Background: It is unclear how DNA-damaging agents target cancer cells over normal somatic cells.
Results: Arf/p53-dependent down-regulation of H2AX enables normal cells to survive after DNA damage.
Conclusion: Transformed cells, which harbor mutations in either Arf or p53, are more sensitive to DNA-damaging agents.
Significance: Cellular transformation renders cells more susceptible to some DNA-damaging agents.
Keywords: ARF, Cancer Therapy, Cell Biology, DNA Damage Response, p53, Arf/p53 Module, H2AX, Camptothecin, Cancer Chemotherapy, γH2AX
Abstract
Anti-cancer drugs generally target cancer cells rather than normal somatic cells. However, the factors that determine this differential sensitivity are poorly understood. Here we show that Arf/p53-dependent down-regulation of H2AX induced the selective survival of normal cells after drug treatment, resulting in the preferential targeting of cancer cells. Treatment with camptothecin, a topoisomerase I inhibitor, caused normal cells to down-regulate H2AX and become quiescent, a process mediated by both Arf and p53. In contrast, transformed cells that harbor mutations in either Arf or p53 do not down-regulate H2AX and are more sensitive to drugs unless they have developed drug resistance. Such transformation-associated changes in H2AX expression rendered cancer cells more susceptible to drug-induced damage (by two orders of magnitude). Thus, the expression of H2AX and γH2AX (phosphorylated form of H2AX at Ser-139) is a critical factor that determines drug sensitivity and should be considered when administering chemotherapy.
Introduction
Cancer chemotherapy drugs usually act by inducing cancer cell death. Although some drugs were specifically developed for targeted cancer chemotherapy (1, 2), most anti-cancer drugs used today are DNA-damaging agents that induce cancer cell death by interfering with checkpoint responses (3–9). Because these anti-cancer drugs are administered without a specialized delivery system, they often cause side effects. However, cancer cells are still preferentially targeted over normal somatic cells. This raises the fundamental question of why cancer cells are more sensitive to drug-induced damage than normal cells. Although it is generally acknowledged that the higher growth rate of cancer cells contributes to their drug sensitivity, it might also be reasonable to consider other, as yet unidentified, mechanisms that may underlie preferential cancer cell killing.
p53 is involved in apoptosis induction. Therefore, cancer cells harboring intact p53 are usually more sensitive to anti-cancer drugs than cells harboring mutated p53 (10–13). However, anti-cancer drugs preferentially kill cancer cells harboring mutations in the Arf/p53 protein module rather than normal somatic cells that possess intact Arf and p53. This is paradoxical and poses many questions, such as how can normal cells survive drug treatment without undergoing p53-mediated cell death?
A recent study showed that the regulation of histone H2AX, which is responsible for efficient DNA damage checkpoint responses, is altered after cellular transformation. This is because down-regulation of H2AX is dependent on regulation by the Arf/p53 protein module, which is widely mutated in transformed cells (14). This suggests a potential mechanism underlying the transformation-coupled alterations in the damage checkpoint response and the resulting sensitivity of cancer cells to anti-cancer drugs. It also poses the following hypothesis. Normal cells survive treatment with anti-cancer drugs because H2AX is down-regulated in an Arf/p53-dependent manner, resulting in impaired DNA checkpoint responses, whereas transformed cells are killed. If this hypothesis is correct, the critical question is whether the sensitivity to DNA damaging drugs changes after cellular transformation involving mutations in the Arf/p53 protein module and the subsequent loss of H2AX regulation.
This study showed that Arf/p53-dependent down-regulation of H2AX was abrogated in transformed cells in the presence of anti-cancer drugs, resulting in an increase in the selective killing of transformed cells by two orders of magnitude. These results highlight a novel mechanism underlying the effects of anti-cancer drugs.
EXPERIMENTAL PROCEDURES
Cell Culture, RNA Interference Experiments, Senescence-associated β-Galactosidase Assay, and FACS Analysis
Arf KO mouse embryonic fibroblasts (MEFs)3 were prepared from Arf KO mice (15). WT and p53 KO MEFs and normal human fibroblasts (NHFs) were prepared as described previously (14) and cultured according to the 3T3 protocol (16). Arf and p53 statuses were also checked by Western blot analysis (supplemental Fig. S1). Normal human mammary epithelial cells (Lonza) were cultured using a MEGM bullet kit (Lonza). MCF7, BT474, HCC1428, HCC38, MDAMB231, Capan1, SW480, and HCT116 cells (ATCC) were cultured in either DMEM or RPMI 1640 supplemented with 10% FBS. The sequences of the siRNAs used for the siRNA experiments have been published previously (17, 18). H2AX overexpression experiments were performed as described previously (17, 18). FACS analysis and double thymidine block were performed as described previously (19). The SA β-galactosidase assay was performed as described previously (14).
Analysis of DNA Damage Induction and Cell Death
DNA damage was induced by camptothecin (CPT), doxorubicin, cisplatin, or hydroxyurea (HU) (Sigma). Survival rates were determined by counting the number of viable cells after 6 days of CPT treatment (experiments shown in Figs. 1–5) or by counting the number of colonies formed after 1-week release from CPT in the presence or absence of PJ34 (ALEXIS) (experiments shown in Fig. 7). The effects of transient H2AX knockdown and overexpression were determined after 2 days of CPT treatment.
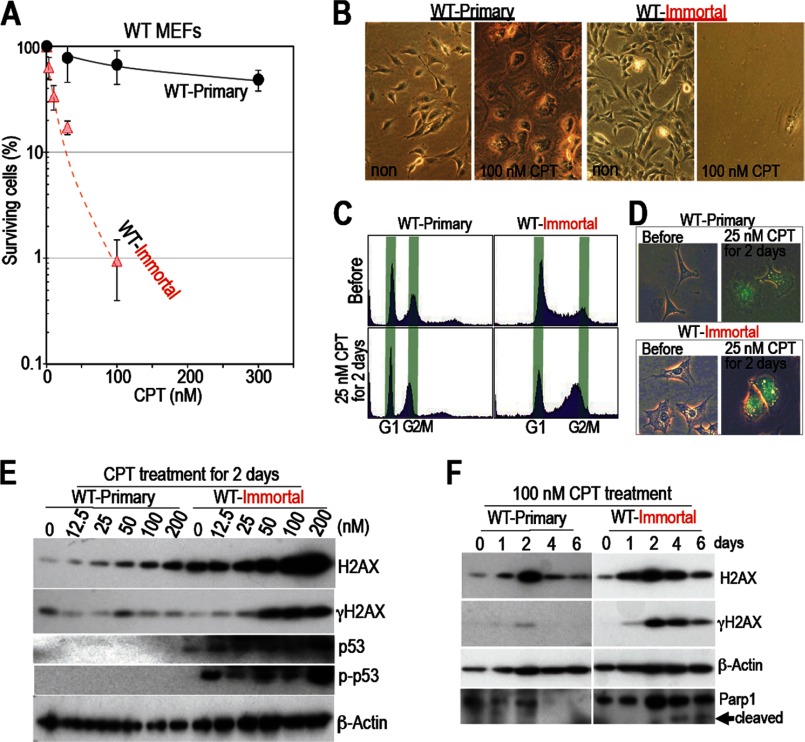
FIGURE 1.
Unlike immortalized MEFs, primary WT MEFs survive in the presence of CPT. A and B, WT MEFs become sensitive to CPT after immortalization. Each type of MEF was treated with CPT for 6 days, and the surviving cells were counted and plotted with surviving percentage compared with the untreated cells (A). Here, 100% survival at 0 nm CPT corresponds to the fractions before CPT treatment. Surviving rates were plotted with the means of three independent experiments and the S.D. Although primary WT MEFs survived, immortalized WT MEFs were sensitive to the drug. Representative images are shown (B). C and D, after CPT treatment, cell cycle arrest and senescence were examined by FACS (C) and a SA-β-galactosidase assay (D). Unlike primary MEFs, immortalized MEFs arrested in G2 phase after CPT treatment and showed increased SA-β-galactosidase activity. E and F, effects of different CPT-doses (E) and different treatment times (F). Unlike primary WT MEFs, which enter a quiescent state after down-regulating H2AX expression, immortalized WT MEFs accumulate H2AX and show increased γH2AX levels, p53 accumulation, increased levels of phosphorylated p53 at Ser-18, and increased signals representing cleaved-Parp1, all of which indicate apoptosis.
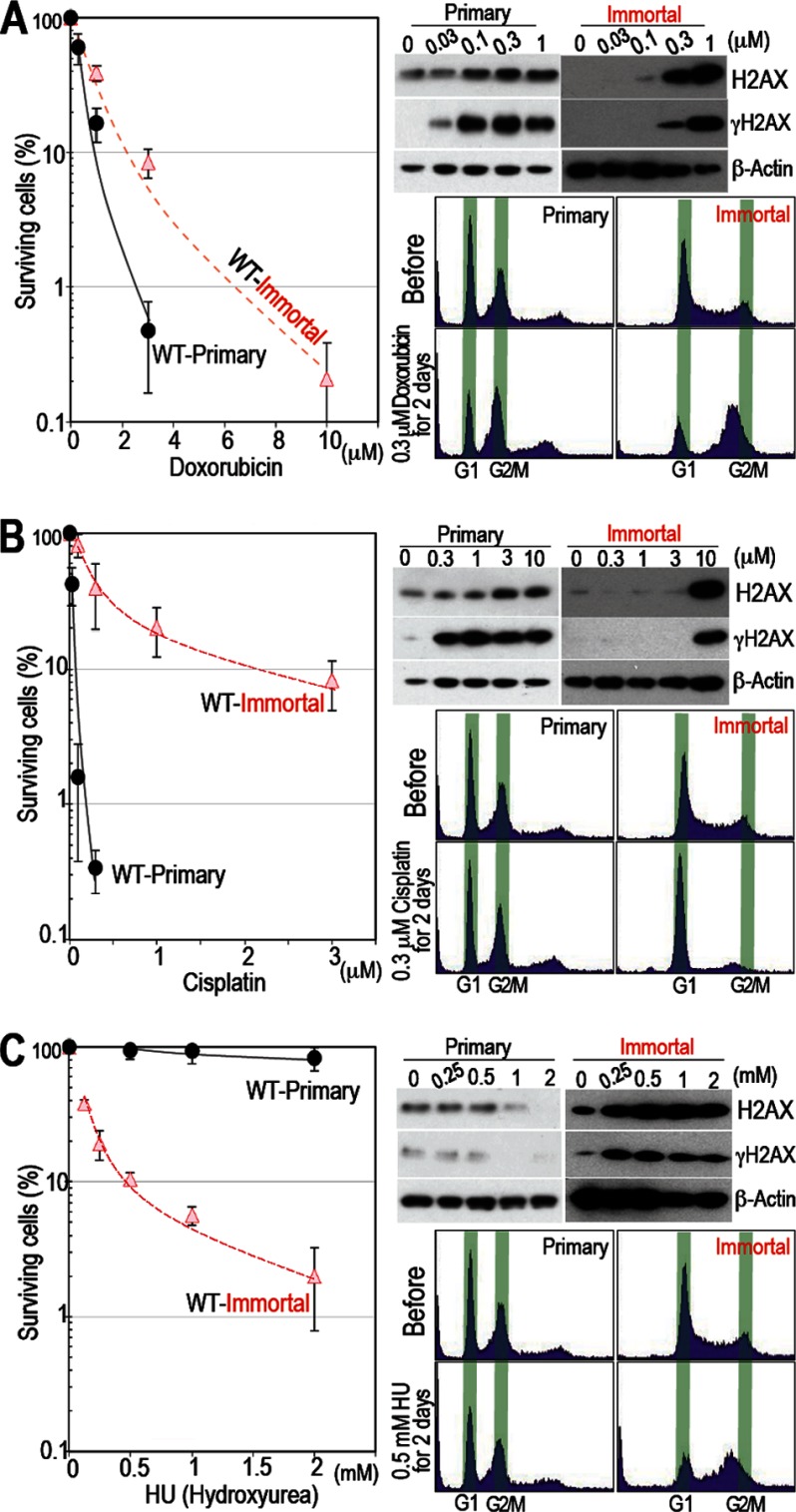
FIGURE 2.
Immortalized MEFs are selectively killed by HU but not by doxorubicin or cisplatin. A and B, primary WT MEFs are sensitive to doxorubicin and cisplatin but not to CPT. Sensitivity to damage and the levels of H2AX and γH2AX in response to doxorubicin (A) and cisplatin (B) were determined as outlined in Fig. 1E. Cell cycle arrest was examined by FACS. Primary MEFs are sensitive to these damaging agents and show increased H2AX accumulation and γH2AX levels. C, similar to their response to CPT, primary WT MEFs survived in the presence of HU. Sensitivity to damage, H2AX and γH2AX levels, and cell cycle arrest were examined as outlined in Fig. 1. Primary WT MEFs are resistant to HU and show no γH2AX signal. Survival rates were plotted as in Fig. 1A.
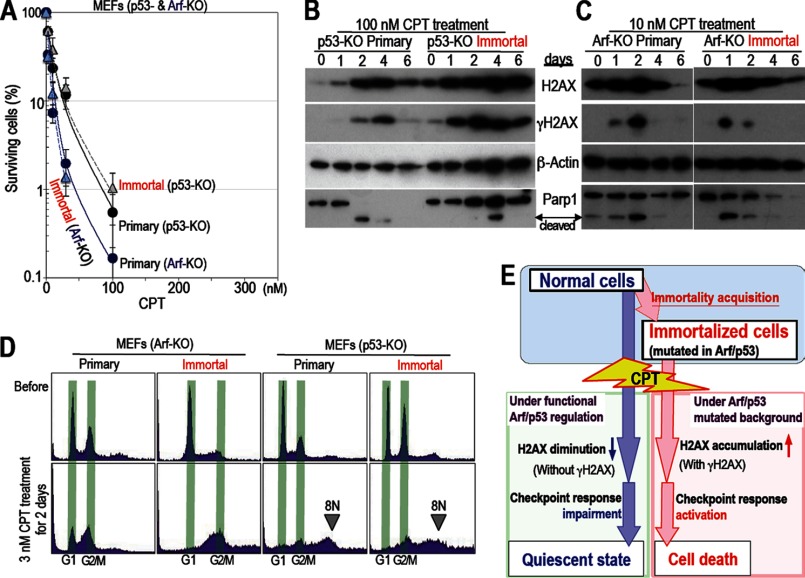
FIGURE 3.
Cells harboring mutations in Arf/p53 are sensitive to CPT. A, MEFs harboring mutations in Arf and p53 are sensitive to CPT. All experiments were performed as outlined in Fig. 1. Both primary and immortalized Arf and p53 KO MEFs were sensitive to CPT (similar to immortalized WT MEFs). Survival rates were plotted as in Fig. 1A. Representative images of the cell cultures are also shown in supplemental Fig. S1A. B and C, the effects of CPT treatment were examined over time. Both Arf and p53 KO MEFs show signals for γH2AX and cleaved Parp1 after CPT treatment. The experiments were performed as outlined in Fig. 1F. Unlike WT MEFs, both primary and immortalized p53 KO MEFs showed increased levels of H2AX and γH2AX and a cleaved-Parp1 signal, suggesting apoptosis induction. Compared with p53 KO MEFs, Arf KO MEFs showed early onset of cell death (see also supplemental Fig. S1B). Therefore, Arf KO MEFs were subsequently treated with 10 nm CPT. D, cell cycle arrest was examined by FACS. Arf KO MEFs arrested in G2 phase, whereas p53 KO MEFs showed an 8N chromosome peak, which often indicates cell death because of mitotic catastrophe. E, model showing the cellular response to CPT. While normal cells reduce their expression of H2AX and become quiescent (with impaired checkpoint responses), immortalized cells expressing γH2AX are killed preferentially.
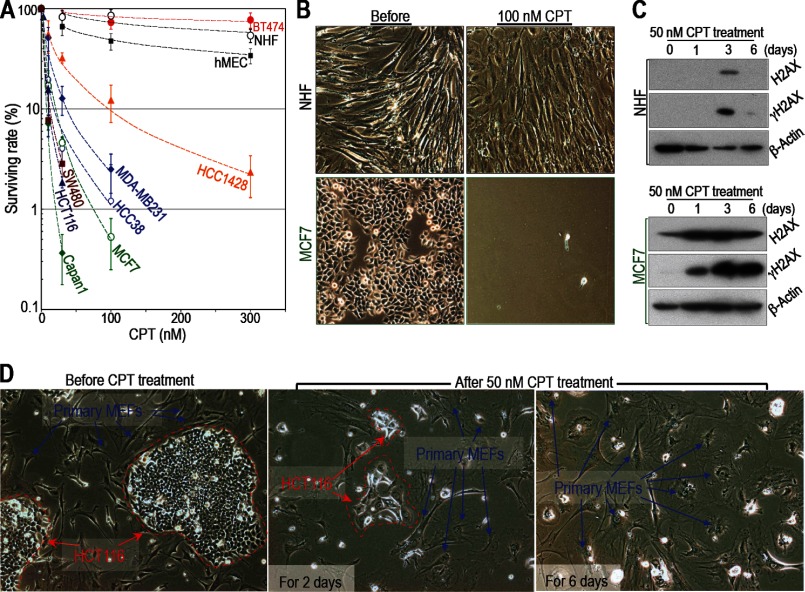
FIGURE 4.
Cancer cells are more sensitive to CPT than normal cells unless they acquire resistance. A–C, cancer cells are generally sensitive to CPT unless they acquire resistance. Cancer cells are more sensitive to CPT than primary NIHs and human mammary epithelial cells (hMEC). However, BT474 breast cancer cells are resistant (A). Sensitivity to damage was determined as outlined in Fig. 1. Survival rates were plotted as in Fig. 1A. B, representative images of NHF and MCF7 cells are shown before and after CPT treatment (other cell lines are shown in supplemental Fig. S3, A and B). C, H2AX and γH2AX levels in MCF7 and NHF cells (levels in other cell lines are shown in supplemental Fig. S3, C and D). The cancer cells used in these experiments harbor mutations in either Arf (HCT116 and MCF7) or p53 (BT474, HCC1428, MDA-MB231, Capan1, HCC 38, and SW480). D, selective killing of cancer cells was examined in a mixed culture of primary WT MEFs and HCT116 (colon cancer) cells. The different cell types are easily distinguished on the basis of morphology and size. HCT116 cells are circled with red dashed lines. Primary WT MEFs are indicated by blue arrows. The images show cells before and after treatment with 50 nm CPT for 2 or 6 days. Both cell types initially showed the flattened and enlarged morphology characteristic of senescent cells (after 2 days). After 6 days, the cancer cells died, but the primary WT MEFs survived.
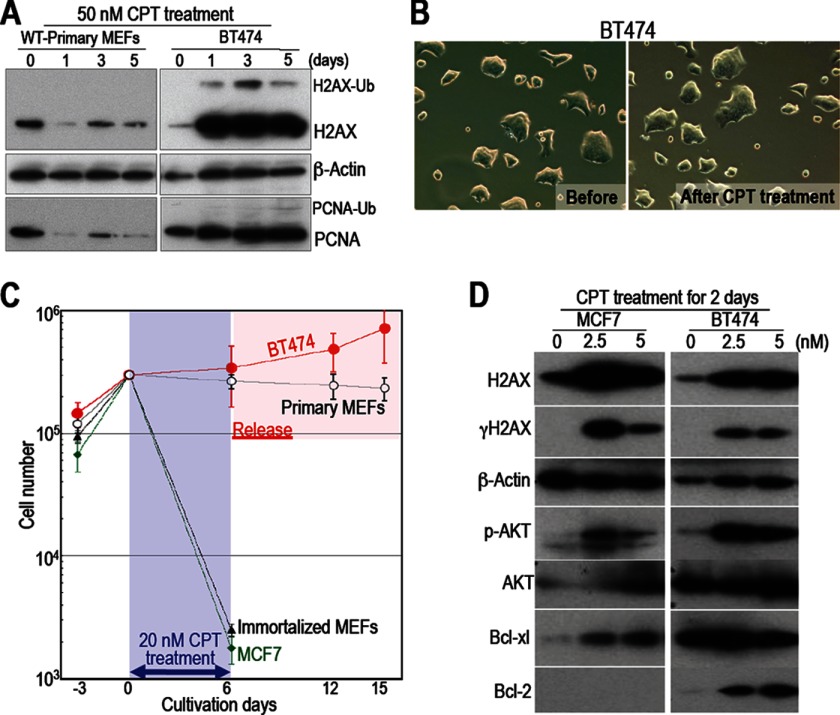
FIGURE 5.
The mechanism underlying the resistance of BT474 cells to CPT is different from that underlying the survival of normal cells. A, the regulatory mechanisms in cancer cells that are resistant to CPT are different from those in surviving normal cells. Responses to 50 nm CPT are shown. Unlike primary WT MEFs, BT474 cells (which are resistant to CPT) accumulated H2AX and maintained their PCNA levels. Ub-H2AX and Ub-PCNA indicate ubiquitinated molecules. B, unlike primary WT MEFs, BT474 cells show morphological changes after CPT treatment. C, each cell type was treated with 20 nm CPT for 6 days, during which most of the immortalized WT MEFs and MCF7 cells died. However, BT474 cells and primary WT MEFs survived. Although BT474 cells recovered growth activity immediately after release from CPT, primary WT MEFs remained quiescent. D, compared with cancer cells that have not developed drug resistance (MCF7 cells), BT474 cells show increased levels of phosphorylated AKT, Bcl-xl, and Bcl2 (all of which promote cell survival).
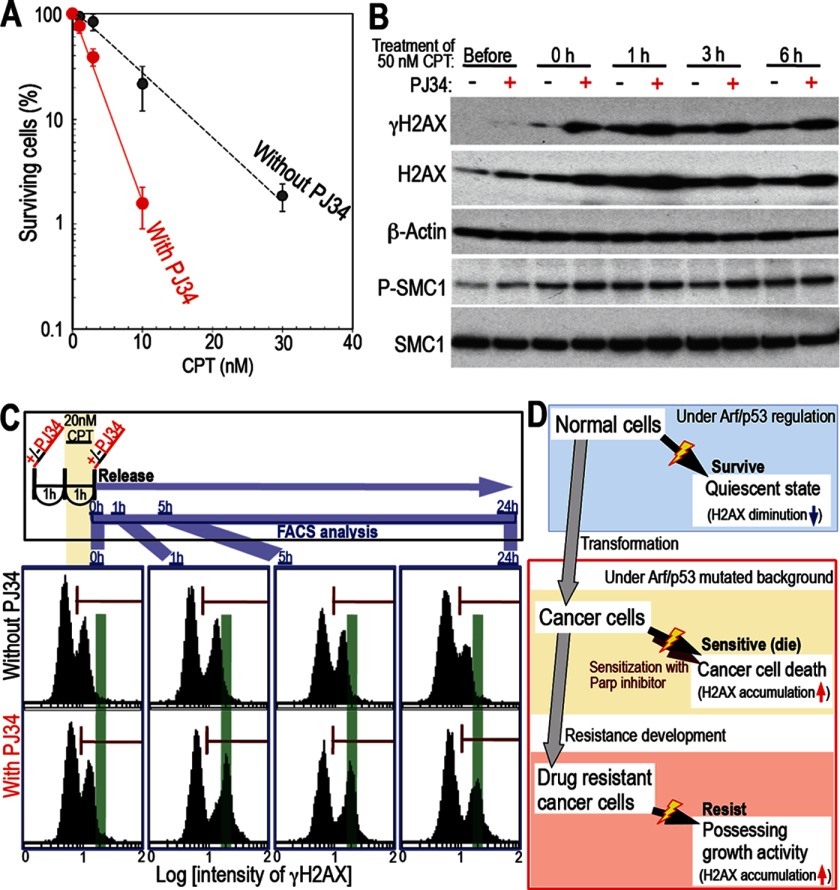
FIGURE 7.
H2AX accumulation induces cancer cell death. A, treatment with a Parp inhibitor increases CPT-induced cancer cell death. The effects of the Parp inhibitor PJ34 were examined in CPT-treated HCT116 cells using colony formation assays. Survival rates were plotted with the means of three independent experiments and the S.D. Treatment with PJ34 caused a significant increase in the level of cell death. B, Parp inhibitors cause increased H2AX accumulation and heightened the checkpoint response in CPT-treated cells. The effects of PJ34 were examined in HCT116 cells synchronized in S phase using a double thymidine block. Cells were released from double thymidine block for 1 h and then treated with CPT in the presence or absence of PJ34. The level of H2AX accumulation was increased significantly in the presence of PJ34, which was associated with increased activation of the checkpoint response (indicated by expression of γH2AX and phosphorylated SMC1). C, Parp inhibition induces increased expression of γH2AX in response to CPT. To address the effects of PJ34 on γH2AX expression, cells were pulsed with CPT for 1 h in the presence or absence of PJ34 and then released. γH2AX levels were then examined by FACS (the top panel shows the experimental scheme). γH2AX levels after 1 h of CPT treatment (0 h) were almost identical in the absence and presence of PJ34, as determined by the number of damaged cells and the intensity of the γH2AX signal (see the signals denoted by the red bar in each panel). At 1–5 h after release from CPT, an increase in γH2AX intensity was observed only in PJ34-treated cells (the peak shifted onto the green line). D, model showing differences in CPT sensitivity in normal cells, cancer cells, and drug-resistant cancer cells. Normal cells survive in the presence of CPT by down-regulating H2AX and becoming quiescent. Transformed cells accumulate H2AX and are killed preferentially unless they develop resistance.
Antibodies and Western blotting
Antibodies against γH2AX (Upstate), H2AX (Bethyl), β-actin (Sigma), p53 (Leica), phosphorylated p53 (Ser-15 (Ser-18 in mice), Cell Signaling Technology, Inc.), SMC1 (Bethyl), phosphorylated SMC1 (Ser-966) (Bethyl), Chk2 (Cell Signaling Technology, Inc.), phosphorylated Chk2 (Thr-68, Cell Signaling Technology, Inc.), PCNA (Santa Cruz Biotechnology, Inc.), Parp1 (Cell Signaling Technology, Inc.), Bcl2 (Abcam), Bcl-xl (Cell Signaling Technology, Inc.), AKT (Cell Signaling Technology, Inc.), and phosphorylated AKT (Thr-308, Cell Signaling Technology, Inc.) were used for Western blot analysis, which was performed as described previously (20).
RESULTS
Unlike Immortalized Cells, Normal Cells Survive in the Presence of CPT by Down-regulating H2AX
MEFs become immortal because of genomic instability (19) and mutations in the Arf/p53 protein module (either Arf or p53) (21), processes similar to those that occur during cancer development (22). Therefore, to test our hypothesis that cellular transformation results in changes in drug sensitivity, we examined the response of MEFs to CPT both before and after immortalization. CPT inhibits topoisomerase I and causes DNA replication stress in a manner similar to that induced by the anti-cancer drugs topotecan and irinotecan (23, 24). Although CPT killed the majority of immortalized MEFs, most primary MEFs survived, showing a flattened and enlarged morphology (Fig. 1, A and B). Sensitivity to CPT increased 100-fold after immortalization (Fig. 1A). Before undergoing cell death, immortalized MEFs arrested at G2 and showed increased SA-β-galactosidase activity (Fig. 1, C and D). In contrast, damaged primary MEFs showed weaker SA-β-galactosidase activity and did not arrest at a specific point in the cell cycle. Thus, treatment with CPT caused primary MEFs to undergo growth arrest and to adopt a flattened and enlarged morphology. However, the cells did not enter a canonical senescent state.
To examine changes in the DNA damage checkpoint response, we compared the damage responses of primary and immortalized MEFs after CPT treatment. The critical difference between primary and immortalized MEFs appeared to be the responses of checkpoint factors, as illustrated by the increased γH2AX signal, p53 accumulation, and the increased levels of phosphorylated p53 (Ser-18 in mice) in immortalized cells (Fig. 1E). Unlike in immortal MEFs, checkpoint factors were rarely activated in primary MEFs. These differences were associated with the levels of H2AX expression. Consistent with the increased checkpoint responses, high levels of H2AX accumulated in immortalized MEFs (Fig. 1E). However, the levels decreased with the appearance of cleaved Parp1 (Fig. 1F). These findings are consistent with apoptosis induction. In contrast, H2AX levels in primary MEFs increased transiently without activating the checkpoint response but, subsequently, decreased again (Fig. 1F), and the cells became growth-arrested and showed a flattened and enlarged morphology (B). This suggests the onset of quiescence in response to the down-regulation of H2AX. Thus, normal cells become quiescent upon down-regulation of H2AX and are able to survive the effects of CPT. This quiescence-associated survival in the presence of CPT is lost upon immortalization.
Cisplatin and Doxorubicin Do Not Preferentially Target Normal Cells, but damaged Cells Accumulate γH2AX and Die
Unlike CPT, primary MEFs did not survive in the presence of doxorubicin or cisplatin (Fig. 2, A and B). In fact, they were more sensitive to these drugs than their immortalized counterparts. FACS analysis was not able to clarify the difference between primary and immortalized MEFs after treatment with cisplatin and doxorubicin. In both cell types, doxorubicin induced G2 arrest (Fig. 2A) but cisplatin did not (B). Importantly, sensitivity to both of these drugs was associated with H2AX accumulation and increased γH2AX expression. Thus, the regulation of cellular H2AX levels is affected by DNA damage, which, importantly, influences cell fate. Cells that down-regulate H2AX become quiescent and are able to survive in the presence of CPT, whereas cells that accumulate H2AX and increase their expression of γH2AX are killed.
Normal Cells Survive Treatment with Drugs That Cause DNA Replication Stress
As with CPT, down-regulation of H2AX and the selective survival of normal cells were observed upon treatment with HU, which induces DNA replication stress by depleting the cellular dNTP pool (Fig. 2C). This implies that H2AX is often down-regulated in response to DNA replication stress-associated damage, conferring a “survival” phenotype upon normal cells. In fact, the major damage caused by CPT occurs in association with DNA replication stress (24–26). Similar to CPT, HU caused G2 phase arrest in immortalized MEFs but not in primary MEFs. These results indicate that normal cells survive in the presence of drugs that induce DNA replication stress by down-regulating H2AX, a process that is regulated by the Arf/p53 protein module. Therefore, cells become sensitive to these drugs after immortalization.
Cells Harboring Mutated Arf or p53 Are Sensitive to CPT
To address whether changes in sensitivity to CPT are the result of mutations in the Arf/p53 protein module, we examined sensitivity to CPT and changes in H2AX expression in Arf and p53 KO MEFs (both primary and immortal). As expected, neither Arf nor p53 KO MEFs showed changes in drug sensitivity as they became immortal. Indeed, they were as sensitive as immortalized WT MEFs (Fig. 3A and supplemental Fig. S2). In addition, both primary and immortal Arf and p53 KO MEFs showed increased accumulation of H2AX and increased expression of γH2AX and cleaved-Parp1 (Fig. 3, B and C). The cells arrested in G2 phase (mainly those harboring Arf mutations) and/or showed the appearance of an 8N chromosome peak (mainly those harboring p53 mutations), which often indicates cell death because of mitotic catastrophe (Fig. 3D). These findings indicate that the survival of normal cells in the presence of CPT is dependent on both Arf and p53. Therefore, the survival of normal cells that have down-regulated their expression of H2AX is abrogated by immortalization because of the associated mutations in the Arf/p53 protein module (Fig. 3E).
Because p53 mediates the induction of cell death, Arf KO MEFs with WT p53 are more sensitive to CPT than cells without p53 (Fig. 3A). However, the difference is much smaller than that observed between normal primary MEFs and MEFs without a functional Arf/p53 protein module (Figs. 1A and 3A).
Cancer Cells Are Preferentially Killed unless They Have Acquired Resistance
A key question is whether alterations in H2AX regulation/expression lead to the preferential killing of cancer cells. To investigate this issue, we examined the killing efficiency of CPT in several types of human cancer cells harboring mutations in the Arf/p53 protein module (supplemental Table S1) and compared it with that in NHFs and human mammary epithelial cells (Fig. 4, A and B, and supplemental Fig. S3, A and B). After exposure to CPT, both NHFs and human mammary epithelial cells became quiescent and survived in the presence of the drug. The cells showed a flattened and enlarged morphology and down-regulated their expression of H2AX. On the other hand, cancer cells increased their expression of H2AX and γH2AX (Fig. 4C and supplemental Fig. S3, C and D), and most of the cells died. However, BT474 cells (which are resistant to the drug) were not killed. We also observed the selective killing of cancer cells when studying a mixed culture containing HCT116 cells (a human colon cancer cell line) and primary WT MEFs (Fig. 4D). Thus, cancer cells harboring mutations in the Arf/p53 protein module are sensitive to CPT unless they have acquired resistance.
Although it is still not clear how cancer cells acquire drug resistance, the mechanism underlying the resistance shown by BT474 cells appears to be completely different from that underlying the survival of normal cells. Damaged normal cells lost PCNA and H2AX expression and became quiescent, showing a flattened and enlarged morphology. However, BT474 cells accumulated high levels of H2AX without losing PCNA expression (Fig. 5A) or showing any of the associated morphological changes (B). In addition, the growth of BT474 cells was immediately restored after release from CPT, whereas primary WT MEFs remained quiescent (Fig. 5C). Thus, the mechanism underlying the resistance shown by BT474 is different from that underlying the survival of normal cells. This implies that drug resistance develops in cancer cells as they acquire other mechanisms that permit them to escape cell death.
Drug resistance can be attributed to efflux (the active pumping of a drug out of the cell) as well as to various survival signals. However, the resistance of BT474 cells is probably not associated with drug efflux because damaged BT474 cells accumulated H2AX and showed an increase in γH2AX signaling and in the levels of ubiquitinated PCNA and H2AX (Fig. 5, A and D). The cells also showed increased levels of phosphorylated AKT, Bcl-xl, and Bcl-2 (all of which promote cell survival) upon CPT treatment, which was not observed in MCF7 cells (Fig. 5D). This suggests that survival signals, rather than drug efflux, promote the survival of these cells. Unlike drug efflux, which acts for multiple drugs, survival signals are dependent on the response to each type of damage. In fact, BT474 is resistant to HU but not to cisplatin and doxorubicin (supplemental Fig. S4). Nevertheless, resistant cancer cells that express the γH2AX signal are more likely associated with survival signal activation.
H2AX Knockdown in Cancer Cells Induces Tolerance to CPT and a Senescent Morphology
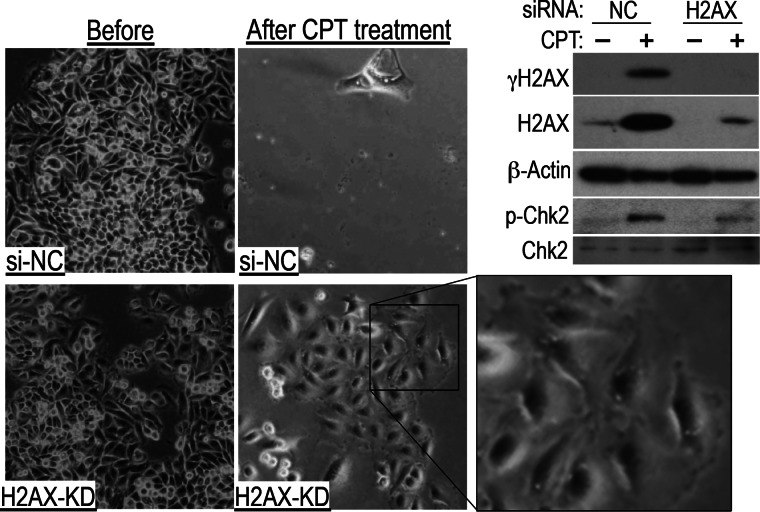
The above results indicate that normal cells resist damage by down-regulating their expression of H2AX, a process regulated by the Arf/p53 protein module. This process is non-functional in cancer cells. However, one question remains. Do reduced H2AX levels directly contribute to resistance? To address this, we knocked down H2AX expression in the colon cancer cell line SW480. SW480 cells treated with a negative control siRNA were killed by CPT. However, SW480 cells treated with siRNA targeting H2AX survived, adopting the flattened and enlarged morphology associated with impairment of DNA damage checkpoint responses (Fig. 6), which was also observed in normal cells. Similar results were obtained for MCF7 and HCT116 cells (supplemental Fig. S5, A and B). In addition, immortalized MEFs were sensitized to CPT treatment upon H2AX overexpression (supplemental Fig. S5C). These results suggest that normal cells survive in the presence of CPT by down-regulating H2AX. This lends further support to the concept that the mechanism underlying the survival of normal cells involves the Arf/p53-dependent down-regulation of H2AX. Thus, cancer cells that are unable to down-regulate H2AX are killed preferentially.
FIGURE 6.
H2AX knockdown in cancer cells induces tolerance to CPT. After H2AX knockdown (KD), SW480 cancer cells treated with 10 nm CPT for 2 days became quiescent and showed a senescent morphology. Representative images are shown. The top right panels show the effects of H2AX knockdown on DNA damage checkpoint activation. A weakened response is indicated by the levels of phosphorylated Chk2 and γH2AX. NC, cell transfected with negative control siRNA.
A Parp Inhibitor Sensitizes Cells to CPT, Resulting in Increased H2AX Accumulation and Enhanced Checkpoint Responses
On the basis of the above results, Arf/p53-dependent down-regulation of H2AX enables cells to survive in the presence of CPT, whereas H2AX accumulation is associated with cell death via the induction of efficient checkpoint responses. This prompted us to investigate the effects of modulating H2AX levels. For this, we used the Parp inhibitor PJ34, which is thought to efficiently induce cancer cell death for the following reasons. Down-regulation of H2AX in normal cells is less efficient during non-homologous end-joining than during homologous recombination (14), suggesting a more efficient checkpoint response during non-homologous end-joining than during homologous recombination, and after damage induced by CPT, non-homologous end-joining becomes the predominant repair mechanism in the absence of Parp1 because of the suppression of homologous recombination (27, 28). Therefore, we tested whether PJ34 enhances the killing efficiency of CPT in HCT116 cells via increased expression of H2AX. As expected, treatment with PJ34 significantly increased the level of CPT-induced cell death (3-fold), which was associated with increased H2AX accumulation and damage-related cell signaling, as indicated by an increase in the levels of γH2AX and phosphorylated SMC1 (Fig. 7, A and B). In addition, cells pulsed with CPT for 1 h, either in the presence or absence of PJ34, showed identical levels of damage (during the first hour), as assessed by the number of γH2AX-positive cells and the γH2AX signal intensity (Fig. 7C, 0 h). This led to a significant increase in γH2AX intensity in PJ34-treated cells (see the shifted peak at 1 h). Furthermore, the γH2AX signal decayed much more slowly in cells treated with PJ34, which is consistent with a deficiency in Parp-dependent repair (25, 29). Thus, Parp inhibition induces H2AX accumulation by modulating the repair system, a process that enhances the checkpoint responses and leads to increased cancer cell death (Fig. 7D).
DISCUSSION
Although DNA-damaging drugs are used widely to treat cancer, it is still unclear how they induce death in cancer cells more efficiently than in normal somatic cells. This study identified a novel regulatory mechanism underlying the selective killing of cancer cells that have not yet acquired drug resistance (Fig. 7D). The mechanism can be outlined as follows. Normal cells down-regulate H2AX in response to DNA replication stress via a process that is regulated by Arf/p53, resulting in defective checkpoint responses (H2AX is required for efficient checkpoint responses), and unlike benign tumors, malignant cancer cells mostly harbor mutations in the Arf/p53 protein module (either Arf or p53). Thus, they are unable to down-regulate H2AX and are sensitive to drugs that induce DNA replication stress (unless they have acquired drug resistance).
Normal cells generally become growth-arrested after several rounds of proliferation in vivo and in vitro, a process that is associated with H2AX down-regulation and is dependent on both Arf and p53. Although it is still unclear how the down-regulation of H2AX is controlled by the Arf/p53 protein module (30), the process only occurs in normal cells under the regulation of both Arf and p53 (14), which prevents transformation (19, 31). The results of this study show that normal cells down-regulate H2AX and enter a quiescent state in response to CPT, which is identical to the growth arrest observed when cells proliferate normally and clearly differently from CPT-resistant BT474 cancer cells (see supplemental Fig. S6 and Fig. 5C). This implies that CPT treatment may simply induce premature growth arrest in normal cells, which then enter a quiescent state (unless the damage is too severe). Normal cells only enter such a state after exposure to mild stress and are protected from immortalization. This process is clearly different from the response to acute damage (32, 33). Thus, cells generally survive in the presence of CPT or HU unless cells are transformed with mutations in the Arf/p53 protein module. This results in aberrant H2AX regulation and changes in the checkpoint response.
p53 is involved in the DNA damage checkpoint response (34–36), during which it is activated by ATM and ATR and plays a role in inducing senescence and apoptosis (37–39). Thus, cancer cells harboring mutations in p53 are less sensitive to drugs than cells that do not harbor p53 mutations. However, chemotherapy kills cancer cells harboring Arf/p53 mutations more efficiently than it kills normal somatic cells that possess functional Arf/p53. The results presented in this study show that Arf/p53-dependent down-regulation of H2AX allows normal cells to survive in the presence of cytotoxic drugs that induce DNA replication stress (Figs. 3E and 7D). This is mainly because cells lacking H2AX show defective activation of checkpoint responses, such as ATM and ATR (40–42), which activate p53-mediated apoptosis. This suggests that the level of H2AX is the critical factor that determines whether cell death is induced. Taken together with the results of the experiments showing that a Parp inhibitor modulates H2AX expression, this strongly suggests that, before embarking upon a course of chemotherapy, H2AX status must be taken into consideration if efficient cell death is to be induced.
Acknowledgments
We thank the RIKEN BRL Cell Bank for the normal human umbilical cord fibroblast (NHF) cells (HUC-F2). We also thank C. J. Sherr for Arf KO mice and H. Esumi, A.-M. Ryden, P. Hsieh, and N. Takamatsu for critical discussions.
This study was supported by National Cancer Center Research and Development Fund Grant 23-C-10, by a grant-in-aid and the Third Term Comprehensive 10-Year Strategy for Cancer Control, and by Grants-in-Aid for Scientific Research MEXT KAKENHI Grant 20770136. This work was also supported by a research resident fellowship from the Foundation for Promotion of Cancer Research (to A. I.).

This article contains supplemental Figs. S1–S6 and Table 1.
- MEF
- mouse embryonic fibroblast
- NHF
- normal human fibroblast
- SA
- senescence-associated
- CPT
- camptothecin
- HU
- hydroxyurea
- PCNA
- proliferating cell nuclear antigen.
REFERENCES
- 1. Druker B. J. (2003) David A. Karnofsky Award lecture. Imatinib as a paradigm of targeted therapies. J. Clin. Oncol. 21, 239s-245s [DOI] [PubMed] [Google Scholar]
- 2. Vanneman M., Dranoff G. (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernier J. (2005) Alteration of radiotherapy fractionation and concurrent chemotherapy. A new frontier in head and neck oncology? Nat. Clin. Pract. Oncol. 2, 305–314 [DOI] [PubMed] [Google Scholar]
- 4. Folprecht G., Köhne C. H. (2005) Drug Insight. Metastatic colorectal cancer. Oral fluoropyrimidines and new perspectives in the adjuvant setting. Nat. Clin. Pract. Oncol. 2, 578–587 [DOI] [PubMed] [Google Scholar]
- 5. Kelland L. (2007) The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584 [DOI] [PubMed] [Google Scholar]
- 6. Midgley R., Kerr D. J. (2005) Adjuvant chemotherapy for stage II colorectal cancer. The time is right! Nat. Clin. Pract. Oncol. 2, 364–369 [DOI] [PubMed] [Google Scholar]
- 7. Reni M., Cereda S., Galli L. (2007) PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) for patients with advanced pancreatic cancer: the ghost regimen. Cancer Lett. 256, 25–28 [DOI] [PubMed] [Google Scholar]
- 8. Vincenzi B., Schiavon G., Pantano F., Santini D., Tonini G. (2008) Predictive factors for chemotherapy-related toxic effects in patients with colorectal cancer. Nat. Clin. Pract. Oncol. 5, 455–465 [DOI] [PubMed] [Google Scholar]
- 9. Redon C. E., Nakamura A. J., Zhang Y. W., Ji J. J., Bonner W. M., Kinders R. J., Parchment R. E., Doroshow J. H., Pommier Y. (2010) Histone γH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin. Cancer Res. 16, 4532–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding H. F., Fisher D. E. (1998) Mechanisms of p53-mediated apoptosis. Crit. Rev. Oncog 9, 83–98 [DOI] [PubMed] [Google Scholar]
- 11. Oren M. (2003) Decision making by p53. Life, death and cancer. Cell Death Differ. 10, 431–442 [DOI] [PubMed] [Google Scholar]
- 12. Zawacka-Pankau J., Krachulec J., Grulkowski I., Bielawski K. P., Selivanova G. (2008) The p53-mediated cytotoxicity of photodynamic therapy of cancer. Recent advances. Toxicol. Appl. Pharmacol. 232, 487–497 [DOI] [PubMed] [Google Scholar]
- 13. Lu C., El-Deiry W. S. (2009) Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis 14, 597–606 [DOI] [PubMed] [Google Scholar]
- 14. Atsumi Y., Fujimori H., Fukuda H., Inase A., Shinohe K., Yoshioka Y., Shikanai M., Ichijima Y., Unno J., Mizutani S., Tsuchiya N., Hippo Y., Nakagama H., Masutani M., Teraoka H., Yoshioka K. (2011) Onset of quiescence following p53 mediated down-regulation of H2AX in normal cells. PLoS ONE 6, e23432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamijo T., Zindy F., Roussel M. F., Quelle D. E., Downing J. R., Ashmun R. A., Grosveld G., Sherr C. J. (1997) Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 [DOI] [PubMed] [Google Scholar]
- 16. Todaro G. J., Green H. (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lukas C., Melander F., Stucki M., Falck J., Bekker-Jensen S., Goldberg M., Lerenthal Y., Jackson S. P., Bartek J., Lukas J. (2004) Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 23, 2674–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dimitrova N., de Lange T. (2006) MDC1 accelerates nonhomologous end-joining of dysfunctional telomeres. Genes Dev. 20, 3238–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ichijima Y., Yoshioka K., Yoshioka Y., Shinohe K., Fujimori H., Unno J., Takagi M., Goto H., Inagaki M., Mizutani S., Teraoka H. (2010) DNA lesions induced by replication stress trigger mitotic aberration and tetraploidy development. PLoS ONE 5, e8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshioka K., Yoshioka Y., Hsieh P. (2006) ATR kinase activation mediated by MutSα and MutLα in response to cytotoxic O6-methylguanine adducts. Mol. Cell 22, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matheu A., Maraver A., Klatt P., Flores I., Garcia-Cao I., Borras C., Flores J. M., Viña J., Blasco M. A., Serrano M. (2007) Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379 [DOI] [PubMed] [Google Scholar]
- 22. Matheu A., Maraver A., Serrano M. (2008) The Arf/p53 pathway in cancer and aging. Cancer Res. 68, 6031–6034 [DOI] [PubMed] [Google Scholar]
- 23. Pommier Y., Leo E., Zhang H., Marchand C. (2010) DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17, 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pommier Y. (2006) Topoisomerase I inhibitors. Camptothecins and beyond. Nat. Rev. Cancer 6, 789–802 [DOI] [PubMed] [Google Scholar]
- 25. Ray Chaudhuri A., Hashimoto Y., Herrador R., Neelsen K. J., Fachinetti D., Bermejo R., Cocito A., Costanzo V., Lopes M. (2012) Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 19, 417–423 [DOI] [PubMed] [Google Scholar]
- 26. Rao V. A., Fan A. M., Meng L., Doe C. F., North P. S., Hickson I. D., Pommier Y. (2005) Phosphorylation of BLM, dissociation from topoisomerase IIIα, and colocalization with γ-H2AX after topoisomerase I-induced replication damage. Mol. Cell. Biol. 25, 8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugimura K., Takebayashi S., Taguchi H., Takeda S., Okumura K. (2008) PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J. Cell Biol. 183, 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hochegger H., Dejsuphong D., Fukushima T., Morrison C., Sonoda E., Schreiber V., Zhao G. Y., Saberi A., Masutani M., Adachi N., Koyama H., de Murcia G., Takeda S. (2006) Parp-1 protects homologous recombination from interference by Ku and Ligase IV in vertebrate cells. EMBO J. 25, 1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y. W., Regairaz M., Seiler J. A., Agama K. K., Doroshow J. H., Pommier Y. (2011) Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 39, 3607–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshioka K., Atsumi Y., Fukuda H., Masutani M., Teraoka H. (2012) The quiescent cellular state is Arf/p53-dependent and associated with H2AX downregulation and genome stability. Int. J. Mol. Sci. 13, 6492–6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujimori H., Shikanai M., Teraoka H., Masutani M., Yoshioka K. (2012) Induction of cancerous stem cells during embryonic stem cell differentiation. J. Biol. Chem. 287, 36777–36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li T., Kon N., Jiang L., Tan M., Ludwig T., Zhao Y., Baer R., Gu W. (2012) Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149, 1269–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brady C. A., Jiang D., Mello S. S., Johnson T. M., Jarvis L. A., Kozak M. M., Kenzelmann Broz D., Basak S., Park E. J., McLaughlin M. E., Karnezis A. N., Attardi L. D. (2011) Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 145, 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liebermann D. A., Hoffman B., Vesely D. (2007) p53 induced growth arrest versus apoptosis and its modulation by survival cytokines. Cell Cycle 6, 166–170 [DOI] [PubMed] [Google Scholar]
- 35. Lowe S. W., Cepero E., Evan G. (2004) Intrinsic tumour suppression. Nature 432, 307–315 [DOI] [PubMed] [Google Scholar]
- 36. Vousden K. H. (2000) p53. Death star. Cell 103, 691–694 [DOI] [PubMed] [Google Scholar]
- 37. Zhou B. B., Elledge S. J. (2000) The DNA damage response. Putting checkpoints in perspective. Nature 408, 433–439 [DOI] [PubMed] [Google Scholar]
- 38. Shiloh Y. (2003) ATM and related protein kinases. Safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 [DOI] [PubMed] [Google Scholar]
- 39. Kastan M. B., Lim D. S. (2000) The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1, 179–186 [DOI] [PubMed] [Google Scholar]
- 40. Bonner W. M., Redon C. E., Dickey J. S., Nakamura A. J., Sedelnikova O. A., Solier S., Pommier Y. (2008) γH2AX and cancer. Nat. Rev. Cancer 8, 957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bassing C. H., Alt F. W. (2004) H2AX may function as an anchor to hold broken chromosomal DNA ends in close proximity. Cell Cycle 3, 149–153 [DOI] [PubMed] [Google Scholar]
- 42. Kinner A., Wu W., Staudt C., Iliakis G. (2008) γ-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 36, 5678–5694 [DOI] [PMC free article] [PubMed] [Google Scholar]