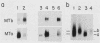Abstract
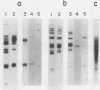
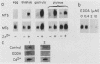
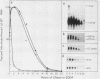
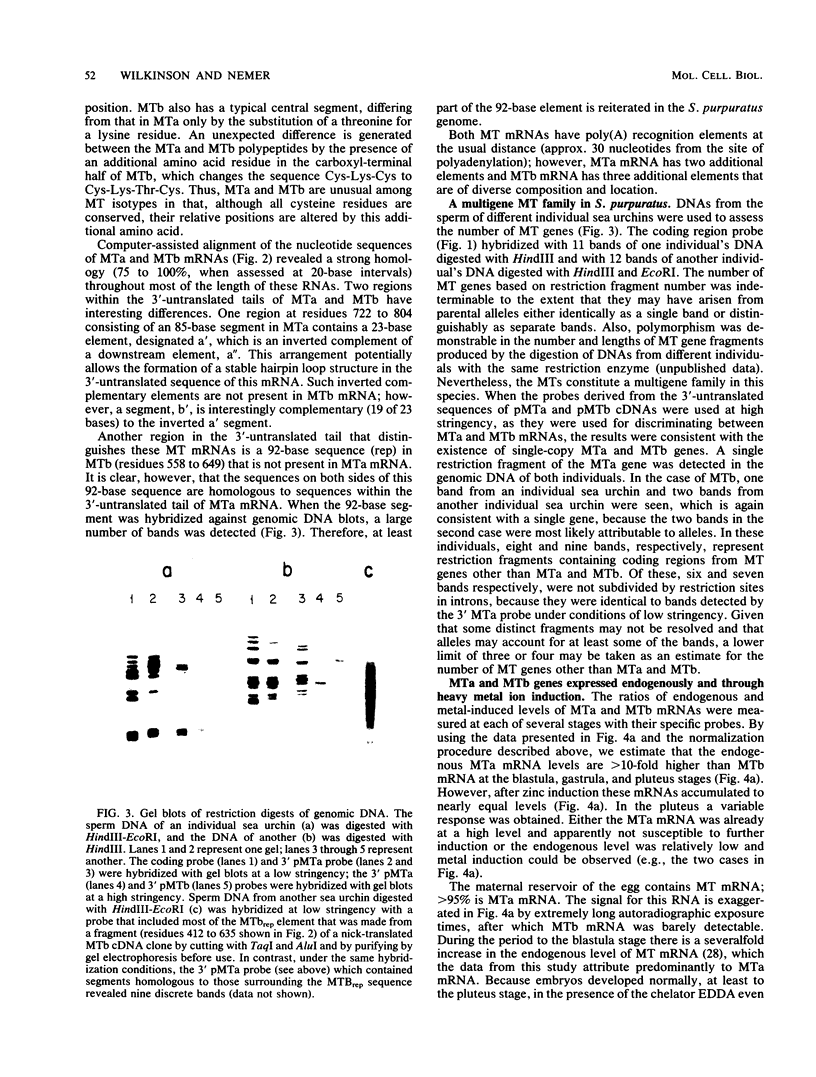
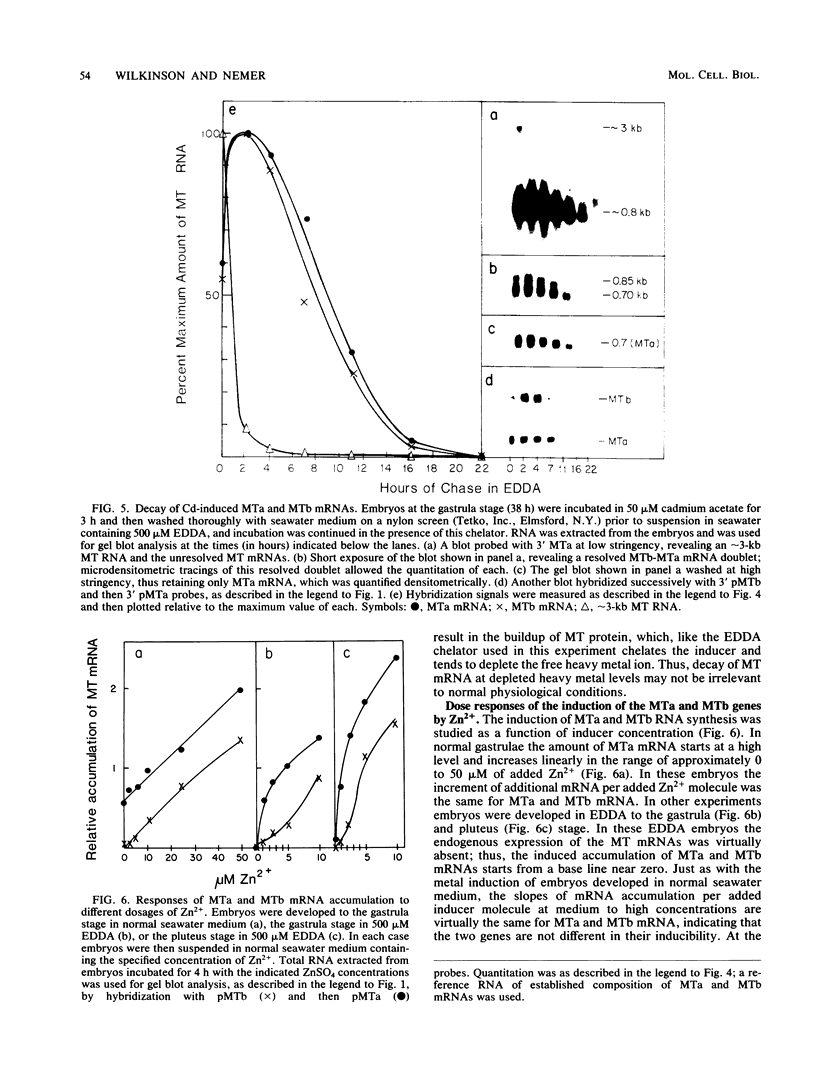
Sea urchin embryo metallothionein (MT) mRNAs MTa and MTb have distinct cDNA sequences and are transcripts of different genes of a multigene family. These MT mRNAs differ in size and in their 3'-untranslated sequences. They encode proteins that are unusual among MT isotypes in that the relative positions of their cysteine residues are partially out of register, suggesting potential differences in function. In pluteus larvae MTa mRNA is expressed abundantly and exclusively in the ectoderm, while MTb mRNA, which is restricted to the endomesoderm at a low endogenous level, can be induced to a high level by heavy metal ions (M2+). MT mRNA is present in the maternal reservoir of the egg and is predominantly (greater than 95%) MTa mRNA. Endogenous expression in the embryo, which is at a much higher level than in the egg, requires M2+ for gene transcription, is developmentally regulated, and is greater than 90% MTa mRNA. When induced by added M2+, however, MTa and MTb mRNAs accumulate to almost equal levels. The differences in the ratios of MTa/MTb expressed endogenously and inductively are not attributable to differences in the stabilities of these MT mRNAs, which were observed under conditions of M2+ depletion, or in their inducibilities, which were observed at moderate to high M2+ levels. We found, instead, that the MTa gene responds to M2+ at a lower threshold level than MTb, so that at very low M2+ concentrations the ratio of induced MTa/MTb mRNA is high and equivalent to the endogenous ratio. Thus, endogenous expression of the MTa gene is selectively enhanced in the ectoderm by determinants that are responsive at low M2+ threshold concentrations.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Angerer L. M., Kawczynski G., Wilkinson D. G., Nemer M., Angerer R. C. Spatial patterns of metallothionein mRNA expression in the sea urchin embryo. Dev Biol. 1986 Aug;116(2):543–547. doi: 10.1016/0012-1606(86)90156-9. [DOI] [PubMed] [Google Scholar]
- Angerer R. C., Davidson E. H. Molecular indices of cell lineage specification in sea urchin embryos. Science. 1984 Dec 7;226(4679):1153–1160. doi: 10.1126/science.6594757. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger Y., Goodman C. M., Forte C. P., Fesik S. W., Armitage I. M. Model for mammalian metallothionein structure. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1501–1505. doi: 10.1073/pnas.80.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. W., Armitage I. M. Evidence for site-selective metal binding in calf liver metallothionein. J Biol Chem. 1982 Feb 10;257(3):1259–1262. [PubMed] [Google Scholar]
- Bruskin A. M., Bedard P. A., Tyner A. L., Showman R. M., Brandhorst B. P., Klein W. H. A family of proteins accumulating in ectoderm of sea urchin embryos specified by two related cDNA clones. Dev Biol. 1982 Jun;91(2):317–324. doi: 10.1016/0012-1606(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Haslinger A., Karin M. Upstream promoter element of the human metallothionein-IIA gene can act like an enhancer element. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8572–8576. doi: 10.1073/pnas.82.24.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heguy A., West A., Richards R. I., Karin M. Structure and tissue-specific expression of the human metallothionein IB gene. Mol Cell Biol. 1986 Jun;6(6):2149–2157. doi: 10.1128/mcb.6.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Vasák M., Lerch K., Gilg D. E., Hunziker P., Bernhard W. R., Good M. Structure of mammalian metallothionein. Environ Health Perspect. 1984 Mar;54:93–103. doi: 10.1289/ehp.54-1568188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Shott R. J., Rose S. J., 3rd, Thomas T. L., Britten R. J., Davidson E. H. Sea urchin actin gene subtypes. Gene number, linkage and evolution. J Mol Biol. 1984 Jan 15;172(2):149–176. doi: 10.1016/s0022-2836(84)80035-2. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Lerch K., Ammer D., Olafson R. W. Crab metallothionein. Primary structures of metallothioneins 1 and 2. J Biol Chem. 1982 Mar 10;257(5):2420–2426. [PubMed] [Google Scholar]
- McClay D. R., Chambers A. F. Identification of four classes of cell surface antigens appearing at gastrulation in sea urchin embryos. Dev Biol. 1978 Mar;63(1):179–186. doi: 10.1016/0012-1606(78)90123-9. [DOI] [PubMed] [Google Scholar]
- Meijlink F., Curran T., Miller A. D., Verma I. M. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Nemer M. An altered series of ectodermal gene expressions accompanying the reversible suspension of differentiation in the zinc-animalized sea urchin embryo. Dev Biol. 1986 Mar;114(1):214–224. doi: 10.1016/0012-1606(86)90397-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Travaglini E. C., Rondinelli E., D'Alonzo J. Developmental regulation, induction, and embryonic tissue specificity of sea urchin metallothionein gene expression. Dev Biol. 1984 Apr;102(2):471–482. doi: 10.1016/0012-1606(84)90212-4. [DOI] [PubMed] [Google Scholar]
- Nemer M., Wilkinson D. G., Travaglini E. C. Primary differentiation and ectoderm-specific gene expression in the animalized sea urchin embryo. Dev Biol. 1985 Jun;109(2):418–427. doi: 10.1016/0012-1606(85)90468-3. [DOI] [PubMed] [Google Scholar]
- Nemer M., Wilkinson D. G., Travaglini E. C., Sternberg E. J., Butt T. R. Sea urchin metallothionein sequence: key to an evolutionary diversity. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4992–4994. doi: 10.1073/pnas.82.15.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettesheim D. G., Engeseth H. R., Otvos J. D. Products of metal exchange reactions of metallothionein. Biochemistry. 1985 Nov 19;24(24):6744–6751. doi: 10.1021/bi00345a003. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H. Cell specificity and an effect of ras on human metallothionein gene expression. Proc Natl Acad Sci U S A. 1986 May;83(10):3346–3350. doi: 10.1073/pnas.83.10.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer H., Haslinger A., Heguy A., Holtgreve H., Karin M. In vivo competition between a metallothionein regulatory element and the SV40 enhancer. Science. 1986 Apr 4;232(4746):76–80. doi: 10.1126/science.3006253. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. W., Rondinelli E., Nemer M. Differences in abundance of individual RNAs in normal and animalized sea urchin embryos. Dev Biol. 1983 Apr;96(2):520–528. doi: 10.1016/0012-1606(83)90189-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney U., Jahroudi N., Foster R., Gedamu L. Structure, organization, and regulation of human metallothionein IF gene: differential and cell-type-specific expression in response to heavy metals and glucocorticoids. Mol Cell Biol. 1986 Jan;6(1):26–37. doi: 10.1128/mcb.6.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Domain nature of metallothionein. J Biol Chem. 1982 Apr 10;257(7):3471–3476. [PubMed] [Google Scholar]
- Winge D. R., Nielson K. B. Formation of the metal-thiolate clusters of rat liver metallothionein. Environ Health Perspect. 1984 Mar;54:129–133. doi: 10.1289/ehp.8454129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]