Abstract
A systematic review of the effectiveness of rehabilitation for persons with unilateral neglect (UN) after stroke was conducted by searching the computerized databases from 1997 through 2012. Randomized controlled trials (RCTs) of neglect treatment strategies for stroke patients which used the Behavioral Inattention Test (BIT) as the primary outcome measure were eligible for inclusion. Out of 201 studies initially identified, 12 RCTs covering 277 participants were selected for analysis. All had the same weakness of low power with smaller samples and limitation in the blinding of the design. Prism Adaptation (PA) was the most commonly used intervention while continuous Theta-burst stimulation (cTBS) appeared to be a new approach. Meta-analysis showed that for immediate effects, the BIT conventional subscore had a significant and large mean effect size (ES = 0.76; 95% CI 0.28–1.23; p = 0.002) whereas the BIT total score showed a modestly significant mean ES (ES = 0.55; 95% CI 0.16–0.94; p = 0.006). No significant mean ES in sensitivity analysis was found for long-lasting effects across all BIT outcomes. PA appeared to be the most effective intervention based on the results of pooled analysis. More rigorous studies should be done on repetitive transcranial magnetic stimulation (rTMS) before it can be concluded that it is a promising treatment for UN.
Keywords: systematic review, stroke, unilateral neglect, rehabilitation, Behavioral Inattention Test
Introduction
Unilateral neglect (UN) is a heterogeneous perceptual disorder that often follows stroke, especially after right hemisphere lesion. Its most typical feature is failure to report or respond to stimuli presented from the contralateral space, including visual, somatosensory, auditory, and kinesthetic sources. Sufferers may even fail to perceive their own body parts (Mesulam, 1999). The reported incidence varies from 10 to 82% following right- and from 15 to 65% following left-hemisphere stroke (Plummer et al., 2003). Subject selection criteria, lesion site, the nature and timing of the assessment, and lack of agreement on assessment methods are all responsible for the variability in these reported rates (Stone et al., 1991; Azouvi et al., 2002). UN has a significant negative impact associated with functional recovery at home discharge (Jehkonen et al., 2006; Mutai et al., 2012).
Different treatment approaches and assessment tools have been developed to evaluate and address UN. The most recent literature shows that rehabilitation can be classified under two types of behavioral approaches: recruiting the hemiplegic limbs to reduce spatial preference for the ipsilesional space, or improving awareness of the contralesional space to promote patients’ attention (Pierce and Buxbaum, 2002; Paci et al., 2010). More than 18 methods using these general approaches have been put into practice (Luauté et al., 2006) with varying results based on a large number of outcome measures. Although the reported quality is moderate for most of the RCTs in neglect rehabilitation (Paci et al., 2010), some interventions appear to be more promising. Comments have also been made that the effects of treatment are often task-specific or transient and cannot be generalized to daily functioning (Pierce and Buxbaum, 2002; Bowen et al., 2007). Due to a lack of evidence, it is also hard to report which approach is the optimal recommendation for clinical practice (Luauté et al., 2006), and interestingly, professional therapists rarely use these scientifically proven interventions (Petzold et al., 2012).
Many RCTs have employed “pencil-and-paper” tasks, including line bisection, cancelation tasks, copying, and drawing, as treatment outcomes for UN. One of the commonest tests, and one that has been used extensively as an outcome measure for UN, is the Behavioral Inattention Test (BIT) (Bowen et al., 1999, 2007). This is a criterion-referenced test for UN or visual inattention in patients suffering from stroke or brain injuries, comprising two parts: the conventional and the behavioral subtests (Halligan et al., 1991). The conventional subtests include six traditional paper-and-pencil tasks: line crossing, letter cancelation, star cancelation, figure copying, line bisection, and representative drawing. The behavioral subtests consist of nine simulated daily living tasks: picture scanning, telephone dialing, menu reading, article reading, telling and setting the time, coin sorting, address and sentence copying, map navigation, and card sorting. Both parts can be used separately in clinical for impairment and function level assessments, and it has been recommended as a good predictor of functional performance in daily living with good construct and predictive validity (Hartmanmaeir and Katz, 1995).
The aim of this study was to develop a systematic review to assess the effectiveness of rehabilitation for UN as measured by the BIT and to evaluate the effects of the interventions reported in the RCTs using a meta-analysis.
Methods
Database
We searched the following electronic databases for trials published in English; PubMed/Medline (1965+ via EbscoHost), PsycINFO (1806+), physiotherapy evidence database (PEDro), Science Direct, CINAHL (Cumulative Index to Nursing and Allied Health Literature, 1982+), and Cochrane Central Register of Controlled Trials (CENTRAL). We also hand-searched the bibliographies of all studies ordered in full text. Date of publication was limited from January 1997 to June 2012 as most of the full-text electronic versions of journal papers are available since 1997.
The terms used in the search were: cerebrovascular accident OR stroke; neglect; visuo-spatial neglect; visual neglect; unilateral neglect; and hemisphere neglect. The search was limited to RCTs involving adults aged 19 or over.
Selection criteria
We included all RCTs that sought to identify the effectiveness of any type of rehabilitation intervention in UN in adult stroke patients diagnosed by clinical examination and/or classical neuropsychological tests. Only studies which reported the BIT (Wilson et al., 1987) as the primary outcome measure were included. The BIT includes a score for the conventional subtest (BIT-C) and/or the behavioral subtest (BIT-B) as well as the total score [BIT (Total)].
We excluded observational studies and case reports as well as cross-over design studies; studies where full text was not available; studies with a sample size of less than five in each group; and those rated as 4 or less out of 10 by the PEDro in the quality assessment described below. Cross-over design studies were excluded in our review as they usually confounded the estimates of the treatment effects with carry-over and learning effects (Leslie and Mary, 2007).
Quality assessment
After the database search, two reviewers assessed the methodological quality of the trials according to the PEDro scale. This was developed specifically for evaluating the quality of studies aiming to compare the effectiveness of rehabilitation (Verhagen et al., 1998; Sherrington et al., 2000) and has been proved to be valid in measuring the methodological quality of clinical trials. There are 11 items in the PEDro scale. The first criterion, item eligibility, is not scored as it is used as a component of external validity; the remaining items yield a total score from 10 (RCT that meets all items) to 0 (RCT that does not meet any item) (Paci et al., 2010). The PEDro scale item scores can be summed to obtain a total score that can be used as interval data for parametric statistical analysis (Bhogal et al., 2005; de Morton, 2009). The PEDro scale classifies studies as high or low quality based on a cut-off score of six (Maher et al., 2003). Articles scoring six or higher are considered of high quality and low-quality studies score less than six.
Data extraction and analysis
Each selected study was carefully assessed against the inclusion criteria, and the necessary information and characteristics summarized in a table. We calculated Cohen’s d on individual treatment effect size (ES) for these studies and compared the effectiveness among different interventions. Meta-analysis on overall treatment effectiveness was done with Review Manager Version 5.0 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2012). The standardized mean difference (SMD) was presented as the ES and its 95% confidence interval (CI) computed. Because of the heterogeneity of the interventions, we could only perform a pooling for meta-analysis for a single intervention reported in two or more trials. The test of heterogeneity was used to assess the potential heterogeneity across studies. If heterogeneity existed, a random-effect model was used. The random-effect approach assumes that the ES from each trial is a random sample from a larger population of possible ES. Otherwise, the fixed-effect model was used. A sensitivity analysis was also used to assess the impact of overall treatment effectiveness by excluding each trial once at a time.
Results
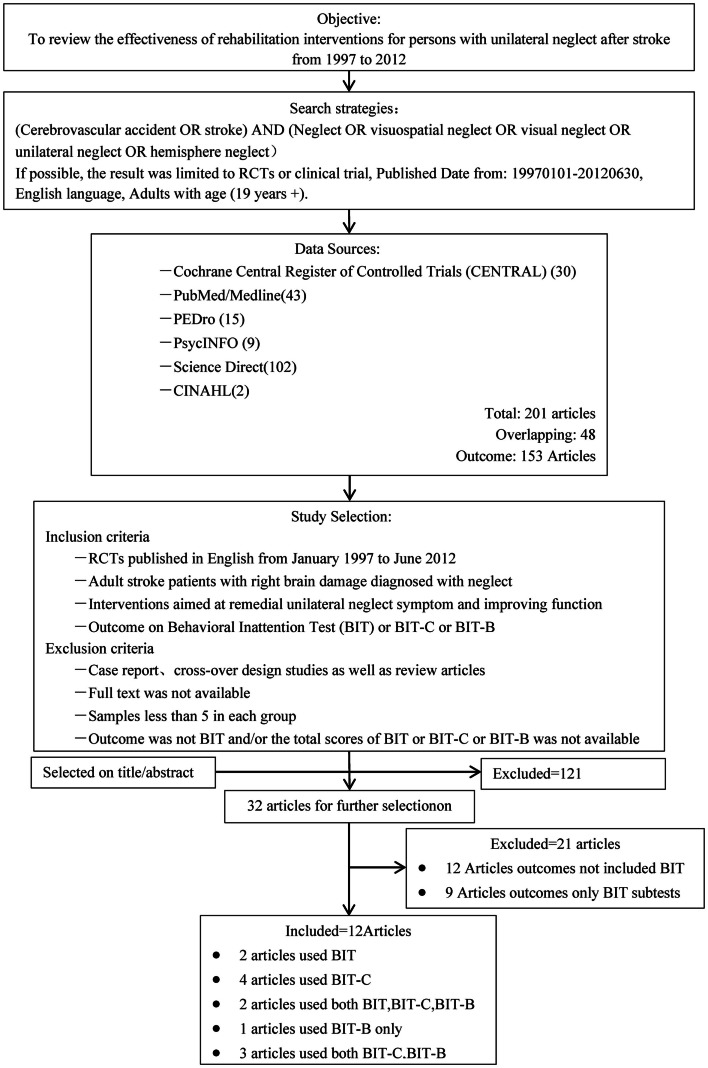
Figure 1 illustrates the selection process. The initial search yielded 201 citations from January 1997 through June 2012. After removing duplicates, 153 citations remained. Based on the title and abstract of the articles, 32 potentially relevant articles were selected. After careful evaluation by the reviewers, we identified 25 clinical trials (Wiart et al., 1997; Robertson et al., 2002; Harvey et al., 2003; Pizzamiglio et al., 2004; Katz et al., 2005; Fong et al., 2007; Nys et al., 2008; Schroder et al., 2008; Ertekin et al., 2009; Luukkainen-Markkula et al., 2009; Polanowska et al., 2009; Serino et al., 2009; Song et al., 2009; Tsang et al., 2009; Saevarsson et al., 2010; Turton et al., 2010; Ferreira et al., 2011; Kamada et al., 2011; Kim et al., 2011; Làdavas et al., 2011; Mizuno et al., 2011; Welfringer et al., 2011; Gorgoraptis et al., 2012; Ianes et al., 2012; Koch et al., 2012) to be included in the final assessment. Of these, 12 articles were included in our final review (Robertson et al., 2002; Harvey et al., 2003; Fong et al., 2007; Nys et al., 2008; Luukkainen-Markkula et al., 2009; Serino et al., 2009; Tsang et al., 2009; Turton et al., 2010; Ferreira et al., 2011; Làdavas et al., 2011; Mizuno et al., 2011; Koch et al., 2012) with the others excluded because the BIT was not used as the primary outcome measure.
Figure 1.
Overview of the search and selection process.
The quality of all 12 RCTs was fair to good (Table 1). Four (33.3%) were identified as of fair quality as their scores were below six in the scale. Two studies (Mizuno et al., 2011; Koch et al., 2012) used double-blind designs whereas others were mostly single-blind.
Table 1.
PEDro scores of included studies.
| Studies | Eligibility | 1, Random allocation | 2, Concealed allocation | 3, Baseline comparability | 4, Blind subjects | 5, Blind therapists | 6, Blind assessors | 7, Adequate follow-up | 8, Intention-to-treat analysis | 9, Between-group comparisons | 10, Point estimates variability | Score | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITEMS | |||||||||||||
| Nys et al. (2008) | Yes | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 6/10 | Good |
| Serino et al. (2009) | Yes | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 5/10 | Fair |
| Turton et al. (2010) | Yes | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6/10 | Good |
| Mizuno et al. (2011) | Yes | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 8/10 | Good |
| Làdavas et al. (2011) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 6/10 | Good |
| Robertson et al. (2002) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6/10 | Good |
| Luukkainen-Markkula et al. (2009) | Yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 5/10 | Fair |
| Fong et al. (2007) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6/10 | Good |
| Tsang et al., 2009 | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6/10 | Good |
| Harvey et al. (2003) | Yes | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5/10 | Fair |
| Koch et al. (2012) | Yes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9/10 | Good |
| Ferreira et al. (2011) | No | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5/10 | Fair |
Characteristics of the studies
Descriptions of the 12 articles reviewed are listed in Table 2. A total of 277 subjects with UN were included in this analysis. All were adults with right brain damage due to stroke; most had a diagnosis of first single right hemisphere stroke. The duration from stroke onset to study covered the period from the acute (≤4 weeks) to the chronic phase (≥6 months), but most studies were conducted in the subacute and chronic phases after stroke. All studies used similar selection criteria.
Table 2.
Characteristics of included studies.
| Studies | Methods |
Interventions |
BIT results |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Study design | Control | Groups subjects (n) | Duration from onset to treatment | Treatment | Regime | Duration | Immediate | Long-term | |
| Nys et al. (2008) | PA | single-blind RCT | Placebo (neutral goggles) | n = 16 PA gp = 10 CT gp = 6 | ≤4 weeks | Wore pair of goggles fitted with wide-field point-to-point prismatic lenses shifted their visual field 10°/0° rightward and do some fast pointing movements | 30 min/session 4-days-in-row sessions | 4 days | BIT-C (−); BIT-B (−); follow-up = 1 month | |
| Serino et al. (2009) | PA | single-blind pseudo-RCT | Placebo (neutral goggles) | n = 20 PA gp = 10 CT gp = 10 | ≥1 month | Wore prismatic lenses, which shifted their visual field 10°/0°rightward and pointing movements | 30 min/Session 10 daily sessions within 2 weeks | 2 weeks | BIT (+) | BIT (+); follow-up = 1 month |
| Turton et al. (2010) | PA | single-blind RCT | Placebo (flat plain glass) | n = 36 PA gp = 17 CT gp = 19 (1 drop-out) (1 drop-out) | ≥20 days | Wore 10 diopter, 6 degree prisms using index finger to touch a bold vertical line on screen | Once a day, each working day | 2 weeks | BIT (−) | BIT (−); follow-up = 8 weeks |
| Mizuno et al. (2011) | PA | double-masked RCT | Placebo (neutral glasses) | n = 38 PA gp = 18 CT gp = 20 | ≤3 months | Wore prism glasses shifted visual field 12° to right and repeat pointing tasks | 20 min/Session bid, 5 days/week | 2 weeks | BIT-C (−); BIT-B (−) | BIT-C (−); BIT-B (−); follow-up until discharge |
| Làdavas et al. (2011) | PA | single-blind pseudo-RCT | Placebo (neutral glasses) | n = 30 TPA gp = 10 CPA gp = 10 CT gp = 10 | ≥2 months | Wore wide-field prismatic lenses inducing a 10° shift visual field to right and repeat pointing tasks | 30 min/Session one per day, 10 sessions | 2 weeks | TPA:BIT-B (+); BIT-C (+); CPA:BIT-C (−); BIT-B (−) | No follow-up |
| Robertson et al. (2002) | LA | single-blind RCT | Dummy device | n = 40 LA + PT = 19 (2 drop-out) PT = 21 (2 drop-out) | LA: 152.8 ± 142.4 PT: 152.1 ± 117.9 | Using a semi-automatic device for limb activation combined with perceptual training | 45 min/Session once a week 12 sessions | 12 weeks | BIT-B (−) | BIT-B (−); follow-up = 18–24 months |
| Luukkainen-Markkula et al. (2009) | LA | single-blind RCT | Conventional visual scanning training | n = 12 LA gp = 6 CT gp = 6 | ≤6 months | Arm activation training (determined by the individual hand and arm motor status assessed by WMFT) | Total 48 h of therapy | 3 weeks | BIT-C (+) | BIT-C (+) follow-up = 6 months |
| Fong et al. (2007) | TRTR + EP | single-blind RCT | Conventional OT | n = 54 TR gp = 19 TR + EP gp = 20 CT gp = 15 | ≤8 weeks | Trunk rotation was performed in three different positions: supine lying on a plinth, unsupported sitting on a plinth, and standing in a standing frame | 1 h/Session 5 times/week | 30 days | BIT-B (−); BIT-C (−); BIT (−) | BIT-B (−); BIT-C (−); BIT (−); follow-up = 60 days |
| Tsang et al. (2009) | EP | single-blind RCT | Conventional OT | n = 34 EP gp = 17 CT: 22.18 ± 15.87 | EP: 21.5 ± 21.67 | Underwent occupational therapy with special glasses blocking the right half visual field | 30 min ADL +30 min NDT for UL/day | 4 weeks | BIT-C (+) | No follow-up |
| CT gp = 17 | ||||||||||
| Harvey et al. (2003) | VF | RCT | Same activities but without feedback | n = 14 VF gp = 7 CT gp = 7 | 5–25 months | Experimenter-administered practice of rod lifting with judge center grids for proprioceptive and visual feedback | 1 h/day with 3 days, then 10 days of home-based intervention | 3 days/2 weeks | BIT-C (+); BIT-B (−) | BIT-C (+); BIT-B (−); follow-up = 1 month |
| Koch et al. (2012) | TBS | double-blind RCT | Sham coil angled 90° | n = 18 TBS gp = 9 CT gp = 9 | ≥1 months (43 ± 16 days) | 3-pulse bursts at 50 Hz repeated every 200 ms for 40 s, 80% AMT over the left PPC | 2 Sessions/day, 15 min interval; 5 days/week | 2 weeks | BIT-B (+); BIT-C (+); BIT (+) | BIT-B (+); BIT-C (+); BIT (+); follow-up = 1 month |
| Ferreira et al. (2011) | MP VST | single-blind RCT | Conventional PT without any treatment for neglect | n = 15 MP gp = 5 VST gp = 5 CT gp = 5 | ≥3 months | VS: the protocol included 4 tasks: 2 directed to the extrapersonal space and 2 addressing peripersonal neglect; MP: included 4 tasks: 2 tasks of motor imagery and 2 of visual imagery | 1 h/Session twice per week | 5 weeks | VST: BIT-C (+); MP: BIT-C (−) | VST: BIT-C (+); MP:BIT-C (−) follow-up = 2 months |
PA, prism adaptation; LA, limb activation; TR, trunk rotation; EP, eye patching; VF, visuomotor feedback; TBS, theta-burst stimulation; MP, mental practice; VST, visual scanning training; BIT (Total), total score on Behavioral Inattention Test; BIT-C, BIT conventional subtest; BIT-B, BIT behavioral subtest; OT, occupational therapy; PT, physiotherapy.
Among the 12 studies, 5 (Nys et al., 2008; Serino et al., 2009; Làdavas et al., 2011; Mizuno et al., 2011) studied the effectiveness of prism adaptation (PA). There were differences in the PA procedure used; one study (Nys et al., 2008) used repetitive PA for a short period while another used different feedback strategies in PA (terminal and concurrent prism adaptation). During terminal PA, only the final part of the pointing movement is visible and PA relies most strongly on a strategic recalibration of visuomotor eye–hand (Làdavas et al., 2011). In contrast, in concurrent PA the second half of the pointing movement is visible, and thus adaptation mainly consists of a realignment of proprioceptive coordinates (Làdavas et al., 2011). All five studies used the same control methods with neutral goggles. Two articles (Robertson et al., 2002; Luukkainen-Markkula et al., 2009) applied limb activation. Other studies used different interventions; visuomotor feedback, virtual reality, repetitive transcranial magnetic stimulation (rTMS), and continuous Theta-burst stimulation (cTBS). Compared to a previous review (Luauté et al., 2006), no new intervention was reported in our review during the time period stated except for cTBS. All studies investigated a single treatment, except for one RCT (Fong et al., 2007) which investigated the effectiveness of a combination of two different methods, namely trunk rotation and eye patching.
The duration of treatment ranged from 4 days (Nys et al., 2008) to 5 weeks (Ferreira et al., 2011), but for half of the studies was 30 min per session for 5 sessions per week over 2 weeks, giving a total of 10 sessions. All the trials were conducted in hospitals except for one (Harvey et al., 2003) which involved self-administered home-based practice for 2 weeks.
Apart from the BIT, the outcome for neglect severity included the Catherine Bergego Scale (CBS), the Bell Cancelation Test, reading, computerized visual search tasks, and paper-and-pencil neglect tests. In all studies, functional outcomes were included, namely the Functional Independence Measure, the Barthel Index, upper limb motor functions (the Wolf Motor Function Test and the Modified Motor Assessment Scale), and the Stroke Impairment Assessment Set.
Three studies (Serino et al., 2009; Turton et al., 2010; Ferreira et al., 2011) used the BIT (Total) only; three (Nys et al., 2008; Làdavas et al., 2011; Mizuno et al., 2011) used both the BIT-C and the BIT-B separately as outcomes; and two (Fong et al., 2007; Koch et al., 2012) used the BIT (Total) and both the BIT-C and BIT-B as outcomes. Only one study (Robertson et al., 2002) used only the BIT-B as the outcome.
Effects of rehabilitation interventions
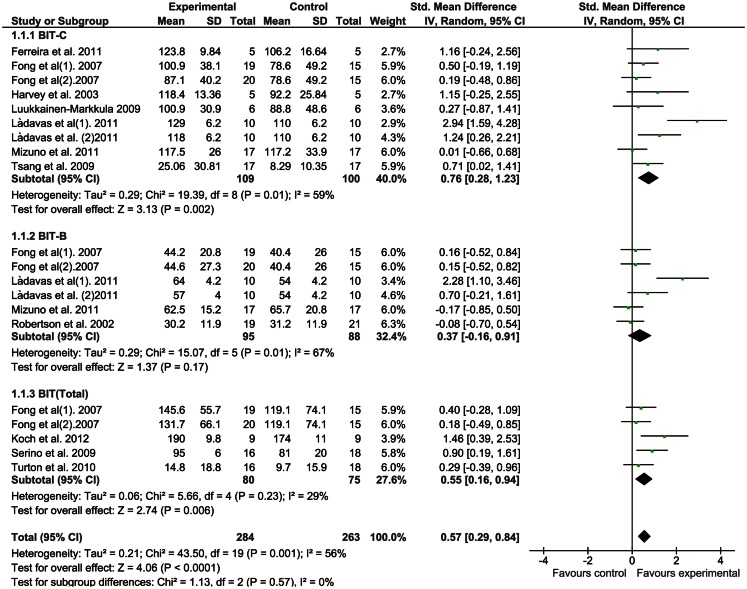
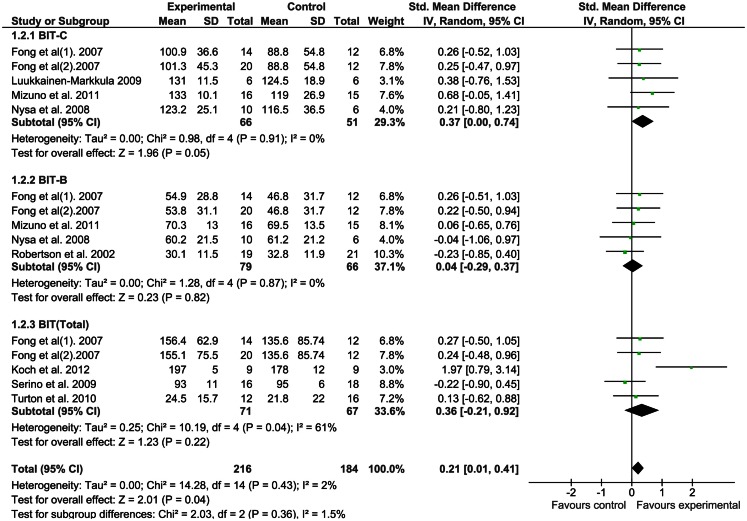
We applied a meta-analysis on all outcomes to calculate SMD and 95% CI using random-effects models. A comparison of the results of both the immediate and long-lasting effects is presented in forest plots (Figures 2 and 3).
Figure 2.
Rehabilitation interventions versus any control, outcome: immediate effects.
Figure 3.
Rehabilitation interventions versus any control, outcome: long-lasting effects.
Immediate effects of interventions
Figure 2 shows the forest plot of the immediate effects of the interventions covered in the 12 studies. The meta-analysis shows that there was significant heterogeneity across the studies, so the random-effect model was chosen. The BIT-C had a significant mean ES of 0.76 (95% CI, 0.28–1.23; p = 0.002). The BIT-B showed an insignificant mean ES of 0.37 (95% CI, −0.19 to 0.91; p = 0.17), and the BIT (Total) a statistically significant mean ES of 0.55 (95% CI, 0.16–0.94; p = 0.006). The sensitivity of each trial on the mean ES was also assessed by excluding each trial one at a time. The overall results were the same even when any single trial was eliminated.
Long-lasting effects of rehabilitation interventions
Figure 3 shows the forest plot of the long-lasting effects of the interventions studied. The meta-analysis shows that none of the ES were significant for the BIT outcomes except the BIT-C (p = 0.05). The sensitivity of each trial on the mean ES was also evaluated by excluding one trial at a time, but the results were not significant (p > 0.05).
To find out the optimal intervention for UN, Cohen’s d was calculated on the individual ES of each approach as the difference between the pre- and posttest means for the single treatment group divided by the SD of the pretest scores. There was more than one paper covering PA, so we pooled the ES of PA in three studies for the BIT-C, two for the BIT-B, and two for the BIT (Total) before conducting a relative comparison of the ES of all studies. The results showed that for immediate effects, after pooling, PA had the highest ES as measured by the BIT-C and the BIT-B, while cTBS had the highest ES measured by the BIT (Total). All interventions showed low ES for long-lasting effects (Tables 3 and 4).
Table 3.
Immediate effect size of each rehabilitation intervention.
| Outcomes | Study | Intervention | Effect size |
|---|---|---|---|
| BIT-C | Làdavas et al. (2011) (1) | PA | 1.31 (−0.26, 2.88) (pooled) |
| Làdavas et al. (2011) (2) | |||
| Mizuno et al. (2011) | |||
| Ferreira et al. (2011) | VST | 1.16 (−0.24, 2.56) | |
| Harvey et al. (2003) | VF | 1.15 (−0.25, 2.55) | |
| Tsang et al. (2009) | EP | 0.71 (0.02, 1.41) | |
| Fong et al. (2007) (1) | TR | 0.50 (−0.19, 1.19) | |
| Luukkainen-Markkula et al. (2009) | LA | 0.27 (−0.87, 1.41) | |
| Fong et al. (2007) (2) | TR + EP | 0.19 (−0.48, 0.86) | |
| BIT-B | Làdavas et al. (2011) (1) | PA | 0.86 (−0.45, 2.18) (pooled) |
| Mizuno et al. (2011) | |||
| Fong et al. (2007) (1) | TR | 0.16 (−0.52, 0.84) | |
| Fong et al. (2007) (2) | TR + EP | 0.15 (−0.52, 0.82) | |
| Robertson et al. (2002) | LA | −0.08 (−0.70, 0.54) | |
| BIT (Total) | Koch et al. (2012) | TBS | 1.46 (0.39, 2.53) |
| Serino et al. (2009) | PA | 0.55 (0.16, 0.94) (pooled) | |
| Turton et al. (2010) | |||
| Fong et al. (2007) (1) | TR | 0.40 (−0.28, 1.09) | |
| Fong et al. (2007) (2) | TR + EP | 0.18 (−0.49, 0.85) |
Table 4.
Long-lasting effect size of each rehabilitation intervention.
| Items | Study | Intervention | Effect size |
|---|---|---|---|
| BIT-C | Mizuno et al. (2011) | PA | 0.52 (−0.07, 1.11) (pooled) |
| Nys et al. (2008) | |||
| Luukkainen-Markkula et al. (2009) | LA | 0.38 (−0.76, 1.53) | |
| Fong et al. (2007) (1) | TR | 0.26 (−0.52, 1.03) | |
| Fong et al. (2007) (2) | TR + EP | 0.25 (−0.47, 0.97) | |
| BIT-B | Fong et al. (2007) (1) | TR | 0.26 (−0.51, 1.03) |
| Fong et al. (2007) (2) | TR + EP | 0.22 (−0.50, 0.94) | |
| Mizuno et al. (2011) | PA | 0.03 (−0.55, 0.60) (pooled) | |
| Nys et al. (2008) | |||
| Robertson et al. (2002) | LA | −0.23 (−0.85, 0.40) | |
| BIT (Total) | Fong et al. (2007) (1) | TR | 0.27 (−0.50, 1.05) |
| Fong et al. (2007) (2) | TR + EP | 0.24 (−0.48, 0.96) | |
| Koch et al. (2012) | TBS | 1.97 (0.79, 3.14) | |
| Serino et al. (2009) | PA | −0.06 (−0.57, 0.44) (pooled) | |
| Turton et al. (2010) |
Pooled effects of PA on UN
The pooled ES of the single intervention PA on each BIT outcome were also analyzed (Table 5). No statistically significant results were found for either immediate or long-lasting effects as reflected in the BIT outcomes with significant heterogeneity.
Table 5.
PA intervention on neglect.
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
|---|---|---|---|---|
| Immediate effects | 5 | 216 | Std. mean difference (IV, random, 95% CI) | 0.89 (0.27, 1.51) |
| BIT-C | 3 | 74 | Std. mean difference (IV, random, 95% CI) | 1.31 (−0.26, 2.88) |
| BIT-B | 3 | 74 | Std. mean difference (IV, random, 95% CI) | 0.86 (−0.45, 2.18) |
| BIT (Total) | 2 | 68 | Std. mean difference (IV, random, 95% CI) | 0.59 (−0.02, 1.19) |
| Long-lasting effects | 4 | 125 | Std. mean difference (IV, random, 95% CI) | 0.15 (−0.20, 0.51) |
| BIT-C | 2 | 47 | Std. mean difference (IV, random, 95% CI) | 0.52 (−0.07, 1.11) |
| BIT-B | 1 | 16 | Std. mean difference (IV, random, 95% CI) | −0.04 (−1.06, 0.97) |
| BIT (Total) | 2 | 62 | Std. mean difference (IV, random, 95% CI) | −0.06 (−0.57, 0.44) |
Discussion
Our systematic review indicates that there is modest evidence for the use of PA to reduce UN in stroke, with immediate and long-lasting effects, and eye patching as shown by BIT-C scores for immediate effects. Other studies obtained positive effects from the use of visual scanning training (Ferreira et al., 2011), visuomotor feedback (Harvey et al., 2003), and TBS (Koch et al., 2012). Since Koch et al. (2012) only report the BIT (Total) and not the BIT-C and BIT-B subscale scores, it is impossible to draw any conclusion that rTMS is better than PA in improving the performance of tasks in the BIT-C and the BIT-B for neglect patients as no comparison could be done.
According to this review, PA is inclined to exhibit the highest ES for immediate effects, but this was not statistically significant as the 95% CI crossed over the zero point. The possible neural mechanism underlying the therapeutic effect of PA is that it reduces spatial neglect by enhancing the recruitment of intact brain areas responsible for visuo-spatial output through short-term sensori-motor plasticity pathways (Rossetti et al., 1998; Luauté et al., 2006). Although this technique has produced some improvement in a wide range of neglect symptoms, especially visual (Shiraishi et al., 2010; Mizuno et al., 2011; Rusconi and Carelli, 2012), some contradictory results have also been reported (Ferber et al., 2003; Rousseaux et al., 2006). The inconsistent results are probably due to the lack of comparability of treatment apparatus, treatment duration, the tasks used to assess PA effects, and post-stroke duration. Similar to PA, hemiplegic half-field eye patching is another compensational intervention for neglect which works by blocking the ipsilesional visual field. The initial study by Tsang et al. (2009) demonstrates a significant result with an ES of 0.71 immediately after intervention. More good-quality RCTs are needed to assess its long-lasting effects on UN.
Transcranial magnetic stimulation is a safe and non-invasive procedure to detect or modulate brain activity by passing a strong brief electrical current through an insulated wired coil placed on the skull which generates a transient magnetic field in the brain (Hummel and Cohen, 2006). TBS is a kind of rTMS using a lower stimulation intensity and a shorter time of stimulation to induce long-lasting effects in the cortex (Cárdenas-Morales et al., 2010) which demonstrates a relatively high ES as measured by the BIT total scores discussed in this review. TMS has become a popular method to stimulate the human brain, with rTMS attracting particular interest for its therapeutic potential to modify cortical excitability (Funke and Benali, 2011), which sheds light on the use of the inter-hemispheric rivalry model in explaining the recovery after neglect disorder in stroke patients. According to the literature, rTMS induces and repairs the inter-hemispheric imbalance (a neglect-like behavior) in the left or right posterior parietal cortex in healthy humans (Kinsbourne, 1977, 1994; Oliveri et al., 2001; Rounis et al., 2007). Based on this model, some studies have explored whether the use of inhibitory rTMS over the contralesional hemisphere to reduce the pathological hyperactivity of either hemisphere may be useful in promoting recovery from neglect after stroke with promising results (Oliveri et al., 2001; Brighina et al., 2003; Shindo et al., 2006; Koch et al., 2008; Nyffeler et al., 2009; Song et al., 2009). Compared to traditional standard cognitive intervention, rTMS can accelerate clinical recovery (Oliveri et al., 2001; Shindo et al., 2006; Song et al., 2009; Paik and Paik, 2010). It seems that patients more severely affected at baseline also benefited more from this intervention. However, the small sample size of the TBS study makes it impossible to draw any conclusion based on robust evidence. There may be a publication bias whereby large studies will report small ES whereas small studies will report large ES.
This review cannot determine the best time to commence neglect rehabilitation interventions, because most participants in the studies included here were recruited in either the subacute or chronic phases. Only two studies implemented rehabilitation within 1 month of stroke (Fong et al., 2007; Nys et al., 2008). As most of the spontaneous recovery after stroke happens in the first month (Kerkhoff and Schenk, 2012), further research is necessary to determine the effects of early but specific intervention for UN compared to conventional rehabilitation in order to avoid the confounding effect of spontaneous recovery. Neglect is the best single predictor of long-term functional impairment and poor rehabilitation outcome in the early stage (Jehkonen et al., 2001; Nys et al., 2005). One study (He et al., 2007) based on neuroimaging shows that 2 weeks after stroke, the normally functional connectivity between the left and right dorsal parietal cortex was disrupted, with the degree of breakdown correlated with the severity of left spatial neglect. It is therefore reasonable that patients should start a neglect intervention as soon as possible in the acute stage, in order to avoid non-use of the hemiplegic limbs, by increasing multisensory inputs or stimulation to the ipsilateral brain regions, and thus slowing down the secondary changes in the brain related to neglect. For further research, we also recommend adequate follow-up to maximize the benefits and monitor the persistence of the effect of neglect rehabilitation interventions.
Limitations of the review
The review has some limitations. It is constrained by the quality of the studies included, none of which scored the intention-to-treat analysis. The blindness design was the biggest weakness of most of these RCTs. The heterogeneity of the studies means that this meta-analysis is less powerful and cannot identify conclusively the optimal treatment approach.
Conclusion
The results of this review confirm that PA appears to be the most common and effective rehabilitation intervention for UN, and that rTMS might be a promising approach for future treatment. As shown by the insignificant long-lasting effects, rehabilitation interventions often had a transient impact and could not be generalized across time to an improvement in daily functioning. All studies faced the same weakness of low power with smaller samples and a limitation in the blindness design. More rigorous studies of various interventions should be done before coming to a firm conclusion.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript is the original work of the authors and has not been submitted for publication before. Part of the material in the manuscript was accepted to be presented at the seventh World Congress of the International Society of Physical and Rehabilitation Medicine on June 16–20, 2013 in Beijing, China. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
References
- Azouvi P., Samuel C., Louis-Dreyfus A., Bernati T., Bartolomeo P., Beis J. M., et al. (2002). Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J. Neurol. Neurosurg. Psychiatr. 73, 160–166 10.1136/jnnp.73.2.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal S. K., Teasell R. W., Foley N. C., Speechley M. R. (2005). The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad Scale in stroke rehabilitation literature. J. Clin. Epidemiol. 58, 668–673 10.1016/j.jclinepi.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Bowen A., Lincoln N., Dewey M. (2007). Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Syst. Rev. CD003586. 10.1002/14651858.CD003586.pub2 [DOI] [PubMed] [Google Scholar]
- Bowen A., McKenna K., Tallis R. C. (1999). Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke 30, 1196–1202 10.1161/01.STR.30.6.1196 [DOI] [PubMed] [Google Scholar]
- Brighina F., Bisiach E., Oliveri M., Piazza A., La Bua V., Daniele O., et al. (2003). 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci. Lett. 336, 131–133 10.1016/S0304-3940(02)01283-1 [DOI] [PubMed] [Google Scholar]
- Cárdenas-Morales L., Nowak D. A., Kammer T., Wolf R. C., Schonfeldt-Lecuona C. (2010). Mechanisms and applications of Theta-burst rTMS on the human motor cortex. Brain Topogr. 22, 294–306 10.1007/s10548-009-0084-7 [DOI] [PubMed] [Google Scholar]
- de Morton N. A. (2009). The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust. J. Physiother. 55, 129–133 10.1016/S0004-9514(09)70043-1 [DOI] [PubMed] [Google Scholar]
- Ertekin ÖA., Gelecek N., Yildirim Y., Akdal G. (2009). Supervised versus home physiotherapy outcomes in stroke patients with unilateral visual neglect: a randomized controlled follow-up study. J. Neurol. Sci. Turk. 26, 325–334 [Google Scholar]
- Ferber S., Danckert J., Joanisse M., Goltz H. C., Goodale M. A. (2003). Eye movements tell only half the story. Neurology 60, 1826–1829 10.1212/01.WNL.0000061478.16239.5C [DOI] [PubMed] [Google Scholar]
- Ferreira H. P., Leite Lopes M. A., Luiz R. R., Cardoso L., André C. (2011). Is visual scanning better than mental practice in hemispatial neglect? Results from a pilot study. Top. Stroke Rehabil. 18, 155–161 10.1310/tsr1802-155 [DOI] [PubMed] [Google Scholar]
- Fong K. N. K., Chan M. K. L., Ng P. P. K., Tsang M. H. M., Chow K. K. Y., Lau C. W. L., et al. (2007). The effect of voluntary trunk rotation and half-field eye-patching for patients with unilateral neglect in stroke: a randomized controlled trial. Clin. Rehabil. 21, 729–741 10.1177/0269215507076391 [DOI] [PubMed] [Google Scholar]
- Funke K., Benali A. (2011). Modulation of cortical inhibition by rTMS – findings obtained from animal models. J. Physiol. (Lond.) 589, 4423–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoraptis N., Mah Y. H., Machner B., Singh-Curry V., Malhotra P., Hadji-Michael M., et al. (2012). The effects of the dopamine agonist rotigotine on hemispatial neglect following stroke. Brain 135, 2478–2491 10.1093/brain/aws154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan P. W., Cockburn J., Wilson B. A. (1991). The behavioural assessment of visual neglect. Neuropsychol. Rehabil. 1, 5–32 10.1080/09602019108402259 [DOI] [Google Scholar]
- Hartmanmaeir A., Katz N. (1995). Validity of the Behavioral Inattention Test (BIT): relationships with Functional Tasks. Am. J. Occup. Ther. 49, 507–516 10.5014/ajot.49.6.507 [DOI] [PubMed] [Google Scholar]
- Harvey M., Hood B., North A., Robertson I. H. (2003). The effects of visuomotor feedback training on the recovery of hemispatial neglect symptoms: assessment of a 2-week and follow-up intervention. Neuropsychologia 41, 886–893 10.1016/S0028-3932(03)00003-4 [DOI] [PubMed] [Google Scholar]
- He B. J., Shulman G. L., Snyder A. Z., Corbetta M. (2007). The role of impaired neuronal communication in neurological disorders. Curr. Opin. Neurol. 20, 655–660 10.1097/WCO.0b013e3282f1c720 [DOI] [PubMed] [Google Scholar]
- Hummel F. C., Cohen L. G. (2006). Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 5, 708–712 10.1016/S1474-4422(06)70525-7 [DOI] [PubMed] [Google Scholar]
- Ianes P., Varalta V., Gandolfi M., Picelli A., Corno M., Di Matteo A., et al. (2012). Stimulating visual exploration of the neglected space in the early stage of stroke by hemifield eye-patching: a randomized controlled trial in patients with right brain damage. Eur. J. Phys. Rehabil. Med. 48, 189–196 [PubMed] [Google Scholar]
- Jehkonen M., Ahonen J. P., Dastidar P., Koivisto A. M., Laippala P., Vilkki J., et al. (2001). Predictors of discharge to home during the first year after right hemisphere stroke. Acta Neurol. Scand. 104, 136–141 10.1034/j.1600-0404.2001.00025.x [DOI] [PubMed] [Google Scholar]
- Jehkonen M., Laihosalo M., Kettunen J. E. (2006). Impact of neglect on functional outcome after stroke – a review of methodological issues and recent research findings. Restor. Neurol. Neurosci. 24, 209–215 [PubMed] [Google Scholar]
- Kamada K., Shimodozono M., Hamada H., Kawahira K. (2011). Effects of 5 minutes of neck-muscle vibration immediately before occupational therapy on unilateral spatial neglect. Disabil. Rehabil. 33, 2322–2328 10.3109/09638288.2011.570411 [DOI] [PubMed] [Google Scholar]
- Katz N., Ring H., Naveh Y., Kizony R., Feintuch U., Weiss P. (2005). Interactive virtual environment training for safe street crossing of right hemisphere stroke patients with unilateral spatial neglect. Disabil. Rehabil. 27, 1235–1244 10.1080/09638280500076079 [DOI] [PubMed] [Google Scholar]
- Kerkhoff G., Schenk T. (2012). Rehabilitation of neglect: an update. Neuropsychologia 50, 1072–1079 10.1016/j.neuropsychologia.2012.01.024 [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Chun M. H., Yun G. J., Song Y. J., Young H. E. (2011). The effect of virtual reality training on unilateral spatial neglect in stroke patients. Annu. Rehabil. Med. 35, 309–315 10.5535/arm.2011.35.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M. (1977). Hemi-neglect and hemisphere rivalry. Adv. Neurol. 18, 41–49 [PubMed] [Google Scholar]
- Kinsbourne M. (1994). Mechanisms of neglect: implications for rehabilitation. Neuropsychol. Rehabil. 4, 151–153 10.1080/09602019408402274 [DOI] [Google Scholar]
- Koch G., Bonní S., Giacobbe V., Bucchi G., Basile B., Lupo F., et al. (2012). è-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology 78, 24–31 10.1212/WNL.0b013e31823ed08f [DOI] [PubMed] [Google Scholar]
- Koch G., Oliveri M., Cheeran B., Ruge D., Lo Gerfo E., Salerno S., et al. (2008). Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain 131, 3147–3155 10.1093/brain/awn273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Làdavas E., Bonifazi S., Catena L., Serino A. (2011). Neglect rehabilitation by prism adaptation: different procedures have different impacts. Neuropsychologia 49, 1136–1145 10.1016/j.neuropsychologia.2011.01.044 [DOI] [PubMed] [Google Scholar]
- Leslie G., Mary P. (2007). Foundations of Clinical Research, 3rd Edn London: Pearson Education [Google Scholar]
- Luauté J., Halligan P., Rode G., Rossetti Y., Boisson D. (2006). Visuo-spatial neglect: a systematic review of current interventions and their effectiveness. Neurosci. Biobehav. Rev. 30, 961–982 10.1016/j.neubiorev.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Luukkainen-Markkula R., Tarkka I. M., Pitkanen K., Sivenius J., Hamalainen H. (2009). Rehabilitation of hemispatial neglect: a randomized study using either arm activation or visual scanning training. Restor. Neurol. Neurosci. 27, 663–672 [DOI] [PubMed] [Google Scholar]
- Maher C. G., Sherrington C., Herbert R. D., Moseley A. M., Elkins M. (2003). Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 83, 713–721 [PubMed] [Google Scholar]
- Mesulam M. M. (1999). Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos. Trans. R. Soc Lond. B Biol. Sci. 354, 1325–1364 10.1098/rstb.1999.1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Tsuji T., Takebayashi T., Fujiwara T., Hase K., Liu M. (2011). Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: a randomized, controlled trial. Neurorehabil. Neural Repair 25, 711–720 10.1177/1545968311407516 [DOI] [PubMed] [Google Scholar]
- Mutai H., Furukawa T., Araki K., Misawa K., Hanihara T. (2012). Factors associated with functional recovery and home discharge in stroke patients admitted to a convalescent rehabilitation ward. Geriatr. Gerontol. Int. 12, 215–222 10.1111/j.1447-0594.2011.00747.x [DOI] [PubMed] [Google Scholar]
- Nyffeler T., Cazzoli D., Hess C. W., Muri R. M. (2009). One session of repeated parietal theta burst stimulation trains induces ling-lasting improvement of visual neglect. Stroke 40, 2791–2796 10.1161/STROKEAHA.109.552323 [DOI] [PubMed] [Google Scholar]
- Nys G. M., de Haan E. H., Kunneman A., de Kort P. L., Dijkerman H. C. (2008). Acute neglect rehabilitation using repetitive prism adaptation: a randomized placebo-controlled trial. Restor. Neurol. Neurosci. 26, 1–12 [PubMed] [Google Scholar]
- Nys G. M. S., van Zandvoort M. J. E., de Kort P. L. M., van der Worp H. B., Jansen B. P. W., Algra A., et al. (2005). The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology 64, 821–827 10.1212/01.WNL.0000152984.28420.5A [DOI] [PubMed] [Google Scholar]
- Oliveri M., Bisiach E., Brighina F., Piazza A., La Bua V., Buffa D., et al. (2001). rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology 57, 1338–1340 10.1212/WNL.57.7.1338 [DOI] [PubMed] [Google Scholar]
- Paci M., Matulli G., Baccini M., Rinaldi L. A., Baldassi S. (2010). Reported quality of randomized controlled trials in neglect rehabilitation. Neurol. Sci. 31, 159–163 10.1007/s10072-010-0316-3 [DOI] [PubMed] [Google Scholar]
- Paik N. J., Paik N. J. (2010). Repetitive transcranial magnetic stimulation for hemispatial neglect in patients after stroke: an open-label pilot study. J. Rehabil. Med. 42, 447–452 10.2340/16501977-0553 [DOI] [PubMed] [Google Scholar]
- Petzold A., Korner-Bitensky N., Salbach N. M., Ahmed S., Menon A., Ogourtsova T. (2012). Increasing knowledge of best practices for occupational therapists treating post-stroke unilateral spatial neglect: results of a knowledge-translation intervention study. J. Rehabil. Med. 44, 118–124 10.2340/16501977-0910 [DOI] [PubMed] [Google Scholar]
- Pierce S. R., Buxbaum L. J. (2002). Treatments of unilateral neglect: a review. Arch. Phys. Med. Rehabil. 83, 256–268 10.1053/apmr.2002.34832 [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L., Fasotti L., Jehkonen M., Antonucci G., Magnotti L., Boelen D., et al. (2004). The use of optokinetic stimulation in rehabilitation of the hemineglect disorder. Cortex 40, 441–450 10.1016/S0010-9452(08)70138-2 [DOI] [PubMed] [Google Scholar]
- Plummer P., Morris M. E., Dunai J. (2003). Assessment of unilateral neglect. Phys. Ther. 83, 732–740 [PubMed] [Google Scholar]
- Polanowska K., Seniow J., Paprot E., Lesniak M., Czlonkowska A. (2009). Left-hand somatosensory stimulation combined with visual scanning training in rehabilitation for post-stroke hemineglect: a randomised, double-blind study. Neuropsychol. Rehabil. 19, 364–382 10.1080/09602010802268856 [DOI] [PubMed] [Google Scholar]
- Robertson I. H., McMillan T. M., MacLeod E., Edgeworth J., Brock D. (2002). Rehabilitation by limb activation training reduces left-sided motor impairment in unilateral neglect patients: a single-blind randomised control trial. Neuropsychol. Rehabil. 12, 439–454 10.1080/09602010244000228 [DOI] [Google Scholar]
- Rossetti Y., Rode G., Pisella L., Farne A., Li L., Boisson D., et al. (1998). Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 395, 166–169 10.1038/25988 [DOI] [PubMed] [Google Scholar]
- Rounis E., Yarrow K., Rothwell J. C. (2007). Effects of rTMS conditioning over the fronto-parietal network on motor versus visual attention. J. Cogn. Neurosci. 19, 513–524 10.1162/jocn.2007.19.3.513 [DOI] [PubMed] [Google Scholar]
- Rousseaux M., Bernati T., Saj A., Kozlowski O. (2006). Ineffectiveness of prism adaptation on spatial neglect signs. Stroke 37, 542–543 10.1161/01.STR.0000198877.09270.e8 [DOI] [PubMed] [Google Scholar]
- Rusconi M. L., Carelli L. (2012). Long-term efficacy of prism adaptation on spatial neglect: preliminary results on different spatial components. Sci. World J. 10.1100/2012/618528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saevarsson S., Kristjansson A., Halsband U. (2010). Strength in numbers: combining neck vibration and prism adaptation produces additive therapeutic effects in unilateral neglect. Neuropsychol. Rehabil. 20, 704–724 10.1080/09602011003737087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder A., Wist E. R., Homberg V. (2008). TENS and optokinetic stimulation in neglect therapy after cerebrovascular accident: a randomized controlled study. Eur. J. Neurol. 15, 922–927 10.1111/j.1468-1331.2008.02229.x [DOI] [PubMed] [Google Scholar]
- Serino A., Barbiani M., Rinaldesi M. L., Làdavas E. (2009). Effectiveness of prism adaptation in neglect rehabilitation: a controlled trial study. Stroke 40, 1392–1398 10.1161/STROKEAHA.108.530485 [DOI] [PubMed] [Google Scholar]
- Sherrington C., Herbert R. D., Maher C. G., Moseley A. M. (2000). PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man. Ther. 5, 223–226 10.1054/math.2000.0372 [DOI] [PubMed] [Google Scholar]
- Shindo K., Sugiyama K., Huabao L., Nishijima K., Kondo T., Izumi S. (2006). Long-term effect of low-frequency repetitive transcranial magnetic stimulation over the unaffected posterior parietal cortex in patients with unilateral spatial neglect. J. Rehabil. Med. 38, 65–67 10.1080/16501970500441807 [DOI] [PubMed] [Google Scholar]
- Shiraishi H., Muraki T., Itou Y. S. A., Hirayama K. (2010). Prism intervention helped sustainability of effects and ADL performances in chronic hemispatial neglect: a follow-up study. NeuroRehabilitation 27, 165–172 [DOI] [PubMed] [Google Scholar]
- Song W., Du B., Xu Q., Hu J., Wang M., Luo Y. (2009). Low-frequency transcranial magnetic stimulation for visual spatial neglect: a pilot study. J. Rehabil. Med. 41, 162–165 10.2340/16501977-0302 [DOI] [PubMed] [Google Scholar]
- Stone S. P., Wilson B., Wroot A., Halligan P. W., Lange L. S., Marshall J. C., et al. (1991). The assessment of visuospatial neglect after acute stroke. J. Neurol. Neurosurg. Psychiatr. 54, 345–350 10.1136/jnnp.54.4.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M. H. M., Sze K. H., Fong K. N. K. (2009). Occupational therapy treatment with right half-field eye-patching for patients with subacute stroke and unilateral neglect: a randomised controlled trial. Disabil. Rehabil. 31, 630–637 10.1080/09638280802240621 [DOI] [PubMed] [Google Scholar]
- Turton A. J., O’Leary K., Gabb J., Woodward R., Gilchrist I. D. (2010). A single blinded randomised controlled pilot trial of prism adaptation for improving self-care in stroke patients with neglect. Neuropsychol. Rehabil. 20, 180–196 10.1080/09602010903040683 [DOI] [PubMed] [Google Scholar]
- Verhagen A. P., de Vet H. C. W., de Bie R. A., Kessels A. G. H., Boers M., Bouter L. M., et al. (1998). The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. J. Clin. Epidemiol. 51, 1235–1241 10.1016/S0895-4356(98)00131-0 [DOI] [PubMed] [Google Scholar]
- Welfringer A., Leifert-Fiebach G., Babinsky R., Brandt T. (2011). Visuomotor imagery as a new tool in the rehabilitation of neglect: a randomised controlled study of feasibility and efficacy. Disabil. Rehabil. 33, 2033–2043 10.3109/09638288.2011.556208 [DOI] [PubMed] [Google Scholar]
- Wiart L., Côme A. B. S., Debelleix X., Petit H., Joseph P. A., Mazaux J. M., et al. (1997). Unilateral neglect syndrome rehabilitation by trunk rotation and scanning training. Arch. Phys. Med. Rehabil. 78, 424–429 10.1016/S0003-9993(97)90236-7 [DOI] [PubMed] [Google Scholar]
- Wilson B., Cockburn J., Halligan P. (1987). Development of a behavioral test of visuospatial neglect. Arch. Phys. Med. Rehabil. 68, 98–102 [PubMed] [Google Scholar]