:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whikehart D.Corneal endothelium: OverviewIn: Dartt DarleneA (eds).Encyclopedia of the eye Academic Press: Oxford, UK; 2010424–434. [Google Scholar]
- Matt N, Dupé V, Garnier JM, Dennefeld C, Chambon P, Mark M, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132 (21:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Graue-Weichers L, Valdez-García J, Ramírez-Luquín T, Claros A. Densidad celular endotelial. Estudio en población general de la Ciudad de México. Rev Mex Oftalmol. 1989;63 (3:91–95. [Google Scholar]
- Wörner CH, Olguín A, Ruíz-García JL, Garzón-Jiménez N. Cell pattern in adult human corneal endothelium. PLoS One. 2011;6 (5:e19483. doi: 10.1371/journal.pone.0019483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38 (3:779–782. [PubMed] [Google Scholar]
- Yee RW, Matsuda M, Schultz RO, Edelhauser HF. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr Eye Res. 1985;4 (6:671–678. doi: 10.3109/02713688509017661. [DOI] [PubMed] [Google Scholar]
- Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95 (1:16–23. doi: 10.1016/j.exer.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DM. The location of the fluid pump in the cornea. J Physiol. 1972;221 (1:43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J, Maurice DM. An update on corneal hydration control. Exp Eye Res. 2004;78 (3:537–541. doi: 10.1016/j.exer.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Srinivas SP. Dynamic regulation of barrier integrity of the corneal endothelium. Optom Vis Sci. 2010;87 (4:E239–E254. doi: 10.1097/OPX.0b013e3181d39464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Zeuthen T, La Cour M, Nagelhus N, Ottersen O, Agre P, et al. Aquaporins in complex tissues: distribution of aquaporins 1-5 in human and rat eye. Am J Physiol. 1998;274 (5 pt 1:C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76 (2:137–143. doi: 10.1016/s0014-4835(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Biswas S, Munier FL, Yardely J, Hart-Holden N, Perveen R, Cousin P, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10 (21:2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell K, Durbeej M, Ghohestani R, et al. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci. 2007;48 (11:4989–4999. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puk O, Dalke C, Calzada-Wack J, Ahmad N, Klaften M, Wagner S, et al. Reduced corneal thickness and enlarged anterior chamber in a novel ColVIIIa2G257D mutant mouse. Invest Ophthalmol Vis Sci. 2009;50 (12:5653–5661. doi: 10.1167/iovs.09-3550. [DOI] [PubMed] [Google Scholar]
- Waring GO, Bourne WM, Edelhauser HF, Kenyon KR. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology. 1982;89 (6:531–590. [PubMed] [Google Scholar]
- Geroski DH, Matsuda M, Yee RW, Edelhauser HF. Pump function of the human corneal endothelium. Effects of age and cornea guttata. Ophthalmology. 1985;92 (6:759–763. doi: 10.1016/s0161-6420(85)33973-8. [DOI] [PubMed] [Google Scholar]
- Böhnke M, Vogelberg K, Engelmann K. Detection of neurone-specific enolase in long-term cultures of human corneal endothelium. Graefes Arch Clin Exp Ophthalmol. 1998;236 (7:522–526. doi: 10.1007/s004170050115. [DOI] [PubMed] [Google Scholar]
- Engelmann K, Bednarz J, Schäfer HJ, Friedl P. Isolation and characterization of a mouse monoclonal antibody against human corneal endothelial cells. Exp Eye Res. 2001;73 (1:9–16. doi: 10.1006/exer.2001.0993. [DOI] [PubMed] [Google Scholar]
- Yokoi T, Seko Y, Yokoi T, Makino H, HAtou S, Yamada N, et al. Establishment of functioning human corneal endothelial cell line with high growth potential. PloS One. 2012;7 (1:e29677. doi: 10.1371/journal.pone.0029677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Bullimore MA. Factors affecting corneal endothelial morphology. Cornea. 2007;26 (5:520–525. doi: 10.1097/ICO.0b013e318033a6da. [DOI] [PubMed] [Google Scholar]
- Polse KA, Brand RJ, Cohen SR, Guillon M. Hypoxic effects on corneal morphology and function. Invest Ophthalmol Vis Sci. 1990;31 (8:1542–1554. [PubMed] [Google Scholar]
- Corkidi G, Marquez J, Usisima R, Toledo R, Valdez J, Graue E. Automated in vivo and online morphometry of human corneal endothelium. Med Biol Eng Comput. 1993;31:432–437. doi: 10.1007/BF02446702. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kato S, Inoue Y, Amano S, Oshika T. The corneal endothelium and thickness in type II diabetes mellitus. Jpn J Ophthalmol. 2002;46 (1:65–69. doi: 10.1016/s0021-5155(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Bourne WM. Biology of the corneal endothelium in health and disease. Eye. 2003;17 (8:912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- Hatou S, Yamada M, Akune Y, Mochizuki H, Shiraishi A, Joko T, et al. Role of insulin in regulation of Na+-/K+-dependent ATPase activity and pump function in corneal endothelial cells. Invest. Ophthalmol Vis Sci. 2010;51 (8:3935–3942. doi: 10.1167/iovs.09-4027. [DOI] [PubMed] [Google Scholar]
- Eghrari AO, Gottsch JD. Fuchs' corneal dystrophy. Expert Rev Ophthalmol. 2010;5 (2:147–159. doi: 10.1586/eop.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L, Kelliher C, Jun AS. Endothelial keratoplasty: historical perspectives, current techniques, future directions. Can J Ophthalmol. 2009;44 (4:401–405. doi: 10.3129/i09-090. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya'ale D. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82 (11:844–851. [PMC free article] [PubMed] [Google Scholar]
- Treviño E, Durán F, Valdez J.IOVS 1993, Vol 34, ARVO Abstract, Program 11761993
- Valdez J, Treviño E, Durán F.IOVS 1993, Vol 34, ARVO Abstract, Program 20371993
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79 (3:214–221. [PMC free article] [PubMed] [Google Scholar]
- Vajpayee RB, Sharma N, Jhanji V, Titiyal JS, Tandon R. One donor cornea for 3 recipients: a new concept for corneal transplantation surgery. Arch Ophthalmol. 2007;125 (4:552–554. doi: 10.1001/archopht.125.4.552. [DOI] [PubMed] [Google Scholar]
- Lawlor M, Kerridge I. Anything but the eyes: culture, identity, and the selective refusal of corneal donation. Transplantation. 2011;92 (11:1188–1190. doi: 10.1097/TP.0b013e318235c817. [DOI] [PubMed] [Google Scholar]
- Valdez-García JE, Pauli A, Madrid-Valero G, Grawe-Weichers E. Análisis morfométrico automatizado del ojo contralateral en queratopatía bulosa pseudofáquica. Rev Mex Oftalmol. 2000;74 (6:267–270. [Google Scholar]
- Valdez-García JE, Graue-Weichers E, Márquez L, Rodríguez-Valdés C. Hallazgos morfométricos endoteliales en cirugía de catarata. Estudio comparativo extracpasular vs. intracapsular. Rev Mex Oftalmol. 2000;74 (4:169–172. [Google Scholar]
- Shao C, Fu Y, Lu W, Fan X. Bone marrow-derived endothelial progenitor cells: a promising therapeutic alternative for corneal endothelial dysfunction. Cells Tissues Organs. 2011;193 (4:253–263. doi: 10.1159/000319797. [DOI] [PubMed] [Google Scholar]
- Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7. doi: 10.1186/1750-1172-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswell R.CorneaIn: Riordan-Eva P, John P (eds).Vaughan & Asbury's General Ophtalmology McGraw Hill: USA; 20041–29. [Google Scholar]
- Allouch C, Borderie V, Touzeau O, Scheer S, Nordmann J, Laroche L. Incidence and factors influencing glaucoma after penetrating keratoplasty. J Fr Ophtalmol. 2003;26 (6:553–561. [PubMed] [Google Scholar]
- Valdez-García JE, Morales-Lozano J, González-González A, Madero-Frech A, Quintanilla-Dieck J. Resultados del transplante de córnea en pacientes con queratopatía bulosa. Rev Mex Oftalmol. 2005;79 (3:242–244. [Google Scholar]
- Engelmann K, Valtink M, Lindemann D, Nitschke M. Transplantation of corneal endothelium--chances and challenges. Klin Monbl Augenheilkd. 2001;228 (8:712–723. doi: 10.1055/s-0029-1245868. [DOI] [PubMed] [Google Scholar]
- Bertelmann E, Pleyer U, Rieck P. Risk factors for endothelial cell loss post-keratoplasty. Acta Ophthalmol Scand. 2006;84 (6:766–770. doi: 10.1111/j.1600-0420.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Proulx S, Brunette I. Methods being developed for preparation, delivery and transplantation of a tissue-engineered corneal endothelium. Exp Eye Res. 2011;95 (1:68–75. doi: 10.1016/j.exer.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Duan D, Klenkler BJ, Sheardown H. Progress in the development of a corneal replacement: keratoprostheses and tissue-engineered corneas. Expert Rev Med Devices. 2006;3 (1:59–72. doi: 10.1586/17434440.3.1.59. [DOI] [PubMed] [Google Scholar]
- Carlsson DJ, Li F, Shimmura S, Griffith M. Bioengineered corneas: how close are we. Curr Opin Ophthalmol. 2003;14 (4:192–197.. doi: 10.1097/00055735-200308000-00004. [DOI] [PubMed] [Google Scholar]
- Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006;47 (4:1387–1396. doi: 10.1167/iovs.05-1199. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23 (8 Suppl:S8–S19. doi: 10.1097/01.ico.0000136666.63870.18. [DOI] [PubMed] [Google Scholar]
- Konomi K, Zhu C, Harris D, Joyce NC. Comparison of the proliferative capacity of human corneal endothelial cells from the central and peripheral areas. Invest Ophthalmol Vis Sci. 2005;46 (11:4086–4091. doi: 10.1167/iovs.05-0245. [DOI] [PubMed] [Google Scholar]
- Peh GS, Toh KP, Wu FY, Tan DT, Mehta JS. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PloS One. 2011;6 (12:e28310. doi: 10.1371/journal.pone.0028310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Rawe I, Joyce NC. Differential protein expression in human corneal endothelial cells cultured from young and older donors. Mol Vis. 2008;14:1805–1814. [PMC free article] [PubMed] [Google Scholar]
- Peh GS, Lee MX, Wu FY, Toh K, Balehosur D, Mehta JS. Optimization of human corneal endothelial cells for culture: the removal of corneal stromal fibroblast contamination using magnetic cell separation. Int J Biomater. 2012;12:601302. doi: 10.1155/2012/601302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41 (10:2930–2935. [PubMed] [Google Scholar]
- Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: a morphologic study. Cornea. 2001;20 (7:731–737. doi: 10.1097/00003226-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, et al. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007;48 (2:614–620. doi: 10.1167/iovs.06-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarz J, Rodokanaki-von Schrenck A, Engelmann K. Different characteristics of endothelial cells from central and peripheral human cornea in primary culture and after subculture. In Vitro Cell Dev Biol Anim. 1998;34 (2:149–153. doi: 10.1007/s11626-998-0097-7. [DOI] [PubMed] [Google Scholar]
- Mimura T, Yamagami S, Yokoo S, Hayashida Y, Chen S, He H, et al. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest. Ophthalmol Vis Sci. 2004;45 (9:2992–2997. doi: 10.1167/iovs.03-1174. [DOI] [PubMed] [Google Scholar]
- Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci. 2004;45 (3:800–806. doi: 10.1167/iovs.03-0016. [DOI] [PubMed] [Google Scholar]
- Hadlock T, Singh S, Vacanti JP, McLaughlin BJ. Ocular cell monolayers cultured on biodegradable substrates. Tissue Eng. 1999;5 (3:187–196. doi: 10.1089/ten.1999.5.187. [DOI] [PubMed] [Google Scholar]
- Schwartzkopff J, Bredow L, Mahlenbrey S, Boehringer D, Reinhard T. Regeneration of corneal endothelium following complete endothelial cell loss in rat keratoplasty. Mol Vis. 2010;16:2368–2375. [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Williams JK, Greven M, Walter KA, Laber PW, Khang G, et al. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31 (26:6738–6745. doi: 10.1016/j.biomaterials.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Mohay J, Wood TO, McLaughlin BJ. Long-term evaluation of corneal endothelial cell transplantation. Trans Am Ophthalmol Soc. 1997;95:131–148. doi: 10.1016/0042-6989(95)90357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SV, Bachman LA, Hann CR, Bahler CK, Fautsch MP. Human corneal endothelial cell transplantation in a human ex vivo model. Invest Ophthalmol Vis Sci. 2009;50 (5:2123–2131. doi: 10.1167/iovs.08-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816–824. [PubMed] [Google Scholar]
- Yokoo S, Yamagami S, Yanagi Y, Uchida S, Mimura T, Usui T, et al. Human corneal endothelial cell precursors isolated by sphere-forming assay. Invest. Ophthalmol Vis Sci. 2005;46 (5:1626–1631. doi: 10.1167/iovs.04-1263. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Mimura T, Yokoo S, Takato T, Amano S. Isolation of human corneal endothelial cell precursors and construction of cell sheets by precursors. Cornea. 2006;25:S90–S92. doi: 10.1097/01.ico.0000247221.95424.d7. [DOI] [PubMed] [Google Scholar]
- Mimura T, Yokoo S, Araie M, Amano S, Yamagami S. Treatment of rabbit bullous keratopathy with precursors derived from cultured human corneal endothelium. Invest Ophthalmol Vis Sci. 2005;46 (10:3637–3644. doi: 10.1167/iovs.05-0462. [DOI] [PubMed] [Google Scholar]
- Mimura T, Yamagami S, Usui T, Seiichi, Honda N, Amano S. Necessary prone position time for human corneal endothelial precursor transplantation in a rabbit endothelial deficiency model. Curr Eye Res. 2007;32 (7-8:617–623. doi: 10.1080/02713680701530589. [DOI] [PubMed] [Google Scholar]
- De Miguel MP, Alio JL, Arnalich-Montiel F, Fuentes-Julian S, de Benito-Llopies L, Amparo F, et al. Cornea and ocular surface treatment. Curr Stem Cell Res Ther. 2010;5 (2:195–204. doi: 10.2174/157488810791268663. [DOI] [PubMed] [Google Scholar]
- Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9. doi: 10.1186/1756-9966-30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah BM, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J Cell Physiol. 2009;218 (1:9–12. doi: 10.1002/jcp.21572. [DOI] [PubMed] [Google Scholar]
- Martínez HR, Zavala-Arcos J, Moreno-Cuevas J, Gutiérrez-Alcalá J, González-Garza MT. XXXIV Reunión Anual de la Academia Mexicana de Neurología. Rev Mex Neurosci. 2010;11 (5:380–437. [Google Scholar]
- Lee RH, Kim B, Choi I, Kim H, Choi H, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14 (4-6:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- Liras A. Future research and therapeutic applications of human stem cells: general, regulatory, and bioethical aspects. J Transl Med. 2010;8:131. doi: 10.1186/1479-5876-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA. The adipose derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21 (11:1783–1787. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–4298. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang J, Liu CY, Wang I, Sieber M, Chang J, et al. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5 (5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Xing C, Han J, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- Du Y, Roh DS, Funderburgh ML, Mann M, Marra K, Rubin J, et al. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680–2689. [PMC free article] [PubMed] [Google Scholar]
- Valdez J, Zavala J, Trevino V, Martinez E.IOVS 2012, ARVO E-Abstract, Program 6008, 2012.
- Valdez JE, Oak SS, Laing RA.IOVS 1994, Vol 35, ARVO Abstract, Program 16041994