Abstract
There is a critical need to identify biomarkers for Systemic Lupus Erythematosus (SLE) which has a high prevalence of renal failure. When urine from patients with lupus nephritis was recently screened for the levels of ∼280 molecules using an exploratory array-based proteomic platform, elevated angiostatin levels were noted. Angiostatin is a bioactive fragment of plasminogen, and has been known to have modulatory function in angiogenesis and inflammation. The significant elevation in urinary angiostatin was next validated in an independent cohort of SLE patients (n = 100) using ELISA. Among patients with SLE, urine angiostatin was significantly increased in active SLE compared with inactive SLE, correlating well with the SLEDAI disease activity index and SLICC renal activity score (r = 0.66, p < 0.0001). ROC curve analysis further confirmed that urinary angiostatin had the capacity to discriminate patients with active SLE from those with inactive disease. Patients with Class IV lupus nephritis exhibited the highest levels of urinary angiostatin. Immunohistochemistry staining localized angiostatin expression to the renal tubular cells in these patients. Finally, when paired urine-kidney samples procured concurrently from patients with LN were next examined, urine angiostatin levels correlated strongly with the renal pathology chronicity index, but not with the activity index. Given that Class IV lupus nephritis and renal pathology chronicity changes forebode poor renal and patient survival, urinary angiostatin emerges as a novel noninvasive marker of renal disease in SLE. Longitudinal studies are in progress to further assess the disease-predictive potential of urinary angiostatin.
Systemic lupus erythematosus (SLE)1 is a chronic autoimmune disease capable of causing devastating clinical manifestations such as kidney failure. Approximately 60% of SLE patients present with lupus nephritis (1); of these, about 10–15% of patients will eventually progress to end-stage renal disease (ESRD) (1). It has been known that early diagnosis of lupus nephritis can offer a better opportunity to control disease progression. Therefore, an early biomarker for diagnosing lupus nephritis is highly desirable in the renal clinic. Currently, renal biopsy is still the gold standard for diagnosing kidney disease because despite its invasive nature, this method allows accurate detection of the severity of renal pathology. Besides the risk of infection and other clinical complications associated with needle biopsy, this method of retrieving renal tissue might also have limitations in acquiring representative kidney specimens reflective of real pathological changes. Serum biomarkers are relatively less invasive; however, serological measurement of anti-dsDNA, C3, C4, and other protein markers does not necessarily correlate well with renal disease.
Urine biomarkers have emerged in recent years and have proven effective in reflecting disease activity in lupus nephritis. Potential biomarkers include IL-6 (2), IL-18 (3), MCP-1 (4), VCAM-1 (5, 6), NGAL (7, 8), and TWEAK (9, 10). Indeed urine may be by far the best source for screening biomarkers for kidney diseases for several reasons. First, urine samples are easily obtained and are noninvasive. Second, because urine is a direct product of the kidney, urine biomarkers may be a direct reflection of renal function. Nevertheless, the ideal urine biomarker for monitoring lupus nephritis (LN) remains elusive.
A limited number of urinary proteomic studies in lupus nephritis have been reported to date. Initial indications are that this approach will open up new avenues for discovery of novel urinary biomarkers of this disease. Mosley et al. (2006) identified unique mass spectral patterns utilizing SELDI-TOF mass spectrometry which could discriminate urine samples from patients with inactive and active lupus nephritis (11). However, the proteins represented by these spectra have not yet been identified. By using a similar technology, Zhang et al. (2008) identified hepcidin as a potential urinary biomarker of lupus nephritis (12). Our previous proteomic study of urine markers in murine immune nephritis included a more comprehensive interrogation of the urinary proteome (13). In that study, a number of potentially important urinary markers were identified by two-dimensional (2D)-gel electrophoresis followed by mass spectrometry (13). Among these urine markers, a number of angiogenesis-related proteins emerged including angiotensinogen, renin, angiostatin, and plasminogen activator inhibitor 1 (13). This is particularly important because angiogenesis-related factors, including VEGF-A (14), VEGFR1 (15–17), VEGFR2 (16), angiopoietin-1, and angiopoietin-2, have been linked to the progression of chronic kidney diseases (CKD) (18).
Angiostatin is a proteolytic fragment of plasminogen, and has been found to be protective in cancer growth through the blockade of angiogenesis via inhibition of migration and proliferation of endothelial cells (19, 20). In addition to the murine studies (13), a second array based study in human lupus nephritis also indicated that urine angiostatin may be elevated in lupus nephritis, as described below. Thus, this study is designed to assess whether elevated urinary angiostatin levels are indicative of renal disease in SLE, using a cross-sectional study design.
MATERIALS AND METHODS
Patients
Patients were recruited from the renal clinics at Parkland and St. Paul University Hospitals of the University of Texas Southwestern Medical Center at Dallas. All patient-related procedures were performed strictly following institution-approved IRB protocols. Five SLE patients were used for a pilot study using a protein screening array, as described below. Validation studies were performed using serum and urine samples from an independent cohort of SLE patients (n = 100) using an orthogonal method. Gender and age-matched healthy volunteers were also recruited for blood draw and urine collection, and used as controls. Patients with other CKD were also recruited and used as disease controls. The inclusion criterion was all SLE patients who fulfilled the ACR classification criteria for SLE. The exclusion criteria were patients in renal failure and children with SLE. Detailed information pertaining to the SLE patients studied, including their demographics, co-morbidities and medications, is listed in Table I. All biofluids were procured and processed as detailed elsewhere (5).
Table I. Demographics and clinical characteristics of patients used for the validation studies. Serum creatinine levels and proteinuria/urine creatinine ratio: CKD controls: 1.85 ± 0.22 mg/dL and 2.73 ± 0.84; Healthy controls: 0.72 ± 0.15 mg/dL and 0.4 ± 0.11.
| No. | 100 |
|---|---|
| Female, no. (%) | 86 (86) |
| Age, mean +/− S.E., years | 35.8 ± 1.1 |
| Race, African American/Hispanic/Caucasian, no. | 45/42/13 |
| SLEDAI, median (interquartile) | 10 (4–16) |
| Renal SLEDAI, median (interquartile) | 4 (4–8) |
| No. of patients with renal SLEDAI = 0 (%) | 20 (20) |
| Protein:creatinine ratio, mg/mg, mean +/− S.E. | 2.0 ± 0.3 |
| Serum Cr, mg/dl, mean +/− S.E. | 1.5 ± 0.1 |
| Comorbidities, no. (%) | |
| Diabetes Mellitus | 10 (10) |
| Hypertension | 75 (75) |
| Dyslipidemia | 58 (58) |
| Cardiovascular disease | 13 (13) |
| Anemia | 67 (67) |
| Antiphospholipid syndrome | 2 (2) |
| Venous thromboembolism | 8 (8) |
| Others | 50 (50) |
| Current medications, no. (%) | |
| Prednisone | 71 (71) |
| Mycophenolic acid | 36 (36) |
| Cyclophosphamide | 7 (7) |
| Azathioprine/MTX | 13 (13) |
| Cyclosporine/Tacrolimus | 3 (3) |
| Hydroxychloroquine | 47 (47) |
| Angiotensin blocking agents | 49 (49) |
Sample Collection and Preparation
Urine sample collection: Midstream clean-catch urine samples were collected. Patients are requested to first cleanse the urethral area with a towelette, then to void the first portion of the urine stream into the toilet. The urine midstream is then collected into a clean BD Vacutainer Brand specimen cup (Franklin Lakes, NJ USA). A urinalysis was performed on the urine samples, and the remaining sample was centrifuged at 10,000 × g for 2 min at 4 °C. The supernatant was divided into 1-ml aliquots and frozen at −80 °C for storage. Each aliquot of urine was retrieved and thawed only once for the assays in this study.
Serum Sample Collection
Whole blood was collected in BD Vacutainer Serum tubes (Cat #: 367812). Tubes were incubated undisturbed at room temperature for 20 min, and then centrifuged at 3000 rpm for 10 min at 4 °C. The supernatant (serum) was divided into 200-μl aliquots and frozen at −80 °C for storage. Each aliquot of serum was retrieved and thawed only once for the assays in this study.
Protein Array
The urine samples used for the initial array-based screen were comprised of three healthy individuals (mean age, 35; three females, all Hispanic), and five patients with SLE (mean age, 40.5; three Hispanic females, one African American female, and one African American male; all five with active renal disease with SLE disease activity index [SLEDAI] ≥16 and renal-related SLE disease activity index [rSLEDAI] ≥8). These urine samples were diluted 5-fold into sample buffer (1% bovine serum albumin in phosphate-buffered saline) and hybridized to glass slide arrays that interrogate the level of 274 different human proteins including angiostatin. Initial biomarker screening was conducted using the RayBio® Human Cytokine Antibody Array G-Series 4000 (Cat# AAH-CYT-G4000–8), which consists of 8 subarrays in one slide and allows for the interrogation of one sample per subarray. Briefly, monoclonal antibodies against various cytokines (or other mediators) were printed on the slides as bait to capture the corresponding cytokines (or other mediators) in urine or serum, and then incubated with a mixture of biotinylated secondary antibodies, and then detected with Cy3 labeled streptavidin. Each analyte was assayed in duplicate. The slides were then scanned using a GenePix 4000B scanner (Molecular Devices). Signals were acquired and transformed to digits using Genepix software. In the array, Positive Control spots (POS1, POS2, POS3) comprised of standardized amounts of biotinylated IgGs printed directly onto the array. All other variables being equal, the Positive Control intensities should be the same for each subarray. This allows for normalization of results from different subarrays (or samples). Also included on the array were Negative Control (NEG) spots consisting of buffer alone (used to dilute antibodies printed on the array). The presence of analytes was marked by signal intensities that exceeded two standard deviations above the mean background signal intensity. GenePix PMT was 33% and gain setting was 550 for all scans in this study.
Validation Assay
Serum and urine samples obtained from the renal clinics at Parkland and St. Paul Hospitals (Dallas, TX) were aliquoted prior to storage at −80 °C. Only one aliquot was retrieved for each assay to avoid multiple freeze/thaw cycles. Urine and serum angiostatin levels were measured using a precoated human angiostatin ELISA kit from Raybiotech Inc. (Norcross, GA). Urine creatinine was measured using a creatinine assay kit from Cayman Chemical (Ann Arbor, MI). Urinary angiostatin was normalized against urine creatinine.
SLICC Renal Activity Score and Estimated Glomerular Filtration Rate (eGFR)
SLICC (Systemic Lupus International Collaborating Clinics) renal activity score was computed as described by Petri M (2008) (21). When calculating the SLICC score, spot urine protein/creatinine ratios were used as estimates of total daily proteinuria, as described elsewhere (21). The SLICC score was computed as described by Petri et al. (22): proteinuria 0.5–1 g/day (3 points), proteinuria >1–3 g/day (5 points), proteinuria >3 g/day (11 points). Also factored in were urine red blood cell count >10/high-power field (3 points), and urine white blood cell count >10/high-power field (1 point). Estimated Glomerular Filtration Rate (eGFR) was calculated using the eGFR calculator from the National Kidney Foundation (http://www.kidney.org).
Immunohistochemistry
Renal biopsies from patients at different stages of kidney disease were collected from the renal clinics at UT Southwestern Medical Center. Totally, 15 patients were evaluated for angiostatin expression in the kidney by immunohistochemistry: controls, n = 3; Class II, n = 4; Class IV, n = 8. Briefly, 10% buffered, formalin-fixed, paraffin-embedded 4 μm tissue sections were prepared. After dewax and rehydration, tissue sections were microwaved for 15 min in 10 mmol/L citrate buffer (pH 6.0). Rabbit anti human Angiostatin pAb (Abcam Inc, Cambridge, MA, USA, ab2904) was used at 1:400 dilutions for overnight incubation at 4 °C. Then the specimens were stained using a biotin-free immunoenzymatic antigen detection system (EXPOSE mouse/Rabbit specific HRP/DAB IHC kit, Abcam Inc, USA). The sections were then counterstained with hematoxylin to visualize cell nuclei. Rabbit isotype control IgG (Abcam Inc, MA, USA, ab27478) at the same dilution was used as control. The expression of angiostatin was quantified by counting the number of positive tubules in 15 random fields for each slide.
Western Blot
Serum and urine levels of angiostatin or other plasminogen fragments were detected using Western blot. Serum and urine samples were denatured in a sample buffer containing 25 mm Tris base, 190 mm glycine and 0.1% SDS, pH8.3 and loaded to SDS-PAGE. Proteins were then transferred to polyvinylidene difluoride membrane followed by incubation with a rabbit polyclonal antibody against Angiostatin (reactive to human) from Abcam, Catalog # 2904. The detailed procedure of Western blot was described elsewhere (23).
Statistics
Data was plotted and analyzed using GraphPad Prism 5 (GraphPad, San Diego, CA) or Medcalc software (Mariakerke, Belgium). A t test was used where the normality test passed; otherwise, the nonparametric Mann-Whitney test was used to analyze the data. Likewise, the Pearson method or the nonparametric Spearman method was used for correlation analyses.
RESULTS
Antibody-based Protein Array Screening Indicated a Dramatic Increase in Urinary Angiostatin in Lupus Nephritis
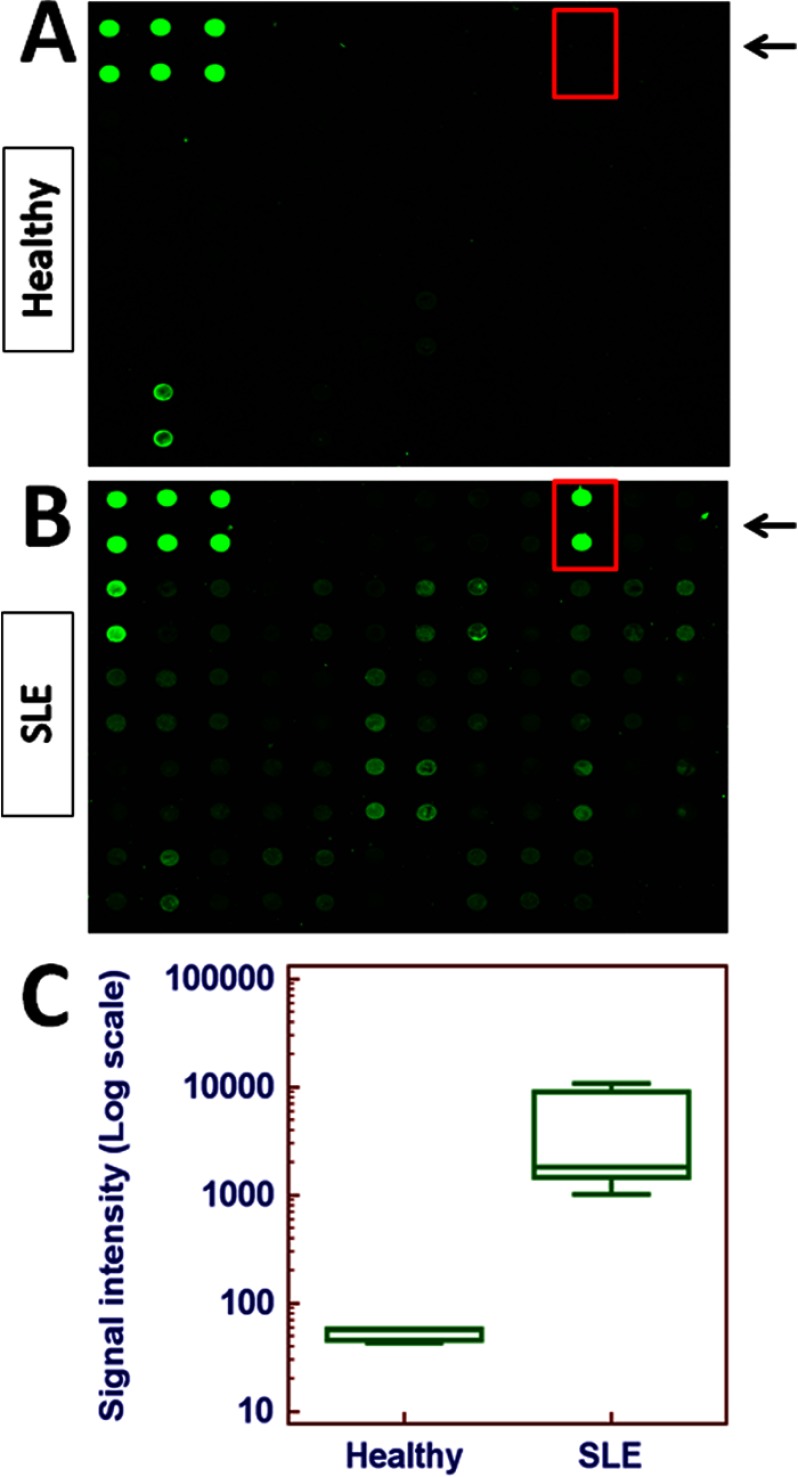
In order to identify novel urinary biomarkers of lupus nephritis, an antibody-based protein array was utilized to scan urine samples from patients with lupus nephritis (n = 5) or healthy controls (n = 3). Among the molecules screened, the levels of angiostatin was increased by almost two orders of magnitude in lupus nephritis samples compared with healthy controls (p = 0.0011) (Fig. 1). Hence, the rest of the study focuses on this molecule.
Fig. 1.
Antibody-based protein array screening of urine samples from patients with lupus nephritis. Representative images of one array containing angiostatin (in red-lined box) are shown for (A) a healthy control and, (B) a patient with lupus nephritis. This array consists of eight sub-arrays that allow detection of 274 proteins of interest simultaneously. Urine samples from SLE patients (n = 5) and healthy controls (n = 3) were applied to the array and the fluorescent signal was captured and quantified using a Genepix 4000B instrument and software. C, Quantitative results were plotted and analyzed using Medcalc software (Mariakerke, Belgium). Expression of angiostatin was increased by almost two orders of magnitude in lupus nephritis samples compared with healthy controls (p = 0.0011).
Validation Study of Elevated Angiostatin in a Larger Panel of SLE Patients
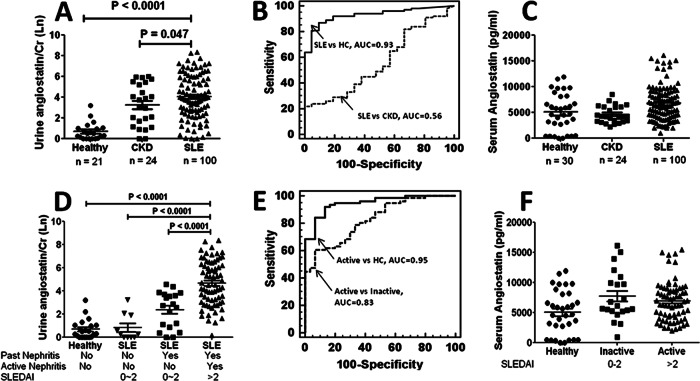
To determine whether angiostatin levels are significantly increased in SLE, an independent cohort of SLE patients (n = 100) was recruited and used to validate the array data shown in Fig. 1. In addition to healthy controls, a CKD control group was also included. Clearly, urinary angiostatin was significantly increased in SLE patients compared with healthy controls (p < 0.0001), (Fig. 2A). In comparison to the urinalysis, there was not a significant increase of angiostatin in the serum of SLE patients compared with healthy controls (Fig. 2C). Not surprisingly, the difference in overall urinary angiostatin levels did not reach significance between SLE and the CKD controls, indicating angiostatin might also be involved in other types of chronic kidney diseases besides lupus nephritis. Receiver Operating Curve analysis (Fig. 2B) further confirmed that urinary angiostatin might be used to discriminate SLE patients from healthy controls (AUC = 0.93), but not from other CKD patients (AUC = 0.56).
Fig. 2.
Validation of urinary angiostatin as a marker in a larger independent cohort of SLE patients (n = 100), chronic kidney disease (CKD) patients (n = 24), and healthy controls (n = 21). A, Urinary angiostatin levels as determined by ELISA are expressed as the natural logarithm of the absolute values of urinary angiostatin (pg/ml) normalized against urine creatinine levels. B, ROC curve analysis was performed and the area-under-the curve (AUC) was used to assess the sensitivity and specificity of urinary angiostatin in discriminating SLE from healthy controls or CKD controls. C, Serum angiostatin levels were measured in samples from the same subjects shown in (A) and (B), with SLE patients (n = 100), CKD controls (n = 24), and healthy controls (n = 30) using ELISA. D, SLE patients were divided into inactive and active groups according to SLEDAI and renal SLEDAI values. Inactive SLE: SLEDAI = 0–2, rSLEDAI = 0; active SLE: SLEDAI > 2, rSLEDAI > 0. Urinary angiostatin levels as determined by ELISA are expressed as the natural log of absolute values of urinary angiostatin (pg/ml) normalized against urine creatinine levels. E, The sensitivity and specificity of urinary angiostatin in discriminating active SLE from inactive SLE or healthy controls were assessed using the AUC in a ROC curve analysis. F, Serum angiostatin levels were also measured in the same SLE patients described above.
Urinary Angiostatin is Able to Discriminate Active SLE from Inactive SLE
After determining that urinary angiostatin is significantly increased in SLE, we next asked whether urinary angiostatin levels reflect disease severity. We further divided the SLE patients into an inactive SLE group (SLEDAI ≤ 2, renal SLEDAI = 0), and an active SLE group (SLEDAI > 2, renal SLEDAI > 0; “lupus nephritis” or “LN”). Within the inactive SLE group, we further distinguished two subgroups: those with past history of nephritis and those without past nephritis. Compared with healthy controls, the urinary angiostatin level was significantly increased among the inactive SLE patients with past nephritis but not in the subgroup without past nephritis (p < 0.0001), (Fig. 2D). More importantly, angiostatin was further increased in active SLE compared with healthy controls (p < 0.0001), inactive SLE without past nephritis (p < 0.0001) and inactive SLE with past nephritis (p < 0.0001) (Fig. 2D), indicating that analysis of urinary angiostatin has the capacity to discriminate SLE patients with kidney damage from SLE patients without kidney damage. These results were further confirmed by ROC curve analysis, where AUC values were all above 0.75 (Active versus HC, 0.95; Active versus inactive, 0.83), (Fig. 2E). However, there was no significant difference in serum angiostatin levels between healthy, inactive SLE, and active SLE groups (Fig. 2F).
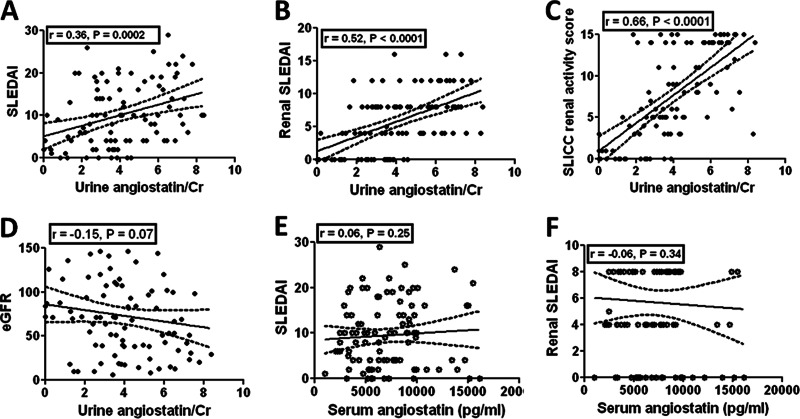
Urinary Angiostatin Positively Correlates With Lupus Disease Severity
The results above clearly demonstrate that the presence of urinary angiostatin is distinct between SLE and healthy subjects. Next, we wanted to ask whether urinary angiostatin could reflect the severity of disease in lupus. The results showed that urinary angiostatin levels positively correlate with SLEDAI (r = 0.36, p = 0.0002, Fig. 3A), renal SLEDAI (r = 0.52, p < 0.0001, Fig. 3B), and SLICC renal activity score (r = 0.68, p < 0.0001, Fig. 3C), but negatively with the estimated Glomerular Filtration Rate (eGFR) (r = −0.15, p = 0.07, Fig. 3D). These results strongly suggest that urinary angiostatin levels could potentially serve as a biomarker of renal disease activity. In comparison, serum angiostatin levels did not correlate with either SLEDAI (Fig. 3E) or the components of renal SLEDAI (Fig. 3F). We also examined the correlation of urine protein/creatinine ratio with disease severity in this cohort of SLE patients. We found that the urine protein/creatinine ratio correlated with SLEDAI (r = 0.28, p = 0.003), rSLEDAI (r = 0.39, p = 0.002), and SLICC renal score (r = 0.51, p < 0.0001).
Fig. 3.
Correlation of urinary angiostatin levels (as determined by ELSIA) with disease activity in SLE. Urinary angiostatin levels in SLE patients were correlated with (A) SLEDAI score, (B) Renal SLEDAI, (C) SLICC renal activity score, and (D) estimated Glomerular Filtration Rate. Plotted is the correlation between (E) serum angiostatin level and SLEDAI score, and (F) renal SLEDAI score. Correlation analysis was performed using Graphpad Prism software. “Renal SLEDAI” is a summation of the renal components of the SLEDAI activity measure.
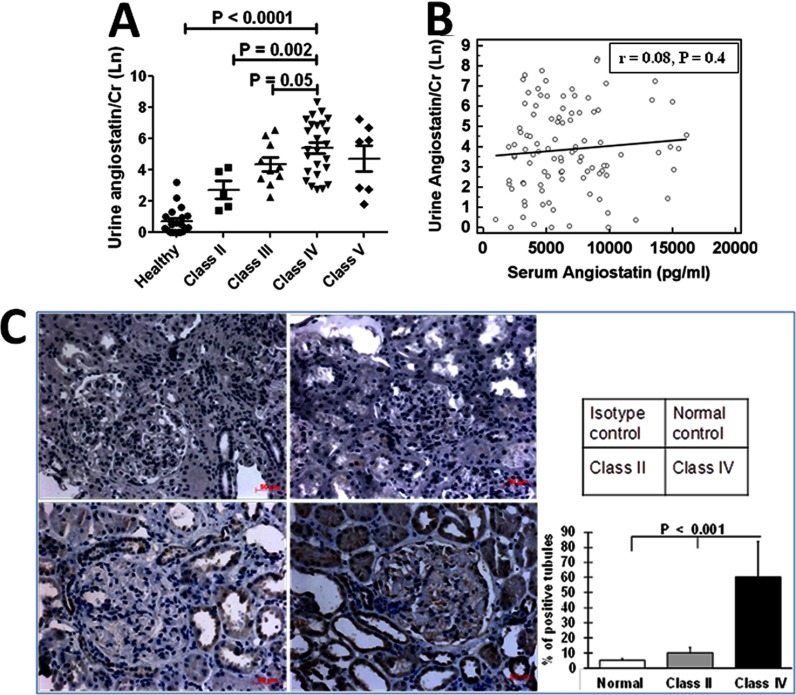
Urinary Angiostatin is Increasingly Elevated with the Deterioration of Renal Pathology
Renal biopsy, although invasive, is still the gold standard for renal disease diagnosis and prognostication. To this end, we wanted to determine whether urinary angiostatin has the capacity to reflect renal pathology and, if so, how well it could predict the degree of renal damage. For this analysis we chose SLE patients whose urine samples were collected within six months of renal biopsy, and then divided these patients into four groups according to their renal pathology; namely class II, class III, class IV (with or without accompanying class V), or class V. It was clear that the urinary angiostatin level is increasingly elevated as renal disease “progresses”, peaking at class IV (Fig. 4A). To investigate the potential source of the increased urine angiostatin in lupus nephritis, we compared urinary angiostatin levels with serum angiostatin levels, and found no correlation (r = 0.008, p = 0.40, Fig. 4B), suggesting that the elevated urine angiostatin is unlikely to be simply serum-derived. Of note, the level of serum angiostatin was not reflective of renal pathology (data not shown). Next, we used a rabbit antihuman angiostatin antibody to stain renal biopsies from patients with lupus nephritis. The renal expression of angiostatin was evaluated by a semi-quantitative analysis, in which the number of positively stained tubules was counted in 15 randomly selected fields for each slide. There was no angiostatin expression in normal human renal tissues (n = 3), and only modest angiostatin expression in Class II LN (n = 4). However, significantly increased angiostatin expression was observed in both the tubules and the glomeruli from Class IV LN renal tissues (n = 8) (Fig. 4C). Taken together, we surmise that serum is not the major source of the increased urine angiostatin. An alternative explanation is that the kidney, particularly the tubular cells, might either produce or enrich angiostatin locally. This molecule may then be excreted into the urine as a consequence of ensuing tubulo-interstitial renal damage.
Fig. 4.
Capacity of urinary angiostatin to reflect renal pathology in lupus nephritis. Forty-seven SLE patients whose urine was collected within six months of renal biopsy were subgrouped into Class II, Class III, Class IV, and Class V, following the WHO classification of renal pathology. ELISA-determined urinary angiostatin level in each class of patients is shown in (A), and the correlation between serum and urine angiostatin levels is shown in (B). C, Angiostatin expression in the kidney was examined using immunohistochemistry staining, using normal human kidney tissue, Class II and class IV lupus nephritis kidneys (n = 3- 4 each group; top left : isotype control; top right : normal human kidney; lower left : Class II LN and lower right: Class IV LN; Original magnification 400×). The angiostatin expression score is plotted in the right panel. There was no angiostatin expression in normal human kidneys and modest expression levels in the tubular epithelial cells in Class II LN. However, significantly enhanced angiostatin expression was found in both tubules and glomeruli from Class IV LN renal tissue (p < 0.001, two-tailed t test).
Effect of Drugs on Urinary Angiostatin Levels
In the current cohort of SLE patients, we have performed multivariate analysis, and we have found that the use of NSAID (nonsteroidal anti-inflammatory drugs) or prednisone was not associated with elevated urinary angiostatin in SLE. There was no significant difference in the urine angiostatin levels between those on prednisone (4.4 ± 2.3, natural log transformed value of urinary angiostatin/urine creatinine, the unit is abbreviated as “NLU” hereafter) versus those not on prednisone (3.6 ± 1.9 NLU), p > 0.05. Likewise, there was no significant difference in urine angiostatin levels between SLE patients on cytoxan/mycopholate/5-azacitidine/methotrexate (range of means: 3.8–4.1 NLU) versus those not on these immunosuppressives (ranges of means: 3.3–5.2 NLU). However, the small subset of patients who were not on any of these medications had significantly higher levels of urine angiostatin compared with those on any form of treatment (5.2 versus 3.6 NLU, p < 0.001), possibly reflecting disease amelioration by the drugs.
Urinary Angiostatin Reflects Renal Chronicity Changes in Lupus Nephritis in Concurrent Biopsy Samples
In order to evaluate precisely how well urinary angiostatin can predict particular changes in renal pathology, we collected urine samples from the patients on the same day renal biopsies were performed. We then measured urinary angiostatin levels and compared them with the renal pathology activity index and the renal pathology chronicity index in these paired urine/biopsy samples collected simultaneously. Renal pathology activity and chronicity indices were computed as described elsewhere (24, 25). The activity index is based on evaluation of six histologic parameters (i.e. glomerular endocapillary proliferation, glomerular leukocyte infiltration, glomerular subendothelial hyaline deposits, glomerular fibrinoid necrosis, or karyorrhexis, cellular crescents and interstitial inflammation), each graded on a scale of 0 to 3. A score of 0 = absent; 1 = <25% glomeruli affected; 2 = 25–50% glomeruli affected and 3 = >50% glomeruli affected. The scores for glomerular necrosis and cellular crescents were double-weighted because of their more ominous prognostic value. The sum (from 0 to 24) of each individual score represents the activity index. Likewise, chronicity index (from 0 to 12) was graded by summating the individual scores of four histologic features—glomerular sclerosis, fibrous crescents, tubular atrophy and interstitial fibrosis.
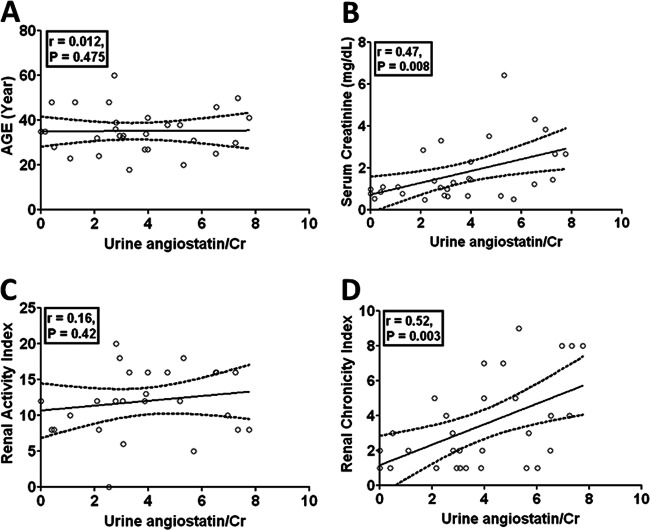
In these concurrent samples, we analyzed the correlation of urinary angiostatin with clinical predictors including age, sex, serum creatinine, renal chronicity index and real activity index. Our results clearly show that there is no association between urinary angiostatin levels and age (r = 0.012, p = 0.475, Fig. 5A) or sex (r = 0.092, p = 0.626) or renal pathology index (r = 0.16, p = 0.42, Fig. 5D). However, urinary angiostatin levels positively correlate with serum creatinine levels (r = 0.47, p = 0.008, Fig. 5B) and the renal pathology chronicity index (r = 0.52, p = 0.003, Fig. 5C). These results suggest that urinary angiostatin may be a convenient marker of chronicity changes in the kidney in patients with LN.
Fig. 5.
Assessment of urinary angiostatin in relation to the renal pathology activity and chronicity changes in concurrent renal biopsy samples. Urine samples were collected from 30 SLE patients at the time of renal biopsy. Urinary angiostatin was measured in these concurrent urine samples using ELISA, and then creatinine-normalized. Urinary angiostatin levels were correlated with age (A), serum creatinine (B), renal pathology activity index (C), and the renal pathology chronicity index (D), as scored by the pathologist in concurrent biopsy samples obtained at the time of urine collection.
DISCUSSION
Currently, there are two major challenges in the clinical management of lupus nephritis. First, there is the need for earlier diagnosis of lupus nephritis, specifically before the development of ESRD, which is irreversible and fatal. Early diagnosis makes it possible for early therapeutic intervention and a better outcome (26, 27). Second, noninvasive diagnosis of lupus nephritis is clearly an urgent need, in place of the traditional needle biopsy of the kidney, which carries the potential risk of infection and other complications. Given the fact that ∼60% of lupus patients eventually develop kidney disease (1), there is an urgent need to find solutions to these challenges. In recent years, there have been several studies demonstrating association of urine biomarkers with lupus nephritis, including CSF-1 (28), ICAM-1 (6); NGAL (8), TWEAK (9), OPN (29) and MCP-1 (29), ET-1 (30), transferring (13, 31), ceruloplasmin (13, 31), alpha1-acid-glycoprotein (31), lipocalin-type prostaglandin-D synthetase (l-PGDS)(13, 31), free light chain Ig (13, 32), VCAM-1 (5, 6, 33), CXCL16 (5), haptoglobin (34), adiponectin (35), and IL-6 (2). Urine biomarkers are attractive candidates as noninvasive alternatives in the diagnosis of lupus nephritis. Preferably, the selected urinary marker(s) must also accurately reflect underlying or ongoing renal pathology. However, most of the markers cited above have not been tested in relation to underlying renal pathology using concurrent kidney/urine paired samples procured at the same time.
To better correlate renal pathology results with urine marker levels, it is ideal to collect urine samples at the time of renal biopsy. However, in reality it is challenging to procure these concurrent samples. In this study, although a subset of the urine samples were obtained at the time of renal biopsy, others were obtained within a time interval of 6 months following the renal biopsy. These patients were on various medications, including a subset that were on immunosuppressives. This is clearly a cofounding factor and a limitation of the part of this study that did not examine concurrent urine/biopsy samples.
In the limited urine/renal biopsy concurrent samples from SLE patients (n = 30), we showed that urinary angiostatin correlate positively with the renal pathology chronicity index (r = 0.52, p = 0.003, Fig. 5A), suggesting that urinary angiostatin could potentially serve as a noninvasive renal pathology chronicity marker. These findings represent the first report of a urinary molecule identified to be a potential noninvasive, alternative marker of renal pathology chronicity in lupus nephritis. In addition, urine angiostatin emerges as a sensitive marker capable of discriminating patients with active disease from inactive SLE or healthy controls, with promising AUC values compared with previous biomarker candidates assessed similarly (8, 9, 29). The next step would be to test the disease predictive value of urine angiostatin in a longitudinal study cohort in order to establish if urine angiostatin is superior at predicting long term renal morbidity and mortality, compared with existing yard sticks and other competing candidates.
There are a couple of limitations in this study. First, although a subset of the urine samples were obtained at the time of renal biopsy, others were obtained within a time interval of 6 months following the renal biopsy. These patients were on various medications, including a subset who were on immunosuppressives. This is clearly a confounding factor and a limitation of the part of this study that did not examine concurrent urine/biopsy samples. It will be optimal to have more concurrent urine/renal biopsy samples, in order to increase the statistical power in the correlation analysis of urine angiostatin with concurrent renal pathology. Second, this study is cross-sectional in nature. It will be important to perform longitudinal studies to test if urine angiostatin can reflect disease activity, and samples are currently being assembled to accomplish this. Finally, it would also be important to expand the spectrum of disease controls examined, including other systemic autoimmune diseases and renal diseases. In this context, because urine angiostatin has been reported to be elevated in some patients with CKD, it may simply be a marker of renal disease, rather than being specific for SLE.
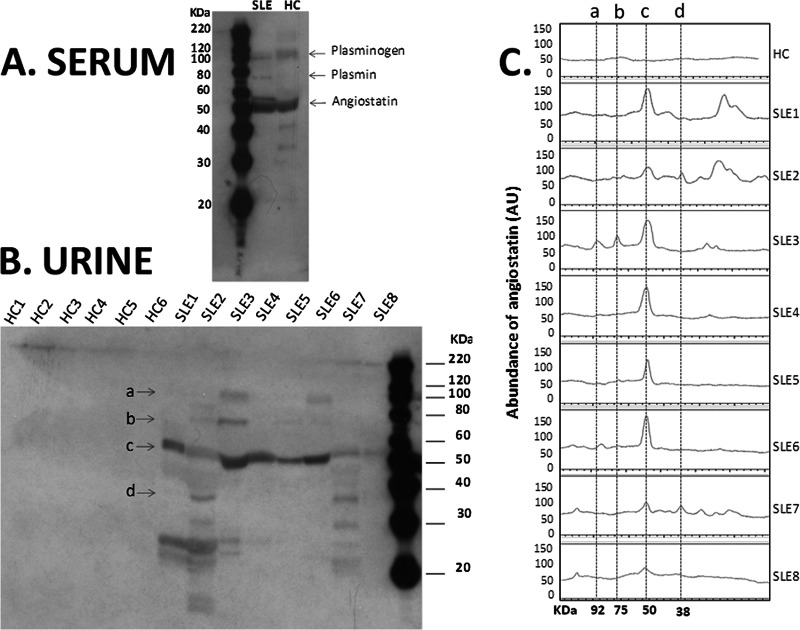
Human plasminogen is composed of heavy chain (amino-terminal) and light chain (carboxyl-terminal), and the heavy chain consists of 5 kringle (K) domains: K1, K2, K3, K4, and K5. On activation, fragments (e.g. plasmin, angiostatin or other kringles) are released from plasminogen (36). The kringle domains facilitate plasminogen binding to large substrates [e.g. fibrinogen (37)] as well as small molecule ligands [e.g. chloride ion (38)]. It has been shown that the activation of human plasminogen by human urinary urokinase is inhibited by Cl− at physiological concentrations (38). When Na+ absorption occurs in renal tubules, Cl− is also absorbed as a counter ion (39). Given the fact that the chloride concentration varies over a wide range during passage of the glomerular filtrate axially along the nephron, the activation of plasminogen via Cl−-binding could be significantly modulated. This in turn could affect the production of plasminogen fragments, such as plasmin and angiostatin in the urine. Although the major species detected in the urine of SLE patients appears to be angiostatin (rather than plasminogen or any of its other derivatives, Fig. 6), it remains unclear if and how variations in ionic concentrations in the glomerular filtrate may shape heightened angiostatin generation in SLE or other CKD.
Fig. 6.
Angiostatin and other plasminogen fragments in the serum and urine of lupus patients. Western blot analysis was performed to examine the serum (A) and urine (B) levels of angiostatin and other plasminogen fragments in lupus patients (SLE) and healthy controls (HC). Four major forms of plasminogen and its derivatives are indicated by the arrows “a” (plasminogen, ∼92 KDa), “b” (plasmin, ∼75 KDa), “c” (angiostatin K1–4, 50 KDa), and “d” (angiostatin K1–3, 38 KDa). Western blot images were further analyzed (C) using an ImageQuant TL software to quantify the peaks of the four major forms (a-d) of plasminogen and its derivatives.
The molecular mechanisms that underlie the secretion of angiostatin into the urine during lupus nephritis remain unknown. Angiostatin was first characterized by Folkman's group as an anti-angiogenesis molecule that inhibits the migration and proliferation of endothelial cells (EC) (19, 20). It is important to note that ectopic ATP synthase on the EC surface can recruit inflammatory cells and induce vascular inflammation. Angiostatin could exert anti-angiogenic effects by inhibiting ectopic ATP synthase on ECs (40). Taken together with the identification of other angiogenesis-related proteins in urine from lupus nephritis patients, such as angiotensinogen, renin, ceruloplasmin, and plasminogen activator inhibitor 1 (13), these findings suggest angiogenesis might play a role in the pathogenesis of renal diseases. Interestingly, other angiogenesis-related factors have also been implicated in the progression of chronic kidney diseases (CKD), including VEGF-A (14), VEGFR1 (15–17), VEGFR2 (16), angiopoietin-1, and angiopoietin-2 (18). Consistent with these findings, Mu et al. have attempted to treat diabetic rats with recombinant adeno-associated viruses expressing angiostatin, and their results indicated that overexpression of angiostatin might have therapeutic effect on diabetic nephropathy (41). Extrapolating from those findings, it is tempting to speculate that angiostatin might also be renoprotective in lupus nephritis, although this needs to be formally evaluated.
Footnotes
* This work is partly supported by NIH R01 DK81872, George M. O'Brien Kidney Research Core Center NIH P30DK079328.
1 The abbreviations used are:
- SLE
- systemic lupus erythematosus
- ESRD
- end stage renal disease
- CKD
- chronic kidney disease
- SLICC
- systemic lupus international collaborating clinics
- eGFR
- estimated glomerular filtration rate.
REFERENCES
- 1. Cameron J. S. (1999) Lupus nephritis. J. Am. Soc. Nephrol. 10, 413–424 [DOI] [PubMed] [Google Scholar]
- 2. Iwano M., Dohi K., Hirata E., Kurumatani N., Horii Y., Shiiki H., Fukatsu A., Matsuda T., Hirano T., Kishimoto T., et al. (1993) Urinary levels of IL-6 in patients with active lupus nephritis. Clin. Nephrol. 40, 16–21 [PubMed] [Google Scholar]
- 3. Migliorini P., Anzilotti C., Pratesi F., Quattroni P., Bargagna M., Dinarello C. A., Boraschi D. (2010) Serum and urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus erythematosus. Eur. Cytokine Netw. 21, 264–271 [DOI] [PubMed] [Google Scholar]
- 4. Marks S. D., Shah V., Pilkington C., Tullus K. (2010) Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr. Nephrol. 25, 2283–2288 [DOI] [PubMed] [Google Scholar]
- 5. Wu T., Xie C., Wang H. W., Zhou X. J., Schwartz N., Calixto S., Mackay M., Aranow C., Putterman C., Mohan C. (2007) Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J. Immunol. 179, 7166–7175 [DOI] [PubMed] [Google Scholar]
- 6. Abd-Elkareem M. I., Al Tamimy H. M., Khamis O. A., Abdellatif S. S., Hussein M. R. (2010) Increased urinary levels of the leukocyte adhesion molecules ICAM-1 and VCAM-1 in human lupus nephritis with advanced renal histological changes: preliminary findings. Clin. Exp. Nephrol. 14, 548–557 [DOI] [PubMed] [Google Scholar]
- 7. Brunner H. I., Mueller M., Rutherford C., Passo M. H., Witte D., Grom A., Mishra J., Devarajan P. (2006) Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 54, 2577–2584 [DOI] [PubMed] [Google Scholar]
- 8. Rubinstein T., Pitashny M., Levine B., Schwartz N., Schwartzman J., Weinstein E., Pego-Reigosa J. M., Lu T. Y., Isenberg D., Rahman A., Putterman C. (2010) Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology 49, 960–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz N., Rubinstein T., Burkly L. C., Collins C. E., Blanco I., Su L., Hojaili B., Mackay M., Aranow C., Stohl W., Rovin B. H., Michaelson J. S., Putterman C. (2009) Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res. Ther. 11, R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z. C., Zhou Q. L., Li X. Z., Yang J. H., Ao X., Veeraragoo P., Zuo X. X. (2011) Elevation of human tumor necrosis factor-like weak inducer of apoptosis in peripheral blood mononuclear cells is correlated with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Cytokine 53, 295–300 [DOI] [PubMed] [Google Scholar]
- 11. Mosley K., Tam F. W., Edwards R. J., Crozier J., Pusey C. D., Lightstone L. (2006) Urinary proteomic profiles distinguish between active and inactive lupus nephritis. Rheumatology 45, 1497–1504 [DOI] [PubMed] [Google Scholar]
- 12. Zhang X., Jin M., Wu H., Nadasdy T., Nadasdy G., Harris N., Green-Church K., Nagaraja H., Birmingham D. J., Yu C. Y., Hebert L. A., Rovin B. H. (2008) Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int. 74, 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu T., Fu Y., Brekken D., Yan M., Zhou X. J., Vanarsa K., Deljavan N., Ahn C., Putterman C., Mohan C. (2010) Urine proteome scans uncover total urinary protease, prostaglandin D synthase, serum amyloid P, and superoxide dismutase as potential markers of lupus nephritis. J. Immunol. 184, 2183–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masuda Y., Shimizu A., Mori T., Ishiwata T., Kitamura H., Ohashi R., Ishizaki M., Asano G., Sugisaki Y., Yamanaka N. (2001) Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am. J. Pathol. 159, 599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sawano A., Iwai S., Sakurai Y., Ito M., Shitara K., Nakahata T., Shibuya M. (2001) Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97, 785–791 [DOI] [PubMed] [Google Scholar]
- 16. Thomas S., Vanuystel J., Gruden G., Rodríguez V., Burt D., Gnudi L., Hartley B., Viberti G. (2000) Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J. Am. Soc. Nephrol. 11, 1236–1243 [DOI] [PubMed] [Google Scholar]
- 17. Hara A., Wada T., Furuichi K., Sakai N., Kawachi H., Shimizu F., Shibuya M., Matsushima K., Yokoyama H., Egashira K., Kaneko S. (2006) Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int. 69, 1986–1995 [DOI] [PubMed] [Google Scholar]
- 18. Futrakul N., Butthep P., Futrakul P. (2008) Altered vascular homeostasis in chronic kidney disease. Clin. Hemorheol. Microcirc. 38, 201–207 [PubMed] [Google Scholar]
- 19. O'Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Moses M., Lane W. S., Cao Y., Sage E. H., Folkman J. (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 [DOI] [PubMed] [Google Scholar]
- 20. O'Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Cao Y., Moses M., Lane W. S., Sage E. H., Folkman J. (1994) Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb. Symp. Quant. Biol. 59, 471–482 [DOI] [PubMed] [Google Scholar]
- 21. Petri M., Kasitanon N., Lee S. S., Link K., Magder L., Bae S. C., Hanly J. G., Isenberg D. A., Nived O., Sturfelt G., van Vollenhoven R., Wallace D. J., Alarcon G. S., Adu D., Avila-Casado C., Bernatsky S. R., Bruce I. N., Clarke A. E., Contreras G., Fine D. M., Gladman D. D., Gordon C., Kalunian K. C., Madaio M. P., Rovin B. H., Sanchez-Guerrero J., Steinsson K., Aranow C., Balow J. E., Buyon J. P., Ginzler E. M., Khamashta M. A., Urowitz M. B., Dooley M. A., Merrill J. T., Ramsey-Goldman R., Font J., Tumlin J., Stoll T., Zoma A. (2008) Systemic lupus international collaborating clinics renal activity/response exercise: development of a renal activity score and renal response index. Arthritis Rheum. 58, 1784–1788 [DOI] [PubMed] [Google Scholar]
- 22. Petri M., Kasitanon N., Singh S., Link K., Magder L., Bae S. C., Hanly J. G., Nived O., Sturfelt G., van Vollenhoven R., Wallace D. J., Alarcon G. S., Adu D., Avila-Casado C., Bernatsky S. R., Bruce I. N., Clarke A. E., Contreras G., Fine D. M., Gladman D. D., Gordon C., Kalunian K. C., Madaio M. P., Rovin B. H., Sanchez-Guerrero J., Steinsson K., Aranow C., Balow J. E., Buyon J. P., Ginzler E. M., Khamashta M. A., Urowitz M. B., Dooley M. A., Merrill J. T., Ramsey-Goldman R., Font J., Tumlin J., Stoll T., Zoma A. (2008) Systemic lupus international collaborating clinics renal activity/response exercise: comparison of agreement in rating renal response. Arthritis Rheum. 58, 1789–1795 [DOI] [PubMed] [Google Scholar]
- 23. Wu T., Qin X., Kurepa Z., Kumar K. R., Liu K., Kanta H., Zhou X. J., Satterthwaite A. B., Davis L. S., Mohan C. (2007) Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J. Clin. Invest. 117, 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin H. A., 3rd, Muenz L. R., Joyce K. M., Antonovych T. A., Kullick M. E., Klippel J. H., Decker J. L., Balow J. E. (1983) Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am. J. Med. 75, 382–391 [DOI] [PubMed] [Google Scholar]
- 25. Singh S., Wu T., Xie C., Vanarsa K., Han J., Mahajan T., Oei H. B., Ahn C., Zhou X. J., Putterman C., Saxena R., Mohan C. (2012) Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis. Arthritis Res. Ther. 14, R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fiehn C., Hajjar Y., Mueller K., Waldherr R., Ho A. D., Andrassy K. (2003) Improved clinical outcome of lupus nephritis during the past decade: importance of early diagnosis and treatment. Ann. Rheum. Dis. 62, 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reyes-Thomas J., Blanco I., Putterman C. (2011) Urinary biomarkers in lupus nephritis. Clin. Rev. Allergy. Immunol. 40, 138–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menke J., Iwata Y., Rabacal W. A., Basu R., Stanley E. R., Kelley V. R. (2011) Distinct roles of CSF-1 isoforms in lupus nephritis. J Am Soc Nephrol 22, 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiani A. N., Johnson K., Chen C., Diehl E., Hu H., Vasudevan G., Singh S., Magder L. S., Knechtle S. J., Petri M. (2009) Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J. Rheumatol. 36, 2224–2230 [DOI] [PubMed] [Google Scholar]
- 30. Dhaun N., Lilitkarntakul P., Macintyre I. M., Muilwijk E., Johnston N. R., Kluth D. C., Webb D. J., Goddard J. (2009) Urinary endothelin-1 in chronic kidney disease and as a marker of disease activity in lupus nephritis. Am. J. Physiol. Renal Physiol 296, F1477–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki M., Wiers K., Brooks E. B., Greis K. D., Haines K., Klein-Gitelman M. S., Olson J., Onel K., O'Neil K. M., Silverman E. D., Tucker L., Ying J., Devarajan P., Brunner H. I. (2009) Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr. Res. 65, 530–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mastroianni-Kirsztajn G., Nishida S. K., Pereira A. B. (2008) Are urinary levels of free light chains of immunoglobulins useful markers for differentiating between systemic lupus erythematosus and infection? Nephron Clin. Pract. 110, c258–263 [DOI] [PubMed] [Google Scholar]
- 33. Molad Y., Miroshnik E., Sulkes J., Pitlik S., Weinberger A., Monselise Y. (2002) Urinary soluble VCAM-1 in systemic lupus erythematosus: a clinical marker for monitoring disease activity and damage. Clin. Exp. Rheumatol. 20, 403–406 [PubMed] [Google Scholar]
- 34. Varghese S. A., Powell T. B., Budisavljevic M. N., Oates J. C., Raymond J. R., Almeida J. S., Arthur J. M. (2007) Urine biomarkers predict the cause of glomerular disease. J. Am. Soc. Nephrol. 18, 913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rovin B. H., Song H., Hebert L. A., Nadasdy T., Nadasdy G., Birmingham D. J., Yung Yu C., Nagaraja H. N. (2005) Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 68, 1825–1833 [DOI] [PubMed] [Google Scholar]
- 36. Castellino F. J., Ploplis V. A. (2005) Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 93, 647–654 [DOI] [PubMed] [Google Scholar]
- 37. Suenson E., Thorsen S. (1981) Secondary-site binding of Glu-plasmin, Lys-plasmin and miniplasmin to fibrin. Biochem. J. 197, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Urano T., Chibber B. A., Castellino F. J. (1987) The reciprocal effects of epsilon-aminohexanoic acid and chloride ion on the activation of human [Glu1]plasminogen by human urokinase. Proc. Natl. Acad. Sci. U.S.A. 84, 4031–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marunaka Y. (1997) Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium. Jpn. J. Physiol. 47, 499–511 [DOI] [PubMed] [Google Scholar]
- 40. Fu Y., Zhu Y. (2010) Ectopic ATP synthase in endothelial cells: a novel cardiovascular therapeutic target. Curr. Pharm. Des. 16, 4074–4079 [DOI] [PubMed] [Google Scholar]
- 41. Mu W., Long D. A., Ouyang X., Agarwal A., Cruz P. E., Roncal C. A., Nakagawa T., Yu X., Hauswirth W. W., Johnson R. J. (2009) Angiostatin overexpression is associated with an improvement in chronic kidney injury by an anti-inflammatory mechanism. Am. J. Physiol. Renal Physiol. 296, F145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]