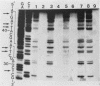
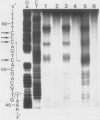
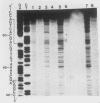
Abstract
We have compared the sites of nucleotide incision on DNA damaged by oxidizing agents when cleavage is mediated by either Escherichia coli endonuclease III or an endonuclease present in bovine and human cells. E. coli endonuclease III, the bovine endonuclease isolated from calf thymus, and the human endonuclease partially purified from HeLa and CEM-C1 lymphoblastoid cells incised DNA damaged with osmium tetroxide, ionizing radiation, or high doses of UV light at sites of pyrimidines. For each damaging agent studied, regardless of whether the E. coli, bovine, or human endonuclease was used, the same sequence specificity of cleavage was observed. We detected this endonuclease activity in a variety of human fibroblasts derived from normal individuals as well as individuals with the DNA repair deficiency diseases ataxia telangiectasia and xeroderma pigmentosum. The highly conserved nature of such a DNA damage-specific endonuclease suggests that a common pathway exists in bacteria, humans, and other mammals for the reversal of certain types of oxidative DNA damage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Bacchetti S., Benne R. Purification and characterization of an endonuclease from calf thymus acting on irradiated DNA. Biochim Biophys Acta. 1975 May 16;390(3):285–297. doi: 10.1016/0005-2787(75)90349-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breimer L. H. A DNA glycosylase for oxidized thymine residues in Drosophila melanogaster. Biochem Biophys Res Commun. 1986 Jan 14;134(1):201–204. doi: 10.1016/0006-291x(86)90547-4. [DOI] [PubMed] [Google Scholar]
- Breimer L. H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984 May 10;259(9):5543–5548. [PubMed] [Google Scholar]
- Breimer L. H. Urea--DNA glycosylase in mammalian cells. Biochemistry. 1983 Aug 30;22(18):4192–4197. doi: 10.1021/bi00287a005. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Properties of a human lymphoblast AP-endonuclease associated with activity for DNA damaged by ultraviolet light, gamma-rays, or osmium tetroxide. Biochemistry. 1983 Sep 13;22(19):4507–4512. doi: 10.1021/bi00288a024. [DOI] [PubMed] [Google Scholar]
- Burton K., Riley W. T. Selective degradation of thymidine and thymine deoxynucleotides. Biochem J. 1966 Jan;98(1):70–77. doi: 10.1042/bj0980070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Weiss B. Endonuclease III (nth) mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jan;82(2):474–478. doi: 10.1073/pnas.82.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P. W., Chan G. L., Haseltine W. A. T4 DNA polymerase (3'-5') exonuclease, an enzyme for the detection and quantitation of stable DNA lesions: the ultraviolet light example. Nucleic Acids Res. 1985 May 10;13(9):3285–3304. doi: 10.1093/nar/13.9.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P. W., Helland D. E., Haseltine W. A. Mechanism of action of a mammalian DNA repair endonuclease. Biochemistry. 1986 Apr 22;25(8):2212–2220. doi: 10.1021/bi00356a054. [DOI] [PubMed] [Google Scholar]
- Gates F. T., Linn S. Endonuclease from Escherichia coli that acts specifically upon duplex DNA damaged by ultraviolet light, osmium tetroxide, acid, or x-rays. J Biol Chem. 1977 May 10;252(9):2802–2807. [PubMed] [Google Scholar]
- Helland D. E., Doetsch P. W., Haseltine W. A. Substrate specificity of a mammalian DNA repair endonuclease that recognizes oxidative base damage. Mol Cell Biol. 1986 Jun;6(6):1983–1990. doi: 10.1128/mcb.6.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland D. E., Raae A. J., Fadnes P., Kleppe K. Properties of a DNA repair endonuclease from mouse plasmacytoma cells. Eur J Biochem. 1985 May 2;148(3):471–477. doi: 10.1111/j.1432-1033.1985.tb08863.x. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Blazka M. E. Hypersensitivity of cultured ataxia-telangiectasia cells to etoposide. J Natl Cancer Inst. 1986 Jun;76(6):1007–1011. [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem. 1982 Oct 10;257(19):11750–11754. [PubMed] [Google Scholar]
- Hentosh P., Henner W. D., Reynolds R. J. Sequence specificity of DNA cleavage by Micrococcus luteus gamma endonuclease. Radiat Res. 1985 Apr;102(1):119–129. [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Male R., Helland D. E., Kleppe K. Purification and characterization of 3-methyladenine-DNA glycosylase from calf thymus. J Biol Chem. 1985 Feb 10;260(3):1623–1629. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nes I. F., Nissen-Meyer J. Endonuclease activities from a permanently established mouse cell line that act upon DNA damaged by ultraviolet light, acid and osmium tetroxide. Biochim Biophys Acta. 1978 Aug 23;520(1):111–121. doi: 10.1016/0005-2787(78)90012-6. [DOI] [PubMed] [Google Scholar]
- Nes I. F. Purification and properties of a mouse-cell DNA-repair endonuclease, which recognizes lesions in DNA induced by ultraviolet light, depurination, gamma-rays, and OsO4 treatment. Eur J Biochem. 1980 Nov;112(1):161–168. doi: 10.1111/j.1432-1033.1980.tb04997.x. [DOI] [PubMed] [Google Scholar]
- Radman M. An endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. J Biol Chem. 1976 Mar 10;251(5):1438–1445. [PubMed] [Google Scholar]
- Royer-Pokora B., Peterson W. D., Jr, Haseltine W. A. Biological and biochemical characterization of an SV40-transformed xeroderma pigmentosum cell line. Exp Cell Res. 1984 Apr;151(2):408–420. doi: 10.1016/0014-4827(84)90391-4. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y., Tabor E., Becker Y. Abnormal response of ataxia-telangiectasia cells to agents that break the deoxyribose moiety of DNA via a targeted free radical mechanism. Carcinogenesis. 1983 Oct;4(10):1317–1322. doi: 10.1093/carcin/4.10.1317. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Tabor E., Becker Y. The response of ataxia-telangiectasia homozygous and heterozygous skin fibroblasts to neocarzinostatin. Carcinogenesis. 1982;3(7):815–820. doi: 10.1093/carcin/3.7.815. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Rosney C. M., Campbell J. B. Unusual sensitivity of ataxia telangiectasia cells to bleomycin. Cancer Res. 1979 Mar;39(3):1046–1050. [PubMed] [Google Scholar]
- Teoule R., Cadet J. Radiation-induced degradation of the base component in DNA and related substances--final products. Mol Biol Biochem Biophys. 1978;27:171–203. doi: 10.1007/978-3-642-81196-8_9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Lancker J. L., Tomura T. Purification and some properties of a mammalian repair endonuclease. Biochim Biophys Acta. 1974 Jun 14;353(1):99–114. doi: 10.1016/0005-2787(74)90101-4. [DOI] [PubMed] [Google Scholar]