Background: Little is known about how bacteria sense or respond to reactive chlorine species, such as bleach.
Results: NemR is a redox-regulated transcription factor which senses bleach.
Conclusion: NemR controls expression of genes encoding electrophile detoxification enzymes, which increase bleach resistance.
Significance: We demonstrate a bleach-sensing bacterial response system and a new mechanism contributing to bacterial bleach survival.
Keywords: Bacteria, Gene Expression, Oxidative Stress, Redox Regulation, Transcription Repressor, GloA, Hypochlorous Acid, NemR, Reactive Chlorine Species
Abstract
Hypochlorous acid (HOCl), the active component of household bleach, also functions as a powerful antimicrobial during the innate immune response. Despite its widespread use, surprisingly little is known about how cells sense or respond to HOCl. We now demonstrate that Escherichia coli NemR is a redox-regulated transcriptional repressor, which uses the oxidation status of HOCl-sensitive cysteine residues to respond to bleach and related reactive chlorine species. NemR controls bleach-mediated expression of two enzymes required for detoxification of reactive electrophiles: glyoxalase I and N-ethylmaleimide reductase. Both enzymes contribute to bacterial bleach survival. These results provide evidence that bleach resistance relies on the capacity of organisms to specifically sense reactive chlorine species and respond with the up-regulation of enzymes dedicated to detoxification of methylglyoxal and other reactive electrophiles.
Introduction
Hypochlorous acid (HOCl)5 is a powerful oxidant and one of the most commonly used disinfectants in the world (1). It is also naturally generated during the microbicidal oxidative burst of neutrophils and appears to play a key role in controlling bacterial colonization of mucosal epithelia (2, 3). Despite this physiological importance, little is known about how bacteria sense or defend themselves against reactive chlorine species (RCS), which include HOCl, chloramines, and other related compounds that are able to chlorinate and oxidize biomolecules (4). Very few transcription factors sensitive to HOCl have been reported to date. YjiE has recently been shown to regulate a variety of genes in Escherichia coli in response to HOCl and to play a role in HOCl survival (5). OhrR of Bacillus subtilis, previously thought to be specific for sensing organic hydroperoxides, has been reported to be involved in HOCl resistance as well (6). Relatively little is known, however, about how these regulators and the genes they control contribute to HOCl survival. This is in contrast to the very large body of research that has been conducted to investigate how bacteria sense and defend themselves against other, less reactive bactericidal oxidants (4, 7) such as hydrogen peroxide (H2O2) or superoxide (O2−) (for recent review, see Refs. 8–11).
Given the importance of HOCl in health and disease, we therefore decided to perform a more detailed investigation of how bacteria sense and respond to bleach. Here, we report our discovery that the widely conserved TetR family repressor NemR functions as a HOCl-responsive transcription factor in E. coli in addition to its previously described role in responding to cysteine-modifying electrophiles (12). In vitro and in vivo studies reveal that HOCl sensitivity is conferred upon NemR by cysteine residues that are highly sensitive toward oxidation by HOCl and related physiologically important RCS, such as N-chlorotaurine, an antimicrobial compound formed when HOCl reacts with taurine (2-aminoethanesulfonic acid) during the oxidative burst of neutrophils (13). RCS oxidation of cysteine residues, which is a fully reversible process in vitro and likely also in vivo, leads to a decrease in NemR DNA binding affinity, causing the derepression of downstream target gene expression. We demonstrate that bleach-mediated up-regulation of the NemR-controlled genes gloA and nemA increases bacterial bleach survival, providing the first evidence that resistance toward bleach relies on the ability of bacteria to detoxify methylglyoxal and reactive electrophiles.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
All strains and plasmids used in this study are listed in Table 1. E. coli was grown at 37 °C in lysogenic broth (LB) or MOPS minimal medium (Teknova, Inc.) containing 0.2% glucose, 1.32 mm K2HPO4, and 10 μm thiamine. Unless specified otherwise, all chemicals were from Fisher or Sigma-Aldrich. N-Chlorotaurine and methylglyoxal were synthesized before each use (14, 15).
TABLE 1.
Strains and plasmids used in this study
Unless otherwise indicated, all strains and plasmids were generated in the course of this work.
| Strain name | Marker(s)a | Relevant genotype | Source |
|---|---|---|---|
| E. coli strains | |||
| XL1-Blue | TcR NxR | endA1 gyrA96(nalR) thi-1 recA1 relA1 lac glnV44 F'[::Tn10(tet+) proAB+ lacIq Δ(lacZ)M15] hsdR17(rK− mK+) | Stratagene |
| BL21(DE3) | F−, ompT gal dcm lon hsdSB (rB− mB−) λ (DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) | Novagen | |
| MG1655 | F−, λ−, rph-1 ilvG− rfb-50 | (40) | |
| MJG011 | CmR | F−, λ−, rph-1 ilvG− rfb-50 nemR::cat+ | |
| MJG012 | CmR | F−, λ−, rph-1 ilvG− rfb-50 nemA::cat+ | |
| MJG017 | CmR | F−, λ−, rph-1 ilvG− rfb-50 gloA::cat+ | |
| MJG043 | F−, λ−, rph-1 ilvG− rfb-50 ΔnemR | ||
| MJG044 | F−, λ−, rph-1 ilvG− rfb-50 ΔnemA | ||
| MJG048 | F−, λ−, rph-1 ilvG− rfb-50 ΔgloA | ||
| MJG244 | ApR | F−, λ−, rph-1 ilvG− rfb-50 ΔnemR/pKD46 (λ Red recombinase+ bla+) | |
| MJG246 | CmR | F−, λ−, rph-1 ilvG− rfb-50 nemR+-cat+ | |
| MJG249 | CmR | F−, λ−, rph-1 ilvG− rfb-50 nemRT61A,T292A,T346A, T445A,T447C,T458A-cat+ (encoding NemR5C-S (C106)) | |
| MJG251 | F−, λ−, rph-1 ilvG− rfb-50 nemR+ | ||
| MJG254 | F−, λ−, rph-1 ilvG− rfb-50 nemRT61A, T292A,T346A,T445A,T447C,T458A (encoding NemR5C-S (C106)) | ||
| MJG262 | CmR | F−, λ−, rph-1 ilvG− rfb-50 nemRG317C,C318T-cat+ (encoding NemRC106S) | |
| MJG263 | CmR | F−, λ−, rph-1 ilvG− rfb-50 nemRT61A,T292A,G317C,C318T,T346A, T445A,T447C,T458A-cat+ (encoding NemR6C-S) | |
| MJG265 | F−, λ−, rph-1 ilvG− rfb-50 nemRG317C,C318T (encoding NemRC106S) | ||
| MJG266 | F−, λ−, rph-1 ilvG− rfb-50 nemRT61A,T292A,G317C,C318T,T346A,T445A,T447C,T458A (encoding NemR6C-S) | ||
| Plasmids | |||
| pET-21b(+) | ApR | C-terminal His6 tag overexpression vector | Novagen |
| pKD3 | CmR ApR | cat+ chloramphenicol resistance cassette donor | (19) |
| pKD46 | ApR | λ Red recombinase+ | (19) |
| pCP20 | CmR ApR | Flp recombinase+ | (19) |
| pBAD30 | ApR | Cloning vector with PBAD arabinose-inducible promoter | (22) |
| pNEMR1 | ApR | nemR+ translational fusion to C-terminal His6 tag | |
| pNEMR2 | ApR | nemR+ | |
| pNEMR3 | ApR | nemRG317C,C318T (encoding NemRC106S) translational fusion to C-terminal His6 tag | |
| pNEMR7 | ApR | nemRT292A (encoding NemRC98S) translational fusion to C-terminal His6 tag for protein purification | |
| pNEMR8 | ApR | nemRT346A (encoding NemRC116S) translational fusion to C-terminal His6 tag for protein purification | |
| pNEMR12 | ApR | nemRT445A,T447C (encoding NemRC149S) translational fusion to C-terminal His6 tag for protein purification | |
| pNEMR13 | ApR | nemRT458A, T460C (encoding NemRC153S) translational fusion to C-terminal His6 tag for protein purification | |
| pNEMR16 | ApR | nemRT61A (encoding NemRC21S) translational fusion to C-terminal His6 tag for protein purification | |
| pNEMR32 | ApR | nemRT61A,T292A,T346A,T445A,T447C,T458A (encoding NemR5C-S (C106)) | |
| pNEMR40 | ApR | nemRT61A,T292A,T346A,T445A,T447C,T458A (encoding NemR5C-S (C106)) translational fusion to C-terminal His6 tag | |
| pNEMR43 | ApR | nemRG317C,C318T (encoding NemRC106S) | |
| pNEMR44 | ApR | nemRT61A,T292A,G317C,C318T,T346A,T445A,T447C,T458A,T460C (encoding NemR6C-S) | |
| pNEMR45 | ApR | nemRT61A,T292A,G317C,C318T,T346A,T445A,T447C,T458A, T460C (encoding NemR6C-S) translational fusion to C-terminal His6 tag | |
a Abbreviations: TcR, tetracycline resistance; NxR, nalidixic acid resistance; CmR, chloramphenicol resistance; ApR, ampicillin resistance.
Microarray Analysis and Data Processing
E. coli MG1655 was grown in MOPS glucose medium at 37 °C with aeration to A600 = 0.4–0.5. HOCl was added to a final concentration of 400 μm. 5-ml samples were collected in liquid nitrogen immediately before, 5 min after, and 10 min after HOCl addition, and total RNA was prepared using the Rneasy® Midi kit (Qiagen). cDNA synthesis, array hybridization to Affymetrix GeneChip E. coli genome 2.0 arrays, and imaging were performed at the Affymetrix and Microarray Core facility at the University of Michigan, Ann Arbor. Quality of raw images and expression values were analyzed using the expresso function and affy package of Bioconductor (16). Three biological replicates were conducted. The TM4 MultiExperiment Viewer (17) was used to identify patterns of gene expression and differentially expressed genes. A total of 5367 MG1655 probes were separated into 25 clusters with 100 iterations. The pattern of each cluster was evaluated based on log2-transformed ratios of treated versus untreated samples. Probes in each cluster have expression patterns similar to each other and dissimilar to those in other clusters.
Construction of E. coli Mutant Strains
DNA manipulations were conducted using standard methods (18). Primers are listed in Table 2. All constructs were confirmed by sequencing (GENEWIZ, Inc.). In-frame replacements of complete coding sequences with chloramphenicol resistance cassettes (19) were constructed for the following genes, using the indicated primers: nemR, [1] and [2]; nemA, [3] and [4]; gloA, [5] and [6]. The chloramphenicol resistance cassettes were resolved (19) to yield nonpolar in-frame deletions.
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| [1] | 5′-ATG AAC AAA CAC ACC GAA CAT GAT ACT CGC GTG TAG GCT GGA GCT GCT TCG-3′ |
| [2] | 5′-CTA AAC GGC AGG CGT CGC AAT AAT GTT TTT CAT ATG AAT ATC CTC CTT AGT TCC-3′ |
| [3] | 5′-CCT GCC GTT TAG CAG GCA TTT TTT ATC ACC AGA CGA CCG GGA GCC TTT ATG GCG GTG TAG GCT GGA GCT GCT TC-3′ |
| [4] | 5′-AAT GTT GTT TTA GTA TAG CGG CGG CTT TAC GCC GCT CGC AAT GTT GGA TTA CAT ATG AAT ATC CTC CTT AG-3′ |
| [5] | 5′-ATA CTA AAA CAA CAT TTT GAA TCT GTT AGC CAT TTT GAG GAT AAA AAG ATG GTG TAG GCT GGA GCT GCT TC-3′ |
| [6] | 5′-TCG ATG CAG TAA AGA TGC GGG CGC GAT GAG TTC ACG CCC GGC AGG AGA TTA CAT ATG AAT ATC CTC CTT AGG-3′ |
| [7] | 5′-ATG CAT ATG AAC AAA CAC ACC GAA CAT G-3′ |
| [8] | 5′-CTT aag ctt act acc gcg tgg cac cag AAC GGC AGG CGT CGC AAT AAT-3′ |
| [9] | 5′-TTC GAA TTC TGG TCT ACT ACA CTC CAA CGC A-3′ |
| [10] | 5′-CTT aag ctt CTA AAC GGC AGG CGT CGC AAT AAT-3′ |
| [11] | 5′-CAT CAG TGG TTC TCT GAC AGT AA-3′ |
| [12] | 5′-TTA CTG TCA GAG AAC CAC TGA TG-3′ |
| [13] | 5′-CAA ACA CTG AAC CAG TTT AGC CAA CAT GGA ACC ATC-3′ |
| [14] | 5′-GAT GGT TCC ATG TTG GCT AAA CTG GTT CAG TGT TTG-3′ |
| [15] | 5′-CTC TGC CGA AGT GAG CGA TCT GTC AG-3′ |
| [16] | 5′-CTG ACA GAT CGC TCA CTT CGG CAG AG-3′ |
| [17] | 5′-GAA AAT GGC CGT GAG AAC CAT AGC TTA ACC TTT TGT GGC GAA CC-3′ |
| [18] | 5′-GAA CCA TTG TTT AAC CTT TAG CGG CGA ACC GCT GCA ACA G-3′ |
| [19] | 5′-GGC GAG CAA CTT AGC CTG CAA CGT G-3′ |
| [20] | 5′-TAG CAA CTT TGC TTG CAC TAG ACC GAC TGG TCT ACT ACA CTC CAA CGC ATG AAC AAA CAC ACC GAA CAT G-3′ |
| [21] | 5′-GAA GCA GCT CCA GCC TAC ACC TAA ACG GCA GGC GTC GCA ATA AT-3′ |
| [22] | 5′-ATT ATT GCG ACG CCT GCC GTT TAG GTG TAG GCT GGA GCT GCT TC-3′ |
| [23] | 5′-TTT TTC AGA TGA CAT AAA GGC TCC CGG TCG TCT GGT GAT AAA AAA TGC CTG CAT ATG AAT ATC CTC CTT AG-3′ |
| [24] | 5′-AAG AAC TTA CCT GGT CTT GAC ATC-3′ |
| [25] | 5′-CAG TTT ATC ACT GGC AGT CTC CTT-3′ |
| [26] | 5′-GGT CCT TCT ATC ACT ACT TTC GCT CT-3′ |
| [27] | 5′-GTT TTA CTG TCA GGC AAC CAC TGA T-3′ |
| [28] | 5′-AGG TGC ATA CAC AGT AGA AAA AGC TG-3′ |
| [29] | 5′-TAC AAC GTC GGG TAA TCG GTA TAG-3′ |
| [30] | 5′-TAA ATA CGA ACT CGG CAC TGC TTA T-3′ |
| [31] | 5′-GCG TCT TTC TCT TCG ATT AAC TCA-3′ |
| [32] | 5′-GAA CCT GAA ATC TAC AAC GCT ATC C-3′ |
| [33] | 5′-ATC AGC AGT CAG GAA GAT AAC CTT AG-3′ |
| [34] | 5′-GAC ATC GAT TTT TTA CGT GGT CAG-3′ |
| [35] | 5′-CGA TTT AGT GGC AGA AGT TAG TGT CT-3′ |
| [36] | 5′-TCT GGA CGG GAA AGA ACT CTA-3′ |
| [37] | 5′-ATC CCG GTG AAT CCA CGT T-3′ |
Sequence Analysis and Building of the NemR Model
Custom Python scripts using Biopython 1.57 were used to search for and sort NemR homologs from the National Center for Biotechnology Information databases (accessed 8/26/11). Alignments were performed using MUSCLE 3.8 (20) and visualized with WebLogo 3.1. NemR structure was modeled with SWISS-MODEL (21).
Construction of nemR Plasmids
The nemR gene, without the stop codon, was amplified from E. coli MG1655 with primers [7] and [8] and cloned into the NdeI and HindIII sites of plasmid pET-21b+ (Novagen) to generate the C-terminally His6-tagged NemR expression plasmid pNEMR1. The nemR gene and stop codon, plus 21 bp of 5′ sequence, were amplified from E. coli MG1655 with primers [9] and [10] and cloned into the EcoRI and HindIII sites of plasmid pBAD30 (22) to generate plasmid pNEMR2. The QuikChangeTM protocol (Stratagene) was used to mutate pNEMR1 with primers [11] and [12], [13] and [14], or [15] and [16]. This yielded plasmids pNEMR3, pNEMR7, and pNEMR8. The QuikChange protocol, modified by using only a single mutagenic primer and 35 cycles of amplification, was used to mutate pNEMR1 with primer [17], [18], or [19]. This yielded plasmids pNEMR12, pNEMR13, and pNEMR16. The QuikChange multisite-directed mutagenesis kit (Stratagene) was used to mutate pNEMR2 with primers [13], [15], [17], and [19]. This yielded plasmid pNEMR32. The single-primer QuikChange procedure was used to mutate pNEMR2 and pNEMR32 with primer [11]. This yielded plasmids pNEMR43 and pNEMR44. The nemR alleles were amplified from pNEMR32 and pNEMR44 with primers [7] and [8] and cloned into the NdeI and HindIII sites of plasmid pET-21b+ to yield plasmids pNEMR40 and pNEMR45.
Construction of Chromosomal nemR Variants
The nemR alleles from pNEMR2, pNEMR32, pNEMR43, and pNEMR44 were amplified using primers [20] and [21]. The cat+ cassette was amplified from plasmid pKD3 (19) using primers [22] and [23]. 200 fmol of nemR PCR product and 200 fmol of cat+ PCR product were mixed and used as template for amplification with primers [20] and [23]. The resulting nemR-cat+ PCR products were gel-purified, transformed into strain MJG244, and selected for chloramphenicol resistance. The cat+ cassettes in these strains were then resolved (19).
Gene Expression Analysis by RT-PCR
E. coli strains were grown at 37 °C with shaking in MOPS glucose medium at 37 °C to A600 = 0.4–0.5, and oxidants were added as indicated. After defined time points, 0.5-ml samples were collected in liquid nitrogen. RNA was prepared using the RNeasy® Mini kit (Qiagen) and DNA-freeTM kit (Ambion). SuperScript® III reverse transcriptase (Invitrogen) was used to generate cDNA, and RT-PCRs were set up with SYBR® GreenERTM qRT-PCR mix (Invitrogen) and a Mastercycler® ep realplex2 real-time PCR system (Eppendorf). Expression ratios were calculated compared with expression of each gene in nonstressed MG1655 cultures by the ΔΔCt method (23) and normalized to expression of rrsD, encoding 16S rRNA, expression of which did not change under our conditions. Primers used for RT-PCR analysis were: rrsD, [24] and [25]; nemR, [26] and [27]; nemA, [28] and [29]; gloA, [30] and [31]; pck, [32] and [33]; yeaU, [34] and [35].
HOCl Survival Assays
E. coli MG1655 and isogenic mutant strains were grown at 37 °C with shaking in 10 ml of MOPS glucose medium to A600 = 0.4–0.6 and harvested by centrifugation. Cells were resuspended to A600 = 0.35 in 10 ml of MOPS glucose medium containing 2 mm HOCl in 125-ml baffled flasks and incubated at 37 °C with shaking (200 rpm). 0.5 ml of cells was harvested by centrifugation immediately before and at defined time points after HOCl addition, then rinsed with MOPS medium containing 10 mm Na2S2O3, but no glucose, K2HPO4, or thiamine. Dilutions in 0.9% NaCl and spot-titering on LB agar were performed using a Precision XS Microplate Sample Processor (Bio-Tek). Each strain was tested at least six times.
Methylglyoxal Survival Assays
E. coli MG1655 and isogenic mutant strains were grown at 37 °C with shaking (200 rpm) in 25 ml of MOPS glucose medium to A600 = 0.4–0.6, then methylglyoxal was added to 150 μm, and cells were harvested and spot-titered as above. To test the ability of HOCl to cross-protect against methylglyoxal, 0.4 mm HOCl was added, and cells were incubated for 10 min before the addition of 0.5 mm Na2S2O3 and methylglyoxal.
Quantification of Intracellular Free Methylglyoxal
Methylglyoxal was measured using a previously described HPLC method (24).
Purification of NemR Variants
C-terminally His-tagged NemR variants were purified by a modification of the procedure of Umezawa et al. (12). NemR was overproduced in E. coli BL21(DE3) (Novagen) and purified using nickel-nitrilotriacetic acid Superflow resin (Qiagen). Purified proteins were stored in 50 mm Tris-HCl buffer (pH 7.5) containing 200 mm KCl, 10 mm MgCl2, 0.1 mm EDTA, 1 mm DTT, and 10% glycerol at −80 °C. NemR was exchanged into DTT-free buffer with P-30 gel chromatography columns (Bio-Rad) before use.
Metal Analysis
The metal content of purified NemR was analyzed using inductively coupled plasma-high resolution mass spectrometry at the Keck Elemental Geochemistry Laboratory, Department of Geological Sciences, University of Michigan.
In Vitro Oxidation and Gel Mobility Shift Assays of NemR Proteins
Purified NemR proteins were incubated for 15 min at 37 °C in 50 mm sodium phosphate (pH 8), 150 mm NaCl with the indicated molar ratio of oxidants, then exchanged into gel shift buffer (10 mm Tris-HCl (pH 7.8 at 4 °C), 150 mm NaCl, 3 mm magnesium acetate, 10% glycerol) using a P-30 gel chromatography column. To assay reversibility of oxidation, N-chlorotaurine-treated samples were incubated with 1 mm DTT for 15 min at 37 °C. Gel mobility shift assays were a modification of the procedure of Umezawa et al. using a 222-bp fragment of E. coli MG1655 genomic DNA containing the nemR promoter region (PnemR), amplified with primers [36] and [37] (12). NemR variants were incubated in gel shift buffer with PnemR for 30 min at 37 °C. Fragments were separated by electrophoresis on 10% TBE-polyacrylamide gels (Bio-Rad), stained with ethidium bromide, visualized by UV fluorescence, and quantified using ImageJ 1.44o (25).
Assays of Cysteine Thiol Status
Reduced cysteines were determined in 5 μm reduced or N-chlorotaurine-oxidized NemR5C-S(C106) using Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid) (26).
In Vivo Thiol Trapping of NemR
E. coli BL21 containing plasmids expressing His-tagged NemR variants were grown at 37 °C in MOPS glucose medium to A600 = 0.4–0.5. NemR expression was induced with 10 μm isopropyl 1-thio-β-d-galactopyranoside for 30 min, after which cells were treated with 1 mm HOCl. At the indicated time points, 1-ml cell aliquots were harvested by centrifugation and resuspended in 100% acetone. After 2 h of incubation at −20 °C, precipitated proteins were pelleted by centrifugation and resuspended in 6 m urea, 200 mm Tris-HCl (pH 8.5), 10 mm EDTA, 0.5% w/v SDS, and supplemented with 0.8 m iodoacetamide. Samples were separated by nonreducing SDS-PAGE and visualized by Western blotting using an anti-His tag antibody (Abcam).
RESULTS
Identification of Bleach-responsive Regulators in E. coli
To identify regulators that might contribute to bacterial bleach defense, we examined gene expression in E. coli before and after a sublethal dose of HOCl (0.4 mm) using a transcriptional microarray, the full results of which are shown in supplemental Table S1. Among the genes significantly up-regulated by HOCl treatment were two electrophile detoxification genes (nemA and gloA) known to be under the control of NemR, a transcriptional repressor of the TetR family (Fig. 1A, inset). NemR, which was also found to be significantly up-regulated in response to HOCl treatment in our microarray analysis, has previously been reported to be sensitive to the electrophilic cysteine alkylation agent N-ethylmaleimide (NEM) (12). NEM is a synthetic reagent that irreversibly alkylates cysteine thiols. In contrast, HOCl is a physiological oxidant that causes the rapid and reversible oxidation of cysteine thiols to sulfenic acids and disulfide bonds (27). We therefore decided to pursue the study of NemR as a potentially bleach-responsive transcriptional regulator.
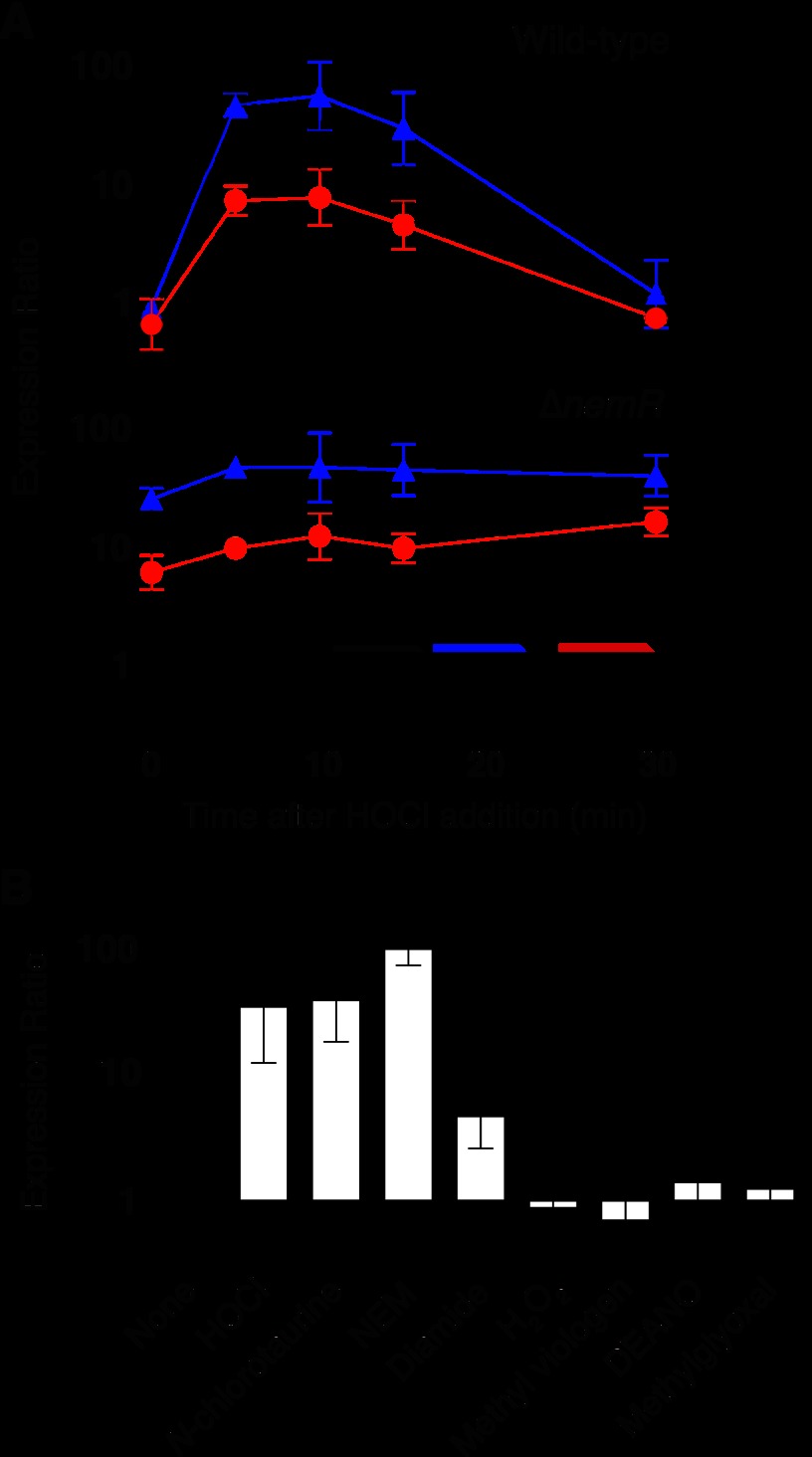
FIGURE 1.
A, E. coli MG1655 (wild-type) and the ΔnemR mutant grown to mid-log phase in MOPS glucose medium and treated with 0. 4 mm HOCl. Expression ratios of nemR (black ■), nemA (blue ▴), or gloA (red ●) were determined by RT-PCR (mean ± S.D. (error bars)). Inset, operon structure of the nem locus using the same color code. B, expression of nemR in E. coli MG1655 upon treatment with 0.4 mm HOCl, 0.2 mm N-chlorotaurine, 0.1 mm N-ethylmaleimide (NEM), 0.5 μm diamide, 2 mm H2O2, 0.4 mm methyl viologen, 0.2 mm diethylamine nitric oxide (DEANO), or 0.2 mm methylglyoxal for 10 min relative to expression in the absence of any treatment (None) (mean ± S.D.).
NemR Is an HOCl-sensing Transcription Factor
Quantitative RT-PCR confirmed our microarray data and revealed approximately 70-fold up-regulation of nemR and 100-fold and 10-fold up-regulation of the downstream genes nemA and gloA, respectively, in response to sublethal HOCl treatment (Fig. 1A). Expression of both nemA and gloA was constitutively induced and almost completely nonresponsive to HOCl in a ΔnemR strain (Fig. 1A), indicating that bleach-mediated expression of both genes was almost entirely dependent on NemR. Although Umezawa et al. (12) identified weak NemR binding sites upstream of the pck and yeaU genes of E. coli, neither of these genes showed NemR-dependent up-regulation upon HOCl treatment (data not shown), suggesting that nemA and gloA are the primary regulatory targets of NemR.
To determine the in vivo oxidant specificity of the NemR response, we treated wild-type E. coli cells with various electrophiles and with reactive oxygen, nitrogen, and chlorine species, utilizing concentrations that induced transient growth arrest under our growth conditions without killing the cells (data not shown). These studies revealed that, in addition to responding to NEM, NemR is sensitive to treatment with either HOCl or N-chlorotaurine, a prominent secondary oxidation product of HOCl in vivo (13). It also responded weakly to the disulfide-generating electrophile diamide (diazenedicarboxylic acid bis(N,N-dimethylamide)) (Fig. 1B) (28). In contrast, no significant NemR-mediated gene expression was observed in response to H2O2, the O2− generator methyl viologen, the nitric oxide source diethylamine nitric oxide (DEANO), or, notably, methylglyoxal, the substrate of the NemR downstream target GloA (glyoxalase I) (29) (Fig. 1B). These data demonstrate that induction of the nemR regulon is sensitive to HOCl and related RCS.
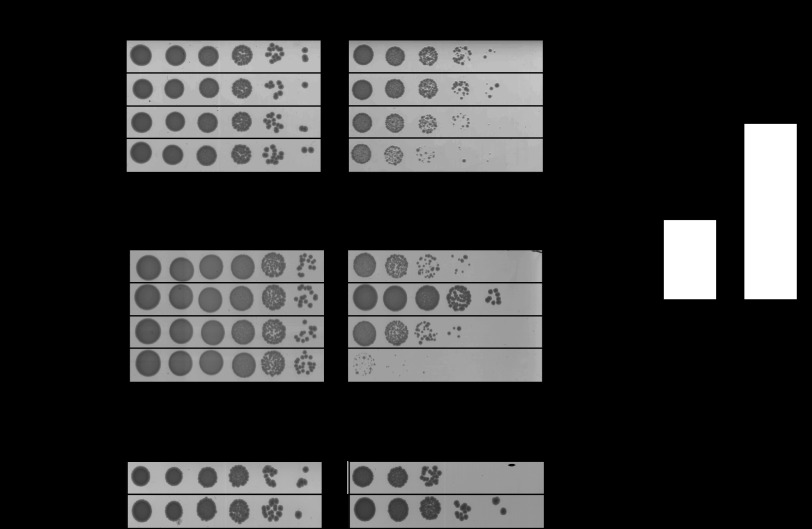
Phenotypic studies revealed that deletion of gloA and, to a lesser extent, nemA, increased the HOCl sensitivity of E. coli cells. In contrast, deletion of nemR had no significant effect on bleach survival (Fig. 2A), indicating that constitutive overexpression of nemA and gloA did not provide additional protection against HOCl. As expected, mutants lacking gloA were more sensitive to methylglyoxal treatment than wild-type E. coli (Fig. 2B) (29). A ΔnemR strain was considerably more resistant to methylglyoxal than wild type presumably due to the constitutive derepression of gloA. Similarly, pretreatment of E. coli with 0.4 mm HOCl protected against methylglyoxal stress (Fig. 2C), as would be expected due to NemR-dependent up-regulation of gloA. ΔnemA mutants showed no defect in survival of methylglyoxal stress, indicating that NemA is more important for HOCl stress tolerance than for resistance to exogenous methylglyoxal (Fig. 2, A and B). Consistent with these results, HPLC analysis of HOCl-treated E. coli revealed substantial accumulation of intracellular methylglyoxal after HOCl treatment (Fig. 2D). These results suggest that NemR is a bacterial repressor which is sensitive to HOCl and related RCS and whose gene products contribute to increased bleach resistance in E. coli by detoxifying reactive electrophiles produced during RCS stress.
FIGURE 2.
A and B, E. coli MG1655 (wild-type) and mutant strains were incubated in MOPS glucose medium containing 2 mm HOCl for 150 min (A) or 150 μm methylglyoxal (MGO) for 120 min (B), then diluted and spot-titered on LB agar. C, E. coli MG1655 was incubated in MOPS glucose medium containing 0.4 mm HOCl for 10 min, followed by the addition of 150 μm methylglyoxal, then, after 150 min, diluted and spot-titered on LB agar. D, E. coli MG1655 was incubated in MOPS glucose medium containing 0.4 mm HOCl. Intracellular free methylglyoxal/cfu) was measured by HPLC (mean ± S.D. (error bars)).
Cysteine 106 Is Conserved among NemR Homologs
The simplest hypothesis explaining these results was that HOCl-mediated modification of NemR leads to the derepression of the nemR operon, causing the up-regulation of genes that increase bacterial resistance to these oxidants. To first investigate whether the NemR bleach sensing mechanism might involve oxidation of redox-sensitive metals (11), we conducted inductively coupled plasma mass spectrometry of the purified NemR protein. However, we were unable to detect any metals associated with NemR (data not shown), indicating that HOCl sensing in NemR is likely mediated by other means. Because some transcription factors, such as the peroxide-sensing OxyR, have been shown to rely on the reactivity of functionally or structurally important cysteine thiols to sense oxidants (9), we aligned NemR homologs from 166 bacterial genera (BLAST e-value < 0.00001) to identify potentially evolutionarily important cysteines. Of the six cysteines in E. coli NemR, only one, Cys-106, is highly conserved (Fig. 3A, E. coli numbering in red). Modeling of the E. coli NemR sequence onto the molecular structure of its Acinetobacter homolog (Protein Data Bank ID code 3KNW, 41% identical, 61% similar to E. coli NemR) (21) predicted that Cys-106 is surface-exposed and faces the predicted DNA binding site (Fig. 3B, green helices). Cys-21 is located directly in the DNA binding helix whereas Cys-116 and Cys-153 are located in the predicted dimerization interface of NemR. None of these other cysteines is, however, particularly well conserved among NemR homologs (Fig. 3A).
FIGURE 3.
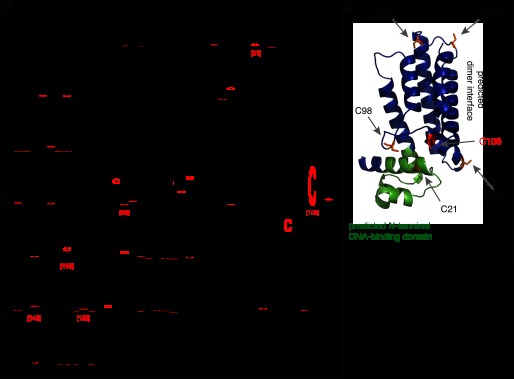
A, alignment of NemR homologs from 166 bacterial genera, with cysteines indicated in red. Locations of cysteines in E. coli NemR numbering are indicated with red bracketed numbers. B, predicted molecular structure of the E. coli NemR monomer. Cysteine residues are in orange, conserved Cys-106 in red, and DNA binding helices in green.
Cys-106 Is Sufficient for NemR RCS-sensing Mechanism
To investigate the role of these cysteines in the ability of NemR to respond to HOCl and related RCS, we constructed strains containing different cysteine mutant alleles of nemR at the native locus in the E. coli chromosome. As a read-out, we monitored their ability to respond to HOCl treatment with the overexpression of nemR using RT-PCR.
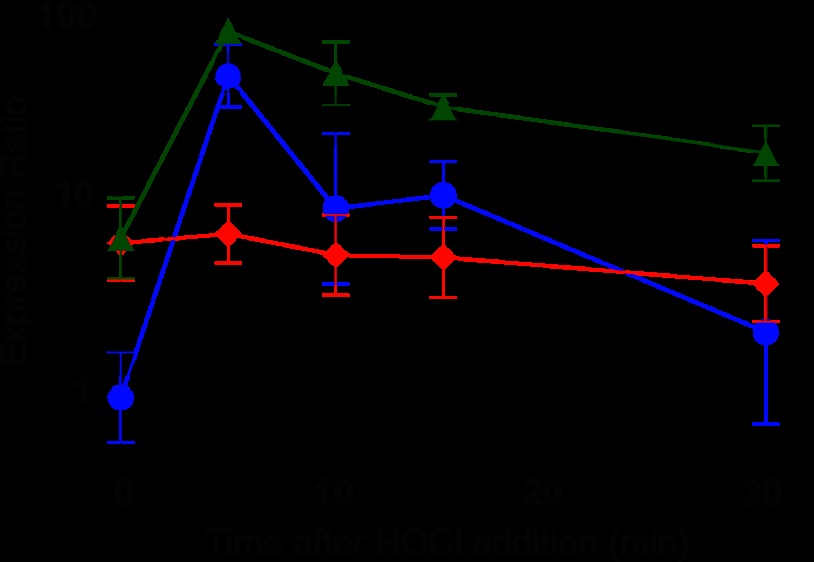
NemR6C-S, in which all six cysteines were mutated to serine, did not respond at all to HOCl treatment (Fig. 4, red trace), strongly indicating that HOCl sensing was cysteine- dependent. Repression of the nemR operon was maintained in these strains, albeit slightly relieved compared with to wild-type strains, indicating that this mutant protein largely maintains its DNA binding capacity despite the lack of all cysteines. A similar relief in basal repression was observed in strains expressing NemR5C-S(C106), which lacked all but the absolutely conserved Cys-106. In contrast to the strain expressing the no-cysteine variant, however, this strain responded to HOCl in a wild-type-like manner (Fig. 4, compare black and green traces). These results suggested that the conserved Cys-106 is sufficient for mediating a wild-type-like HOCl response in NemR.
FIGURE 4.
Expression of chromosomal nemR alleles encoding wild-type NemR (black ■), NemRC106S (blue ●), NemR5C-S(C106) (green ▴), or NemR6C-S (red ♦) before and after 0.4 mm HOCl treatment (mean ± S.D. (error bars)).
Notably, cells expressing NemRC106S, a variant that lacks the conserved Cys-106 but contains all five other nonconserved cysteines, were also able to mount a HOCl response (Fig. 4, blue trace). However, gene expression rapidly declined in these bacteria, suggesting that Cys-106 might be crucial for maintaining a sustained HOCl response. This result was not completely unexpected given that some of the nonconserved cysteines are predicted to be in structurally or functionally critical locations of NemR and their oxidation might lead to derepression as well. Nevertheless, the fact that Cys-106 is absolutely conserved among members of the NemR family and is sufficient to confer the observed bleach response suggests that Cys-106 plays the central role in the NemR RCS-sensing mechanism.
NemR DNA Binding Activity Is RCS-sensitive in Vitro
To investigate the effects of RCS treatment on the DNA binding activity of NemR in vitro, we purified wild-type NemR and cysteine-mutated NemR variants and conducted in vitro gel shift assays using a DNA fragment containing the promoter region of nemR (12). We found that reduced wild-type NemR binds strongly to its own promoter region (Fig. 5A, top left). Incubation of NemR with a 1:1 molar ratio of N-chlorotaurine, used as an HOCl substitute in in vitro experiments because it causes many fewer nonspecific oxidation artifacts than HOCl (30), substantially decreased the NemR DNA binding affinity (Fig. 5A, top right). Incubation of NemR with a 1:1 molar ratio of H2O2 had much less effect on its DNA binding (Fig. 5A, bottom left), consistent with the failure of H2O2 to induce nemR expression in vivo (Fig. 1B). These results supported our in vivo studies and suggested that RCS oxidation of NemR causes its dissociation from DNA, therefore leading to the observed derepression of NemR target gene expression. Most importantly, incubation of oxidized inactive NemR with excess amounts of the thiol-specific reducing agent DTT fully restored DNA binding (Fig. 5A, bottom right), indicating that, unlike the irreversible inactivation of NemR by NEM alkylation (12), oxidative inactivation of NemR is a fully reversible process in vitro.
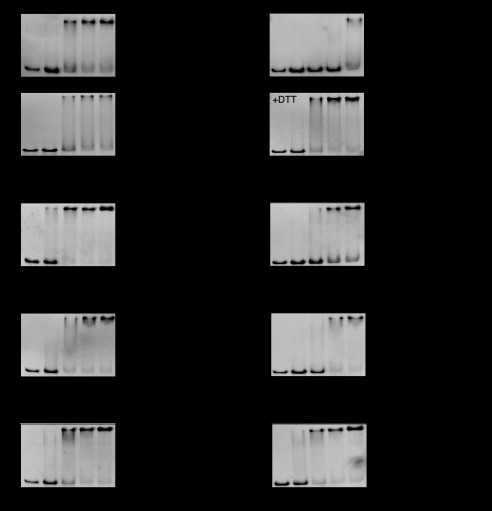
FIGURE 5.
Purified wild-type NemR (A), NemR5C-S(C106) (B), NemRC106S (C), or NemR6C-S (D), before or after incubation with a 1:1 molar ratio of N-chlorotaurine (NCT) or H2O2 were incubated with a DNA fragment containing the nemR promoter (10 nm), visualized by PAGE, and quantified by densitometry. Representative gels are shown, along with the results of quantification (mean ± S.D. (error bars)). To test the reversibility of NemR inactivation, oxidized wild-type NemR was treated with 1 mm DTT for 15 min to reduce reversible thiol modifications before incubation with DNA (A, lower right panel).
In vitro DNA binding analysis of the three NemR cysteine variants yielded results that were also fully consistent with our in vivo findings. The mutant variant NemR5C-S(C106), which contains only the conserved Cys-106, revealed a decrease in in vitro DNA binding affinity in response to N-chlorotaurine treatment that was very similar in relative extent to the decrease observed in N-chlorotaurine-treated wild-type NemR (Fig. 5B). The presence of all cysteines except Cys-106 also resulted in in vitro RCS sensitivity (Fig. 5C), supporting our in vivo results suggesting that one or more of the remaining nonconserved cysteines of E. coli NemR are RCS-sensitive as well (Fig. 4). In contrast, the cysteine-free NemR6C-S protein remained bound to DNA independent of its pretreatment, indicating that absence of all six cysteines did not affect the NemR DNA binding but eliminated its ability to sense reactive chlorine species (Fig. 5D).
Monitoring the in Vitro and in Vivo Thiol Oxidation Status of NemR
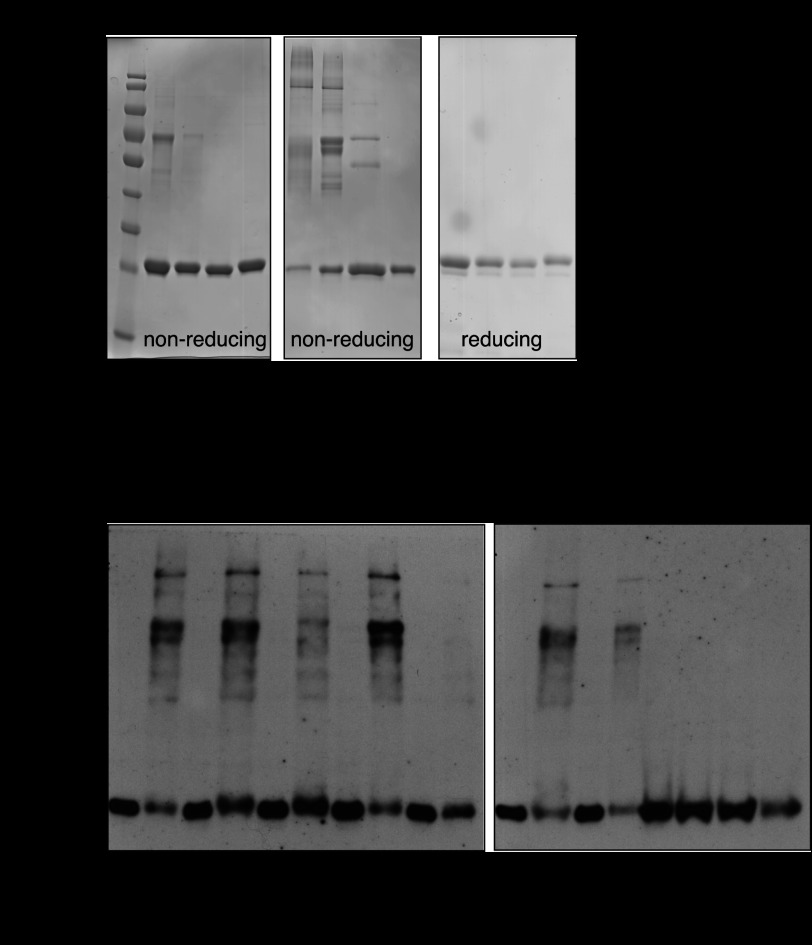
To further examine the role that cysteine residues play in the RCS response of NemR, we examined the thiol oxidation status of our purified NemR wild-type and the three cysteine variants on nonreducing SDS-PAGE before and after treatment with N-chlorotaurine. In the absence of oxidants, all purified NemR variants migrated predominantly as monomeric proteins, although some disulfide-bonded species were already observed in untreated wild-type NemR (Fig. 6A). Upon treatment with a 1:1 molar ratio of N-chlorotaurine, however, both wild-type NemR and NemRC106S, which contains all but the conserved Cys-106, formed a variety of higher molecular mass species, which disappeared as soon as reducing conditions were reestablished (Fig. 6A). These results suggested that NemR forms intermolecular disulfide bonds with other NemR molecules upon treatment with RCS in vitro. As expected, NemR6C-S, which does not contain any cysteine, remained fully monomeric (albeit with a slightly different migration behavior than cysteine-containing variants), further excluding the involvement of any noncysteine modifications in the HOCl-sensing mechanism of NemR. Importantly, HOCl-treated NemR5C-S(C106), which contains only the highly conserved Cys-106 and whose in vivo and in vitro response to HOCl mimics that of wild-type NemR, remained largely monomeric upon treatment with N-chlorotaurine (Fig. 6A). These results suggested that intermolecular disulfide bond formation is not required for NemR functional regulation in vitro. Indeed, Ellman's assay, which we used to monitor the oxidation status of the single Cys-106 in NemR5C-S(C106), revealed that of the 80% of reduced molecules in the untreated NemR5C-S(C106) preparation, all but 8% were oxidized by N-chlorotaurine. Most notably, incubation of oxidized NemR5C-S(C106) with 5 mm DTT for 30 min reduced almost 60% of all molecules, excluding the presence of irreversible overoxidation products (such as sulfinic or sulfonic acids). The simplest hypothesis to explain this result was that, in the absence of other cysteines, Cys-106 might undergo reversible sulfenic acid formation, the immediate consequence of RCS-mediated chlorination of thiol groups (4). Both reduced thiols and sulfenic acids can be detected using the probe NBD-Cl, which forms adducts with absorption maxima of 420 nm for thiols and 350 nm for sulfenic acid (31). We recorded the UV spectrum of N-chlorotaurine-oxidized NemR5C-S(C106) in the presence of NBD-Cl but were unable to detect any significant sulfenic acid adducts in our sample (data not shown). Depending on the microenvironment of the cysteine, however, the nucleophilicity of the sulfur in sulfenic acid may not be sufficient to react with the electrophilic NBD-Cl reagent. We therefore decided to conduct a chymotryptic digest of reduced and N-chlorotaurine-oxidized NemR5C-S(C106) and perform mass spectrometric analysis of the peptides. In contrast to the reduced NemR5C-S(C106) sample, for which 100% coverage was obtained, we were unable to identify the Cys-106-containing peptide in the oxidized sample despite using an error-tolerant search with the latest version of Unimod (32). Future studies are clearly needed to identify the nature of the reversible thiol oxidation product in oxidized NemR.
FIGURE 6.
A, purified NemR variants (2 μg), with and without incubation with a 1:1 molar ratio of N-chlorotaurine (NCT), were visualized by nonreducing or reducing SDS-PAGE, as indicated. B, E. coli BL21 expressing His-tagged NemR variants were grown to mid-log phase, induced with 10 μm isopropyl 1-thio-β-d-galactopyranoside for 30 min, then treated with 1 mm HOCl and harvested at indicated time points. Reduced cysteine thiols were irreversibly alkylated with iodoacetamide, and NemR was visualized by Western blotting using nonreducing SDS-PAGE.
In vivo thiol trapping experiments fully supported our in vitro findings and revealed the formation of intermolecular disulfide bonds in HOCl-treated cells expressing either wild-type NemR or the NemR variants lacking Cys-21, Cys-106, and Cys-149 (Fig. 6B). Variants lacking Cys-98 or Cys-153 formed substantially fewer higher molecular mass species, whereas the variant lacking Cys-116 formed none. These results indicate that Cys-116, located within the predicted dimer interface of NemR (Fig. 3B), plays the primary role in the observed intermolecular disulfide formation after HOCl stress in vivo. Neither the redox-active NemR5C-S(C106) variant nor the inactive NemR6C-S variant formed any disulfide bonded species in vivo. These results confirm that intermolecular disulfide bond formation is not required for a sustained NemR-mediated RCS response in E. coli and suggest that reversible oxidative modification of the absolutely conserved Cys-106 plays the central role in the sensing of and response to RCS.
DISCUSSION
Our study demonstrates that NemR is a transcriptional regulator that uses RCS-sensitive cysteine residues to rapidly induce expression of specific reductases involved in the detoxification of reactive electrophiles. Our data suggest a model in which NemR uses the reversible oxidation of its highly conserved Cys-106 as a trigger for conformational rearrangements that cause its dissociation from the DNA and subsequently the activation of nemA and gloA expression. Because in vitro exposure of wild-type NemR to an equimolar amount of N-chlorotaurine led not only to the oxidation of Cys-106, but also to the formation of a variety of intermolecularly disulfide-bonded oligomers (Fig. 6), it is likely that RCS-mediated chlorination of Cys-106 not only leads to oxidation of Cys-106 but also to an oxidative cascade, chlorinating and oxidizing other cysteine residues in NemR (4). In vivo analysis demonstrated that Cys-116, predicted to be near the dimer interface (Fig. 3B), is involved in the formation of intermolecular disulfide bonds (Fig. 6C) and may be responsible for the inactivation of NemR by RCS in the absence of Cys-106 (Figs. 4 and 5). However, the NemR5C-S(C106) mutant, which lacks Cys-116 (as well as all other cysteines apart from Cys-106), is able to respond to RCS in a wild-type manner both in vivo and in vitro, indicating that Cys-106, the only conserved cysteine in NemR, is sufficient for wild-type-like HOCl sensing in vivo. The additional nonconserved cysteine residues appear to be able to partially substitute for its absence. This result is reminiscent of the peroxide-sensing transcription factor OxyR, which uses reversible peroxide-mediated sufenic acid formation at its absolutely conserved Cys-199 for sensing but is able to undergo disulfide bond formation with the less conserved Cys-208 (9). At this point, we do not know the nature of the reversible thiol modification that occurs at NemR Cys-106 and which leads to the loss in DNA binding affinity. Based on our preliminary mass spectrometric results, the modification appears to be distinct from the reported sulfenamide formation or S-bacillithiolation that occurs at Cys-15 of B. subtilis repressor OhrR. Like NemR, OhrR is inactivated by HOCl, leading to the derepression of its target gene, peroxiredoxin OhrA (33, 34). YjiE, another HOCl-sensing transcription factor in E. coli, contains no conserved cysteines (5), and the mechanism by which YjiE senses HOCl remains unknown. The role of the NemR nonconserved cysteines, and the properties of NemR that confer its specificity for certain electrophiles and RCS are currently under investigation.
Our studies revealed that NemA and particularly GloA are critical to bacterial bleach survival. NemA is a flavin-dependent reductase, which is active on a variety of electrophilic compounds in vitro, including NEM. However, its physiological substrate is so far unknown (35). The function of GloA is much better understood. GloA is broadly conserved among prokaryotic and eukaryotic species as the primary methylglyoxal-detoxifying enzyme (29). Methylglyoxal is a reactive ketoaldehyde capable of damaging proteins and nucleic acids and is formed by dephosphorylation of the glycolytic intermediate dihydroxyacetone phosphate (36). Numerous oxidative stress conditions have been associated with the accumulation of methylglyoxal and other reactive electrophiles in both bacteria and eukaryotes; however, the precise relationship between oxidative stress and methylglyoxal accumulation remains poorly understood (37). Our results demonstrate that increased methylglyoxal production is a direct consequence of HOCl stress and suggest that detoxification of reactive electrophiles is a critical component of bacterial RCS defense. The pathway(s) responsible for HOCl-induced methylglyoxal production are currently being investigated in our laboratory. The fact that NemR does not respond directly to methylglyoxal implies that E. coli uses NemR to mount a proactive response, anticipating an increase in methylglyoxal levels in response to RCS.
Previous work on NemR has shown that it responds to cysteine-modifying electrophiles, including NEM, showdomycin, and, more weakly, iodoacetamide (12). In combination with the results presented here, this suggests that NemR is an RCS- and electrophile-sensing regulator important for defense against a subset of stresses that result in toxic modification of cellular thiols. NemR homologs are broadly conserved among both Gram-negative and Gram-positive bacteria. The E. coli-like nemR operon structure is conserved among Enterobacteriacea (38), suggesting that the NemR-controlled gloA and nemA expression response is conserved throughout this group. In other clades, genes encoding NemR homologs are commonly found in loci containing genes potentially involved in response to oxidative or electrophilic stress, including glutathione S-transferases, oxidoreductases, and alcohol dehydrogenases. Supporting the idea that sensing of RCS may be a common function among NemR homologs, the Rhodococcus rhodochrous NemR homolog DhaR (31% identical, 51% similar to E. coli NemR) has been shown to regulate breakdown of a variety of chlorinated compounds, including 1-chlorobutane and 1,3-dichloropropene, although the detailed mechanism and specificity of DhaR have not been investigated (39).
In conclusion, our studies identified NemR as a broadly conserved bacterial transcription factor that relies on cysteine thiol oxidation to sense and respond to both RCS and reactive electrophiles. In E. coli, NemR regulates expression of electrophile detoxification genes that play an important role in surviving bleach stress.
Acknowledgment
We thank Emily Schwessinger for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-GM065318 and R21-AI097893 (to U. J.).

This article contains supplemental Table S1.
Raw microarray data have been deposited in the ArrayExpress database (accession no. E-MEXP-3639, http://www.ebi.ac.uk/arrayexpress/).
- HOCl
- hypochlorous acid
- RCS
- reactive chlorine species.
REFERENCES
- 1. Rutala W. A., Weber D. J. (1997) Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 10, 597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klebanoff S. J. (2005) Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625 [DOI] [PubMed] [Google Scholar]
- 3. Bae Y. S., Choi M. K., Lee W. J. (2010) Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 31, 278–287 [DOI] [PubMed] [Google Scholar]
- 4. Deborde M., von Gunten U. (2008) Reactions of chlorine with inorganic and organic compounds during water treatment: kinetics and mechanisms. A critical review. Water Res. 42, 13–51 [DOI] [PubMed] [Google Scholar]
- 5. Gebendorfer K. M., Drazic A., Le Y., Gundlach J., Bepperling A., Kastenmüller A., Ganzinger K. A., Braun N., Franzmann T. M., Winter J. (2012) Identification of a hypochlorite-specific transcription factor from Escherichia coli. J. Biol. Chem. 287, 6892–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi B. K., Gronau K., Mäder U., Hessling B., Becher D., Antelmann H. (2011) S-Bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol. Cell. Proteomics, 10.1074/mcp.M111.009506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winterbourn C. C., Kettle A. J. (2013) Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 18, 642–660 [DOI] [PubMed] [Google Scholar]
- 8. Imlay J. A. (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77, 755–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vázquez-Torres A. (2012) Redox active thiol sensors of oxidative and nitrosative stress. Antioxid. Redox Signal. 17, 1201–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crack J. C., Green J., Hutchings M. I., Thomson A. J., Le Brun N. E. (2012) Bacterial iron-sulfur regulatory proteins as biological sensor-switches. Antioxid. Redox Signal. 17, 1215–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spiro S., D'Autréaux B. (2012) Non-heme iron sensors of reactive oxygen and nitrogen species. Antioxid. Redox Signal. 17, 1264–1276 [DOI] [PubMed] [Google Scholar]
- 12. Umezawa Y., Shimada T., Kori A., Yamada K., Ishihama A. (2008) The uncharacterized transcription factor YdhM is the regulator of the nemA gene, encoding N-ethylmaleimide reductase. J. Bacteriol. 190, 5890–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagl M., Hess M. W., Pfaller K., Hengster P., Gottardi W. (2000) Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. Antimicrob. Agents Chemother. 44, 2507–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kellum M. W., Oray B., Norton S. J. (1978) A convenient quantitative synthesis of methylglyoxal for glyoxalase I assays. Anal. Biochem. 85, 586–590 [DOI] [PubMed] [Google Scholar]
- 15. Peskin A. V., Winterbourn C. C. (2001) Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic. Biol. Med. 30, 572–579 [DOI] [PubMed] [Google Scholar]
- 16. Gentleman R. C., Carey V. J., Bates D. M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A. J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J. Y., Zhang J. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saeed A. I., Sharov V., White J., Li J., Liang W., Bhagabati N., Braisted J., Klapa M., Currier T., Thiagarajan M., Sturn A., Snuffin M., Rezantsev A., Popov D., Ryltsov A., Kostukovich E., Borisovsky I., Liu Z., Vinsavich A., Trush V., Quackenbush J. (2003) TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34, 374–378 [DOI] [PubMed] [Google Scholar]
- 18. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 19. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiefer F., Arnold K., Künzli M., Bordoli L., Schwede T. (2009) The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 37, D387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subedi K. P., Choi D., Kim I., Min B., Park C. (2011) Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol. Microbiol. 81, 926–936 [DOI] [PubMed] [Google Scholar]
- 25. Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riddles P. W., Blakeley R. L., Zerner B. (1983) Reassessment of Ellman's reagent. Methods Enzymol. 91, 49–60 [DOI] [PubMed] [Google Scholar]
- 27. Winter J., Ilbert M., Graf P. C., Ozcelik D., Jakob U. (2008) Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell 135, 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kosower N. S., Kosower E. M. (1995) Diamide: an oxidant probe for thiols. Methods Enzymol. 251, 123–133 [DOI] [PubMed] [Google Scholar]
- 29. MacLean M. J., Ness L. S., Ferguson G. P., Booth I. R. (1998) The role of glyoxalase I in the detoxification of methylglyoxal and in the activation of the KefB K+ efflux system in Escherichia coli. Mol. Microbiol. 27, 563–571 [DOI] [PubMed] [Google Scholar]
- 30. Chapman A. L., Winterbourn C. C., Brennan S. O., Jordan T. W., Kettle A. J. (2003) Characterization of noncovalent oligomers of proteins treated with hypochlorous acid. Biochem. J. 375, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poole L. B. (2008) Measurement of protein sulfenic acid content. Curr. Protoc. Toxicol. Chapter 17, Unit 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Creasy D. M., Cottrell J. S. (2004) Unimod: protein modifications for mass spectrometry. Proteomics 4, 1534–1536 [DOI] [PubMed] [Google Scholar]
- 33. Chi B. K., Roberts A. A., Huyen T. T., Bäsell K., Becher D., Albrecht D., Hamilton C. J., Antelmann H. (2013) S-Bacillithiolation protects conserved and essential proteins against hypochlorite stress in firmicutes bacteria. Antioxid. Redox Signal. 18, 1273–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J. W., Soonsanga S., Helmann J. D. (2007) A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc. Natl. Acad. Sci. U.S.A. 104, 8743–8748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueller N. J., Steuckler C., Hauer B., Baudendistel N., Housden H., Bruce N. C., Faber K. (2010) The substrate spectra of pentaerythritol tetranitrate reductase, morphinone reductase, N-ethylmaleimide reductase and estrogen-binding protein in the asymmetric bioreduction of activated alkenes. Adv. Synthesis Catalysis 352, 387–394 [Google Scholar]
- 36. Booth I. R., Ferguson G. P., Miller S., Li C., Gunasekera B., Kinghorn S. (2003) Bacterial production of methylglyoxal: a survival strategy or death by misadventure? Biochem. Soc. Trans. 31, 1406–1408 [DOI] [PubMed] [Google Scholar]
- 37. Thornalley P. J. (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems: role in ageing and disease. Drug Metabol. Drug Interact 23, 125–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Markowitz V. M., Chen I. M., Palaniappan K., Chu K., Szeto E., Grechkin Y., Ratner A., Anderson I., Lykidis A., Mavromatis K., Ivanova N. N., Kyrpides N. C. (2010) The integrated microbial genomes system: an expanding comparative analysis resource. Nucleic Acids Res. 38, D382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poelarends G. J., Kulakov L. A., Larkin M. J., van Hylckama Vlieg J. E., Janssen D. B. (2000) Roles of horizontal gene transfer and gene integration in evolution of 1,3-dichloropropene- and 1,2-dibromoethane-degradative pathways. J. Bacteriol. 182, 2191–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blattner F. R., Plunkett G., 3rd, Bloch C. A., Perna N. T., Burland V., Riley M., Collado-Vides J., Glasner J. D., Rode C. K., Mayhew G. F., Gregor J., Davis N. W., Kirkpatrick H. A., Goeden M. A., Rose D. J., Mau B., Shao Y. (1997) The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462 [DOI] [PubMed] [Google Scholar]