Abstract
Development of liver disease is associated with the appearance of multiply fucosylated glycoforms of haptoglobin. To analyze the disease-related haptoglobin glycoforms in liver cirrhosis and hepatocellular carcinoma, we have optimized an LC-MS-multiple reaction monitoring (MRM) workflow for glycopeptide quantification. The final quantitative analysis included 24 site-specific glycoforms generated by treatment of a tryptic digest of haptoglobin with α(2–3,6,8)-neuraminidase and β(1–4)-galactosidase. The combination of LC-MS-MRM with exoglycosidase digests allowed resolution of isobaric glycoforms of the haptoglobin-T3 glycopeptide for quantification of the multiply fucosylated Lewis Y-containing glycoforms we have identified in the context of liver disease. Fourteen multiply fucosylated glycoforms of the 20 examined increased significantly in the liver disease group compared with healthy controls with an average 5-fold increase in intensity (p < 0.05). At the same time, two tri-antennary glycoforms without fucoses did not increase in the liver disease group, and two tetra-antennary glycoforms without fucoses showed a marginal increase (at most 40%) in intensity. Our analysis of 30 individual patient samples (10 healthy controls, 10 cirrhosis patients, and 10 hepatocellular carcinoma patients) showed that these glycoforms were substantially increased in a small subgroup of liver disease patients but did not significantly differ between the groups of hepatocellular carcinoma and cirrhosis patients. The tri- and tetra-antennary singly fucosylated glycoforms are associated with a MELD score and low platelet counts (p < 0.05). The exoglycosidase-assisted LC-MS-MRM workflow, optimized for the quantification of fucosylated glycoforms of haptoglobin, can be used for quantification of these glycoforms on other glycopeptides with appropriate analytical behavior.
N-Glycosylation is a common modification that controls protein folding and multiple functions of mature glycoproteins (1). This co-translational process is controlled by the activity of glycosyltransferases and glycosidases localized in the endoplasmic reticulum and Golgi compartments (2), and its importance increases with complexity of the organism (3, 4). Changes in the abundance and microheterogeneity of glycosylation have been associated with several diseases, including cancer. For example, increased core fucosylation, branching, and terminal sialylation of proteins were associated with carcinogenesis in multiple publications (5–7). In this study, we focus on the quantitative monitoring of changes in N-glycosylation of haptoglobin (Hp)1 in patients with liver disease.
Quantitative changes in protein glycosylation have been analyzed on the level of enzymatically detached N-glycans and on the level of glycopeptides, but the analyses of detached glycans are better developed. Almost all reported quantifications are based on relative quantities partly because quantitative standards are not readily accessible and because changes in relative distributions are considered more important than absolute quantities of the analytes. The only paper we are aware of reporting HPLC-based absolute quantification of glycans used a standard isolated from hen egg yolk for analysis of enzymatically detached N-glycans in rheumatoid arthritis (8).
Multiple workflows for relative (semi)quantitative analysis of detached N-glycans, using various analytical techniques, were described (9–13). Perhaps the most widely used profiling methods are normal phase HPLC of fluorescently labeled glycans (14, 15) and MALDI/TOF mass spectrometric screening of permethylated glycans (16). Chromatographic resolution of the fluorescently labeled complex glycan mixtures can be somewhat limited, although MALDI/TOF screening has lower quantitative accuracy, but both methods have provided good evidence for quantitative shifts in the distribution of protein-associated N-glycans in cancer diseases (17, 18). Optimized LC-MS/MS analysis of glycans and even glycopeptides allows improved resolution of isomers and further supports the presence of disease-related protein glycoforms that could impact protein function and disease progression (19–21). Improved quantification of a limited set of de-sialylated fluorescently labeled glycans, separated on a capillary sequencer, was applied to quantification in complex samples or to the analysis of glycosylation of isolated hemopexin (22, 23). Improvements in the mass spectrometric quantification of permethylated glycans based on the introduction of D or 13C isotopic labels were proposed but have not yet reached wide acceptance (24–26). Elegant isotopic labeling of glycans in cell culture, similar to SILAC labeling of peptides, was also developed but remains to be used more widely (24, 26). Methods for relative quantification of detached glycans based on the labeling of the reducing end or sialic acids represent further efforts to improve mass spectrometric quantification of detached N-glycans in this fast growing field of science (11, 27–30).
The continued developments in the quantification of detached glycans in samples ranging from complex mixtures to isolated proteins represent significant advances and document the need to improve the quantitative workflows for the assessment of glycosylation changes in biological systems. However, the N-glycan-associated proteins, or site specificity in the case of isolated proteins, cannot be determined by these methods. Site-specific quantitative workflows for the analysis of glycopeptides are developed to a lesser degree due to the complexity of the micro-heterogeneous glycoproteins and associated technical difficulties (19). These limitations stimulated our interest in the LC-MS-MRM quantification of glycopeptides. Our major goal was to achieve a reliable quantitative comparison of site-specific Hp glycoforms between relevant liver disease states (HCC and CIR), a workflow complementary to the methods analyzing distribution of glycoforms at a glycosylation site. Our analysis of haptoglobin (Hp), described in the accompanying article, identified liver disease-related site-specific glycoforms with an unusually high degree of fucosylation (up to six fucoses per glycoform) associated with the Hp-T3 glycopeptide. We describe an exoglycosidase-assisted LC-MS-MRM method using a Q-TRAP instrument for quantification of site-specific glycoforms, and we demonstrate its utility on the analysis of liver disease-related multiply fucosylated glycoforms of Hp-T3.
EXPERIMENTAL PROCEDURES
Study Population
All participants were enrolled under protocols approved by the Institutional Review Board at Georgetown University. The HCC (n = 10) and CIR (n = 10) patients were recruited into the study in collaboration with the Department of Hepatology and Liver Transplantation, Georgetown University Hospital, Washington D. C. All participants with chronic liver disease evaluated for hepatobiliary surgery or liver transplantation were included in the study. Diagnosis of HCC was made by the attending physician based on liver imaging and/or liver biopsy. All the HCC patients were classified using the 7th Edition of the American Joint Committee on Cancer staging manual and represent primarily early stage disease (Table I). The two groups of liver patients were selected to be similar to healthy controls in age and gender and to each other in the etiology of liver disease (hepatitis C virus, hepatitis B virus, or NASH) and the degree of liver damage measured by MELD scores (INR, bilirubin, and creatinine). Participants were selected to have detectable haptoglobin on a Western blot of their plasma samples. Samples were collected using EDTA plasma vacutainer tubes (366643, BD Biosciences) prior to surgery or chemotherapy; the plasma samples were collected according to a standard protocol and frozen within 6 h of blood collection. All plasma samples were collected between 2007 and 2010 and were stored at −80°C until used; haptoglobin isolations were carried out from aliquots at the second thaw.
Table I. Demographic and clinical characteristics of the participants in the HCC, CIR, and CTL groups.
Numbers in parentheses indicate number of participants in each group. HCV, hepatitis C virus; HBV, hepatitis B virus; NA, not applicable.
| HCC (10) | CIR (10) | CTL (10) | p value | |
|---|---|---|---|---|
| Male gender | 9 | 8 | 9 | 0.16 |
| Age (mean) | 56.3 | 53.6 | 54.6 | 0.36 |
| Meld score (mean) | 9.2 | 10.3 | NA | 0.97 |
| INR | 1.19 | 1.24 | NA | 0.42 |
| Bilirubin | 1.3 | 1.23 | NA | 0.45 |
| Creatinine | 0.87 | 0.97 | NA | 0.92 |
| Etiology | ||||
| HCV | 8 | 8 | ||
| HBV | 1 | 1 | ||
| NASH | 1 | 1 | ||
| TNM | ||||
| T1N0M0 | 7 | |||
| T2N0M0 | 2 | |||
| T3N0M0 | 1 | |||
Hp Isolation from Plasma Samples
Hp was isolated by hemoglobin-Sepharose affinity from plasma samples (125 μl) diluted with PBS (pH 7.4) to 500 μl. After a 2-h incubation at room temperature, the resin was washed five times with 500 μl of PBS; proteins were eluted three times with 300 μl of 0.1 m glycine (pH 2.5) and neutralized. Guanidine HCl (Sigma-Aldrich) was added to the combined elution fractions to a 6 m final concentration, and the samples were loaded on an HPLC ProSwift RP-1S column (Dionex, Sunnyvale, CA) in mobile phase A (2% ACN, 0.08% TFA) and separated at 30°C under a gradient of 1–75% B (98% ACN, 0.05% TFA) in 18 min at a 1 ml/min flow rate. The Hp fraction was collected on a SpeedVac and diluted in distilled H2O for further analysis.
Quantification of Hp Glycopeptides
Hp isolated from patient samples (2.5 μg based on peak area of the RP HPLC) was added to the labeled internal standard (VVLHPNYSQVDIGLIK), reduced with DTT, alkylated with iodoacetamide, dissolved in 50 mm ammonium bicarbonate buffer (Sigma-Aldrich) containing 0.05% RapiGest, and digested with trypsin (Promega, Madison, WI) using a Barocycler NEP2320 (Pressure BioSciences, South Easton, MA) at 37°C. Tryptic digests were first treated with α(2–3,6,8)-neuraminidase (New England Biolabs, Ipswich, MA) at 37°C in 50 mm sodium acetate, 5 mm CaCl2 (pH 5.5) (New England Biolabs). When appropriate, the digest with α(2–3,6,8)-neuraminidase was followed by β(1–4)-galactosidase (New England Biolabs) overnight at 37°C in 50 mm sodium acetate, 5 mm CaCl2 (pH 5.5) (New England Biolabs). We have verified that each glycosidase reaction reaches completeness by inspection of the spectra (disappearance of the peaks) of known glycoforms. To ensure reliable quantification, glycopeptides were not enriched for the LC-MS-MRM assays by hydrophilic interaction chromatography. Quantification was achieved by RP chromatography (NanoAcquity, Waters Associates, Milford, MA) on a Symmetry C18 (3 μm × 180 μm × 20 mm) trap column and UPLC capillary column (BEH 300A, 1.7 μm × 150 mm × 0.75 μm) (Waters Associates) interfaced with a 4000 Q-TRAP mass analyzer (AB Sciex, Framingham, MA). Glycopeptide analysis was carried out by a 3-min trapping/washing step using 2% ACN, 0.1% formic acid at a 15 μl/min flow rate followed by a 40-min gradient elution of 0.1% formic acid in ACN. Starting conditions were set to the following: 3% ACN, 0.1% formic acid; 3–50% ACN, 0.1% formic acid for 5–30 min; 50–98% ACN, 0.1% formic acid for 30–35 min; 98% ACN, 0.1% formic acid for 35–40 min. For all optimization and quantification runs, we have injected 1 μl (2 pmol) of sample directly after enzymatic treatment. Q-TRAP conditions were set to curtain gas 13, ion spray voltage 2400 V, ion source gas 130, interface heater temperature 180°C, entrance potential 10 V, and collision exit potential 10. Declustering potential (DP) and collision energy (CE) were optimized for each analyte as described below. Oxonium ions at m/z 204.1, 366.1, 512.2 and peptide-GlcNAc fragments were selected as the monitored MRM transitions, and the data were processed as described below.
Optimization of DP, CE, and Isolation Windows
Tryptic digest of Hp (2 pmol on column) and de-sialylated tryptic glycopeptides were used for the optimization of DP and CE. Transition lists were created in csv format for all identified glycoforms and measured in triplicate. The parameters were optimized on the glycoforms of the T3-peptide. DPs (at the precursor level) and CEs (at the MRM transition level) were optimized using a 5-V CE step followed by a fine optimization with a 2-V CE step. Starting CEs were calculated using default equations recommended by the Q-TRAP manufacturer for the analysis of peptides. We checked all combinations of high and normal (Q1_Q2) isolation window settings for optimal selectivity of precursor and fragment ions without loss of sensitivity by injection of 2 pmol of Hp tryptic digest using the optimized collision parameters. Equations relating precursor ion mass with CE, for each product ion, were derived from the analysis of glycoforms of the T3 glycopeptide (Fig. 5).
Fig. 5.
Plot of linear dependence of optimized fragment-specific (m/z 204, 366, and 512) CE on precursor ion m/z used for quantification of the Hp-T3 glycopeptides.
Normalization Peptide, Labeled Internal T3-Peptide Standard, and Site Occupancy
Peptides for normalization were selected based on the data obtained from the analysis of 2 pmol of Hp tryptic digest on a Q-TOF mass spectrometer (QSTAR Elite, AB Sciex) operated in data-dependent mode. We have identified 15 haptoglobin tryptic peptides by using Protein Pilot 2.0 software (AB Sciex) and the Mascot search engine; 6 of the 15 peptides were selected for further optimization. To compare the stability of the six selected normalization peptides in different disease groups and under relevant sample preparation workflows, including exoglycosidases treatments, three transitions of each of the peptides were optimized using Skyline software and the 4000 Q-TRAP mass spectrometer.
Labeled T3 peptide (VVLHPNYSQVDIGLIK, Leu14 with 6× 13C) (New England Peptide, Gardner, MA) was added to each sample before reduction and alkylation step for standardization of all procedures and determination of site occupancy. Site occupancy was determined as the difference in the quantity of total Hp proteins and quantity of the nonglycosylated T3 peptide (asparagine). Total Hp was determined by UV absorbance of HPLC peak in the RP protein chromatogram on a ProSwift RP-1S column (see above). Hp was quantified against a standard curve established by injections of known amounts of Hp standard. LC-MS-MRM was used to quantify the nonglycosylated T3 peptide (599.0 → 658.4 transition) in each patient's sample tryptic digest spiked with the labeled Hp-T3 peptide standard (601.4 → 665.4 transition). The labeled Hp-T3 standard was added to the samples at a concentration equal to the concentration of the Hp protein. The LC-MS-MRM conditions were identical to the quantification of glycopeptides described above with CE for the peptides set at 35 V; all the Hp peptide concentrations were within the linear calibration range of the Hp-T3 internal standard. We have verified on pooled samples that this site occupancy determination provides results identical (within 3%) to the often used peptide: N-glycosidase F de-glycosylation method (32).
Data Processing and Statistical Analysis
LC-MS-MRM chromatograms were processed using Multi Quant 2.0 software (AB Sciex), and the results were exported as text files for data normalization and quality control analysis. Peak intensities for the glycopeptide's precursor → 204.1 transition (the most intense transition under the optimized CE conditions, see Fig. 2) were used for the quantification of all glycoforms after normalization by the intensity of the (460.8 → 629.4) transition of the GSFPWQAK peptide. The remaining glycoform transitions were used for quality control analysis confirming the specificity of detection by the presence of the peptide-HexNAc fragment, constant ratios of the measured transitions, and uniform retention time of the glycopeptides.
Fig. 2.

Schematic of glycopeptide MRM represented by MS/MS CID spectrum of the A3G1F1 glycoform of Hp-T3. Fragment ions selected for MRM transitions are labeled, and their chromatograms are inserted.
For the quantitative analysis of haptoglobin, we have selected the Hp-T3 tryptic glycopeptide (Fig. 1). First, we have analyzed pooled plasma of healthy individuals, CIR, and HCC patients; this was followed by analysis of individual samples of Hp isolated from healthy individuals (n = 10), CIR (n = 10), and HCC (n = 10) patients. We have examined two datasets comparing duplicate measurements of glycoforms after neuraminidase treatment (n = 10 glycoforms) and after a combined neuraminidase/galactosidase treatment (n = 14 glycoforms). The obtained datasets were analyzed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC). We have tested the hypothesis that some of the Hp-T3 glycoforms change in abundance between healthy controls and liver disease patients and that the glycoforms also change between the liver cirrhosis and hepatocellular carcinoma groups. Assessment of intra-class correlation coefficients showed high reproducibility of the duplicate measurement in all datasets (intra-class correlation coefficients of >0.90). We have therefore used the average of the duplicate measurements in the group comparisons. Because of the non-normal distribution of the datasets with a relatively small sample size, we have used the nonparametric Kruskal-Wallis test to compare the differences in glycoforms among the three groups classified by participants' liver diseases. Because all the participants in the HCC group also have chronic liver disease, with liver damage (MELD scores) comparable with the CIR group, we have grouped the HCC and CIR into one liver disease group for analyses using the Mann-Whitney test. The p values of these comparisons were adjusted for multiple comparisons by the false discovery rate procedure. Correlations between glycoforms were evaluated by Spearman Correlation Coefficients, and associations between glycoform quantities and clinical variables (MELD scores, bilirubin, etc.) were analyzed by mixed models with repeated measurements.
Fig. 1.
Sequence of the β subunit of Hp. Hp-T3 glycopeptide is in italics, bold and underlined; the peptide selected for normalization is underlined, and N-glycosylation sites are labeled red.
RESULTS
Haptoglobin has four glycosylation sites, and its tryptic cleavage produces three glycopeptides. We have selected the Hp-T3 glycopeptide for the LC-MS-MRM quantification of glycoforms between the healthy and liver disease groups (Fig. 1). This is the glycosite of Hp most frequently analyzed in the literature and, in our experience, the most informative Hp glycopeptide because of the large number of hyper-fucosylated glycoforms observed on this site in liver disease (32, 40–42). The Hp-T3 peptide has an appropriate length, chromatographic, and fragmentation behavior that makes it a good candidate for optimization of the MRM method. In contrast, the tryptic T2 glycopeptide is doubly glycosylated, which complicates resolution of the site-specific glycoform structures. A double digest with trypsin and GluC separates the two glycosites, but the resulting short hydrophilic NATAK glycopeptide is not quantifiable by RP chromatography; in addition, the GluC digest in the close proximity of two glycosites is not quantitatively reliable. We have therefore not quantified the T2 glycoforms. We have also not quantified the T1 glycoforms because they are less informative. We can detect a maximum of three fucoses associated with the T1 glycopeptide, and quantitative estimates based on XIC of the precursor ions show typically less than 50% increase in the fucosylated forms in HCC compared with CIR on the Hp-T1, although the XIC of precursor ions in the Hp-T3 glycoforms was typically increased greater than 2-fold.
LC-MS-MRM is an established workflow for the quantification of peptides (31). The CID fragmentation of glycopeptides is, however, dominated by glycan fragments (oxonium ions), and the considerations for MRM design differ significantly from the established methods of peptide analysis. Here, we present an MRM method for the quantification of Hp-T3 glycoforms developed on the basis of glycopeptide fragmentation observed on a Q-TOF mass spectrometer. The MS/MS fragmentation spectrum of glycopeptides (number of peaks and their relative ratio) depends on the fragmentation (collision) energy and type of fragmentation cell used (quadrupole, IT, etc.). We have optimized quantification of the glycopeptides on two mass analyzers, Q-TOF (QStar Elite XL) and Q-TRAP (4000), both manufactured by AB Sciex, with a similar design of the fragmentation cell (quadrupole) and ion optics. This increases our confidence that the quantitative Q-TRAP measurements are optimized for the fragmentation behavior of the glycopeptides.
The following requirements were used for the selection of MRM transitions: presence in all observed fragmentation spectra with good intensity (sensitivity) and at least one detectable specific glycopeptide ion (specificity). We have selected to monitor four MRM transitions per glycopeptide (Fig. 2). Two oxonium ions, m/z 204.1 (1+), 366.1 (1+), are common to all glycoforms and, as the most intense fragments, achieve excellent sensitivity. The m/z 512.2 (1+) oxonium ion is specific for fucosylated glycopeptides. The peptide-glycan fragment ion HexNAc peptide (multiply charged) is specific to Hp but less abundant; it is used for quantification only in case of sufficient abundance. Other glycopeptide or peptide backbone fragments of the Hp-T3 are typically not intense enough to include in the MRM method. The smallest isolation window on the Q-TRAP and Q-TOF instruments is 0.7 Da, which may not be selective enough for complex mixtures of glycopeptides. To ensure that our method of quantification is specific, we isolate Hp as starting material. We have tested the MRM method with different combinations of resolution on both quadrupoles and did not find significant differences in sensitivity between the high-high selected for our study (0.7-Da isolation windows on both quadrupoles) and the other combinations. Chromatographic retention time, presence of specific peptide HexNAc fragment, and ratio of MRM transition signals are considered as additional quality control parameters.
A stable isotope-labeled version of the nonglycosylated Hp-T3 peptide was used for standardization of the procedure and for the estimation of site occupancy. We have established a calibration curve for the Hp-T3 peptide and determined basic characteristics, including linearity, limit of detection, and limit of quantification (supplemental Fig. 1). The nonglycosylated Hp-T3 was determined in tryptic digests of all samples; its concentrations were in the linear dynamic range of the Hp-T3 standard curve and were used to estimate site occupancy by comparison with the protein concentration determined by HPLC-UV. Site occupancy of the Hp-T3 in all samples was in the interval 93–97% in agreement with previously published results (32). We therefore did not adjust for site occupancy in the final workflow.
Our optimization focused on relative quantification of the Hp-T3 glycoforms between disease states because synthetic glycopeptide standards for absolute quantification are not readily available. We have therefore tested six nonglycosylated tryptic peptides from the β subunit of Hp to select the ideal normalization peptide (Fig. 3). These peptides were chosen from the tryptic Hp digest on the basis of their retention time, sequence (lack of methionine etc.), and fragmentation. MRM transitions (three transitions per peptide) for the six peptides were optimized by the usual MRM optimization methods, and the stability of the peptides across the comparison groups (healthy, CIR, and HCC) in the α(2–3,6,8)-neuraminidase and β(1–4)-galactosidase digest was evaluated. Fig. 3 summarizes the retention time of the six peptides and their mean intensity/standard deviation across 30 samples (inset); the two bars represent measurements before (left bar)/after (right bar) galactosidase treatment. The peptide closest in retention time to the quantified Hp-T3 glycoforms, with the highest intensity and stability across the comparison groups (GSFPWQAK), was selected for normalization.
Fig. 3.

Representative chromatogram comparing RT of six tryptic Hp peptides (1, DIAPTLTLYVGK; 2, VGYVSGWGR; 3, VTSIQDWVQK; 4, TEGDGVYTLNNEK; 5, GSFPWQAK; and 6, QLVEIEKK) and de-sialylated Hp-T3 glycoforms (overlapping peaks with RT 22 min). Stability of the peptides in de-sialylated (left bar) and de-sialylated/de-galactosylated (right bar) samples is presented in the inset showing mean intensity/standard deviation of the six peptides (same color code as the chromatogram) across the samples of all 30 participants. The GSFPWQAK peptide was selected for normalization.
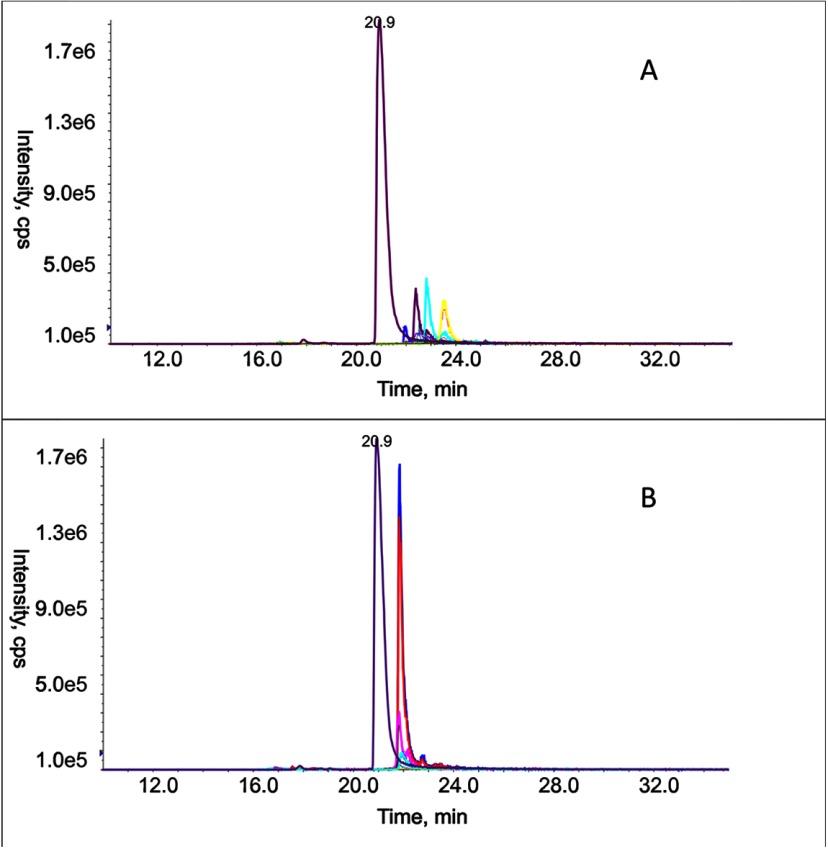
Because we are interested specifically in the changes in fucosylation of the Hp-T3 glycoforms in liver disease, we have quantified the peptides after de-sialylation (Table II). Nonspecific neuraminidase cleaves all sialic acids with α(2–3,6,8)-linkage; removal of the negative charges standardizes retention properties of glycopeptides on the RP column and increases the limit of detection in positive ionization mode (14). The advantages of de-sialylation are documented by comparison of the chromatograms before (Fig. 4A) and after (Fig. 4B) neuraminidase treatment. The intensity of the glycopeptide transitions after de-sialylation becomes comparable with the intensity of transition of the normalization peptide (RT 20.9 min), which documents the improved sensitivity of detection. In addition, the retention behavior of the glycoforms becomes uniform, which reduces matrix effects and improves specificity of detection. A further advantage, and a particularly important point for our analysis, is that we can combine the de-sialylation with galactosidase cleavage for selective detection of specific linkage isomers that would otherwise remain unresolved. We take advantage of the fact that β(1–4)-galactosidase activity is inhibited by outer arm fucosylation (33, 34), which leads to resolution of the glycoforms based on the number of protected outer arm galactoses (Table II).
Table II. Changes in the de-sialylated and de-sialylated/de-galactosylated glycoforms of the Hp-T3 peptide in liver disease.
HCC, cirrhotic patients with HCC (n = 10); CIR, cirrhotic patients without HCC (n = 10); DIS, combined HCC and CIR groups (n = 20); CTL, healthy controls (n = 10). The numbers represent average ± S.D. f the normalized MRM peak areas of the 204 transition. Kruskal-Wallis (CTL:CIR, CTL:HCC, and CIR:HCC) and Mann-Whitney (CTL:DIS) tests with adjustment for multiple comparisons (false discovery rate (FDR)) were used to derive p values in the group comparisons.
| Glycoform | CTL (average ± S.D.) | CIR (average ± S.D.) | HCC (average ± S.D.) | p value CTL:HCC | p value CTL:CIR | p value CIR:HCC | p value CTL:DIS | FDR CTL:DIS |
|---|---|---|---|---|---|---|---|---|
| De-sialylated Hp-T3 glycoforms | ||||||||
| A3G3 | 42.9/6.01 | 40.6/12.1 | 41.9/5.83 | 0.999 | 0.852 | 0.852 | 0.91 | 0.91 |
| A3G3F1 | 7.41/2.13 | 17.4/12.4 | 14.9/10.3 | 0.069 | 0.138 | 0.852 | 0.05 | 0.06 |
| A3G3F2 | 1.51/0.40 | 6.49/5.58 | 3.20/2.71 | 0.028 | 0.059 | 0.437 | 0.02 | 0.04 |
| A3G3F3 | 0.14/0.04 | 0.86/1.23 | 0.87/1.93 | 0.017 | 0.028 | 0.481 | 0.01 | 0.04 |
| A4G4 | 8.25/1.60 | 10.6/4.00 | 11.1/3.10 | 0.038 | 0.157 | 0.852 | 0.04 | 0.05 |
| A4G4F1 | 1.89/0.84 | 9.87/7.64 | 5.37/3.75 | 0.038 | 0.059 | 0.320 | 0.02 | 0.04 |
| A4G4F2 | 0.49/0.49 | 10.1/10.9 | 3.13/3.83 | 0.051 | 0.059 | 0.395 | 0.02 | 0.04 |
| A4G4F3 | 1.90/0.46 | 6.44/2.75 | 3.82/5.34 | 0.044 | 0.015 | 0.395 | 0.01 | 0.04 |
| A4G4F4 | 0.09/0.07 | 0.35/0.45 | 1.05/2.11 | 0.682 | 0.028 | 0.437 | 0.11 | 0.12 |
| A4G4F5 | 0.20/0.15 | 0.63/0.84 | 0.57/0.94 | 0.069 | 0.024 | 0.737 | 0.02 | 0.04 |
| De-sialylated de-galactosylated Hp-T3 glycoforms | ||||||||
| A3 | 45.4/11.2 | 52.9/14.3 | 50.3/8.17 | 0.320 | 0.227 | 0.629 | 0.19 | 0.19 |
| A3G1F1 | 6.72/2.98 | 22.5/19.0 | 18.5/14.3 | 0.060 | 0.178 | 0.852 | 0.05 | 0.06 |
| A3G1F2 | 0.14/0.06 | 0.76/0.56 | 1.06/1.94 | 0.051 | 0.002 | 0.178 | 0.00 | 0.01 |
| A3G2F2 | 0.48/0.28 | 2.83/3.77 | 8.70/14.9 | 0.044 | 0.038 | 0.682 | 0.02 | 0.02 |
| A3G2F3 | 0.09/0.05 | 0.36/0.25 | 1.49/2.79 | 0.020 | 0.017 | 0.970 | 0.01 | 0.01 |
| A4 | 11.3/4.10 | 14.9/5.67 | 14.0/4.70 | 0.157 | 0.178 | 0.682 | 0.10 | 0.11 |
| A4G1F1 | 1.88/1.48 | 12.5/11.3 | 11.0/9.47 | 0.008 | 0.060 | 0.970 | 0.01 | 0.02 |
| A4G1F2 | 0.64/0.37 | 3.46/4.12 | 11.4/23.3 | 0.009 | 0.009 | 0.629 | 0.00 | 0.01 |
| A4G2F2 | 0.39/0.25 | 0.94/1.18 | 0.70/0.94 | 0.024 | 0.069 | 0.970 | 0.02 | 0.02 |
| A4G2F3 | 0.42/2.84 | 0.82/0.65 | 1.34/1.76 | 0.069 | 0.157 | 0.852 | 0.05 | 0.06 |
| A4G3F3 | 0.20/0.23 | 0.54/0.47 | 1.51/2.47 | 0.060 | 0.033 | 1.000 | 0.02 | 0.02 |
| A4G3F4 | 0.20/0.18 | 0.63/0.62 | 3.31/7.15 | 0.009 | 0.138 | 0.481 | 0.19 | 0.19 |
| A4G4F4 | 0.25/0.08 | 0.47/0.23 | 0.55/0.49 | 0.020 | 0.008 | 0.528 | 0.05 | 0.06 |
| A4G4F5 | 0.69/0.34 | 1.52/1.14 | 1.59/1.07 | 0.006 | 0.051 | 0.629 | 0.00 | 0.01 |
Fig. 4.
Chromatogram of the normalization peptide (RT 20.9 min) and Hp-T3 glycopeptides before (A) and after (B) neuraminidase treatment of a pooled HCC sample.
Hp-T3 glycopeptides generated in the tryptic digest and the de-sialylated Hp-T3 glycopeptides were used for the optimization of CE. This is critical because glycoform-specific optimization of CE is required to achieve adequate sensitivity of detection. Skyline software and automatic mode of the AB Sciex mass spectrometer were used at first to calculate CE according to the mass and charge of the precursor. The calculated CE was used as a median value for CE optimization for individual glycoforms of the Hp-T3 peptide as described under “Experimental Procedures.” The experimentally optimized CEs were used to create fragment-specific equations based on precursor mass (Fig. 5). MS/MS spectra of glycopeptides require fragment-specific optimization, which is typically not done for peptides where major MS/MS fragment ratios do not change substantially with the CE adjustment. The ratios of oxonium ions, in the case of glycopeptides, are more variable and require optimization for each of the fragments. We have derived the following general equations for the 204 (CE = 0.0349·(m/z) + 14.877), 366 (CE = 0.0688·(m/z) − 37.825), and 512 (CE = 0.0303·(m/z) − 0.08182) fragment ions. The equations, derived for the Hp-T3 glycoforms, can be applied to complex N-glycoforms of other peptides with quite broad applicability. In our study, the equations derived on the sialylated and de-sialylated Hp-T3 glycoforms were applied to predict the CE for the de-sialylated/de-galactosylated Hp-T3 glycopeptides. The absolute intensities of the measured de-sialylated/de-galactosylated transitions were comparable with the de-sialylated glycoforms using the predicted CE without the need for further CE adjustment. We have also used the equations to optimize MRM analysis of glycoforms of the tryptic T1 and T2 glycopeptides, and the calculated CE was invariably within 3 V of the optimal value (data not shown).
Comparison of ratios of all transitions, used for analysis of two different pooled samples (HCC and CIR), are presented in supplemental Table 1. Here, we do not consider the 512 ion that is specific to the outer arm-fucosylated glycoforms. The results show that the ratios of the 204 and 366 fragments are constant across virtually all de-sialylated and de-sialylated/de-galactosylated Hp-T3 glycoforms. In the case of the glycoforms with low abundance (A4G4F4 and A4G4F5), we are reaching the limit of detection, and the ratios begin to deviate. A similar trend was observed for the peptide-HexNAc transition that can quantify the more abundant glycoforms but becomes less reliable for glycoforms of lower intensity, especially the de-sialylated/de-galactosylated glycoforms. These glycoforms cannot be quantified using the peptide-HexNAc but are still quantifiable using the oxonium ions. This is the reason to use the 204 transition for quantification and the peptide-HexNAc transition, typically above the limit of detection but below the limit of quantification of the minor glycoforms for the confirmation of detection specificity.
To evaluate differences in glycoform quantities between the CTL, CIR, and HCC groups, we have first analyzed pooled patient samples using the optimized exoglycosidase-assisted LC-MS-MRM workflow. Our analysis of site occupancy shows that Hp-T3 is occupied between 93–97% in all 30 patient samples without differences in occupancy between groups. We therefore did not adjust the quantitative comparisons between groups for site occupancy. Quantification of 12 fucosylated glycoforms of the Hp-T3, treated with neuraminidase and galactosidase, by the fucosylation-specific 512.2 transition is presented in Fig. 6. We have included the treatment with β(1–4)-galactosidase because it resolves linkage of isobaric disease-associated glycoforms of Hp-T3 (see accompanying article). Relative quantification of the 12 fucosylated glycoforms in pools of the HCC, CIR, and CTL groups showed that all glycoforms bearing more than one outer arm fucose are significantly (p value <0.0001) elevated in HCC and CIR compared with controls. We have not observed a difference between HCC and CIR in glycoforms with a smaller or equal number of fucoses and galactoses (except A4G4F4). But we have observed a significant difference in the pooled HCC and cirrhosis samples when the number of fucoses is greater than the number of galactoses, which is consistent with the presence of Le(y) structures as described in detail in the accompanying article.
Fig. 6.
MRM of 12 fucosylated glycoforms of the Hp-T3 peptide based on quantification of normalized areas of the fucosylation-specific 512.2 transition. Black column, pooled HCC patients; white column, pooled CIR patients; striped column, pooled CTL. Samples were analyzed in triplicate after treatment with neuraminidase and galactosidase. All glycoforms are significantly (p value <0.0001) different between control and disease groups; glycopeptides marked by an asterisk are significantly different between HCC and CIR (p value < 0.0001) based on Kruskal-Wallis test.
Quantitative comparisons of the Hp-T3 glycoforms in Hp isolated from individual healthy controls (n = 10), CIR patients (n = 10), and patients with HCC (n = 10) are presented in Table II. The standard deviation (S.D.) across the measurements is higher in the CIR and HCC disease groups (average S.D. 5.25) compared with the healthy controls (average S.D. 1.25), which reflects the increased inter-individual variability in the distribution of the glycoforms associated with liver disease. We have used nonparametric methods to compare the normalized glycoform intensities because the data are not normally distributed. Although we do not observe any significant differences between the CIR and HCC groups, the majority of the fucosylated glycoforms is borderline or significantly increased in the HCC and CIR disease groups compared with the healthy controls. This is confirmed in the control versus liver disease (grouped CIR and HCC) comparison, including adjustment for multiple comparisons.
The differences in the distributions of the glycoforms (de-sialylated/de-galactosylated) with the general formula (AxGyFy + 1) and (AxGyFy), observed in the pooled samples, are documented on the A4G1F2 and A4G2F2 (de-sialylated/de-galactosylated) glycoforms, which both derive from the A4G4F2 (de-sialylated) mixture of unresolved “precursor” glycoforms (Fig. 7, A–C). Although the A4G2F2 increases consistently in the HCC and CIR groups compared with controls, several high measurements (outliers) in the case of the A4G1F2 glycoform increase the average value in the HCC group. The A4G1F2 difference is significant in the pooled samples but is insufficiently frequent for a significant increase when individual samples are quantified. The low frequency means that this observation is not likely to improve early detection of HCC. We do not know whether the patients with elevated A4G1F2 represent a subgroup with some specific clinical attributes, but these participants do not stand out in terms of liver damage assessed by the MELD scores. Our study was not designed to follow disease outcomes. The elevated (AxGyFy + 1) glycoforms are infrequent, but as a preliminary observation, we examined association of ∼50 clinical parameters with the intensity of the (AxGyFy + 1) glycoforms. The analysis suggests association with reported family history of cancer (any cancer), antibodies to both hepatitis C and A viruses, elevated aspartate aminotransferase and alanine aminotransferase (highest values among the participants), and elevated alkaline phosphatase. Our data on viral infections (viral load, etc.) is limited, however, and further studies would have to examine the association with viral infection in detail. Because we have observed elevation of the fucosylated glycoforms in the CIR and HCC groups, we have examined association of the fucosylated glycoforms with MELD scores. Among the de-sialylated glycoforms, A3G3F1 is associated with MELD score (p = 0.01); among the de-sialylated/de-galactosylated glycoforms, A3G1F1 (p = 0.01) and A4G1F1 (p = 0.05) are associated with MELD score. These glycoforms were also associated with low platelet count. Ten of 19 participants with liver disease had less than 160 × 103 platelets per mm3, which would indicate that cirrhotic liver damage contributes to the elevation of these glycoforms. Correlation studies among the glycoforms show that nonfucosylated A3G3 and A4G4 glycoforms (p = 0.69), as well as the de-galactosylated A3 and A4 glycoforms (p = 0.83), are significantly correlated (p < 0.05). These nonfucosylated glycoforms are not correlated with any fucosylated glycoforms, some of which correlate with each other.
Fig. 7.

MRM quantification of normalized areas of the 204 transitions of three glycoforms of Hp-T3 in the groups of healthy controls (CTRL, n = 10), CIR (n = 10), and HCC (n = 10) patients. A, de-sialylated A4G4F2 glycoform of Hp-T3; B, de-sialylated/de-galactosylated A4G1F2 glycoform of Hp-T3; and C, de-sialylated/de-galactosylated A4G2F2 glycoform of Hp-T3.
DISCUSSION
Cancer diseases are associated with changes in N-glycosylation of proteins (17, 35, 36). Glycosylation in a protein/site-specific fashion (20, 37–39). Recent literature, including analysis of Hp glycoforms, suggests that the disease-related changes in glycosylation are site-specific as well (32, 40–42). Increase in fucosylation of proteins was described in the context of several cancers, including HCC (43). We have therefore examined site-specific changes in glycoforms of Hp during the progression of chronic liver disease to HCC, and we identified an unusually high degree of Hp fucosylation. In our analyses, the Hp-T3 peptide carries the highest variety of multiply fucosylated glycoforms, which include the Lewis Y-type tumor-associated carbohydrate antigen (44, 45). Quantification of the Hp-T3 glycoforms is therefore a natural progression of our attempt to analyze the liver disease-associated site-specific changes in Hp glycoforms.
Microheterogeneity of the protein glycoforms and ionization/fragmentation behavior of glycopeptides complicate quantification of the site-specific glycoforms (46). These analyses are particularly challenging in the clinical context where the starting material is typically limiting (39). The majority of the reported site-specific glycopeptide analyses rely on the quantification of precursor masses using XIC which suffers from the well known limitations of label-free LC-MS quantification (32, 42, 47). We are aware of one paper that describes the use of LC-MS/MS quantification of glycopeptides of recombinant human protein C produced in a transgenic pig mammary gland (30). We have therefore decided to optimize an exoglycosidase-assisted LC-MS-MRM workflow for relative quantification of the site-specific protein glycoforms under biologically relevant disease conditions, and we report its application to the quantification of the fucosylated Hp-T3 glycoforms in patient samples.
MRM is the most sensitive quantitative LC-MS workflow with a broad linear dynamic range. In this workflow, typically conducted on triple quadrupole mass analyzers (e.g. QTRAP), both quadrupoles work in a selected ion monitoring mode with the first quadrupole set to the precursor mass, and the second quadrupole set to the product ion mass. MRM was developed for quantification of small molecules and successfully applied to the quantification of proteins and peptides. Quantification of typically three transitions (product ions) per peptide defines specificity of the method for peptide quantification, and inclusion of stable isotope-labeled standards allows reliable absolute quantification of proteins. But this is challenging in the case of glycopeptides where CID fragmentation presents a unique set of challenges, and labeled standards are not available.
MRM quantification of glycopeptides requires analyte-specific adjustments and has inherent limitations discussed below. To achieve quantification of the Hp-T3 glycoforms, we have selected intense oxonium ions for quantification and glycoform-selective oxonium and peptide-HexNAc fragments to ensure specificity of detection (Fig. 2). Because the specificity of these transitions would be difficult to ensure in complex (glyco)peptide mixtures, we begin with purification of Hp from patient plasma. Our analyses show that Hp is associated primarily with complex glycans that are multiply fucosylated in the liver disease context. Because we want to quantify the site-specific changes in fucosylation of Hp isolated from limited amounts of patient material, we have de-sialylated the peptides with nonspecific neuraminidase. We and others have observed decreased ionization efficiency of the sialylated complex glycopeptides and their less uniform retention on RP columns (14). De-sialylation minimizes interferences and increases the sensitivity of detection (Fig. 4). In addition, it allows further structural resolution of the isoforms by a second enzymatic treatment with galactosidase (Table II) as documented on the resolved A4G1F2 and A4G2F2 glycoforms (Fig. 7). We have selected for quantification a total of 10 de-sialylated glycoforms and 14 de-sialylated/de-galactosylated glycoforms of the Hp-T3 based on their ability to distinguish HCC and CIR from healthy controls and each other in the analysis of pooled samples. The quantified de-sialylated/de-galactosylated glycoforms include the detectable minor over-fucosylated glycoforms (AxGyFy + 1) separated by the galactosidase activity from their isomers (AxGyFy). These structures had higher intensity in our analyses of pooled HCC compared with CIR and are associated with the presence of the Lewis Y epitope (see accompanying article). The quantities of these minor glycoforms are, however, low, and their detection required glycoform-specific optimization of the MRM analyses.
Glycoform-specific optimization of CE, as well as selection of an appropriate normalization standard, was essential to achieve reliable quantification of the Hp-T3 glycopeptides. The ratio of oxonium ion intensities is more dependent on CE than the intensity of MS/MS fragments of peptides, which is the reason for separate optimization of fragmentation energies for all selected oxonium or peptide-HexNAc ions. The optimized glycoform-specific CEs were used to derive fragment ion-specific equations for CE optimization with applicability to the complex N-glycopeptides as verified on other T3 glycoforms and Hp glycopeptides (Fig. 5). The presented CE optimization may be structure- and instrument-specific but represents an optimization workflow readily adaptable to other LC-MS-MRM contexts, which we have verified on the Hp-T3 glycoforms. It is important to note that the LC-MS-MRM workflow is designed for between group quantitative comparisons. The analyte-specific CE optimization limits its usefulness to profiling of glycoforms occupying a given glycosite; other complementary methods are more appropriate for this purpose.
The Hp-T3 peptide, similar to the other three Hp glycosites, is ∼95% occupied by glycans irrespective of liver disease states. The Hp-T3 glycoforms have appropriate size and properties for LC-MS-MRM analysis. Its analysis provides most informative differences in glycoform intensities between the cirrhosis and HCC groups. The T1 glycopeptide carries a less diverse set of glycoforms. The T2 peptide is doubly glycosylated, and separation of the glycosites with proteinase GluC does not produce reliable and quantifiable results; we have therefore focused on the quantification of Hp-T3. To achieve reliable quantification of the Hp-T3 glycoforms without synthetic isotopically labeled standards, we have evaluated all tryptic Hp peptides as candidates for normalization. We have selected the tryptic peptide with closest on column RT to the glycoforms of interest and with highest stability across the treatments/comparison groups (Fig. 3). RT of the glycopeptides on the RP column depends primarily on the peptide backbone; the Hp-T3 glycoforms and the normalization peptide elute within a narrow RT window, which improves quantification. The peptide is stable under the neuraminidase and galactosidase treatment conditions, which further justifies its use for normalization (supplemental Table 1).
The optimized LC-MS-MRM method was used for the analysis of Hp-T3 in pooled samples of control, CIR, and HCC groups and for quantification of Hp-T3 glycoforms in individual samples of 10 healthy people and 20 patients with liver disease (CIR and HCC). All fucosylated glycoforms increase in the pooled CIR and HCC samples compared with healthy controls (Fig. 6). The subset consistent with Le(y)-type structure (general formula of de-galactosylated glycans AxGyFy + 1) increased in the pooled HCC sample compared with CIR, even though the pools have a comparable degree of liver damage (MELD score). The analysis of individual samples confirms a significant increase in the majority of the fucosylated glycoforms in liver disease compared with controls, but it shows that the increase in Le(y)-type structures in the pooled samples is driven by few high intensity HCC participants and remains insignificant in the analysis of individual samples (Table II). The majority of the fucosylated glycoforms increase borderline or significantly in the CIR and HCC groups compared with healthy controls with an average 5-fold elevation. At the same time, the A3G3 (and the de-galactosylated A3) glycoform does not change significantly (change within 10% intensity) and the A4G4 (and the de-galactosylated A4) glycoform changes at most marginally (change within 40%). This marginal increase may be consistent with the previously reported up-regulation of GnT-V in liver cancer (48), but the increases in glycoform intensity in CIR and HCC individuals compared with healthy controls are dominated by the fucosylated glycoforms. At this point, we do not know whether the subset of patients with Le(y)-type structures could be related to altered disease outcome or specific clinical parameters. The study was not designed to follow disease outcome, and future studies will have to be designed to examine this point.
CONCLUSION
We have developed an exoglycosidase-assisted LC-MS-MRM workflow for quantification of glycoforms of Hp-T3 in liver disease context. The incorporation of exoglycosidase digests into the LC-MS-MRM workflow allows separation of isobaric fucosylated glycoforms associated with cirrhosis and HCC. The fucosylated glycoforms of Hp-T3 are up-regulated on average 5-fold in liver disease, but the Lewis Y-type glycoforms increase in perhaps 10–20% of the patients. This is not sufficient for a consistent detection of the patients with HCC on the background of liver cirrhosis. Additional appropriately designed studies are needed to determine whether this subset of patients a has different prognosis or shares common clinical characteristics. Glycopeptide MRM assays require analyte-specific optimization. To facilitate this task, we suggest a workflow, including fragment-specific CE calculations, for optimization of the LC-MS-MRM analysis of complex N-glycopeptides. Applicability of the workflow is at present limited to isolated N-glycoproteins or their simple mixtures to allow resolution of glycoforms monitored by the common oxonium ions. Although general applicability of the assays has limitations, we expect that the exoglycosidase-assisted LC-MS-MRM will provide an excellent tool for verification studies comparing site-specific glycoforms of interest in relevant disease conditions.
Supplementary Material
Acknowledgments
We thank Drs. Tomas Rejtar and Marina Hincapie for introduction to the concepts of site-specific glycopeptide quantification.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grants U01 CA168926 and RO1 CA135069 (to R.G.) and CCSG Grant P30 CA51008 (to Lombardi Comprehensive Cancer Center supporting the Proteomics and Metabolomics Shared Resource).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- Hp
- haptoglobin
- CE
- collision energy
- CIR
- cirrhosis
- CTL
- control
- DP
- declustering potential
- HCC
- hepatocellular carcinoma
- HexNAc
- N-acetylhexosamine
- MRM
- multiple reaction monitoring
- Q-TRAP
- quadrupole ion trap hybrid mass spectrometer
- RP
- reversed phase
- XIC
- extracted ion chromatogram
- RT
- retention time.
REFERENCES
- 1. Dove A. (2001) The bittersweet promise of glycobiology. Nat. Biotechnol. 19, 913–917 [DOI] [PubMed] [Google Scholar]
- 2. Helenius A., Aebi M. (2001) Intracellular functions of N-linked glycans. Science 291, 2364–2369 [DOI] [PubMed] [Google Scholar]
- 3. Zielinska D. F., Gnad F., Wiśniewski J. R., Mann M. (2010) Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 [DOI] [PubMed] [Google Scholar]
- 4. Park C., Zhang J. (2011) Genome-wide evolutionary conservation of N-glycosylation sites. Mol. Biol. Evol. 28, 2351–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyagi T., Wada T., Yamaguchi K., Hata K. (2004) Sialidase and malignancy: A minireview. Glycoconj. J. 20, 189–198 [DOI] [PubMed] [Google Scholar]
- 6. Zhao Y. Y., Takahashi M., Gu J. G., Miyoshi E., Matsumoto A., Kitazume S., Taniguchi N. (2008) Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 99, 1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harduin-Lepers A., Krzewinski-Recchi M. A., Colomb F., Foulquier F., Groux-Degroote S., Delannoy P. (2012) Sialyltransferases functions in cancers. Front. Biosci. 4, 499–515 [DOI] [PubMed] [Google Scholar]
- 8. Kita Y., Miura Y., Furukawa J., Nakano M., Shinohara Y., Ohno M., Takimoto A., Nishimura S. (2007) Quantitative glycomics of human whole serum glycoproteins based on the standardized protocol for liberating N-glycans. Mol. Cell. Proteomics 6, 1437–1445 [DOI] [PubMed] [Google Scholar]
- 9. Wollscheid B., Bausch-Fluck D., Henderson C., O'Brien R., Bibel M., Schiess R., Aebersold R., Watts J. D. (2009) Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol. 27, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruhaak L. R., Zauner G., Huhn C., Bruggink C., Deelder A. M., Wuhrer M. (2010) Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 397, 3457–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gil G. C., Iliff B., Cerny R., Velander W. H., Van Cott K. E. (2010) High Throughput quantification of N-glycans using one-pot sialic acid modification and matrix assisted laser desorption ionization time-of-flight mass spectrometry. Anal. Chem. 82, 6613–6620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mechref Y., Novotny M. V. (2006) Miniaturized separation techniques in glycomic investigations. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 841, 65–78 [DOI] [PubMed] [Google Scholar]
- 13. Jensen P. H., Karlsson N. G., Kolarich D., Packer N. H. (2012) Structural analysis of N- and O-glycans released from glycoproteins. Nat. Protoc. 7, 1299–1310 [DOI] [PubMed] [Google Scholar]
- 14. Guile G. R., Rudd P. M., Wing D. R., Prime S. B., Dwek R. A. (1996) A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal. Biochem. 240, 210–226 [DOI] [PubMed] [Google Scholar]
- 15. Vanhooren V., Laroy W., Libert C., Chen C. (2008) N-Glycan profiling in the study of human aging. Biogerontology 9, 351–356 [DOI] [PubMed] [Google Scholar]
- 16. Kang P., Mechref Y., Klouckova I., Novotny M. V. (2005) Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 19, 3421–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adamczyk B., Tharmalingam T., Rudd P. M. (2012) Glycans as cancer biomarkers. Biochim. Biophys. Acta 1820, 1347–1353 [DOI] [PubMed] [Google Scholar]
- 18. Goldman R., Ressom H. W., Varghese R. S., Goldman L., Bascug G., Loffredo C. A., Abdel-Hamid M., Gouda I., Ezzat S., Kyselova Z., Mechref Y., Novotny M. V. (2009) Detection of hepatocellular carcinoma using glycomic analysis. Clin. Cancer Res. 15, 1808–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolarich D., Jensen P. H., Altmann F., Packer N. H. (2012) Determination of site-specific glycan heterogeneity on glycoproteins. Nat. Protoc. 7, 1285–1298 [DOI] [PubMed] [Google Scholar]
- 20. Hua S., Nwosu C. C., Strum J. S., Seipert R. R., An H. J., Zivkovic A. M., German J. B., Lebrilla C. B. (2012) Site-specific protein glycosylation analysis with glycan isomer differentiation. Anal. Bioanal. Chem. 403, 1291–1302 [DOI] [PubMed] [Google Scholar]
- 21. Hua S., Lebrilla C., An H. J. (2011) Application of nano-LC-based glycomics toward biomarker discovery. Bioanalysis 3, 2573–2585 [DOI] [PubMed] [Google Scholar]
- 22. Vanderschaeghe D., Szekrényes A., Wenz C., Gassmann M., Naik N., Bynum M., Yin H., Delanghe J., Guttman A., Callewaert N. (2010) High-throughput profiling of the serum N-glycome on capillary electrophoresis microfluidics systems: toward clinical implementation of GlycoHepatoTest. Anal. Chem. 82, 7408–7415 [DOI] [PubMed] [Google Scholar]
- 23. Debruyne E. N., Vanderschaeghe D., Van Vlierberghe H., Vanhecke A., Callewaert N., Delanghe J. R. (2010) Diagnostic value of the hemopexin N-glycan profile in hepatocellular carcinoma patients. Clin. Chem. 56, 823–831 [DOI] [PubMed] [Google Scholar]
- 24. Botelho J. C., Atwood J. A., Cheng L., Alvarez-Manilla G., York W. S., Orlando R. (2008) Quantification by isobaric labeling (QUIBL) for the comparative glycomic study of O-linked glycans. Int. J. Mass Spectrom. 278, 137–142 [Google Scholar]
- 25. Kang P., Mechref Y., Kyselova Z., Goetz J. A., Novotny M. V. (2007) Comparative glycomic mapping through quantitative permethylation and stable-isotope labeling. Anal. Chem. 79, 6064–6073 [DOI] [PubMed] [Google Scholar]
- 26. Orlando R., Lim J. M., Atwood J. A., 3rd, Angel P. M., Fang M., Aoki K., Alvarez-Manilla G., Moremen K. W., York W. S., Tiemeyer M., Pierce M., Dalton S., Wells L. (2009) IDAWG: Metabolic incorporation of stable isotope labels for quantitative glycomics of cultured cells. J. Proteome Res. 8, 3816–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang H., Li X. J., Martin D. B., Aebersold R. (2003) Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling, and mass spectrometry. Nat. Biotechnol. 21, 660–666 [DOI] [PubMed] [Google Scholar]
- 28. Walker S. H., Budhathoki-Uprety J., Novak B. M., Muddiman D. C. (2011) Stable isotope-labeled hydrophobic hydrazide reagents for the relative quantification of N-linked glycans by electrospray ionization mass spectrometry. Anal. Chem. 83, 6738–6745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowman M. J., Zaia J. (2010) Comparative glycomics using a tetraplex stable-isotope coded tag. Anal. Chem. 82, 3023–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gil G. C., Velander W. H., Van Cott K. E. (2009) N-Glycosylation microheterogeneity and site occupancy of an Asn-X-Cys sequon in plasma-derived and recombinant protein C. Proteomics 9, 2555–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson N. L., Anderson N. G., Pearson T. W., Borchers C. H., Paulovich A. G., Patterson S. D., Gillette M., Aebersold R., Carr S. A. (2009) A human proteome detection and quantitation project. Mol. Cell. Proteomics 8, 883–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang D., Hincapie M., Rejtar T., Karger B. L. (2011) Ultrasensitive characterization of site-specific glycosylation of affinity-purified haptoglobin from lung cancer patient plasma using 10 μm i.d. porous layer open tubular liquid chromatography-linear ion trap collision-induced dissociation/electron transfer dissociation mass spectrometry. Anal. Chem. 83, 2029–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lucka L., Fernando M., Grunow D., Kannicht C., Horst A. K., Nollau P., Wagener C. (2005) Identification of Lewis x structures of the cell adhesion molecule CEACAM1 from human granulocytes. Glycobiology 15, 87–100 [DOI] [PubMed] [Google Scholar]
- 34. Royle L., Campbell M. P., Radcliffe C. M., White D. M., Harvey D. J., Abrahams J. L., Kim Y. G., Henry G. W., Shadick N. A., Weinblatt M. E., Lee D. M., Rudd P. M., Dwek R. A. (2008) HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 376, 1–12 [DOI] [PubMed] [Google Scholar]
- 35. Taniguchi N., Miyoshi E., Gu J., Jianguo G., Honke K., Matsumoto A. (2006) Decoding sugar functions by identifying target glycoproteins. Curr. Opin. Struct. Biol. 16, 561–566 [DOI] [PubMed] [Google Scholar]
- 36. Abbott K. L., Pierce J. M. (2010) Lectin-based glycoproteomic techniques for the enrichment and identification of potential biomarkers. Methods Enzymol. 480, 461–476 [DOI] [PubMed] [Google Scholar]
- 37. Valmu L., Alfthan H., Hotakainen K., Birken S., Stenman U. H. (2006) Site-specific glycan analysis of human chorionic gonadotropin β-subunit from malignancies and pregnancy by liquid chromatography-electrospray mass spectrometry. Glycobiology 16, 1207–1218 [DOI] [PubMed] [Google Scholar]
- 38. Wu S. L., Kim J., Bandle R. W., Liotta L., Petricoin E., Karger B. L. (2006) Dynamic profiling of the post-translational modifications and interaction partners of epidermal growth factor receptor signaling after stimulation by epidermal growth factor using extended range proteomic analysis (ERPA). Mol. Cell. Proteomics 5, 1610–1627 [DOI] [PubMed] [Google Scholar]
- 39. Mann B. F., Goetz J. A., House M. G., Schmidt C. M., Novotny M. V. (2012) Glycomic and proteomic profiling of pancreatic cyst fluids identifies hyperfucosylated lactosamines on the N-linked glycans of overexpressed glycoproteins. Mol. Cell. Proteomics 11, M111.015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miyoshi E., Nakano M. (2008) Fucosylated haptoglobin is a novel marker for pancreatic cancer: Detailed analyses of oligosaccharide structures. Proteomics 8, 3257–3262 [DOI] [PubMed] [Google Scholar]
- 41. Lin Z., Simeone D. M., Anderson M. A., Brand R. E., Xie X., Shedden K. A., Ruffin M. T., Lubman D. M. (2011) Mass spectrometric assay for analysis of haptoglobin fucosylation in pancreatic cancer. J. Proteome Res. 10, 2602–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park S. Y., Lee S. H., Kawasaki N., Itoh S., Kang K., Hee Ryu S., Hashii N., Kim J. M., Kim J. Y., Hoe Kim J. (2012) a1–3/4 fucosylation at Asn 241 of ss-haptoglobin is a novel marker for colon cancer: A combinatorial approach for development of glycan biomarkers. Int. J. Cancer 130, 2366–2376 [DOI] [PubMed] [Google Scholar]
- 43. Miyoshi E., Moriwaki K., Nakagawa T. (2008) Biological function of fucosylation in cancer biology. J. Biochem. 143, 725–729 [DOI] [PubMed] [Google Scholar]
- 44. Heimburg-Molinaro J., Lum M., Vijay G., Jain M., Almogren A., Rittenhouse-Olson K. (2011) Cancer vaccines and carbohydrate epitopes. Vaccine 29, 8802–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drake P. M., Cho W., Li B., Prakobphol A., Johansen E., Anderson N. L., Regnier F. E., Gibson B. W., Fisher S. J. (2010) Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin. Chem. 56, 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao Y., Jia W., Wang J., Ying W., Zhang Y., Qian X. (2011) Fragmentation and site-specific quantification of core fucosylated glycoprotein by multiple reaction monitoring-mass spectrometry. Anal. Chem. 83, 8802–8809 [DOI] [PubMed] [Google Scholar]
- 47. Bantscheff M., Schirle M., Sweetman G., Rick J., Kuster B. (2007) Quantitative mass spectrometry in proteomics: a critical review. Anal. Bioanal. Chem. 389, 1017–1031 [DOI] [PubMed] [Google Scholar]
- 48. Zhao Y. Y., Takahashi M., Gu J. G., Miyoshi E., Matsumoto A., Kitazume S., Taniguchi N. (2008) Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 99, 1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.