Abstract
Objective
Duloxetine hydrochloride is a selective serotonin (5-hydroxytryptamine) and norepinephrine reuptake inhibitor. It is approved for effective treatment for major depressive disorder. The pharmacokinetics (PK) of duloxetine has been studied, but few pharmacokinetics properties in Chinese subjects are available. This study explored the dose proportionality and determined duloxetine levels in human plasma by comparing the PK properties after administration of single or multiple doses in healthy volunteers.
Methods
Thirty-six subjects were divided randomly into three groups and received a single dose of 15, 30, or 60 mg duloxetine. Those who received 30 mg continued on to the multiple-dose phase and received 30 mg daily for 7 days. Liquid chromatography/mass spectroscopy was applied to determine concentrations. The PK properties were calculated and included maximum plasma concentration (Cmax), time when maximum plasma concentration was reached (Tmax), time when half-maximum plasma concentration was reached (t1/2), area under the plasma concentration-time curve from time zero to the last measurable concentration (AUC0-t), mean concentration levels (AUC0-∞), and apparent total clearance of the drug from plasma after oral administration (CL/F).
Results
The standard calibration curve was linear in the concentration range 0.11-112 ng/ml (r>0.992). Linear PK properties were found at doses of 15-60 mg. The Cmax and AUC were proportional to dose, but the Tmax and t1/2 did not increase with increasing dose.
Conclusion
No significant differences in the PK parameters were found among the three groups during the single-dose phase. The AUC and Cmax were greater in the multiple-dose phase, indicating duloxetine accumulation following multiple-dose administration.
Keywords: Duloxetine, Pharmacokinetics
INTRODUCTION
Duloxetine hydrochloride, (+)-(s)-N-methyl-(naphtyloxy)-2-thiophenepropylamine hydrochloride, is a selective serotonin (5-hydroxytryptamine [5-HT]) and norepinephrine (NE) reuptake inhibitor developed by Eli Lily Company.1) Its molecular formula is C18H19NOS · HCI, and the molecular weight is 333.88. Duloxetine enhances 5-HT and NE function in the central nervous system and is an effective treatment for major depressive disorder (MDD), pain, and stress urinary incontinence.2,3) Duloxetine has low in vitro affinity to dopaminergic, serotonergic, cholinergic, adrenergic, histaminergic, and γ-aminobutyrate receptors but does not inhibit monoamine oxidase.
Duloxetine is extensively metabolized hepatically by cytochrome P450 (CYP) isozymes 1A2 and 2D6.4,5) It has high affinity for plasma protein and is primarily conjugated with albumin and α1 acid glycoprotein.6) The main biological conversion pathway is combined naphthyl epoxidation and oxidation. The time when half-maximum plasma concentration was reached (t1/2) is about 12 hours (range, 8-17 hours) and the drug reaches a steady plasma drug concentration within 3 days after initial administration. Maximum plasma concentration (Cmax) is reached in 6 hours after oral administration, with no effect of food, but food can delay the peak time to 6-10 hours and decrease the degree of absorption by 10%. If duloxetine is administered at night, absorption lags about 3 hours compared with morning administration, and apparent clearance increases by one-third. The estimated apparent volume of distribution is 1,640 L.7,8) Various clinical trials have indicated that duloxetine is effective for treating MDD at doses of 40-120 mg daily. Common adverse effects are nausea, vomiting, dry mouth, drowsiness, decreased appetite, and increased sweating. No definite clinical data are available to indicate its effect on blood pressure, heart rate, or QT interval.
This study was designed to evaluate the pharmacokinetic (PK) properties and safety of duloxetine in Chinese healthy volunteers after single and multiple-dose administration.
METHODS
Experimental Methods
This single-center, open-label study was designed to determine the PK properties of duloxetine after single and multiple-dose administration. Thirty-six subjects were selected to participate from among 44 Chinese volunteers based on medical history, a physical examination, electrocardiogram (ECG), and clinical laboratory screening criteria assessments. The examinations ensured that the participants had no history of cardiovascular, gastrointestinal, respiratory, renal, endocrinal, psychiatric, or nervous system diseases. Subjects were excluded if they had substance or alcohol abuse problems or used any kind of drugs (including Chinese herbal drugs) during 1 week prior to the study.
This study protocol was reviewed and approved by the Institutional Review Board of Shanghai Mental Health Center. Written informed consent was obtained from all subjects prior to any study-related activity.
Study Design
The study included single and multiple-dose phases. Thirty-six subjects were randomly divided into low, middle, and high dose groups (n=12/group) for the single-dose phase, and duloxetine was administered orally once at 15, 30, and 60 mg.
All subjects were hospitalized the night before the study and fasted 10 hours before drug administration. All single-dose phase participants received a single dose of 15, 30, or 60 mg duloxetine with 200 ml water at 8:00 AM. Subjects assigned to the duloxetine middle group in the single-dose phase continued to the multiple-dose phase with an interval of 7 days and received 30 mg duloxetine daily for 7 days. Standard meals were provided at 8:30, 12:30, and 18:00, and calorie intake did not exceed 2,200 kcal. No strenuous physical activity was permitted during the 24-hour period after drug administration. Smoking and consumption of alcohol or caffeine-containing beverages were prohibited throughout the study.
In the single-dose phase, sequential blood samples (3 ml each) were collected from an indwelling venous catheter at 0, 1, 2, 4, 5, 6, 8, 12, 15, 24, 36, 48, and 72 hours after drug administration. Samples of venous blood for the multiple-dose phase were drawn before drug administration and on days 4, 5, and 6 to determine the minimum steady-state plasma drug concentration during a dosage interval (Cssmin). On day 7, blood samples were drawn at 0, 1, 2, 3, 4, 5, 6, 8, 12, 15, 24, 36, 48, and 60 hours after drug administration. All other experimental conditions were the same as those during the single-dose phase.
PK Analysis
Duloxetine plasma concentrations were detected by liquid chromatography/mass spectroscopy. Individual PK data were analyzed according to the mono-compartmental method. The PK parameters analysis was performed using the SAS 9.1.3 (SAS Institute Cary, NC, USA), DAS 3.0 (NCES, Alexandria, VA, USA), and Winnonlin statistics program (Pharsight, Phoenix, AZ, USA). The following PK parameters were determined for each subject in the single-dose phase: Cmax, time to maximum plasma concentration (Tmax), t1/2, area under the plasma concentration-time curve from time zero to the last measurable concentration (AUC0-t), mean concentration levels (AUC0-∞), apparent total clearance of the drug from plasma after oral administration (CL/F), and apparent volume of distribution (Vdβ/F). Cmax, Tmax, t1/2, AUC0-t, CL/F, and AUC0-t on day 7/day 1 were determined for each subject in the multiple-dose phase.
Tolerability Evaluation
Adverse events were elicited from the subjects by means of spontaneous reporting and specific questioning. Clinical laboratory tests (serum chemistry, hematology, and urinalysis), and physical examinations including vital signs and ECG, findings were recorded.
Statistical Analysis
Descriptive statistics were used to summarize the duloxetine PK parameters by dose. Untransformed and log-transformed data for Cmax and AUC were analyzed using an analysis of variance regression model to establish dose linearity and dose proportionality. The statistical tests were two-sided at the 0.05 level of significance. The t-test was used to assess gender differences. The 95% confidence intervals (CIs) for each difference were calculated.
RESULTS
Subjects
Thirty-six healthy volunteers were enrolled in the single-dose phase of the study. Their mean ages were 24.08±3.15, 24.83±2.62, and 22.92±3.26 years in the low, middle, and high dose groups, respectively. Mean body weights were 58.50±6.22, 57.88±7.81, and 59.08±6.00 kg, and heights were 165.42±5.98, 165.25±8.53, and 163.08±7.83 cm in the three groups, respectively.
Method Validation
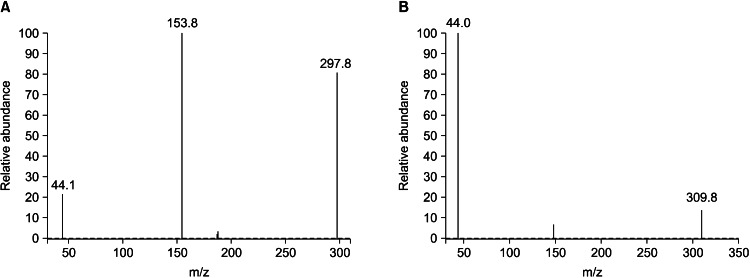
The chromatographic results are shown in Fig. 1. The duloxetine retention time was 4.3 minutes. No interference was observed at the duloxetine retention time.
Fig. 1.
The selective reaction detection of ion scan mass spectra of duloxetine and the internal standard, fuloxetine. (A) Product ion scan mass spectra of duloxetine. (B) Product ion scan mass spectra of fuloxetine.
The duloxetine standard calibration curve was linear (range, 0.11-112.00 ng/ml) (r>0.992). The limit of quantification was 0.11 ng/ml and was defined at a signal-to-noise ratio of >10. The methodological and extraction recoveries were 85-115%. The intra-day and inter-day relative standard deviations (RSDs) were <10%. Stability tests showed that the plasma sample and stock solutions were stable (RSD<10%; percent different <10%) after short-term storage (0, 4, 8, and 12 hours at 25℃ and 20 hours at 4℃), three freeze/thaw cycles (-20-25℃), and long-term storage (60 days at -20℃).
PKs
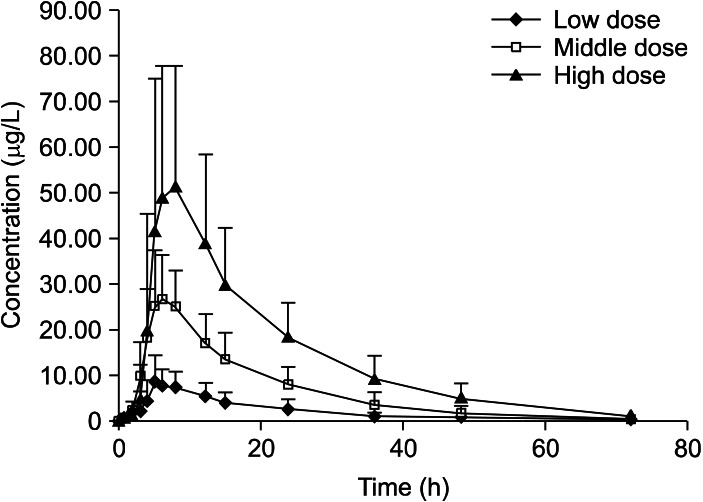
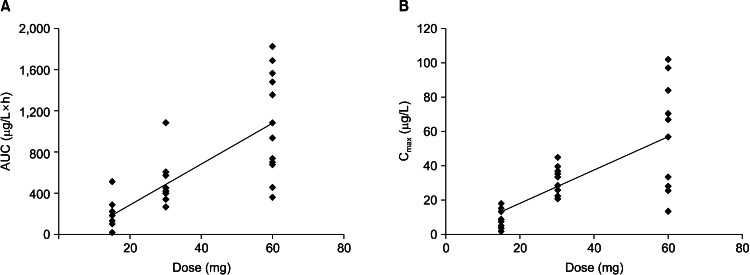
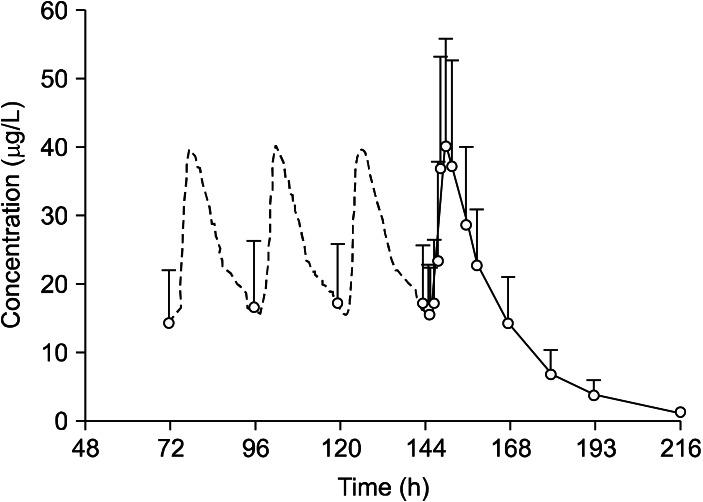
The mean plasma duloxetine concentration-time profiles after a single oral dose of 15, 30, or 60 mg are shown in Fig. 2, and the PK parameters are summarized in Table 1. No significant differences in the PK parameters were found among the three dose groups (t1/2: p=0.074; V1/F: p=0.150; CL/F: p=0.081; lag time [Tlag]: p=0.502). In the 15-60 mg range, Cmax and AUC were proportional to dose (r=0.771 and 0.730, respectively; p<0.001), and Tmax and t1/2 did not increase with increasing dose. Linear PK properties were found for duloxetine enteric-coated tablets at doses of 15-60 mg in vivo (Fig. 3). The slopes of the Cmax and AUC0-∞ plots were 0.98 and 19.90, and the 90% CIs of the slopes were 0.980-1.300 for Cmax and 19.900-25.638 for AUC0-∞ (p<0.000). Subjects in the middle dose group in the single-dose phase of the PK study continued to the multiple-dose phase of the study and received 30 mg duloxetine for 7 days. The PK agreed with the single-compartment model. Mean plasma duloxetine concentration-time profiles after multiple oral doses are shown in Fig. 4, and the PK parameters are summarized in Table 1. A steady-state concentration was achieved after administering duloxetine for 3 consecutive days. Mean plasma concentrations on days 4, 5, 6, and 7 before dosing were 13.95±8.10, 16.43±9.70, 17.09±9.00, and 17.21±8.50 ng/ml, respectively. No significant differences in PK parameters were observed between males and females (t1/2: p=0.821; Ke: p=0.144; V1/F: p=0.166; CL/F: p=0.247; AUC0-t: p=0.723; Ka: p=0.108; Tlag: p=0.427). Accumulation ratio (the AUC0-t on day 7/AUC0-t on day 1) was 1.7±0.4 (1.10±0.04 after logarithmic transformation).
Fig. 2.
Mean plasma concentration-time curve after a single oral dose of 15, 30 or 60 mg of duloxetine.
Table 1.
Pharmacokinetic parameters of duloxetine after a single dose including the 3 dose groups and multiple dosage in Chinese volunteers
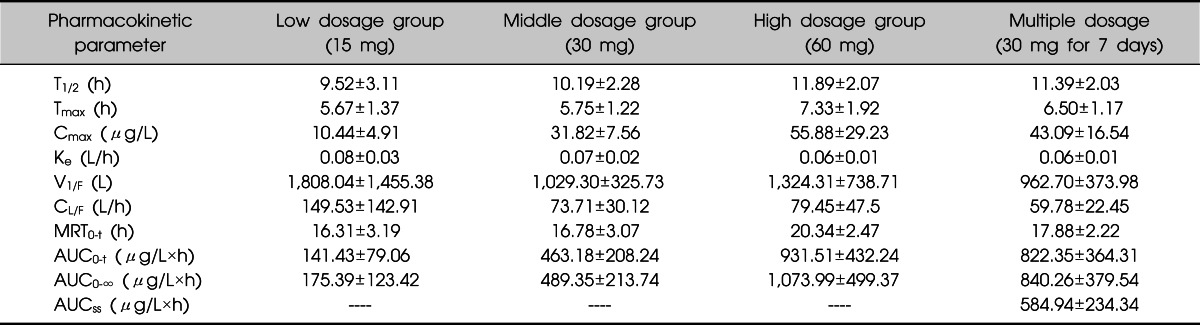
Values are presented as mean±standard deviation.
T1/2, elimination half-life; Tmax, time to Cmax; Cmax, maximum observed concentration; Ke, the rate of elimination; V1/F, initial volume of distribution; CL/F, oral system clearance; MRT0-t, the mean residence time of 0-120 min; AUC0-t, area under concentration time curve (AUC) from time zero to definite time; AUC0-∞, AUC from time zero to infinity; AUCss, AUC in steady-state.
Fig. 3.
Analyses of dose linearity with duloxetine. (A) Area under the plasma concentration time curve (AUC)0-∞/dose. (B) Maximum plasma concentration (Cmax)/dose.
Fig. 4.
Mean plasma concentration-time curve after multiple dose of 30 mg of duloxetine.
Tolerability
No serious or unexpected adverse events were reported during the PK study. The incidences of adverse events during the single-dose phase were 33.3% (4/12), 41.7% (5/12), and 66.7% (8/12) in the three dose groups, respectively, and the most common adverse event related to duloxetine was nausea (12/36; 33.3%), followed by dizziness (6/36; 16.7%) and decreased appetite (3/36; 8.3%). A tendency toward an increased incidence of adverse events was observed in the 60 mg dose group, but no difference was found among the three groups (p=0.250). We also observed a case of hypertension in the 60 mg dose group and considered that the drug caused the increase, so we terminated the subject from the study. The most common adverse events in the multiple-dose phase were nausea (6/12; 50%) and dizziness (2/12; 16.7%). All adverse events were mild or moderate in intensity and disappeared after discontinuation of duloxetine. The results of vital signs, ECG, clinical laboratory assessments, and physical examinations were within normal limits for all subjects, and no clinically significant differences were detected among the groups during the study.
DISCUSSION
Duloxetine was approved for treating adults with MDD in 2004 and is also used to treat pain related to diabetic peripheral neuropathy and stress urinary incontinence. The primary purpose of this study was to assess the safety, tolerability, and PK of duloxetine after administration of single and multiple oral doses in Chinese healthy volunteers.
The 15, 30, and 60 mg oral doses were used in the single-dose phase and agreed with the one-compartment PK model. Serum duloxetine Cmax and AUC increased linearly after administration. A steady state was reached after administering duloxetine for 3 consecutive days in the multiple-dose phase, in agreement with the one-compartment PK model. No differences in Tmax or t1/2 values were observed between the single- and multiple-dose phases. The AUC in steady-state (AUCss) was approximately 1.7 times that of the single-dose phase, indicating an accumulation of duloxetine after multiple-dose administration.
The duloxetine doses used in this study were clinically recommended doses, gender had no significant effect on the PK parameters, and the results agreed with those from another report.9) The main PK results were consistent with a study conducted by Zhao et al.10) in Chinese healthy volunteers. Chan et al.11) compared the single- and multiple-dose PK of duloxetine between healthy Japanese and Caucasians and found no significant difference in Cmax, AUC, or t1/2. Our results were similar to those of the Japanese, suggesting that the PK properties of duloxetine are similar between races.
Duloxetine is extensively metabolized in the liver by CYP1A2 and CYP2D6.5) The variability in AUC and Cmax values may due to different CYP1A2 and CYP2D6 isozyme phenotypes.11) The AUC and Cmax coefficients of variation ranged from 42.24% to 51.32% and from 23.76% to 52.30%, respectively, in this study; however, differences in CYP1A2 and CYP2D6 activity are difficult to detect in a clinical study. The coefficient of variation also indicates the importance of monitoring blood concentrations and preparing individualized doses to ensure safety and effectiveness.
In conclusion, duloxetine administered orally to Chinese healthy volunteers at 15-60 mg was safe and well tolerated. The PK properties of duloxetine were linear. The AUC and Cmax were higher in the multiple-dose phase than in the single-dose phase, indicating that duloxetine accumulated following multiple-dose administration of 30 mg. Gender had no significant effect on the PK parameters.
Acknowledgments
This study was supported by National Major Project for IND, Clinical tech-platform for evaluation of new drug in psychiatry (No. 2012ZX09303-003) and Shanghai Health talent professional project (No. XBR2011049).
References
- 1.Westanmo AD, Gayken J, Haight R. Duloxetine: a balanced and selective norepinephrine- and serotonin-reuptake inhibitor. Am J Health Syst Pharm. 2005;62:2481–2490. doi: 10.2146/ajhp050006. [DOI] [PubMed] [Google Scholar]
- 2.Waitekus AB, Kirkpatrick P. Duloxetine hydrochloride. Nat Rev Drug Discov. 2004;3:907–908. doi: 10.1038/nrd1564. [DOI] [PubMed] [Google Scholar]
- 3.Cruz MP, Gonzales ME, Jacobs J, LaFave MC. Duloxetine HCI (Cymbalta) for the treatment of depression, neuropathic pain, fibromyalgia, and stress urinary incontinence. Drug Forecast. 2006;31:84–97. [Google Scholar]
- 4.Skinner MH, Kuan HY, Pan A, Sathirakul K, Knadler MP, Gonzales CR, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther. 2003;73:170–177. doi: 10.1067/mcp.2003.28. [DOI] [PubMed] [Google Scholar]
- 5.Lantz RJ, Gillespie TA, Rash TJ, Kuo F, Skinner M, Kuan HY, et al. Metabolism, excretion, and pharmacokinetics of duloxetine in healthy human subjects. Drug Metab Dispos. 2003;31:1142–1150. doi: 10.1124/dmd.31.9.1142. [DOI] [PubMed] [Google Scholar]
- 6.Bymaster FP, Beedle EE, Findlay J, Gallagher PT, Krushinski JH, Mitchell S, et al. Duloxetine (Cymbalta), a dual inhibitor of serotonin and norepinephrine reuptake. Bioorg Med Chem Lett. 2003;13:4477–4480. doi: 10.1016/j.bmcl.2003.08.079. [DOI] [PubMed] [Google Scholar]
- 7.Eli Lilly and Co. Cymbalta (duloxetine) product information. [cited 2012 Jul]. Available from http://pi.lilly.com/us/cymbalta-pi.pdf.
- 8.Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol. 2000;40:161–167. doi: 10.1177/00912700022008810. [DOI] [PubMed] [Google Scholar]
- 9.Guo XF, Zhao JP, Chen JD. Duloxetine: a novel antidepressant. Chin J New Drugs Clin Rem. 2006;25:552–555. [Google Scholar]
- 10.Zhao RK, Cheng G, Tang J, Song J, Peng WX. Pharmacokinetics of duloxetine hydrochloride enteric-coated tablets in healthy Chinese volunteers: a randomized, open-label, single- and multiple-dose study. Clin Ther. 2009;31:1022–1036. doi: 10.1016/j.clinthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Chan C, Yeo KP, Pan AX, Lim M, Knadler MP, Small DS. Duloxetine pharmacokinetics are similar in Japanese and Caucasian subjects. Br J Clin Pharmacol. 2007;63:310–314. doi: 10.1111/j.1365-2125.2006.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]