Background: Tet methylcytosine dioxygenase converts 5-mC to 5-hmC in DNA.
Results: Ascorbate significantly and specifically enhances Tet-mediated generation of 5-hmC.
Conclusion: Our findings suggest that ascorbate enhances 5-hmC generation, most likely by acting as a co-factor for Tet methylcytosine dioxygenase to generate 5-hmC.
Significance: The availability of ascorbate could have significant consequences for health and diseases by modulating the epigenetic control of genome activity.
Keywords: DNA Methylation, Enzymes, Epigenetics, Transport, Vitamin C, 5-Hydroxymethylcytosine, 5-Methylcytosine, Phloretin, Tet Methylcytosine Dioxygenases, Mouse Embryonic Fibroblast
Abstract
Ascorbate (vitamin C) is best known for its role in scurvy, in which the hydroxylation of collagen catalyzed by dioxygenases is incomplete due to ascorbate deficiency. Here, we report a novel function of ascorbate in the hydroxylation of 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC) in DNA catalyzed by Tet (ten-eleven translocation) methylcytosine dioxygenase. The content of 5-hmC is extremely low in mouse embryonic fibroblasts cultured in ascorbate-free medium. Additions of ascorbate dose- and time-dependently enhance the generation of 5-hmC, without any effects on the expression of Tet genes. Treatment with another reducer glutathione (GSH) does not change the level of 5-hmC. Further, blocking ascorbate entry into cells by phloretin and knocking down Tet (Tet1, Tet2, and Tet3) expression by short interference RNAs (siRNA) significantly inhibit the effect of ascorbate on 5-hmC. These results suggest that ascorbate enhances 5-hmC generation, most likely by acting as a co-factor for Tet methylcytosine dioxygenase to hydroxylate 5-mC. Thus, we have uncovered a novel role for ascorbate in modulating the epigenetic control of genome activity.
Introduction
The epigenome constitutes the interface of the dynamic environment and the genome. Identifying enzymes and co-factors catalyzing epigenetic modifications is the key to understanding the molecular connections between the epigenome and the environment. DNA methylation at the 5-position of cytosine is the major covalent modification of mammalian DNA and plays a critical role in epigenetic regulation of genome activity. Recently, Tet2 family proteins have been shown to convert the covalent epigenetic mark 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC) in DNA (1, 2). This newly discovered conversion of 5-mC to 5-hmC by Tet family proteins is so far the most important and consistent mechanism underlying the active demethylation of DNA. Tet proteins can further oxidize 5-hmC to 5-formylcytosine and 5-carboxylcytosine, which could eventually be removed from the genome (3).
Tet proteins belong to the iron- and 2-oxoglutarate-dependent dioxygenase superfamily, which catalyzes the hydroxylation of a diverse variety of substrates including methylated nucleic acids and proteins (4, 5). These dioxygenases utilize Fe2+ as a co-factor and 2-oxoglutarate as a co-substrate, and some of them require ascorbate as another co-factor for full catalytic activity. One classical member of this family is collagen prolyl 4-hydroxylase (P4H), well known for its involvement in scurvy, in which ascorbate deficiency causes incomplete hydroxylation of residues in collagen, resulting in characteristic signs of the disease.
Several lines of evidence demonstrated that like P4H, the catalytic activity of Tet is also dependent on Fe2+ and 2-oxoglutarate. Mutations at the iron-binding sites in Tet proteins, as well as supplementation of 2-hydroxyglutarate, a competitive inhibitor of 2-oxoglutarate, suppress their catalytic activity of converting 5-mC to 5-hmC (2, 6, 7). Further, recombinant Tet proteins require both Fe2+ and 2-oxoglutarate to hydroxylate 5-mC to 5-hmC in test tubes (2), strongly supporting that 2-oxoglutarate and Fe2+ act as co-substrate and co-factor, respectively, to initiate the conversion of 5-mC to 5-hmC.
The requirement for ascorbate as an additional co-factor for P4H and other dioxygenases suggests a potential role for this reducing co-factor in Tet-mediated hydroxylation of 5-mC to 5-hmC. In the absence of ascorbate, the initial prolyl hydroxylation catalyzed by collagen P4H can proceed at a maximal rate. However, uncoupled decarboxylation of 2-oxoglutarate results in catalytically inactive oxidized iron species and enzyme inactivation. Thus, to maintain the enzyme in full active form, ascorbate is needed to reduce the oxidized iron species (8). Recombinant Tet1 has been shown to convert 5-mC to 5-hmC in vitro in the absence of ascorbate (2). As expected, Fe2+ and 2-oxoglutarate can satisfy the need of Tet proteins to initiate the hydroxylation of 5-mC, as they do for P4H. However, the role of ascorbate as a co-factor to sustain and complete the 5-mC to 5-hmC conversion in vivo has not been systematically analyzed.
Interestingly, studies have repeatedly shown that the presence of ascorbate dramatically modifies the status of DNA methylation. In embryonic stem cells, ascorbate caused widespread DNA demethylation of nearly 2,000 genes (9). Ascorbate also enhanced the generation of induced pluripotent stem cells from terminally differentiated cells, which was accompanied by genome-wide demethylation (10, 11). These studies suggest that ascorbate indeed facilitates DNA demethylation in vivo. However, it remains unknown whether the effect of ascorbate on DNA demethylation is due to an enhanced hydroxylation of 5-mC. We tested the hypothesis that ascorbate participates, possibly as a co-factor, in Tet-mediated generation of 5-hmC in cells.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatments
Mouse embryonic fibroblasts (MEFs), derived from a wild type C57BL/6 mouse, were maintained in DMEM (Invitrogen) containing no ascorbate in its formula. MEFs were cultured in 6-well plates with or without coverslips. When confluency was reached, cells were treated with l-ascorbate (Sigma) at different concentrations or for varying times. Each treatment group contained three wells in all experiments. Each experiment was repeated at least three times. To block the entry of ascorbate into cells, MEFs were pretreated with phloretin (Sigma) or vehicle for 3 h before ascorbate administration.
Dot-blot Assay
Genomic DNA was extracted from MEFs using QIAamp DNA mini kits according to the manufacturer's instructions (Qiagen). A Qubit fluorometer (Life Technologies) was used to quantify DNA concentration. The procedure of dot-blot followed the published methods (12). Briefly, DNA samples were diluted with 2 n NaOH and 10 mm Tris·Cl, pH 8.5, and then loaded on Hybond N+ nylon membrane (GE Healthcare) using a 96-well dot-blot apparatus (Bio-Rad). After being baked in 80 °C for 30 min and blocked by 5% nonfat milk for 1 h at room temperature, the membrane was incubated in a polyclonal anti-5-hmC antibody (Active Motif 39769, 1:10,000) at 4 °C overnight. 5-hmC was visualized by using chemiluminescence. The density of dots was captured by AlphaImager. To ensure equal loading, the membrane was stained with methylene blue after immunoblotting. Statistical significance of differences in 5-hmC content between different treatments was assessed by Student's t test, at α = 0.05.
Immunostaining
Immunostaining of 5-hmC was performed using the published methods (12). Briefly, after treatments, MEFs were fixed with 4% paraformaldehyde and then incubated with 1 n HCl at 37 °C for 30 min. After washing with PBS and blocking with 3% serum in PBS for 1 h, cells were incubated with anti-5-hmC antibody (Active Motif 39769, 1:10,000) at 4 °C overnight. Cy3-conjugated donkey anti-rabbit IgG (1:400; Jackson ImmunoResearch Laboratories) was used as an immunofluorescent secondary antibody. Cells were then counterstained with DAPI. Double fluorescence images were acquired with a Zeiss LSM710 confocal microscope.
Gene Silencing
siGENOME Tet1/Tet2/Tet3 siRNAs were purchased from Dharmacon. Each siRNA pool contains four different sets of siRNAs targeting one Tet gene. MEFs were transfected with the combined three siRNA pools (75 pmol of Tet1 siRNA + 75 pmol of Tet2 siRNA + 75 pmol of Tet3 siRNA per well) or the same amount of control siRNA using Lipofectamine RNAiMAX (Invitrogen) following the manufacturer's instructions. After transfection for 24 h, MEFs were treated with or without ascorbate for another 24 h before cell harvest or fixation.
RT-PCR
RNA was extracted from MEFs using the RNeasy kit (Qiagen). A NanoDrop 8000 photospectrometer was used to measure the yield of RNA extraction. The SuperScript III first-strand synthesis system (Invitrogen) was used for reverse transcription (RT) according to the manufacturer's instructions. The PCR was applied by the following four pairs of primers: Tet1 forward, 5′-AACAAGAGGCCCCAGAG-3′; Tet1 reverse, 5′-TTCTTCCCCATGACCAC-3′; Tet2 forward, 5′-CTCCTGGTGAACAAAGTCAGAATGG-3′; Tet2 reverse, 5′-CTAATAGCTGCCAGATCAGGACC-3′; Tet3 forward, 5′-CCGGATTGAGAAGGTCATCTAC-3′; Tet3 reverse, 5′-AAGATAACAATCACGGCGTTCT-3′; GAPDH forward, 5′-TTAGCACCCCTGGCCAAGG-3′; GAPDH reverse, 5′-CTTACTCCTTGGAGGCCATG-3′. The PCR products were separated in a 2% agarose gel. The gel image was captured using the AlphaImager gel documentation system.
Quantitative PCR
Quantitative PCR was performed using an ABI HP7900. Within the plate, samples were plated in duplicate in adjacent wells within the column: one for amplification of each Tet (Tet1, Tet2, and Tet3) and the other one for the housekeeper gene GAPDH as the internal control. Each sample was repeated three times at different locations in the 384-well plate. After the PCR run was complete, quantitative gene expression data were acquired and analyzed using the ABI Prism 7900HT sequence detection system (RQ Manager). The experiment was repeated twice. Statistical significance of differences in Tet (Tet1, Tet2, and Tet3) levels between different treatments was assessed by Student's t test, at α = 0.05.
RESULTS
We first evaluated the expression of Tet in MEFs derived from a wild type C57BL/6 mouse. In agreement with previous studies (13, 14), we found that MEFs express Tet (Tet1, Tet2, and Tet3) mRNAs at low but detectable levels, with Tet3 expressed at a higher level than Tet1 and Tet2 (data not shown), thus constituting an appropriate experimental system to analyze their enzymatic requirements.
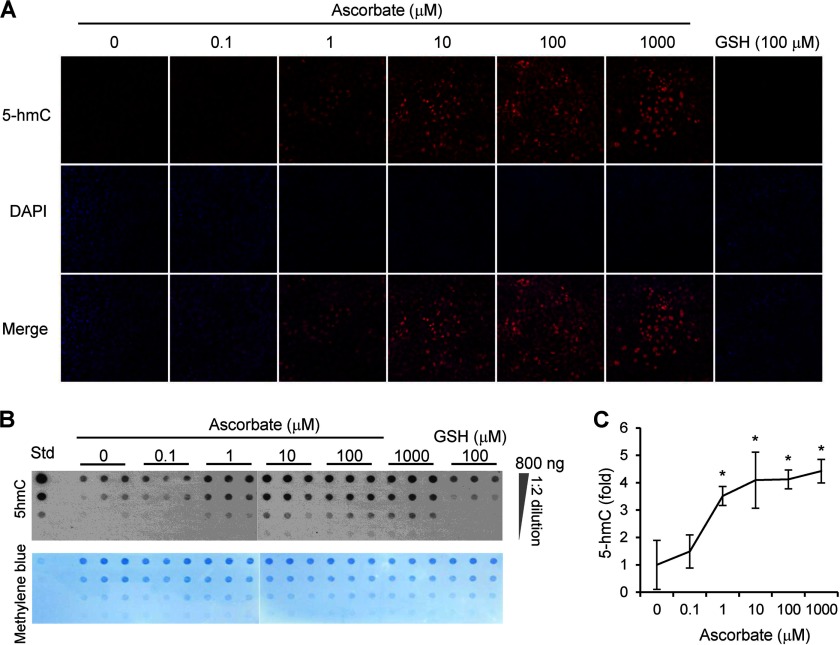
Standard cell culture media usually lack ascorbate. However, when available, ascorbate can effectively enter into cells through different transporters. We thus examined the effects of ascorbate administration to MEFs. After the addition of ascorbate for 24 h, cells were either harvested for extraction of genomic DNA followed by dot-blot analysis of 5-hmC content or fixed for 5-hmC immunostaining. In the absence of ascorbate, a low 5-hmC signal was detectable by dot-blot assays, and faint 5-hmC immunoreactivity in MEFs was observed. After treatment for 24 h, ascorbate (0–1,000 μm) dose-dependently increased 5-hmC content, as exhibited in immunostaining (Fig. 1A) and dot-blot determinations (Fig. 1B). No obvious change in cell culture growth was observed after the treatment with ascorbate. Semiquantitative analysis of dot-blots indicated that ascorbate (1–1,000 μm) enhanced the generation of 5-hmC up to 4-fold above basal levels (Fig. 1C). This result suggests that ascorbate enhances 5-hmC generation by potentially activating endogenous Tet proteins in MEFs.
FIGURE 1.
Ascorbate dose-dependently enhances the generation of 5-hmC in MEFs. A, immunostaining shows that ascorbate (0–1,000 μm) dose-dependently increases 5-hmC signal. Without treatment, 5-hmC signal (shown by Cy3 labeling) is absent or negligible, whereas the presence of 5-hmC is obvious after treatment with 1 μm of ascorbate for 24 h. Treatment with ascorbate (10–1,000 μm) further increases 5-hmC signal. In contrast, there is no obvious 5-hmC signal in MEFs after incubation with 100 μm GSH for 24 h. B, a representative dot-blot shows that ascorbate (0–1,000 μm) dose-dependently increases the content of 5-hmC, whereas GSH (100 μm) does not have any effects on 5-hmC. Std: 5-hmC standard (Active Motif) at 2.5, 0.5, 0.1, and 0.02 ng (from top to bottom). C, semiquantitative analysis of the dot-blot indicates that ascorbate (1–1,000 μm) increases 5-hmC up to 4-fold of the basal level (*, p < 0.05 assessed by Student's t test; data are represented as mean ± S.E.).
To examine the specificity of this effect, we checked whether other reducers could also influence the generation of 5-hmC. MEFs were treated with glutathione (GSH, 100 μm) for 24 h. We found that GSH did not obviously change the level of 5-hmC (Fig. 1, A and B), suggesting that the effect of ascorbate on 5-hmC could not be attributed to its role as a general reducer. Furthermore, the effect of ascorbate on 5-hmC was ubiquitous, not limited to MEFs. For example, ascorbate (10 μm) also caused a significant increase of 5-hmC in the human non-fibroblastic HEK-293T cells and HeLa cells (data not shown).
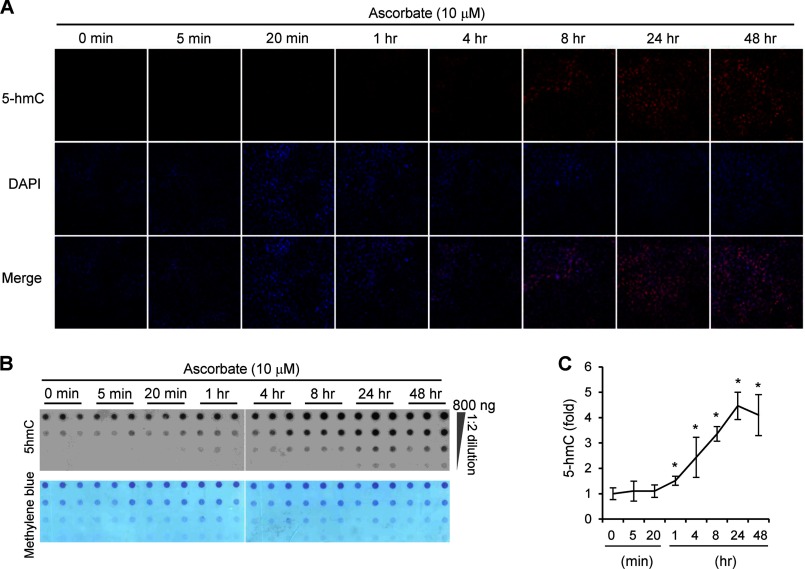
MEFs were then treated with 10 μm ascorbate for varying times to observe the time-course response of ascorbate. Surprisingly, the effect of ascorbate on 5-hmC is quite rapid. The generation of 5-hmC in MEFs can be induced by ascorbate in as little as 1 h (Fig. 2, A–C). The rapid effect suggests that no protein synthesis is required, but that intracellular accumulation of ascorbate is enough to enhance the activity of enzymes, most probably Tet in MEFs, poised to generate 5-hmC. Indeed, the addition of ascorbate (10–1,000 μm) did not significantly change the mRNA of Tet genes (p > 0.05; data not shown), suggesting that the effect of ascorbate is not caused by alteration in Tet expression. Furthermore, the effect of ascorbate on 5-hmC was also sustained for a long time. After treatment for 48 h, the level of 5-hmC was only slightly decreased as compared with treatments for 8 or 24 h (Fig. 2C). Additionally, variations in the concentrations of iron and glucose in the medium did not obviously change the effect of ascorbate on 5-hmC (data not shown).
FIGURE 2.
Ascorbate time-dependently enhances the generation of 5-hmC in MEFs. A, immunostaining shows that 10 μm ascorbate (0–48 h) time-dependently enhances the generation of 5-hmC in MEFs. There is no obvious 5-hmC signal in controls (0 min) and cells incubated with ascorbate for 5 or 20 min. Treatment with ascorbate for 1–48 h causes a gradual and dramatic increase of 5-hmC signal in MEFs. B, a representative dot-blot shows that ascorbate (10 μm) time-dependently (0–48 h) increases the content of 5-hmC. C, semiquantitative analysis of the dot-blot indicates that the incubation of ascorbate for 1–48 h increases 5-hmC levels (*, p < 0.05 assessed by Student's t test; data are represented as mean ± S.E.).
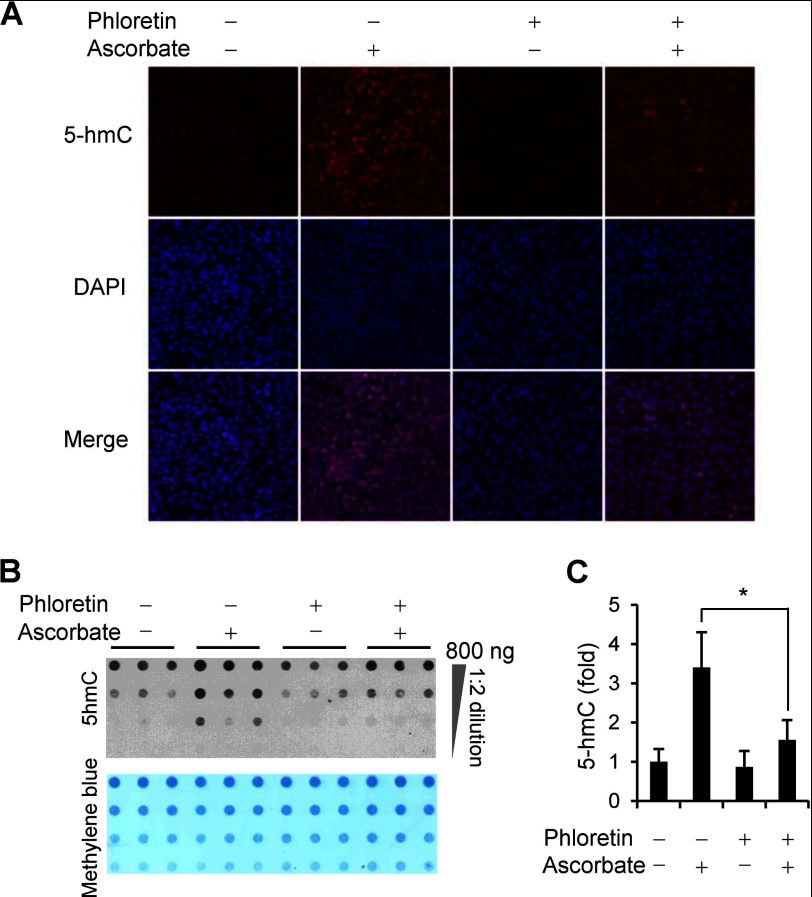
Most mammals, with the exception of primates, guinea pigs, and fruit bats, can synthesize ascorbate de novo in the liver. Both dietary and hepatic ascorbate enter and accumulate in tissues mainly through sodium-dependent vitamin C transporters (SVCTs) and glucose transporters (GLUTs) (15). To test whether blocking the transporter-dependent entry of ascorbate into cells could abolish or decrease the effect of ascorbate on 5-hmC, we pretreated MEFs with phloretin (100 μm), which has been shown to inhibit the uptake of ascorbate by nearly 70% (16). The data showed that phloretin itself did not alter the 5-hmC content in ascorbate-free MEFs. However, the addition of phloretin reduced the generation of 5-hmC induced by ascorbate treatment approximately to basal levels (Fig. 3, A–C). This result suggests that the generation of 5-hmC in MEFs indeed requires the entry of ascorbate into cells.
FIGURE 3.
The effect of ascorbate on 5-hmC is blocked by phloretin. A, immunostaining shows that phloretin (100 μm) does not change 5-hmC signal in ascorbate-free MEFs but does block the induction of 5-hmC by ascorbate (10 μm) treatment for 24 h. B, a representative dot-blot shows that phloretin (100 μm) inhibits the effects of ascorbate (10 μm) on 5-hmC content. C, semiquantitative analysis of the dot-blot indicates that phloretin inhibits the induction of 5-hmC by ascorbate (*, p < 0.05 assessed by Student's t test; data are represented as mean ± S.E.).
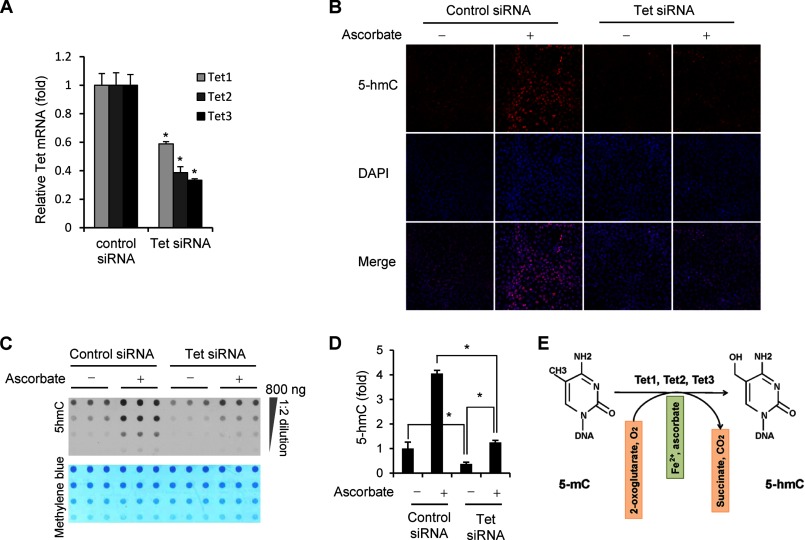
To confirm that the effect of ascorbate on 5-hmC is through Tet proteins, we simultaneously knocked down the expression of Tet (Tet1, Tet2, and Tet3) in MEFs using short interference RNAs (siRNAs). The mRNA level of Tet genes was decreased to ∼60% for Tet1, ∼40% for Tet2, and ∼35% for Tet3 as compared with MEFs being transfected with control siRNAs (Fig. 4A). The basal level of 5-hmC in ascorbate-free MEFs in which Tet (Tet1, Tet2, and Tet3) mRNAs have been down-regulated was decreased to ∼40% of the controls. The addition of ascorbate (10 μm) still enhanced the generation of 5-hmC to more than 3-fold above basal levels in these MEFs with reduced Tet expression (Fig. 4, B and C). However, the induction of 5-hmC by ascorbate in Tet-deficient cells was attenuated as compared with its effects in the control cells (Fig. 4D). Possible off-target effects of the siRNA mixture cannot be completely excluded. However, the fact that Tet-mediated oxidation of 5-mC seems to be the only source of 5-hmC suggests that the decreased 5-hmC after siRNA treatment is most likely due to the reduced expression of Tet genes. Overall, these results suggest that the enhancing effect of ascorbate on 5-hmC generation was indeed mediated by Tet in MEFs.
FIGURE 4.
The effect of ascorbate on 5-hmC generation is mediated by Tet methylcytosine dioxygenase. A, siRNAs targeting Tet (Tet1, Tet2, and Tet3) decrease the level of Tet mRNAs to, ∼60% for Tet1, ∼40% for Tet2, and ∼35% for Tet3 as compared with control siRNA shown by quantitative RT-PCR. (*, p < 0.05 assessed by Student's t test; data are represented as mean ± S.E.) B, immunostaining shows that knocking down the expression of Tet genes decreases the basal level of 5-hmC signal and attenuates the induction of 5-hmC by ascorbate (10 μm) treatment for 24 h. C, a representative dot-blot shows that cells with less Tet1–3 reduce the generation of 5-hmC by ascorbate (10 μm) treatment. D, semiquantitative analysis of the dot-blot indicates that Tet1–3 partially depleted cells exhibit a decrease in the basal level of 5-hmC (∼40% as compared with the controls). Ascorbate still induces an increase (∼3-fold) of 5-hmC content in Tet1–3 partially depleted cells, but this effect is much smaller as compared with its effect in control cells (*, p < 0.05 assessed by Student's t test; data are represented as mean ± S.E.). E, in addition to Fe2+ as a co-factor and 2-oxoglutarate as co-substrate, ascorbate is suggested as an additional co-factor for Tet to hydroxylate 5-mC to 5-hmC.
DISCUSSION
Our results suggest that ascorbate enhances 5-hmC generation, most likely by acting as a co-factor for Tet methylcytosine dioxygenase to hydroxylate 5-mC to 5-hmC (Fig. 4E). Although the role of 5-hmC is not completely understood, in addition to its role as an intermediate in DNA demethylation, 5-hmC has been proposed to have gene regulatory functions involved in processes such as development, pluripotency, and regulation of RNA splicing (17). To the best of our knowledge, this is the first time that ascorbate has been shown to enhance the generation of 5-hmC in cells. In addition to MEFs, we also observed a similar effect of ascorbate in HEK-293T cells and HeLa cells. The effect of ascorbate on 5-hmC appeared to reach a plateau at about 10 μm in MEFs. The ascorbate concentration in normal human and mouse plasma is at the ∼50 μm range and could reach the mm range in many tissues (18), suggesting that the physiological ascorbate level is high enough to affect the generation of 5-hmC in vivo. Tet proteins are the major known enzymes to convert 5-mC to 5-hmC. It should be noted that due to the low expression of Tet genes in MEFs, ascorbate at a relatively low level might fulfill the requirement of Tet methylcytosine dioxygenase to reach their maximal enzymatic activity. However, the expression of Tet genes in embryonic tissues is high especially at early developmental stages (17), suggesting that a higher level of ascorbate is required for the 5-hmC-involved deprogramming and reprogramming during pregnancy. Furthermore, Tet genes also express at a relatively higher level in some terminally differentiated cells such as Purkinje cells and other brain cells in adults (19, 20), suggesting a role of ascorbate in regulating the epigenome of those cells.
The effect of ascorbate on 5-hmC is quite rapid in MEFs. It is known that Tet proteins need Fe2+ as a co-factor and 2-oxoglutarate as a co-substrate to hydroxylate 5-mC in DNA (2, 6, 7). The components of the culture medium used in our experiments, as well as their metabolites, could provide both Fe2+ and 2-oxoglutarate for Tet proteins. However, the generation of 5-hmC without ascorbate treatment remains extremely low. In contrast, the addition of ascorbate could rapidly induce significant generation of 5-hmC in as little as 1 h, the time needed for ascorbate to be transported into cells and reach Tet proteins and their substrate, co-substrate, and co-factor. This rapid response of 5-hmC to ascorbate treatment suggests that in addition to Fe2+ and 2-oxoglutarate, ascorbate could be directly involved in the catalytic activity of Tet methylcytosine dioxygenase. Furthermore, the effect of ascorbate on 5-hmC was sustained. After treatment for 48 h, the level of 5-hmC was only slightly decreased as compared with treatments for 8 or 24 h. As a co-factor for P4H, ascorbate is oxidized during the hydroxylation of Pro residues (8). Likewise, ascorbate is probably oxidized in Tet-mediated 5-mC hydroxylation. In this case, the regeneration of ascorbate from dehydroascorbate by intracellular redox systems could underlie its longer term effect on 5-hmC generation. Alternatively, the initial promotion of 5-hmC generation could have generated a relatively stable epigenetic modification in the cell.
Our results further support that the effect of ascorbate on 5-hmC is specific and likely mediated by Tet proteins. First, another reducer, such as GSH, does not affect the generation of 5-hmC, suggesting that the effect of ascorbate on 5-hmC cannot be attributed to ascorbate being a general reducer. Secondly, the passive diffusion of ascorbate into cells is at a minimal level (15). The treatment of ascorbate transporter blocker phloretin inhibits the effect of ascorbate on 5-hmC, suggesting that ascorbate enhances the generation of 5-hmC only after being transported into cells. Thirdly, ascorbate does not obviously affect the expression of Tet genes. Fourthly, knocking down the expression of Tet genes decreases the effect of ascorbate on 5-hmC, suggesting that this effect is through activating the enzymatic activity of Tet proteins, not by increasing the expression of Tet genes. Although a detailed mechanism of how ascorbate affects the catalytic activity of Tet remains to be elucidated, it is likely that, as in P4H-mediated hydroxylation (8), ascorbate may reconstitute Tet by reducing the inactive Fe3+ state to the active Fe2+ state after Tet is inactivated by uncoupled 2-oxoglutarate turnover.
Thus, we have uncovered a novel role for ascorbate in regulating 5-hmC generation in DNA and further modulating the epigenetic control of genome activity. The impaired generation of 5-hmC has been recently associated with malignancies (21, 22). Our findings may help develop novel therapies for these conditions by rescuing the impaired generation of 5-hmC using ascorbate treatments in the future.
CONCLUSION
This study identified a novel function of ascorbate in promoting Tet-mediated generation of 5-hmC, suggesting that the availability of ascorbate will have a profound effect on many cellular functions. Our data support ascorbate as a critical mediator of the interface between the genome and environment. Thus, genetic and environmental factors that influence the synthesis, absorption, transportation, and metabolism of ascorbate could have significant consequences for genome integrity, development, pluripotency, and ultimately health and disease by modulating the epigenetic control of genome activity and cellular homeostasis.
Acknowledgments
We thank E. Dikici (University of Miami) for technical assistance in dot-blot. We also thank P. Jing (Emory University) for sharing a 5-hmC immunostaining protocol.
This research was supported, in part, by a James and Esther King Biomedical Research Program award (3KN08) and a BrightFocus Foundation grant (M2012048) (to G. W.)
This article was selected as a Paper of the Week.
- Tet
- ten-eleven translocation
- 5-mC
- 5-methylcytosine
- 5-hmC
- 5-hydroxymethylcytosine
- P4H
- prolyl 4-hydroxylase
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Kriaucionis S., Heintz N. (2009) The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tahiliani M., Koh K. P., Shen Y., Pastor W. A., Bandukwala H., Brudno Y., Agarwal S., Iyer L. M., Liu D. R., Aravind L., Rao A. (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu H., Zhang Y. (2011) Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 25, 2436–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westbye M. P., Feyzi E., Aas P. A., Vågbø C. B., Talstad V. A., Kavli B., Hagen L., Sundheim O., Akbari M., Liabakk N. B., Slupphaug G., Otterlei M., Krokan H. E. (2008) Human AlkB homolog 1 is a mitochondrial protein that demethylates 3-methylcytosine in DNA and RNA. J. Biol. Chem. 283, 25046–250056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng G., Dahl J. A., Niu Y., Fedorcsak P., Huang C. M., Li C. J., Vågbø C. B., Shi Y., Wang W. L., Song S. H., Lu Z., Bosmans R. P., Dai Q., Hao Y. J., Yang X., Zhao W. M., Tong W. M., Wang X. J., Bogdan F., Furu K., Fu Y., Jia G., Zhao X., Liu J., Krokan H. E., Klungland A., Yang Y. G., He C. (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ito S., D'Alessio A. C., Taranova O. V., Hong K., Sowers L. C., Zhang Y. (2010) Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S. H., Ito S., Yang C., Wang P., Xiao M. T., Liu L. X., Jiang W. Q., Liu J., Zhang J. Y., Wang B., Frye S., Zhang Y., Xu Y. H., Lei Q. Y., Guan K. L., Zhao S. M., Xiong Y. (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorres K. L., Raines R. T. (2010) Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 45, 106–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung T. L., Brena R. M., Kolle G., Grimmond S. M., Berman B. P., Laird P. W., Pera M. F., Wolvetang E. J. (2010) Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 28, 1848–1855 [DOI] [PubMed] [Google Scholar]
- 10. Esteban M. A., Wang T., Qin B., Yang J., Qin D., Cai J., Li W., Weng Z., Chen J., Ni S., Chen K., Li Y., Liu X., Xu J., Zhang S., Li F., He W., Labuda K., Song Y., Peterbauer A., Wolbank S., Redl H., Zhong M., Cai D., Zeng L., Pei D. (2010) Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 [DOI] [PubMed] [Google Scholar]
- 11. Stadtfeld M., Apostolou E., Ferrari F., Choi J., Walsh R. M., Chen T., Ooi S. S., Kim S. Y., Bestor T. H., Shioda T., Park P. J., Hochedlinger K. (2012) Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat. Genet. 44, 398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szulwach K. E., Li X., Li Y., Song C. X., Wu H., Dai Q., Irier H., Upadhyay A. K., Gearing M., Levey A. I., Vasanthakumar A., Godley L. A., Chang Q., Cheng X., He C., Jin P. (2011) 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 14, 1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doege C. A., Inoue K., Yamashita T., Rhee D. B., Travis S., Fujita R., Guarnieri P., Bhagat G., Vanti W. B., Shih A., Levine R. L., Nik S., Chen E. I., Abeliovich A. (2012) Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature 488, 652–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koh K. P., Yabuuchi A., Rao S., Huang Y., Cunniff K., Nardone J., Laiho A., Tahiliani M., Sommer C. A., Mostoslavsky G., Lahesmaa R., Orkin S. H., Rodig S. J., Daley G. Q., Rao A. (2011) Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson J. X. (2005) Regulation of vitamin C transport. Annu. Rev. Nutr. 25, 105–125 [DOI] [PubMed] [Google Scholar]
- 16. May J. M., Qu Z. C. (2005) Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch. Biochem. Biophys. 434, 178–186 [DOI] [PubMed] [Google Scholar]
- 17. Tan L., Shi Y. G. (2012) Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 139, 1895–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsao C. S., Leung P. Y., Young M. (1997) Effect of dietary ascorbic acid intake on tissue vitamin C in mice. J. Nutrition 117, 291–297 [DOI] [PubMed] [Google Scholar]
- 19. Guo J. U., Su Y., Zhong C., Ming G. L., Song H. (2011) Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khare T., Pai S., Koncevicius K., Pal M., Kriukiene E., Liutkeviciute Z., Irimia M., Jia P., Ptak C., Xia M., Tice R., Tochigi M., Moréra S., Nazarians A., Belsham D., Wong A. H., Blencowe B. J., Wang S. C., Kapranov P., Kustra R., Labrie V., Klimasauskas S., Petronis A. (2012) 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat. Struct. Mol. Biol. 19, 1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ko M., Huang Y., Jankowska A. M., Pape U. J., Tahiliani M., Bandukwala H. S., An J., Lamperti E. D., Koh K. P., Ganetzky R., Liu X. S., Aravind L., Agarwal S., Maciejewski J. P., Rao A. (2010) Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468, 839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lian C. G., Xu Y., Ceol C., Wu F, Larson A., Dresser K., Xu W., Tan L., Hu Y., Zhan Q., Lee C. W., Hu D., Lian B. Q., Kleffel S., Yang Y., Neiswender J., Khorasani A. J., Fang R., Lezcano C., Duncan L. M., Scolyer R. A., Thompson J. F., Kakavand H., Houvras Y., Zon L. I., Mihm M. C., Jr., Kaiser U. B., Schatton T., Woda B. A., Murphy G. F., Shi Y. G. (2012) Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150, 1135–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]