Abstract
Lipid droplets (LDs) are important organelles in energy metabolism and lipid storage. Their cores are composed of neutral lipids that form a hydrophobic phase and are surrounded by a phospholipid monolayer that harbors specific proteins. Most well-established LD proteins perform important functions, particularly in cellular lipid metabolism. Morphological studies show LDs in close proximity to and interacting with membrane-bound cellular organelles, including the endoplasmic reticulum, mitochondria, peroxisomes, and endosomes. Because of these close associations, it is difficult to purify LDs to homogeneity. Consequently, the confident identification of bona fide LD proteins via proteomics has been challenging. Here, we report a methodology for LD protein identification based on mass spectrometry and protein correlation profiles. Using LD purification and quantitative, high-resolution mass spectrometry, we identified LD proteins by correlating their purification profiles to those of known LD proteins. Application of the protein correlation profile strategy to LDs isolated from Drosophila S2 cells led to the identification of 111 LD proteins in a cellular LD fraction in which 1481 proteins were detected. LD localization was confirmed in a subset of identified proteins via microscopy of the expressed proteins, thereby validating the approach. Among the identified LD proteins were both well-characterized LD proteins and proteins not previously known to be localized to LDs. Our method provides a high-confidence LD proteome of Drosophila cells and a novel approach that can be applied to identify LD proteins of other cell types and tissues.
One of the most pressing issues in eukaryotic cell biology is the need to determine the dynamic composition of organelles that allow for the coordinated execution of many different simultaneous processes. Such knowledge of organelle composition provides the basis for understanding protein functions and their coordination in different organelles. Therefore, comprehensive and highly accurate inventories of organelle proteins are essential.
Rapid advances in mass spectrometry (MS)-based1 proteomics are revolutionizing cell biology (1–3). Subcellular fractionation combined with MS enables the systematic determination of organelle composition. Because the sensitivity of MS has increased, even less abundant proteins of an organelle can now be identified. However, the increased sensitivity also identifies more, and less abundant, contaminant proteins, resulting in longer proteome lists for an experiment and complicating the identification of genuine proteins of an organelle.
This analytic problem is particularly apparent for lipid droplets (LDs). LD architecture differs from that of other organelles in that they have hydrophobic cores of neutral lipids, primarily triacylglycerol and sterol esters, rather than an aqueous lumen. The LD core is shielded from the cytosol by a phospholipid monolayer, into which specific proteins are embedded (4–6). The primary function of LDs is to store lipids as reservoirs of metabolic energy and membrane precursors. Additionally, LDs participate in host–pathogen interactions (7) and play central roles in numerous physiological processes and linked pathologies. For example, obesity is characterized by the overaccumulation of LDs in tissues, and LD accumulation in macrophages is a hallmark of atherosclerosis (8, 9). Given the diverse distribution of LDs in cell types and processes, their protein composition likely varies among cell types and is dynamic. Knowledge of LD compositions in different cell types will lay the foundation for functional characterization of this organelle and could help to reveal novel mechanisms contributing to metabolic diseases.
LD protein composition has been analyzed in over a dozen studies that employed different methodologies to study different cell types ranging in evolutionary complexity from yeast to human (10–21). These studies have provided long lists of putative LD proteins, but apart from the consistent identification of several LD marker proteins (e.g., perilipins), LD proteomes have been plagued by the identification of contaminants that co-purify with LDs. This is due in part to the tight interactions of LDs with other organelles. Microscopy analyses revealed intimate connections between LDs and the endoplasmic reticulum (ER) and mitochondria (12, 22–26), and LDs can form contacts with peroxisomes and different types of endosomes (27, 28). Not surprisingly, therefore, ER and mitochondrial proteins are found abundantly in reported LD proteomes.
To overcome these limitations of LD protein identification, we designed and applied a quantitative high-resolution MS-based proteomics strategy that employs protein correlation profiles (PCPs) to confidently identify LD proteins in Drosophila cells. PCPs provide a quantitative approach for determining the correlation profiles of purified proteins relative to known organelle markers (29). PCP studies have yielded some of the most reliable inventories of organelles (30–33), but such an approach has not previously been applied to LDs.
EXPERIMENTAL PROCEDURES
Drosophila S2 Cells—Drosophila S2 cells were cultured in Schneider's Drosophila medium (Invitrogen) supplemented with 10% fetal bovine serum and antibiotics (100 unit/ml penicillin and 100 μg/ml streptomycin) at 27 °C as described elsewhere (34).
For protein localization in S2 cells, expression vectors (actin promoter) were cloned with the Gateway system (Invitrogen) for the following proteins: Alg14-mCherry (CG6308), Alg1-mCherry (CG18012), Alg2-mCherry (CG1291), Alg11-mCherry (CG11306), Alg10-mCherry (CG32076), mCherry-Seipin (CG9904), selenoprotein SELT-mCherry (CG3887), MBOAT-mCherry (CG5926), Lunapark-mCherry (CG8735), mCherry-steroid binding protein (CG9066), mCherry-sec14 homolog (CG13848), lyso-PA acyltransferase (CG32699), mCherry-short chain dehydrogenase (CG2064), lipase-mCherry (CG17292), mCherry-phospholipase D (CG7718), oxysterol binding protein-mCherry (CG1513), Tango 14-mCherry (CG4775), mCherry-FAS associated factor 2 (CG10372), fatty acid transferase-mCherry (CG7400), CGI-58-mCherry (CG1882), and mCherry-HSL (CG11055). Transfection of S2 cells was performed using Effectene reagent (Qiagen) according to manufacturer's instructions. Transfected cells were stained with 1 μg/ml BODIPY493/503 (Invitrogen) for 10 min and imaged with a spinning-disk confocal microscope (TiLL iMIC CSU22, Andor) with a back-illuminated electron-multiplying charge-coupled device camera (iXonEM 897, Andor) and a 100 × 1.4 NA oil immersion objective (Olympus); 16-bit images were collected with Image iQ (version 1.9, Andor), deconvoluted (Huygens, SVI), and cropped (ImageJ).
LD Purification
For LD purification, ten 75-cm2 cell culture dishes of Drospohila S2 cells were used. Stable isotope labeling of amino acids in cell culture (SILAC) of the S2 cells was performed as previously described, and the labeling efficiency was >98% before the last passage (35). LD formation was induced by incubating confluent cells with 1 mm oleate complexed to BSA (36) overnight. LD formation was confirmed via microscopy. Cells were harvested, washed twice with ice-cold PBS, resuspended in 2 ml of buffer (200 mm Tris/HCL, pH 7.5, 2 mm magnesium diactetate, and Complete Protease Inhibitor (Roche)), and lysed on ice with a 2-ml tissue grinder (Kontes) with 20 strokes by hand. The resulting cell lysate was fractionated via three 1-h centrifugation steps at 3000g, 20,000g, and 100,000g at 4 °C in thin-wall polyallomer (2.2 ml, 11 mm × 35 mm) tubes (Beckman Coulter) with an S-55S swinging bucket rotor in a Sorvall MTX 150 micro-ultracentrifuge (Thermo Scientific). The supernatant was adjusted to 1 m sucrose in buffer, and the resulting 2 ml fraction was layered under a 12-ml sucrose step-gradient with 2-ml steps (0.75, 0.5, 0.25, 0.125, 0 m) and centrifuged for 12 h at 200,000g at 4 °C in 13.2-ml thin-wall polyallomer tubes (Beckman Coulter) with a TH-641 swinging bucket rotor in a Sorvall WX80 ultracentrifuge (Thermo Scientific). The floating white LD fraction was collected with a tube slicer (Beckman Coulter), and the other 2-ml fractions were collected with a pipette. Six fractions of the gradient and the three pellet fractions of the differential centrifugation steps were analyzed via MS-based proteomics.
Protein Digestion
One hundred micrograms of each heavy SILAC sucrose gradient fraction were mixed with equal amounts of light SILAC LD standard. Combined samples were precipitated with 2 ml of ice-cold acetone at 4 °C overnight. Precipitated proteins were collected via centrifugation, dried at room temperature, dissolved in 50 μl 6 m urea, 2 m thiourea, and 10 mm Tris, and subjected to in-solution digestion. Protein samples were then reduced with 5 μl 1 mm DTT for 45 min at room temperature, alkylated with 5 μl 5.5 mm iodoacetamide for 30 min, and digested with 4 μg endopeptidase Lys-C (Waco) for 3 h. The resulting peptide mixtures were diluted with 200 μl 50 mm ammonium bicarbonate and digested with 4 μg sequencing-grade modified trypsin (Promega) per protein overnight at room temperature. Trypsin was inactivated via acidification with 0.5 μl trifluoroacetic acid (pH <3), and subsequently 5 μg of peptides was concentrated and desalted on reversed-phase C18 STAGE tips (37, 38).
Liquid Chromatography–MS/MS Analysis
Peptides were eluted from STAGE tips by 30 μl buffer B (80% acetonitrile in 0.5% acetic acid) solution into a 96-well sample plate (Abgene), concentrated in a SpeedVac until the organic solvent was removed, and reconstituted with a one-to-one mix of buffer A (0.5% acetic acid) and buffer A* (2% acetonitrile in 0.1% trifluoroacetic acid). Eluted peptides were analyzed using a nanoflow high-pressure liquid chromatography system (Agilent Technologies or Proxeon Biosystems) coupled on-line via a nanoelectrospray ion source (Proxeon Biosystems) to an LTQ-Orbitrap mass spectrometer (Thermo Scientific). Peptide samples were loaded onto a C18 reversed-phase column (15 cm long, 75 μm inner diameter, packed in-house with ReproSil-Pur C18-AQ 3-μm resin) in buffer A (0.5% acetic acid) with a flow rate of 500 nl/min and eluted with a linear gradient from 2% to 40% buffer B (80% acetonitrile and 0.5% acetic acid solution) at a flow rate of 250 nl/min over 2 h.
Mass spectra were acquired in the positive ion mode, applying a data-dependent automatic switch between the acquisition of one Orbitrap survey scan (mass range of m/z 300–1700) and MS/MS of the five most intense ions in the LTQ (“top 5” method).
The target value in the LTQ-Orbitrap was 1,000,000 for the survey scan at a resolution of 60,000 at m/z 400 with lock masses. Fragmentation in the LTQ was performed by means of collision-induced dissociation with a target value of 5000 ions. The ion selection threshold was 1000 counts. Selected sequenced ions were dynamically excluded for 90 s.
MS Data Analysis
Raw mass spectrometric data were analyzed with MaxQuant (version 1.2.0.11) (39). The raw data and MaxQuant output files are available from the Tranche proteome repository with the following access codes: raw data: DgrMLzEv/6uaJd0L+QG1eqTzvRM0sAVU9N7VkL1urdh4cEd9s81j2LyAZtnIMVNaxi oxMS8GExz8Cqwi0pojr8uY57EAAAAAAAASFg = = MaxQuant output files: cIEbTExJn93219yD BeDHPxk0P7i0nbSYed2mj340buWup2jDDy9B2i+0kIP3u9nOGSysN934kMXZASqabMdLwf/XkqQAAAAAAAAHLQ = = Summary output file: 2LQWICE+HalrmoOv5bTVH2IY4kP2wx63wYf3jJ8 eCSepID8BDExjH8OIDiKUUS2idpX9ia5ZSAxZXJzhArn4XKRvlHsAAAAAAAABVg = =.
The peak list was searched against FlyBase (version 5.24), containing 15,016 proteins combined with common contaminants and concatenated with the reversed versions of all sequences using Andromeda, a search engine incorporated into the MaxQuant framework (40). Precursor and fragment ions were searched with a maximal initial mass deviation of up to 7 ppm or 0.5 Da, respectively. In the peptide search, trypsin, allowing for cleavage N-terminal to proline, was used as the enzyme specificity. Cysteine carbamidomethylation was selected as a fixed modification, and protein N-terminal acetylation and methionine oxidation were chosen as variable modifications. Depending on a priori knowledge about the number of Arg and Lys residues in the precursor ion determined by MaxQuant before the search, Arg10 and Lys8 were used as additional fixed or variable modifications. A maximum of two missed cleavages and three labeled amino acids were allowed.
MaxQuant automatically quantified and normalized peptides and proteins based on the light SILAC LD standard. Quantification of SILAC pairs was performed by MaxQuant with standard settings using a minimum ratio count of 2. A false discovery rate of 0.01 was required for proteins and peptides with a minimum length of six amino acids. Hierarchical clustering and gene ontology annotation analysis were performed using Perseus software, version 1.2.0.10. Soft clustering was performed using Mfuzz, a software package implemented in the statistical program language R (41).
RESULTS
A Protein Correlation Profile Strategy to Analyze the LD Proteome
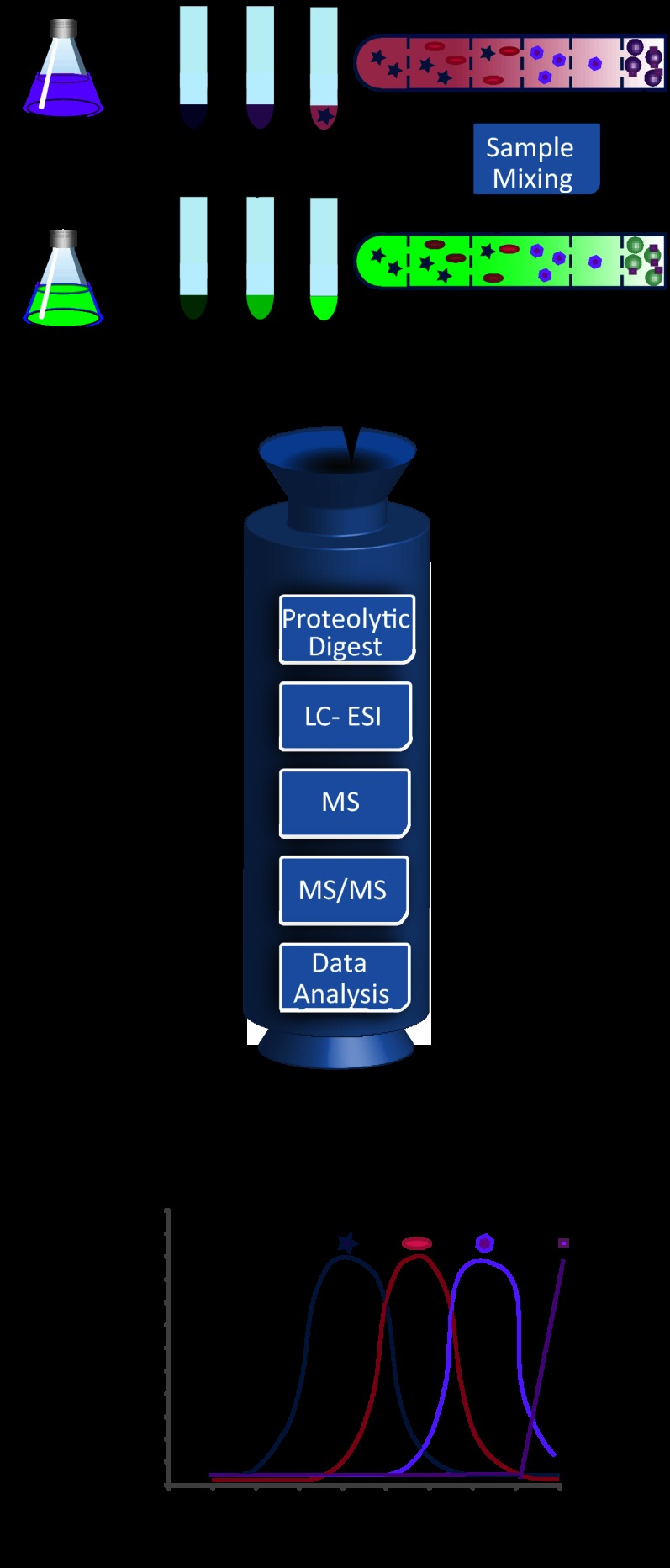
We used Drosophila S2 cells, for which abundant functional data on LD biology exist, to develop an LD PCP. The PCP strategy requires two components that are each labeled with different isotope-containing amino acids: a purified LD sample (the target organelle or “LD standard”) and all fractions of a separate LD purification (Fig. 1).
Fig. 1.
Experimental scheme of quantitative analysis of an LD proteome via protein correlation profiling. LDs are purified from two populations of Drosophila S2 cells, one unlabeled (Arg0Lys0) (purple) and one labeled with heavy amino acids Arg10 and Lys8 (green). After three steps of differential centrifugation (centrifugation pellets are fractions 9–7), cell lysates are further fractionated with a sucrose step gradient (fractions 6–1). LDs are large round structures in fraction 1. The partitioning of schematic sample proteins is indicated. For quantitative analysis, the top (LD-containing) fraction of the light sample is mixed with each of the six fractions of the sucrose gradient and the three pellet fractions of the heavy samples. Mixed fractions are de-lipidated, and proteins are precipitated. After in-solution digestion of the proteins, samples are separately analyzed via liquid chromatography on-line coupled to high-resolution MS/MS. The heavy/light ratio (H/L) for the proteins of each fraction was normalized by dividing it by the highest ratio among the fractions, giving a value of 0 to 1 (H/L) for each fraction. Profiles show values plotted against each fraction for a number of example proteins represented schematically.
To obtain these fractions, we purified LDs from cells that were treated with oleate and metabolically labeled with heavy, non-radioactive isotope-containing lysine and arginine (SILAC (42)). Of several tested protocols, a combination of sequential differential centrifugations followed by a sucrose density gradient purification proved to be most effective. To reduce the possibility of contamination of the LD fraction by other organelles or vesicles of disrupted organelles during cell homogenization, we first used three differential centrifugation steps to efficiently remove nuclei, mitochondria, and cellular membranes (fractions 7–9). These centrifugation steps efficiently removed many heavy organelles from the LDs, optimizing our protocol for the analysis of LD proteins, but this procedure precludes the distinction of, for example, mitochondrial and nuclear proteins in the resulting protein correlation profile. In addition, these centrifugation steps decrease the protein complexity of the resulting samples, which enables more comprehensive MS analysis. Samples were then subjected to sucrose density gradient centrifugation, which is commonly used to isolate LDs (36). We modified this part of the purification protocol with a sucrose step gradient (fractions 6–1), instead of a continuous sucrose gradient, in order to increase the focusing of protein complexes from different organelles at the interphases of the density steps. To generate the LD standard for PCP analysis, we collected the LD fraction (fraction 1) from a similar purification scheme of oleate-loaded cells that were labeled with light isotope-containing lysine and arginine.
The PCP method is based on spiking a light amino-acid-labeled LD standard in each of the heavy isotope-labeled fractions of the purification. Upon analysis, therefore, the ratio of heavy- and light-labeled versions of each protein in each sample reflects its abundance in this fraction relative to the abundance in the LD fraction. Assembling these abundance ratios for each protein in all fractions yields a plot reflecting the purification and enrichment of each of the identified proteins (shown conceptually in Fig. 1).
To determine the relative abundance of the proteins in each fraction, we subjected the mixed samples to liquid chromatography coupled on-line to high-resolution MS (Fig. 1). The resulting spectra were analyzed with the MaxQuant software suite (39, 40), leading to an overall identification of 2703 proteins. For the LD fraction, this resulted in the identification of 1481 proteins. The number of identified proteins in the other fractions ranged from 1946 (fraction 8) to 2126 (fraction 9), and in each case, more than 99% were quantified with SILAC ratios (see Table I for proteomics results, supplemental Table S2 for complete proteomics results, and supplemental Fig. S1 for annotated spectra for all single peptide identifications).
Table I. Identified proteins.
| Fraction | Proteins identified | Protein SILAC ratio | Protein SILAC ratio (%) | Peptides identified | Razor peptides | Unique peptides | Proteins with >5 valid values |
|---|---|---|---|---|---|---|---|
| 1 | 1481 | 1480 | 99.93 | 10,410 | 9849 | 9370 | 1389 |
| 2 | 1972 | 1969 | 99.85 | 14,400 | 13,673 | 13,044 | 1823 |
| 3 | 2035 | 2029 | 99.71 | 15,104 | 14,379 | 13,748 | 1866 |
| 4 | 2087 | 2076 | 99.47 | 15,422 | 14,799 | 14,256 | 1823 |
| 5 | 2055 | 2052 | 99.85 | 15,253 | 14,647 | 14,114 | 1814 |
| 6 | 2112 | 2101 | 99.48 | 15,407 | 14,767 | 14,201 | 1848 |
| 7 | 2041 | 2038 | 99.85 | 14,047 | 13,429 | 12,892 | 1781 |
| 8 | 1946 | 1944 | 99.90 | 13,728 | 13,101 | 12,551 | 1703 |
| 9 | 2126 | 2119 | 99.67 | 14,477 | 13,859 | 13,315 | 1827 |
| Total | 2703 | 2698 | 99.82 | 26,961 | 25,969 | 25,081 | 1981 |
Table indicates the numbers of proteins identified in total and in each of the purification fractions of MS measurements before and after filtering for at least five valid values in all nine fractions, as well as the number and percentage of proteins identified with the SILAC ratio and the numbers of peptides, unique peptides, and razor peptides in each fraction.
To assess the reproducibility of the LD PCP measurements, we compared the results of two different mass spectrometric analyses of the purification fractions mixed with the LD standard. Plotting the protein abundance ratios from the two technical replicates revealed a high correlation between ratios of peptide abundance in a fraction of the two different experiments (Fig. 2), indicating that our measurements were highly reproducible. Considering this high correlation, most of the variation in the ratios in fraction 1 (average heavy/light ratio (H/L) of 2.9 ± 1.7 S.D.) likely derives from variation in either biological samples or different preparations. Our analysis also revealed that the average ratio in fraction 1 did not center on 1 (Fig. 2A). This systematic error is likely due to the complications of measuring protein concentrations in an LD fraction containing large amounts of triacylglycerol. Importantly, because the same amount of LD standard was added to each fraction, the measured ratios of proteins to this standard were comparable across all measured samples. The use of the same internal standard in all measurements thus further adds to the robustness of the PCP approach.
Fig. 2.
High reproducibility of MS measurements for the LD PCP. The ratios of the heavy and light labeled versions of each protein from two independent measurements are plotted against each other for each fraction of the purification. The high correlation of the data points in the scatterplots indicates that differences in the amounts of proteins from light versus heavy labeled samples can be detected with high reproducibility. Pearson correlations and regression lines are shown in red.
LD PCP Yields Distinct Purification Profiles for LDs and Other Organelles
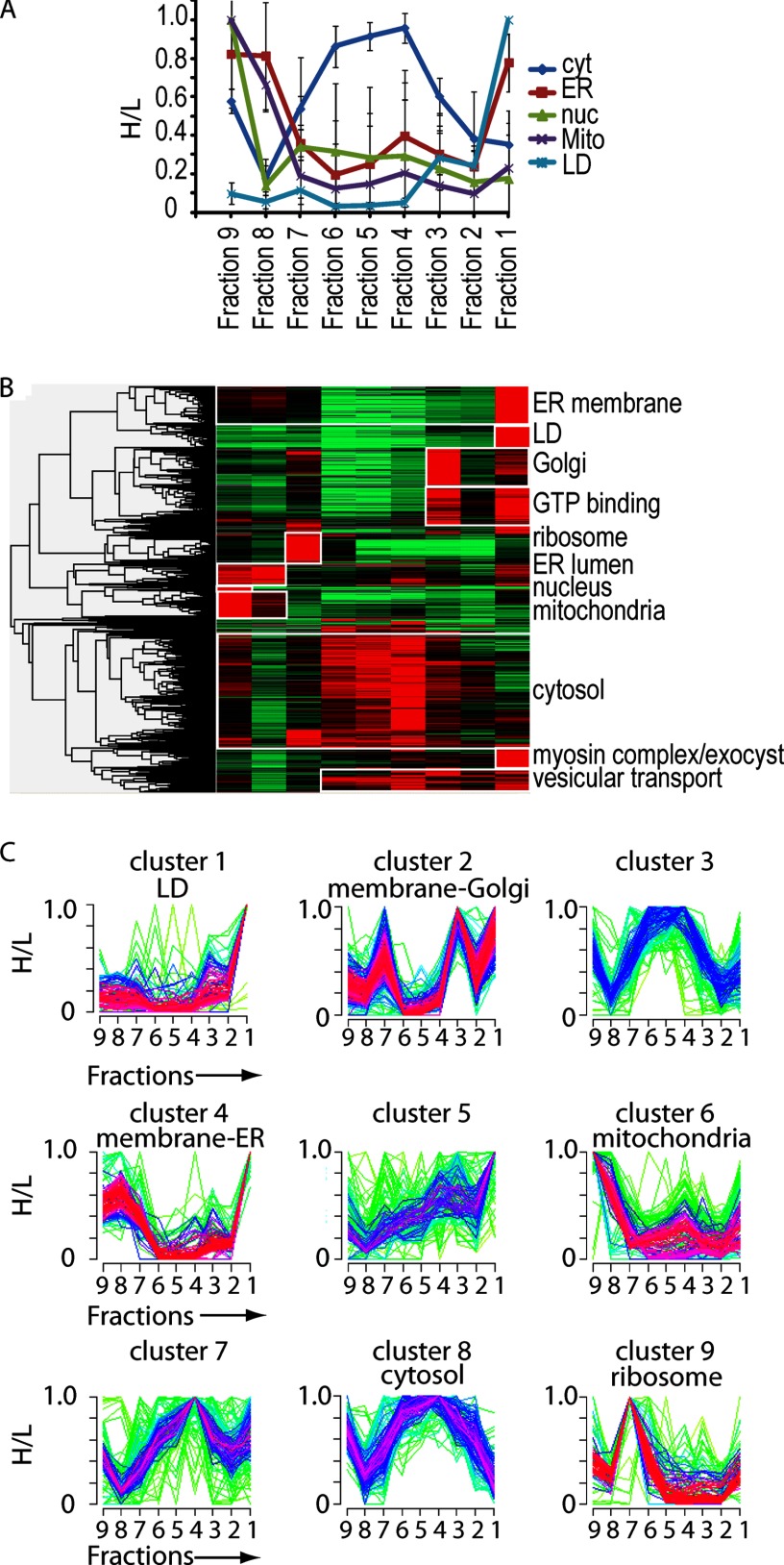
We next tested whether the LD PCP segregated organelles into distinct fractions. To compare the profiles of individual proteins, we set the maximum ratio obtained to 1 for each protein and normalized the ratios in the remaining fractions relative to this (H/L). We first analyzed the averaged profiles of known marker proteins for abundant organelles, including the nuclei, the ER, mitochondria, and LDs. The individual abundance profiles of the marker proteins are shown in supplemental Table S2 and are plotted in supplemental Fig. S2. Each of the organelle profiles was distinct from each of the others and from profiles of cytosolic proteins (Fig. 3A). Moreover, LDs peaked in the top fraction of the gradient centrifugation, as expected, whereas proteins of organelles characterized by higher density, such as the ER and Golgi apparatus, peaked in the denser fractions. We cannot exclude the possibility that fragments of these organelles might float with LDs. However, as proteins of other organelles have their main abundance in other fractions, such contaminants are nonetheless efficiently subtracted from bona fide LD proteins.
Fig. 3.
Identification of LD proteins in S2 cells via LD PCP. A, averaged fractionation profiles for different organelle marker proteins. The average normalized ratios for marker proteins of the indicated cellular organelles, as determined via quantitative LC-MS/MS, are shown for fractions of an LD purification. Values are mean ± S.D. of five proteins. B, hierarchical clustering of H/L for proteins identified in fraction 1 of LC-MS/MS analysis of all fractions of a LD purification with Perseus (39). At least five of nine valid values per protein were required. The color code represents the protein ratio H/L normalized by the maximum value among the fractions. Gray indicates that the protein was not identified in that fraction. Clusters enriched for proteins of certain organelles are marked with white boxes. Cellular organelles whose proteins were enriched in a certain cluster are indicated on the right. C, soft clustering of proteins identified in fraction 1 of LC-MS/MS analysis of all fractions of an LD purification with Mfuzz in R (41). Proteins identified in the LD fraction that were identified in at least five of the nine fractions were analyzed. A normalized H/L was used. The cluster number C was set at 9, and cluster stability m = 1.75. Clusters that showed enrichment of proteins of a certain organelle or function are indicated. Proteins with a minimal membership value of 0.3 for LD cluster 1 are shown in supplemental Table S2.
We next analyzed the PCP results for the LD fraction. Because MS analysis identifies the most abundant peaks in a spectrum, the lower abundance of some proteins can result in stochastic absences of measurements of their peptides in some fractions. To offset this potential problem, we filtered our data for proteins that were detected in at least five of the nine purification fractions. Of the 1481 proteins identified in the LD fraction, 1389 met these filtering criteria.
To assess the relative behavior of different proteins with the LD PCP method, we applied hierarchical clustering of the filtered profiles (peptides in at least five fractions). This analysis revealed specific clusters with distinct purification profiles (Fig. 3B), as expected for proteins purifying as part of an organelle. This contrasts with a continuous spectrum of purification profiles, as would be expected if proteins distributed independently of one another. Analysis of the clusters by gene ontology showed that many of them had an enrichment of marker proteins of a predominant organelle (Fig. 3B).
Among the proteins in the LD fraction, we found some that peaked in abundance in a different fraction and thus likely were part of a different organelle. Particularly, ER membrane proteins were abundant in the LD fraction (fraction 1), possibly because of the close association between ER and LDs, but they also had a major peak in fraction 8. In contrast, ER luminal proteins had a peak with cytosolic proteins (fractions 2–4), in addition to the ER membrane peak in fraction 8, likely indicating the release of luminal proteins into the cytosol during purification.
The application of hierarchical clustering to LD PCP profiles has a limitation: the process assigns a protein to one organelle class, even if there are two different localizations of the protein (e.g. LDs and ER). To overcome this limitation, we analyzed our data via soft clustering, which generates clusters of typical profiles and assigns to each protein a membership value for each cluster (41). The membership value (a value between 0 and 1) provides a measurement of the similarity of each protein profile to the core proteins of the cluster. Thus, it measures the likelihood that a protein belongs to a cluster. For example, the similarity of a protein's profile to the LD profile is expressed as an “LD membership value” for each protein, which is an indication of the likelihood that a particular protein is an LD protein (supplemental Table S3).
LD PCP Reliably Identifies LD Proteins
Soft clustering identified a profile (“cluster 1”) that was typical of LD proteins (Fig. 3C, supplemental Table S3). Of the 1389 proteins identified in the LD fraction with more than five valid values in all fractions, only 111 showed an LD-specific purification profile with a membership value > 0.3 for the LD cluster. This indicates that only 7.5% of the proteins identified in this fraction were candidates for bona fide LD proteins, corresponding to roughly 0.74% of all Drosophila genes. Soft clustering additionally identified groups of proteins representing other organelles (e.g. “cluster 2” contains primarily proteins of the Golgi apparatus).
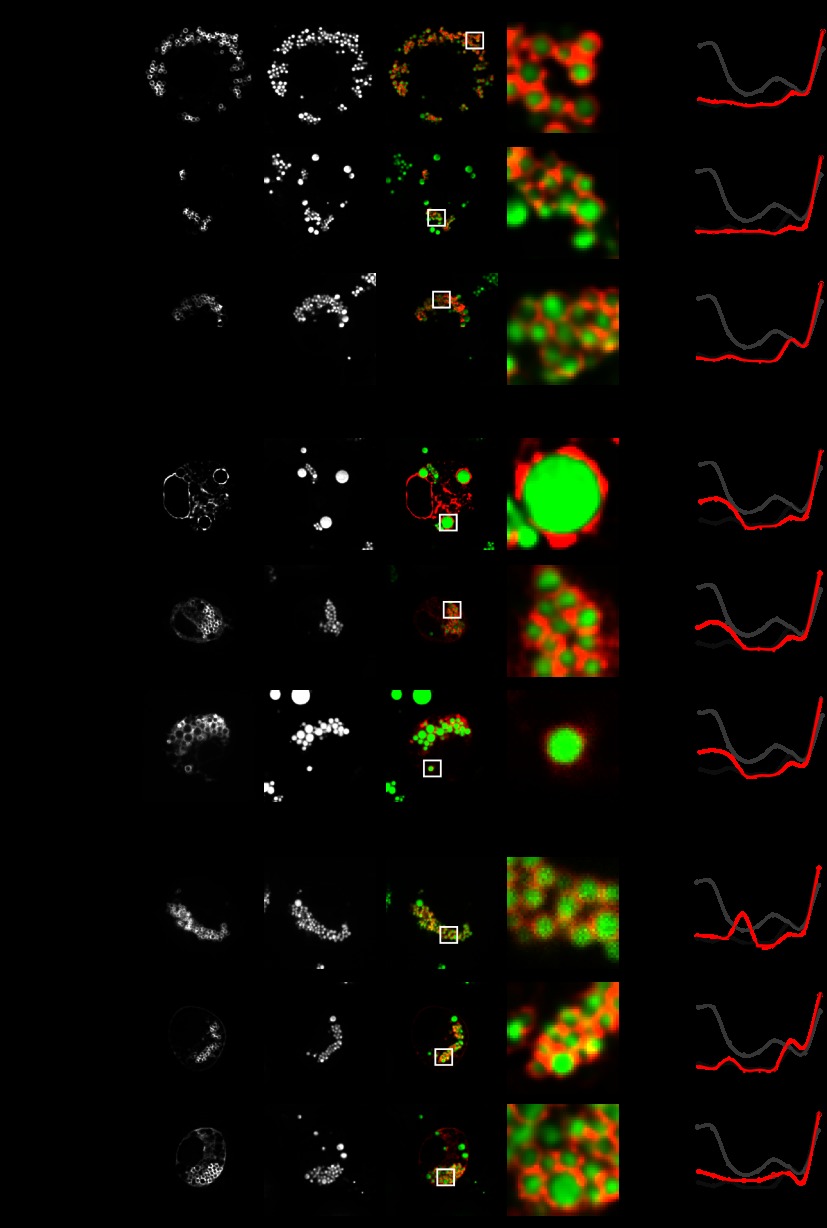
To test whether the proteins identified by PCP as LD proteins were indeed LD proteins, we analyzed their subcellular distribution by means of fluorescence microscopy of expressed proteins in Drosophila S2 cells. Of the 111 candidates, we randomly picked 20 proteins (supplemental Table S4) for analysis. Confirming the PCP analysis, 18 of the 20 proteins (90%) showed a characteristic ring-like staining pattern around LDs (examples can be seen in Figs. 4A–4C). The other two proteins, encoded by CG5014 (VAP33) and CG33113 (reticulon), showed fluorescent aggregates in most cells and a reticular ER pattern in a few cells. It is not clear whether the observed aggregation and localization to other organelles might be due to tagging and/or overexpression of these proteins, rather than a reflection of their normal subcellular distribution. Alternatively, these two proteins might not be genuine LD proteins. Moreover, considering that the overexpression of fluorescently tagged proteins might lead to spill-over to other organelles, it is difficult to distinguish bona fide LD proteins from proteins with dual cellular localization. Therefore, PCP might be advantageous over microscopy for tagged proteins, as it measures the distribution of endogenous proteins.
Fig. 4.
Localization of a subset of proteins found in the LD proteome as determined via fluorescent microscopy of expressed proteins. The indicated fluorescent mCherry-tagged enzymes were transiently expressed in S2 cells (left-hand panels, red) loaded with 1 mm oleate for 12 h. LDs were stained with BODIPY (middle panels, green). The overlays of the two channels and zoomed views of a representative LD section are shown (rightmost two panels). Bar = 5 μm (overview) or 1 μm. Protein correlation profiles for the tagged proteins are shown in the right-hand panels in red. Dark gray lines indicate averaged PCPs for LD proteins, and bright gray lines indicate averaged protein correlation profiles for ER proteins (Fig. 3A). A, proteins localizing exclusively to LDs. B, proteins localizing to LDs and ER. C, proteins localizing to LDs and any other cellular compartment.
Of the remaining 18 proteins, 4 showed only LD-ring signal in fluorescent microscopy, and we classified these as having an “LD-only” localization (Fig. 4A, supplemental Fig. S3A). In contrast, nine other proteins contained an additional small peak in the fraction containing mostly ER proteins and also showed a reticular pattern, likely representing an ER signal, under fluorescence microscopy. We classified these proteins as “LD + ER” (Fig. 4B, supplemental Fig. S3B). In these cases, tagged proteins might localize to both the ER and LDs, either because they have a dual localization or because the LD surface is saturated and cannot accommodate additional protein. Alternatively, LD localization in these cases might be due to the spill-over of overexpressed protein from the ER. The remaining five proteins showed a fluorescence signal around LDs, but also some signal that likely represented an organelle other than the ER, such as the plasma membrane (see Fig. 4C or supplemental Fig. S3C CG17292 for an example), and had small additional peaks in the LD PCP that did not overlap with the ER fraction. We classified those proteins as localized to “LD + other” organelles.
Comparing the LD PCP and Other LD Proteome Studies
Several proteomics studies have analyzed the composition of LDs in different cell types and species (10, 11, 13, 16–19, 21). No data are available on the LD composition of Drosophila cell lines, although proteomic data were generated by two conventional MS-based proteomics from LDs purified from Drosophila larval fat body or embryos (10, 11). Relative to the other Drosophila studies, the sensitivity of our measurements was much greater (in total, 1481 proteins identified in the LD fraction, in comparison to 645 and 247 proteins). In contrast, however, our LD PCP strategy filtered out contaminants and thereby defined with high confidence a much smaller set of LD proteins (111). This suggests that more than 90% of the proteins typically identified in an LD fraction via MS are likely contaminants. Not surprisingly, therefore, the two LD proteomes of Drosophila fat body and larvae overlap by only 14.4% (112 of a total 780 proteins). In comparing our results with the previously published Drosophila studies, we found little overlap (Fig. 5A, Table II). For example, we found 10 and 24 proteins overlapping with those identified in the studies of Beller et al. and Cermelli et al., respectively (10, 11).
Fig. 5.
Comparison of the LD PCP with other proteomic studies and analysis for enriched pathways. A, comparison of the LD proteome with published Drosophila LD proteomes (10, 11). Venn diagram shows overlap between the three studies. B, overrepresentation of specific pathways among LD-protein-associated functions. Groups of proteins with related functions detected on LDs are shown.
Table II. Overlap between LD proteomes.
| Protein name/function | CG |
|---|---|
| Cermelli et al., 2006 (11) | |
| 44DEa, fatty-acid ligase activity | CG8732 |
| Saccheropin dehydrogenase | CG5167 |
| Atlastin | CG6668 |
| Abhydrolase 1, CGI-58 | CG1882 |
| Saccheropin dehydrogenase | CG2604 |
| Hormone sensitive lipase | CG11055 |
| Brummer | CG5295 |
| Amidase | CG8839 |
| Lipase | CG9186 |
| Glycosyltransferase | CG1291 |
| Amidase | CG5112 |
| Kugelkem | CG5175 |
| Toutatis | CG10897 |
| Prenyltransferase | CG10778 |
| Fatp | CG7400 |
| Fatty acyl-CoA reductase | CG8303 |
| Vap-33–1 | CG5014 |
| Rab8 | CG8287 |
| SNF4A, Loechrig, kinase | CG17299 |
| Rab7 | CG5915 |
| Beller et al., 2006 (10) | |
| Reticulon | CG33113 |
| Saccheropin dehydrogenase | CG5167 |
| Saccheropin dehydrogenase | CG2604 |
| Brummer | CG5295 |
| Lipase | CG9186 |
| Alpha esterase | CG1112 |
| Fatty acid desaturase | CG5887 |
| Cytochrome b5 | CG2140 |
| Fatty-acid ligase activity | CG3961 |
| All three studies | |
| Saccheropin dehydrogenase | CG5167 |
| Saccheropin dehydrogenase | CG2604 |
| Brummer | CG5295 |
| Lipase | CG9186 |
Proteins identified in LD PCP and in one of the published Drosophila LD proteome studies.
Comparing the LD PCP Proteome with Genes Identified via Functional Screens
We and others reported high-content genome-wide screens for LD phenotypes after depletion of each Drosophila protein from cell lines via RNAi (34, 43). In addition, screens in Drosophila larvae (44) and adult flies (45) identified genes that function in fat storage. We expected that some of the LD proteins we identified would perform important functions for normal LD morphology and/or fat storage. To identify such proteins, we analyzed the data from our LD PCP proteome for overlap with genes previously found in screens for LD-related phenotypes (34, 43–45). We found relatively little overlap between the data sets (Table III), showing that many proteins influencing LD morphology are not localized to the organelle. Reciprocally, many LD proteins function in cellular processes that are independent of LD morphology. Moreover, proteins playing crucial roles in LD function might have redundantly functioning homologs, precluding a strong LD phenotype when only one of the proteins is depleted.
Table III. LD proteins with LD phenotype.
| Protein name | CG | Phenotype | Study |
|---|---|---|---|
| SelT-like protein | CG3887 | Moderately altered LD morphology in S2 cells; more, smaller, more dispersed LDs | Guo et al., 2008 (34) |
| CCT1 | CG1049 | Dramatically altered LD morphology in S2 cells; fewer, larger LDs | Guo et al., 2008 (34) |
| CCT2 | CG18330 | Dramatically altered LD morphology in S2 cells; fewer, larger LDs | Guo et al., 2008 (34) |
| Rab8 | CG8287 | Altered mean signal in automated analysis in S2 | Guo et al., 2008 (34) |
| Brummer | CG5295 | Overstorage in S2 cells | Beller et al., 2008 (43) |
| Mes2 | CG11100 | Overstorage in S2 cells | Beller et al., 2008 (43) |
| Lyso-PA acyltransferase | CG32699 | Increased fat storage in Drosophila larvae | Reis et al., 2010 (44) |
| Fatp | CG7400 | Increased fat storage in Drosophila larvae | Reis et al., 2010 (44) |
| Cytochrome P450 6a2 | CG9438 | Reduced triacylglycerol levels in adult Drosophila | Pospisilik et al., 2010 (45) |
| Alpha-esterase-7 | CG1112 | Reduced triacylglycerol levels in adult Drosophila | Pospisilik et al., 2010 (45) |
| Myosin V | CG2146 | Elevated triacylglycerol levels in adult Drosophila | Pospisilik et al., 2010 (45) |
Proteins identified in the LD PCP whose deletion leads to an alteration in LD morphology or lipid metabolism found in different published screens.
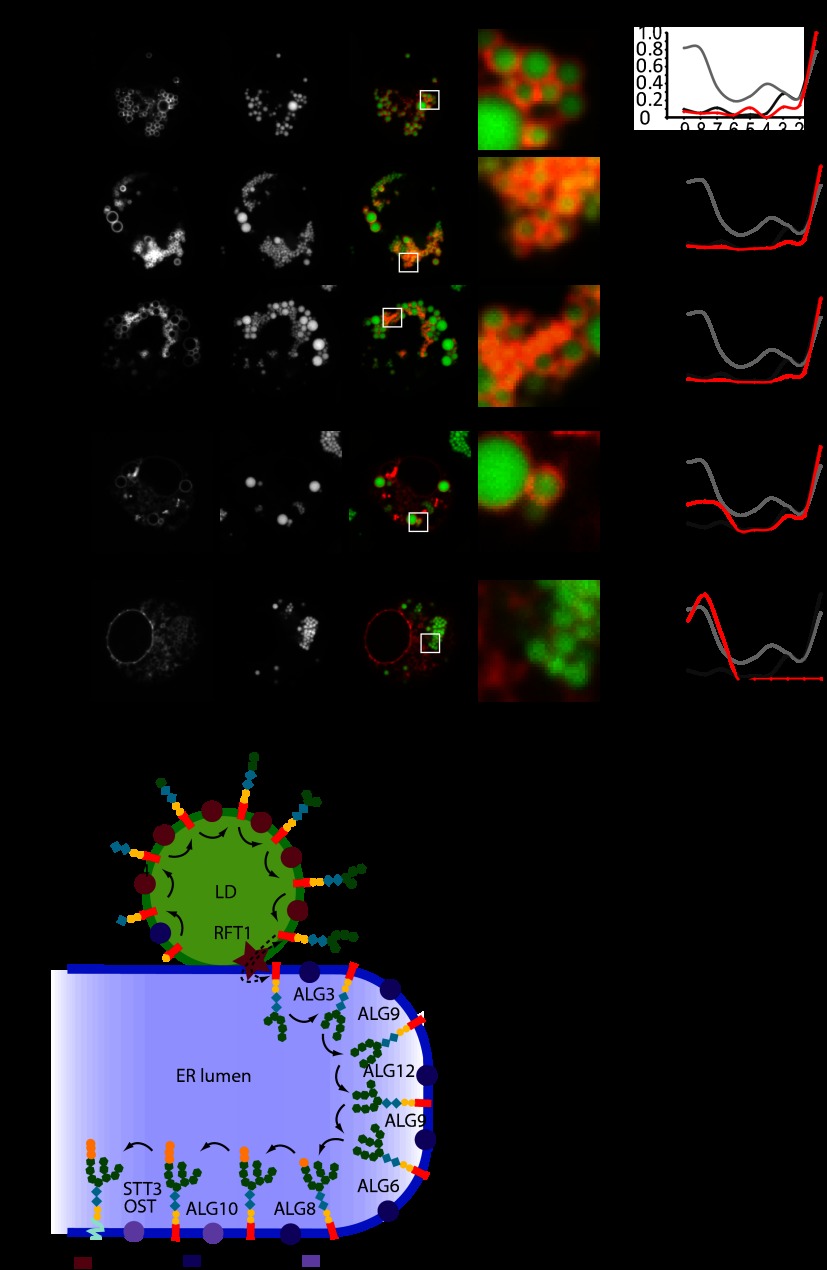
Our LD PCP identified groups of proteins participating in the biological processes that have not been previously linked to LDs (Fig. 5B). For example, we found a transcription factor, enzymes of lysine metabolism, and parts of the machinery generating dolichol-linked glycans for protein glycosylation on LDs.
To further test the reliability of the LD PCP and to assay for the localization of the N-glycan synthesis machinery, we fluorescently tagged all enzymes of dolichol-linked glycan synthesis indentified via MS and compared the localization of the tagged proteins with their profiles generated via PCP (Fig. 6A). In each of the tested cases, localization of the tagged enzymes via fluorescence microscopy correlated well with their assignment to LDs or the ER based on the correlation profiles of the LD PCP. Alg1, Alg2, and Alg14 had a clear LD profile in the LD PCP and exclusively localized to LDs via fluorescence microscopy. Alg11, showing a strong peak in the LD and a smaller peak in the ER fraction, showed dual localization between LDs and ER. In contrast, Alg10, which had an ER profile in the LD PCP, was present in a reticular pattern excluded from LDs, likely representing the ER. Alg3 and Alg9, which were not detected in the LD PCP, also localized in an ER pattern (data not shown). These findings show that specifically, enzymes catalyzing the early steps of glycan synthesis for N-linked protein modification localize to LDs. Furthermore, these data further confirm that our approach reliably distinguishes between LD and ER proteins.
Fig. 6.
Several N-glycan biosynthesis enzymes localize to LDs. A, the indicated mCherry-tagged proteins of the N-glycan synthesis pathway were expressed and analyzed as described for Fig. 4. PCPs of the indicated proteins are shown on the left. B, organization of the N-glycan biosynthesis on LDs and ER.
DISCUSSION
In this paper, we describe a quantitative proteomics approach based on LD purification and MS that employs SILAC and PCP, similar to what has been employed for other organelles (29), to efficiently determine an LD proteome with a high degree of confidence. The combination of a careful purification scheme and PCP methodology enabled us to extract from the complete LD-containing fraction a set of just over 100 LD proteins with significant confidence. This approach overcomes the limitations of classical fractionation approaches that assign proteins to an organelle based solely on their presence in a purified organelle fraction, rather than co-enrichment with an organelle. Importantly, although our approach relies on cellular fractionation, which can yield false organelle protein identification as result of changes in the behavior of disrupted membranes, we were able to confirm almost all tested candidate proteins for LD localization via fluorescence microscopy, increasing our confidence in our methodology. Our results included some previously identified proteins, validating our approach, and the procedure excluded other proteins as contaminants and identified novel proteins that might function at the LDs.
We focused our analysis on LDs in Drosophila S2 cells as proof of principle for the methodology. These cells provide a well-established system for studying LD cell biology and have been used in high-content, microscopy-based RNAi screens for LD phenotypes (34, 43). Moreover, the ease and efficiency of gene expression knockdown by RNAi in S2 cells allows future analyses of LD proteomes after the depletion of various cellular factors.
Our LD PCP results yielded several expected proteins with important LD functions. For example, we found CCT1 and CCT2, the isoenzymes catalyzing the rate-limiting step of de novo phosphatidylcholine synthesis, which we had previously found to localize to LDs (46). Similarly, as expected, we identified Brummer, the Drosophila homolog of the major triglyceride lipase ATGL. In addition, we identified a number of proteins that were not previously known as LD proteins but which are functionally connected to LDs. For example, our LD PCP results identified lysophosphatidic acid acyltransferase, which was among 66 genes that resulted in increased larval fat mass when inactivated (44). In addition, the overlay of hit lists from genetic screens and our LD PCP identified several proteins not previously implicated in LD biology. For example, we identified SELT, a glycosyltransferase, which results in smaller, more dispersed LDs when depleted (34), and Mes2, a transcription factor highly expressed in fat body (47, 48) that leads to triacylglycerol over-storage when depleted (43). The localization of Mes2 on LD suggests a signaling function in transmitting information from LDs to the nucleus. We also identified Rab8, a small GTPase involved in vesicular trafficking that was found in a genome-wide screen of genes involved in LD morphology (43).
A comparison of our proteomes with conventional proteomic studies of Drosophila larval fat body or total larvae LDs (10, 11) showed little overlap between the identified proteins. This could be due to either variations between larval and S2 cell LD proteomes or a larger number of contaminants in the conventional proteomes than in the LD PCP. The latter possibility is supported by the presence of many proteins in the conventional proteome results that have been localized primarily to other organelles, such as ribosomal components or mitochondrial proteins. However, assessing a priori whether a protein is a contaminant based on its known function is challenging. This is highlighted by the unexpected finding that histones, with a well-defined nuclear function, are important LD proteins in developing Drosophila embryos (11).
In addition to expected proteins, such as CCT and Brummer, we found a number of interesting proteins that had been reported in other studies. For example, the LD PCP and conventional proteomes found an as yet uncharacterized protein with homology to lipases (CG9186 (11)). Moreover, prenyltransferase and fatty acid desaturases were detected among other lipid metabolism enzymes by means of both LD PCP and conventional proteomics. Most likely, these enzymes are abundant and thus have been identified in a number of proteomic studies. Intriguingly, all three LD proteomics studies identified saccheropin dehydrogenase on LDs, suggesting a role for LDs in lysine metabolism. We also found several proteins that were previously not known to localize to LDs. Among them, CG17292 is particularly interesting, as it contains a lipase motif and is highly expressed in Drosophila oenocytes, a cell type that functions similarly to hepatocytes (49). This finding, together with data suggesting that the enzyme has retinyl esterase activity in vitro,2 suggests a function for CG17292 in lipid metabolism.
Unexpectedly, the enzymes catalyzing the early steps of the N-linked protein glycosylation pathway were found in the LD PCP, and we confirmed their localization via fluorescence microscopy. In this pathway, glycans are first sequentially added to a dolichol lipid moiety. This process is thought to occur on the cytosolic side of the ER. The resulting glycolipid then flips across the membrane in a poorly characterized reaction to function as a substrate for oligosaccharyltransferase, which transfers the glycan to asparagine residues of ER-lumen exposed proteins. Here, we found specifically that the enzymes that build the glycan tree on dolichol were enriched on LDs (Fig. 6B). In addition, we detected the homolog of Rft1, proposed to be involved in flipping the dolichol-glycan across the ER bilayer (50), only in LD fraction 1, and not any other fraction, suggesting that it might also be enriched strongly on LDs. Previously, these enzymes were thought to catalyze their reactions on the cytoplasmic side of the ER (51). Interestingly, a previous study in yeast found Dpm1, the enzyme catalyzing the first step of glycan synthesis, on LDs (52). These data suggest a link between N-linked glycosylation and LD biology. Of note, dolichol isoprenoid sidechains are particularly extended lipids that might be more easily accommodated at LDs than at the ER bilayer membrane.
In addition to proteins that we found to be exclusively enriched with LDs, our dataset also revealed proteins that have a dual localization. Such proteins have a pool localized on the LD surface, leading to an abundance peak in the LD fraction, and an additional pool acting at a different cellular site, leading to an additional peak in a different fraction of the purification. Examples of such proteins are the members of the COPI machinery, the lysosomal NPC2 protein, and epsin, which thus might have dual localizations in the cell, with a pool of each protein localized to LDs. The identification of such proteins might aid in elucidating the processes that mediate the interaction between organelles and might shed light on the role of such junctions in lipid metabolism.
The LD PCP methodology described herein can be easily adapted for the analysis of LD proteomes of a wide range of samples. Also, LD PCP can be combined with dynamic measurements (e.g. to determine changes in proteome composition after the treatment of cells with different stimuli). In such an experiment, one can include another LD fraction, with a third SILAC condition. Changes in the abundance ratio between heavy and medium versions of a protein would then indicate changes in the LD proteome. For determining LD proteomes in tissues, SILAC labeling of the sample is not easily feasible, although it has been achieved for whole animals (53, 54). Alternatively, for such cases an LD standard can be obtained from a closely related cell line. This approach, using a labeled hepatocyte cell line, was productive in the analysis of the phosphoproteomic response to liver insulin signaling (55). In cases when a single cell type does not reflect the composition of the tissue cells well, a “Super-SILAC” approach, in which extracts of several cell lines are mixed to reflect the tissue proteome composition (56), can also be used to generate an LD standard for use in an LD PCP of tissue samples. Thus, the LD PCP is an approach with which one can confidently indentify LD proteins that are isolated from a wide range of sources, including cells and tissues, and studied under dynamic conditions.
In summary, we have established a robust method with which to identify LD proteins based on the subtraction of contaminants using PCP combined with subcellular fractionation. By applying this LD PCP method to Drosophila cells, we have revealed expected LD proteins, but we also have identified novel LD proteins and uncovered potential links between LDs and other cellular processes, such as protein glycosylation. We expect that this methodology will be widely applicable to LD research and will help define the dynamic proteome of this important metabolic organelle in different tissues and metabolic states.
Supplementary Material
Acknowledgments
We thank Nadin Neuhauser and Nagarjuna Nagaraj for assistance in data processing and members of the Walther and Farese laboratories for critical comments on the manuscript, as well as Gary Howard for editorial assistance.
Footnotes
* This work was supported by Grant No. R01 GM097194 to T.C.W. R.V.F. was supported by Grant No. RO1 GM099844 and the Gladstone Institutes. F.W. is a fellow of the Boehringer Ingelheim Fonds.”
 This article contains supplemental material.
This article contains supplemental material.
2 N. Krahmer, T. C. Walter, M. Kumari, and R. Zechner, unpublished observation.
1 The abbreviations used are:
- ER
- endoplasmic reticulum
- LD
- lipid droplet
- MS
- mass spectroscopy
- PCP
- protein correlation profile
- SILAC
- stable isotope labeling of amino acids in cell culture.
REFERENCES
- 1. Bensimon A., Heck A. J., Aebersold R. (2012) Mass spectrometry-based proteomics and network biology. Annu. Rev. Biochem. 81, 379–405 [DOI] [PubMed] [Google Scholar]
- 2. Yan W., Aebersold R., Raines E. W. (2009) Evolution of organelle-associated protein profiling. J. Proteomics 72, 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walther T. C., Mann M. (2010) Mass spectrometry-based proteomics in cell biology. J. Cell. Biol. 190, 491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farese R. V., Jr., Walther T. C. (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139, 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujimoto T., Parton R. G. (2011) Not just fat: the structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 3, a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brasaemle D. L., Wolins N. E. (2012) Packaging of fat: an evolving model of lipid droplet assembly and expansion. J. Biol. Chem. 287, 2273–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herker E., Ott M. (2011) Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol. Metab. 22, 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenberg A. S., Coleman R. A., Kraemer F. B., McManaman J. L., Obin M. S., Puri V., Yan Q. W., Miyoshi H., Mashek D. G. (2011) The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 121, 2102–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bozza P. T., Viola J. P. (2010) Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fatty Acids 82, 243–250 [DOI] [PubMed] [Google Scholar]
- 10. Beller M., Riedel D., Jänsch L., Dieterich G., Wehland J., Jäckle H., Kühnlein R. (2006) Characterization of the Drosophila lipid droplet subproteome. Mol. Cell. Proteomics 5, 1082–1094 [DOI] [PubMed] [Google Scholar]
- 11. Cermelli S., Guo Y., Gross S. P., Welte M. A. (2006) The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 16, 1783–1795 [DOI] [PubMed] [Google Scholar]
- 12. Pu J., Ha C. W., Zhang S., Jung J. P., Huh W. K., Liu P. (2011) Interactomic study on interaction between lipid droplets and mitochondria. Protein Cell 2, 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279, 46835–46842 [DOI] [PubMed] [Google Scholar]
- 14. Ding Y., Wu Y., Zeng R., Liao K. (2012) Proteomic profiling of lipid droplet-associated proteins in primary adipocytes of normal and obese mouse. Acta Biochim. Biophys. Sin. (Shanghai) [DOI] [PubMed] [Google Scholar]
- 15. Larsson S., Resjo S., Gomez M. F., James P., Holm C. (2012) Characterization of the lipid droplet proteome of a clonal insulin-producing beta-cell line (INS-1 832/13). J. Proteome Res. 11, 1264–1273 [DOI] [PubMed] [Google Scholar]
- 16. Zhang H., Wang Y., Li J., Yu J., Pu J., Li L., Zhang S., Peng G., Yang F., Liu P. (2011) Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J. Proteome Res. 10, 4757–4768 [DOI] [PubMed] [Google Scholar]
- 17. Blouin C. M., Le Lay S., Eberl A., Kofeler H. C., Guerrera I. C., Klein C., Le Liepvre X., Lasnier F., Bourron O., Gautier J. F., Ferre P., Hajduch E., Dugail I. (2010) Lipid droplet analysis in caveolin-deficient adipocytes: alterations in surface phospholipid composition and maturation defects. J. Lipid Res. 51, 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartz R., Zehmer J. K., Zhu M., Chen Y., Serrero G., Zhao Y., Liu P. (2007) Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 6, 3256–3265 [DOI] [PubMed] [Google Scholar]
- 19. Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. (2004) Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 279, 3787–3792 [DOI] [PubMed] [Google Scholar]
- 20. Wu C. C., Howell K. E., Neville M. C., Yates J. R., III, McManaman J. L. (2000) Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis 21, 3470–3482 [DOI] [PubMed] [Google Scholar]
- 21. Zhang P., Na H., Liu Z., Zhang S., Xue P., Chen Y., Pu J., Peng G., Huang X., Yang F., Xie Z., Xu T., Xu P., Ou G., Zhang S. O., Liu P. (2012) Proteomic study and marker protein identification of caenorhabditis elegans lipid droplets. Mol. Cell. Proteomics, 11, 318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blanchette-Mackie E. J., Scow R. O. (1983) Movement of lipolytic products to mitochondria in brown adipose tissue of young rats: an electron microscope study. J. Lipid Res. 24, 229–244 [PubMed] [Google Scholar]
- 23. Shaw C. S., Jones D. A., Wagenmakers A. J. M. (2008) Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem. Cell Biol. 129, 65–72 [DOI] [PubMed] [Google Scholar]
- 24. Jacquier N., Choudhary V., Mari M., Toulmay A., Reggiori F., Schneiter R. (2011) Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Sci. 124, 2424–2437 [DOI] [PubMed] [Google Scholar]
- 25. Ozeki S., Cheng J., Tauchi-Sato K., Hatano N., Taniguchi H., Fujimoto T. (2005) Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J. Cell Sci. 118, 2601–2611 [DOI] [PubMed] [Google Scholar]
- 26. Martin S., Driessen K., Nixon S. J., Zerial M., Parton R. G. (2005) Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J. Biol. Chem. 280, 42325–42335 [DOI] [PubMed] [Google Scholar]
- 27. Liu P., Bartz R., Zehmer J. K., Ying Y. S., Zhu M., Serrero G., Anderson R. G. (2007) Rab-regulated interaction of early endosomes with lipid droplets. Biochim. Biophys. Acta 1773, 784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Binns D., Januszewski T., Chen Y., Hill J., Markin V. S., Zhao Y., Gilpin C., Chapman K. D., Anderson R. G., Goodman J. M. (2006) An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 173, 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andersen J. S., Mann M. (2006) Organellar proteomics: turning inventories into insights. EMBO Rep. 7, 874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen J. S., Lam Y. W., Leung A. K., Ong S. E., Lyon C. E., Lamond A. I., Mann M. (2005) Nucleolar proteome dynamics. Nature 433, 77–83 [DOI] [PubMed] [Google Scholar]
- 31. Andersen J. S., Lyon C. E., Fox A. H., Leung A. K., Lam Y. W., Steen H., Mann M., Lamond A. I. (2002) Directed proteomic analysis of the human nucleolus. Curr. Biol. 12, 1–11 [DOI] [PubMed] [Google Scholar]
- 32. Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426, 570–574 [DOI] [PubMed] [Google Scholar]
- 33. Foster L. J., de Hoog C. L., Zhang Y., Xie X., Mootha V. K., Mann M. (2006) A mammalian organelle map by protein correlation profiling. Cell 125, 187–199 [DOI] [PubMed] [Google Scholar]
- 34. Guo Y., Walther T. C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J. S., Vale R. D., Walter P., Farese R. V. (2008) Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453, 657–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonaldi T., Straub T., Cox J., Kumar C., Becker P. B., Mann M. (2008) Combined use of RNAi and quantitative proteomics to study gene function in Drosophila. Mol. Cell 31, 762–772 [DOI] [PubMed] [Google Scholar]
- 36. Brasaemle D. L., Wolins N. E. (2006) Isolation of lipid droplets from cells by density gradient centrifugation. Curr. Protoc. Cell. Biol. Chapter 3, Unit 3 15 [DOI] [PubMed] [Google Scholar]
- 37. Rappsilber J., Ishihama Y., Mann M. (2003) Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 [DOI] [PubMed] [Google Scholar]
- 38. Rappsilber J., Mann M., Ishihama Y. (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 39. Cox J., Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 40. Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V., Mann M. (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 [DOI] [PubMed] [Google Scholar]
- 41. Futschik M. E., Carlisle B. (2005) Noise-robust soft clustering of gene expression time-course data. J. Bioinform. Comput. Biol. 3, 965–988 [DOI] [PubMed] [Google Scholar]
- 42. Ong S. E., Blagoev B., Kratchmarova I., Kristensen D. B., Steen H., Pandey A., Mann M. (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 43. Beller M., Sztalryd C., Southall N., Bell M., Jäckle H., Auld D. S., Oliver B. (2008) COPI complex is a regulator of lipid homeostasis. PLoS Biol. 6, e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reis T., Van Gilst M. R., Hariharan I. K. (2010) A buoyancy-based screen of Drosophila larvae for fat-storage mutants reveals a role for Sir2 in coupling fat storage to nutrient availability. PLoS Genet. 6, e1001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pospisilik J. A., Schramek D., Schnidar H., Cronin S. J., Nehme N. T., Zhang X., Knauf C., Cani P. D., Aumayr K., Todoric J., Bayer M., Haschemi A., Puviindran V., Tar K., Orthofer M., Neely G. G., Dietzl G., Manoukian A., Funovics M., Prager G., Wagner O., Ferrandon D., Aberger F., Hui C. C., Esterbauer H., Penninger J. M. (2010) Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140, 148–160 [DOI] [PubMed] [Google Scholar]
- 46. Krahmer N., Guo Y., Hilger M., Lingrell S., Wilfling F., Heger K., Newman H. W., Schmid-Supprian M., Vance D. E., Mann M., Farese R. V., Jr., Walther T. C. (2011) Localized activation of CTP: phosphocholine cytidyltransferase (CCT) is required for phosphotidylcholine synthesis during lipid droplet expansion. Cell. Metab. 14(4), 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chintapalli V. R., Wang J., Dow J. A. (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715–720 [DOI] [PubMed] [Google Scholar]
- 48. Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., Yang L., Artieri C. G., van Baren M. J., Boley N., Booth B. W., Brown J. B., Cherbas L., Davis C. A., Dobin A., Li R., Lin W., Malone J. H., Mattiuzzo N. R., Miller D., Sturgill D., Tuch B. B., Zaleski C., Zhang D., Blanchette M., Dudoit S., Eads B., Green R. E., Hammonds A., Jiang L., Kapranov P., Langton L., Perrimon N., Sandler J. E., Wan K. H., Willingham A., Zhang Y., Zou Y., Andrews J., Bickel P. J., Brenner S. E., Brent M. R., Cherbas P., Gingeras T. R., Hoskins R. A., Kaufman T. C., Oliver B., Celniker S. E. (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gutierrez E., Wiggins D., Fielding B., Gould A. P. (2007) Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445, 275–280 [DOI] [PubMed] [Google Scholar]
- 50. Helenius J., Ng D. T., Marolda C. L., Walter P., Valvano M. A., Aebi M. (2002) Translocation of lipid-linked oligosaccharides across the ER membrane requires Rft1 protein. Nature 415, 447–450 [DOI] [PubMed] [Google Scholar]
- 51. Schwarz F., Aebi M. (2011) Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582 [DOI] [PubMed] [Google Scholar]
- 52. Takeda Y., Nakano A. (2008) In vitro formation of a novel type of membrane vesicles containing Dpm1p: putative transport vesicles for lipid droplets in budding yeast. J. Biochem. 143, 803–811 [DOI] [PubMed] [Google Scholar]
- 53. Kruger M., Moser M., Ussar S., Thievessen I., Luber C. A., Forner F., Schmidt S., Zanivan S., Fassler R., Mann M. (2008) SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell 134, 353–364 [DOI] [PubMed] [Google Scholar]
- 54. Sury M. D., Chen J. X., Selbach M. (2010) The SILAC fly allows for accurate protein quantification in vivo. Mol. Cell. Proteomics 9, 2173–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Monetti M., Nagaraj N., Sharma K., Mann M. (2011) Large-scale phosphosite quantification in tissues by a spike-in SILAC method. Nat. Methods 8, 655–658 [DOI] [PubMed] [Google Scholar]
- 56. Geiger T., Cox J., Ostasiewicz P., Wisniewski J. R., Mann M. (2010) Super-SILAC mix for quantitative proteomics of human tumor tissue. Nat. Methods 7, 383–385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.