Background: Plant pathogens secrete enzymes that degrade plant cell walls to enhance infection and nutrient acquisition.
Results: A novel endotransglucosylase catalyzes cleavage and transfer of β-glucans and decreases the physical strength of plant cell walls.
Conclusion: Endotransglucosylation causes depolymerization and polymerization of β-glucans, depending on substrate molecular size.
Significance: Enzymatic degradation of plant cell walls is required for wall loosening, which enhances pathogen invasion.
Keywords: Cell Wall, Cellulase, Enzyme Purification, Enzymes, Glycobiology, Cell Wall-degrading Enzymes
Abstract
A Magnaporthe oryzae enzyme, which was encoded by the Mocel7B gene, was predicted to act on 1,3–1,4-β-glucan degradation and transglycosylation reaction of cellotriose after partial purification from a culture filtrate of M. oryzae cells, followed by liquid chromatography-tandem mass spectrometry. A recombinant MoCel7B prepared by overexpression in M. oryzae exhibited endo-typical depolymerization of polysaccharides containing β-1,4-linkages, in which 1,3–1,4-β-glucan was the best substrate. When cellooligosaccharides were used as the substrate, the recombinant enzyme generated reaction products with both shorter and longer chain lengths than the substrate. In addition, incorporation of glucose and various oligosaccharides including sulforhodamine-conjugated cellobiose, laminarioligosaccharides, gentiobiose, xylobiose, mannobiose, and xyloglucan nonasaccharide into β-1,4-linked glucans were observed after incubation with the enzyme. These results indicate that the recombinant enzyme acts as an endotransglucosylase (ETG) that cleaves the glycosidic bond of β-1,4-glucan as a donor substrate and transfers the cleaved glucan chain to another molecule functioning as an acceptor substrate. Furthermore, ETG treatment caused greater extension of heat-treated wheat coleoptiles. The result suggests that ETG functions to induce wall loosening by cleaving the 1,3–1,4-β-glucan tethers of plant cell walls. On the other hand, use of cellohexaose as a substrate for ETG resulted in the production of cellulose II with a maximum length (degree of polymerization) of 26 glucose units. Thus, ETG functions to depolymerize and polymerize β-glucans, depending on the size of the acceptor substrate.
Introduction
Many plant pathogens secrete a battery of cell wall-degrading enzymes (CWDEs)2 to hydrolyze plant cell wall components. The actions of these enzymes likely contribute to produce glucose as a carbon source and to break plant cell walls to enhance pathogen invasion. Endoglucanase, cellobiohydrolase, and β-glucosidase are significantly involved in the degradation of cellulose and hemicellulosic polysaccharides. The combined actions of these enzymes show a synergistic effect (1–3). In addition, substrate-specific enzymes such as xyloglucan-specific endoglucanase, 1,3–1,4-β-glucanase, xylanase, and polygalacturonase are thought to play important roles in the degradation of plant cell walls (4–7).
Some CWDEs exhibit transglycosylation activity, namely the transfer of substrate to another molecule, as well as hydrolytic activity. β-Glucosidases release glucose from β-glucans and simultaneously catalyze transglycosylation reactions to generate reaction products with higher molecular weights than the starting substrate and with various glycosidic bonds (8, 9).
The transglycosylation reaction can be used to produce cellulose using β-cellobiosyl and β-lactosyl fluorides (10, 11). An amino acid alteration in the hydrolytic enzyme, Cel7B from Humicola insolens, enhanced polymerization by the transglycosylation reaction (12, 13). Trichoderma reesei enzyme (TrCel12A), a member of glycoside hydrolase family (GH) 12, catalyzed a transglycosylation reaction (14, 15); however, this enzyme degraded the substrate into glucose, cellobiose, and cellotriose after long incubation times (supplemental Fig. S1). Endoglucanase I from T. reesei produced cellulose by a transglycosylation reaction from cellotriose, but cellulose likely disappears after long incubations (16). The current hypothesis is that the produced cellulose was digested into water-soluble oligosaccharides. Thus, these enzymes possess transglycosylation activity but appear to ultimately degrade β-glucans.
The involvement of CWDEs of plant pathogens in the infection of host plants has been investigated. Enhanced activity of 1,3–1,4-glucanase (MoCel12A) caused increased spore formation of Magnaporthe oryzae (4). Deletion of genes encoding polygalacturonases in Botrytis cinerea reduced its virulence on tomato (17, 18). Disruption of multigenes encoding GH6 and GH7 by RNAi gene silencing resulted in reduced virulence of M. oryzae (19). Therefore, the activities of CWDEs are believed to be involved in pathogen infection. However, the effects of CWDEs from pathogens on changes in cell wall structure and physical properties of the wall remain unresolved.
Plant cell walls are formed by composite polymers that provide rigidity and flexibility. The structure maintains cell shape and functions as a physical barrier to avoid pathogen invasion. On the other hand, cell walls of growing plant cells are loosened by cleaving the tethers formed between cellulose microfibrils through the actions of plant-derived proteins when cell enlargement occurs (20–22). Similarly, pathogen-derived enzymes may function to loosen plant cell walls by cleaving the tethers when pathogens start their invasion.
M. oryzae is a pathogen that causes rice blast, the most devastating disease of rice. M. oryzae is thought to secret a variety of CWDEs during infection. The mixed-linkage 1,3–1,4-β-glucan is an important hemicellulosic polymer in Poaceous plants, including rice, that provides wall strength by forming tethers between cellulose microfibrils (23). The synthesis of 1,3–1,4-β-glucan was observed in rice seedlings (24). Cleavage of the tethers is thought to cause wall loosening and subsequently wall strength is decreased during cell growth. Similarly, M. oryzae is proposed to produce enzymes to degrade 1,3–1,4-β-glucan tethers to enhance hyphal penetration. In the present study, we identify and characterize a M. oryzae endotransglucosylase (ETG), a member of the GH7 family (MoCel7B), that cleaves β-1,4-glucans as donor substrates and transfers the cleaved glucans to other molecules as acceptor substrates. Notably, the endotransglucosylation reaction of ETG, which enhances 1,3–1,4-β-glucan depolymerization in the presence of glucose and the increased extension of inactivated wheat coleoptiles, is demonstrated in detail. Furthermore, evidence for in vitro cellulose synthesis by the endotransglucosylation reaction is presented. These findings provide significant information about 1,3–1,4-β-glucan degradation during infection of M. oryzae and the utilization of the enzymatic reaction for efficient cellulose synthesis.
EXPERIMENTAL PROCEDURES
Materials
Polysaccharides tested as substrates included: cellulose (Sigmacell 20, Sigma), phosphoric acid-swollen cellulose made from Sigmacell 20, barley 1,3–1,4-β-glucan (Megazyme), laminarin (Sigma), glucomannan from konjac (Megazyme), mannan from Saccharomyces cerevisiae (Sigma), and tamarind xyloglucan (Megazyme).
Strains, Media, Culture Growth Conditions, and M. oryzae Inoculation
M. oryzae (Strain; Ina72) mycelia were cultured in YG medium (0.5% (w/v) yeast extract and 2% (w/v) glucose) for 4 days at 25 °C with gyratory shaking at 130 rpm. M. oryzae spores that formed on oatmeal agar plates after incubation at 23 °C for 5 days under dark-blue light were suspended in ddH2O (3 × 105 spores/ml) and sprayed on rice (Oryza sativa) leaves of cultivar “Shin-2” at the four- to five-leaf stage.
Purification and LC/MS/MS of 1,3–1,4-β-Glucan-degrading Enzyme
A culture filtrate of M. oryzae grown in YG medium for 4 days was concentrated and desalted by ultrafiltration (10 kDa cut-off) and subjected to 65% (w/v) anmonium sulfate precipitation. The precipitate was equilibrated with 10 mm sodium phosphate buffer (pH 7.0) and sequentially fractionated on a strong anion-exchange column (MonoQ, GE Healthcare), a gel permeation column (Superdex 75 10/300 GL, GE Healthcare), and a second anion-exchange column (MonoQ) as described in supplemental Methods. Proteins were subjected to SDS-PAGE followed by Coomassie Brilliant Blue staining. Protein bands from the second MonoQ separation were digested with trypsin and analyzed by liquid chromatography-tandem mass spectrometry (LC/MS/MS) (supplemental Methods).
PCR and Cloning of the 1,3–1,4-β-Glucan-degrading Enzyme
A cDNA pool was synthesized using Superscript III reverse transcriptase (Invitrogen) and oligo(dT20) primer from total RNA of M. oryzae-infected rice leaves using a Plant RNA Extraction kit (Qiagen, Hilden, Germany). Cloning of the Mocel7B gene was performed by PCR using PrimeStar GXL DNA polymerase (Takara Bio), forward primer 1, 5′-ATGCCATCCTCGCATCCGAGGCACAC-3′ and reverse primer 1, 5′-CAGCCGCCTAGCACCGCCCTGGAAAG-3′.
Gene Expression Analysis in Rice Leaves Infected with M. oryzae
Rice leaves at 4 days postinoculation with M. oryzae were used for gene expression analysis by SuperSAGE as described previously (25). Double-stranded cDNAs were synthesized from total RNA using a Superscript double-stranded cDNA synthesis kit (Invitrogen). The resulting 26-mer oligonucleotides were sequenced by a DNA sequencer (Genome Analyzer IIx, Illumina, San Diego, CA). Obtained DNA sequences were analyzed sequentially by blastx searches based on the M. oryzae genome sequence and the blastp program at the National Center for Biotechnology Information (ncbi.nlm.nih.gov/).
Preparation of Recombinant Enzyme
Overexpression of a recombinant MoCel7B fused with seven contiguous histidine residues at the carboxyl terminus in M. oryzae, purification using a polyhistidine-binding resin (TALON metal affinity resin, Clontech), and immunoblotting using a horseradish peroxidase-conjugated monoclonal antibody against the histidine tag (Qiagen) were conducted as described previously (4). Protein concentration was determined using a Bradford protein assay kit (ThermoScientific) with BSA (Sigma) as a standard.
Assay for Enzymatic Activities
Increases in reducing power were assayed using p-hydroxybenzoic hydrazide-HCl (PAHBAH method, Ref. 26) after reaction mixtures (60 μl) containing substrate (0.5% (w/v) for polysaccharides, and 5 mm for oligosaccharides), 50 mm sodium phosphate buffer (pH 6.0), and purified recombinant enzyme (0.01–0.05 μg) were incubated at 40 °C for 20 min. A unit of activity was defined as the amount of enzyme required to produce 1 μmol of reducing power equivalent to glucose in 1 min. Viscometric changes were assayed periodically in 1-ml reaction mixtures containing 1,3–1,4-β-glucan (2%, w/v), 50 mm sodium phosphate buffer (pH 6.0), and recombinant enzyme (1 μg) after incubation at 40 °C in a semi-microviscometer (Kusano Science, Tokyo, Japan). Molecular weight distributions of 1,3–1,4-β-glucan after incubation with the enzyme for 18 h at 40 °C was determined by gel permeation chromatography on Sepharose CL-6B followed by staining with 0.5% (w/v) anthrone in H2SO4 (27). Reaction products generated from oligosaccharides were analyzed by an HPLC (Dionex ICS-3000) equipped with an anion-exchange column (Carbopak PA-1, 4 × 250 mm, Dionex) eluted with a linear gradient of sodium acetate (0–200 mm) for 20 min in the presence of 100 mm NaOH at a flow rate of 0.5 ml/min. For assaying acceptor substrate specificity of the enzyme, the reaction products were developed by thin layer chromatography (TLC) on silica gel plates (60 F254, Merck) in butan-1-ol:acetic acid:water (2:1:1, v/v) and stained with 0.5% (w/v) thymol in EtOH:H2SO4 (19:1, v/v).
Assay for Transfers to Sulforhodamine Conjugates
Sulforhodamine-conjugated cellotetraose (SR-C4) was synthesized using cellotetraose and lissamine rhodamine B sulfonyl chloride (Roche Applied Science) as described previously (28). SR-C4 was digested with T. reesei endoglucanase and separated by paper chromatography in butan-1-ol:acetic acid:water (2:1:1). The fluorescent spot corresponding to sulforhodamine-conjugated cellobiose (SR-C2) was excised and dissolved in water. A mixture (20 μl) containing recombinant enzyme (0.02 μg), SR-C2 (2.5 mm), polysaccharide (0.1%, w/v) or cellooligosaccharide (5 mm) and 50 mm sodium phosphate buffer (pH 6.0) was incubated at 40 °C and the reaction products were developed by TLC in butan-1-ol:acetic acid:water (2:1:1, v/v). Fluorescent spots were observed under UV light (254 nm) in a Luminescent Image Analyzer LAS-4000 (Fujifilm, Tokyo, Japan). The fluorescence intensity of liquids was measured at an excitation wavelength of 564 nm and an emission wavelength of 612 nm in a fluorimetric plate reader (SpectraMax Gemini XS, Molecular Devices, CA).
Extension Assay
Wheat (Triticum aestivum L. cv. Nambu) was grown in soil for 4 days at 25 °C in the dark. Coleoptiles were excised, abraded with carborundum, inactivated in boiling water for 1 min, and 10 specimens were incubated in 600 μl of 50 mm sodium phosphate buffer (pH 6.0) containing recombinant enzyme (5.0 μg) and 300 mm glucose or BSA (5.0 μg) as a control. Specimens were fixed between two clamps about 5 mm apart and loaded at a linear increasing tension of 500 mN/min for 36 s in an extensometer (TMA/SS6000, Seiko Instruments, Tokyo, Japan). The length was automatically recorded per second. Strain was calculated by (Lt − L0)/L0 (Lt, length of coleoptiles at each time points; L0, length of coleoptiles at time 0).
Cellulose Synthesis by ETG
Reaction mixtures containing 5 mm cellohexaose, recombinant enzyme (0.5 μg), and 50 mm sodium phosphate buffer (pH 6.0) with or without 50 mm glucose were incubated at 40 °C. The mixtures were divided into soluble and insoluble fractions by centrifugation at 22,000 × g for 5 min. The amount of insoluble material was measured using 0.5% (w/v) anthrone in H2SO4 after the insoluble material was dissolved in ice-cold 72% (v/v) H2SO4.
Electron Microscopy and Electron Diffraction
Electron microscopic observations and electron diffraction of insoluble material (synthesized cellulose) were performed as described previously (29). The insoluble product was applied to a carbon-coated copper grid that was glow-discharged before use. The sample grid was stained by 2% (w/v) uranyl acetate for morphological observation by negative staining, and dried without any post-treatment for electron diffraction. An electron microscope (JEM-2000EX II, Jeol Co. Ltd., Tokyo, Japan) was operated at 100 kV in both modes of bright-field observation and diffraction. Images of negatively stained sample were taken by a side-mounted CCD camera (Megaview G2, Olympus Soft Imaging Solutions GmbH). Electron diffraction was carried out with a 20-μm condenser lens aperture inserted, and the first condenser lens was excited maximally (spot size 8). Diffraction diagrams were recorded on MEM film (Mitsubishi Paper Milling, Tokyo, Japan) and developed with Correctol (Fujifilm, Tokyo, Japan). The diffractograms were digitized with a MegaPlus camera (Eastman Kodak) and analyzed with ImageJ to calculate d-spacings from Debye rings; 0.2355 nm for the (111) plane of Au was used as a reference for calibration.
MALDI-TOF MS of Synthesized Cellulose II
Insoluble material was suspended in a mixture of dimethyl sulfoxide and N,N-dimethylformamide (1:1, v/v).
After matrix solution (0.5 μl), a mixture (10:1, v/v) of 1% (w/v) 2,5-dihydroxybenzoic acid (DHB) in 40% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid and 0.2% (w/v) sodium trifluoroacetate in the same solvent was applied onto a ground steel target plate and air-dried. The sample preparation (0.5 μl) was dried by hot air and then the matrix solution (0.5 μl) was applied and dried at room temperature. The mixture (analyte) was applied to an Autoflex III instrument (Bruker Daltonics, Bremen, Germany) equipped with a smartbeam laser in reflector positive ion mode. The acceleration voltage and reflector mirror were adjusted to 19 and 21 kV, respectively. Peptide calibration standard II (Bruker Daltonics, Bremen, Germany) was used for calibrating the standard curve.
RESULTS
Identification of a Protein Acting on 1,3–1,4-β-Glucan Degradation and Transglycosylation
Results from analyses of 1,3–1,4-β-glucan degrading activity and cellooligosaccharide transglycosylation activity using column fractionated samples of M. oryzae culture filtrate suggested an occurrence of an ETG reaction that catalyzes endo-typical cleavage and transfer of β-glucans (supplemental Fig. S2). To identify the M. oryzae ETG, fractions 20–22 from the last anion-exchange column chromatography were subjected to SDS-PAGE followed by Coomassie Brilliant Blue staining (Fig. 1). Fifteen protein bands from the active fractions were excised, digested with trypsin, and analyzed by LC/MS/MS. Major proteins in each band were laccase-2 (number 1), para-nitrobenzyl esterase (number 2), pepsin (number 3), endoglucanase (number 4), minor extracellular protease vpr (number 5), laccase (number 6), peroxidase superfamily protein (number 7), aminopeptidase Y (number 8), endoglucanase (number 9), cell surface spherulin 4 superfamily protein (number 10), GAT1 superfamily protein (number 11), ThiJ/PfpI family protein (number 12), extracellular protease vpr (number 13), protein with no candidate (number 14), and aminopeptidase Y (number 15). Endoglucanase detected in protein band numbers 4 and 9 was found to belong to the GH7 family (MoCel7B). No other proteins are likely to be involved in plant cell wall degradation. These results suggest that the enzyme is a key protein that degrades 1,3–1,4-β-glucan during M. oryzae culture.
FIGURE 1.

Fractionation of 1,3–1,4-β-glucan degrading activity. Fractions numbers 18–23 including active fraction numbers 18–20 from the last anion-exchange column were subjected to SDS-PAGE followed by staining with Coomassie Brilliant Blue. Protein bands indicated with arrowheads were digested with trypsin, and the resulting peptides were analyzed by LC/MS/MS.
Enzymatic Properties of the Recombinant Enzyme
A Mocel7B gene was cloned from a M. oryzae cDNA pool, fused with a heptahistidine-tagged DNA sequence, and overexpressed in M. oryzae. The recombinant MoCel7B was purified by histidine tag affinity chromatography (supplemental Fig. S3) and used in the analyses of enzymatic properties. When polymer degrading activity was assayed by measuring increases in reducing power, the enzyme exhibited high activity toward 1,3–1,4-β-glucan, glucomannan, and carboxymethyl cellulose (CMC), but had low activity toward xyloglucan, cellulose, and phosphoric acid-swollen cellulose (Table 1). No activity was observed toward β-1,3-linked laminarin and mannan. β-1,4-Glucosyl linkages of 1,3–1,4-β-glucan and glucomannan could be cleaved by the enzyme. These results suggest that the recombinant enzyme preferentially cleaves water-soluble glucans with β-1,4-linkages but without substitutions. The activity toward 1,3–1,4-β-glucan reached a maximal level at pH 5.5–6.0 adjusted with sodium phosphate (supplemental Fig. S4A). The activity increased at temperatures up to 50 °C but rapidly decreased at 60 °C (supplemental Fig. S4B).
TABLE 1.
Polysaccharide degrading activity of the recombinant enzyme encoded by Mocel7B
The reaction mixture (60 μl) containing recombinant enzyme (0.05 μg) and polysaccharides (0.3 mg) or cellulose (3.0 mg) in 50 mm sodium phosphate buffer (pH 6.0) was incubated at 40 °C for 20 min. Hydrolytic activity was determined by measuring the increase in reducing power. Data are the mean ± S.E. of three determinations.
| Substrate | Specific activity | Relative activity |
|---|---|---|
| units/mg | % | |
| 1,3–1,4-β-Glucan | 4.20 ± 0.04 | 100.0 |
| Glucomannan | 0.86 ± 0.07 | 20.5 |
| Mannan | NDa | |
| CMC | 0.72 ± 0.05 | 17.1 |
| Xyloglucan | 0.01 ± 0.01 | <1 |
| Laminarin | ND | |
| Cellulose | 0.02 ± 0.01 | <1 |
| PSCb | 0.14 ± 0.03 | 3.3 |
a ND, values were not determined due to low activity.
b PSC, phosphoric acid-swollen cellulose.
Transglucosylation of Cellooligosaccharides
To explore the detailed reaction mechanism of the recombinant enzyme encoded by Mocel7B, the reaction products generated from cellooligosaccharides were analyzed by HPLC (Fig. 2). The main products were glucose and cellopentaose from cellotriose, cellobiose and cellohexaose from cellotetraose, cellotriose and predicted celloheptaose from cellopentaose, and cellotetraose and predicted cellooctaose from cellohexaose. Thus, reaction products with both lower and higher molecular weights than the starting substrates were generated by the reaction with the recombinant enzyme. In contrast, no reaction products were detected when cellobiose or glucose was used as a substrate (data not shown).
FIGURE 2.
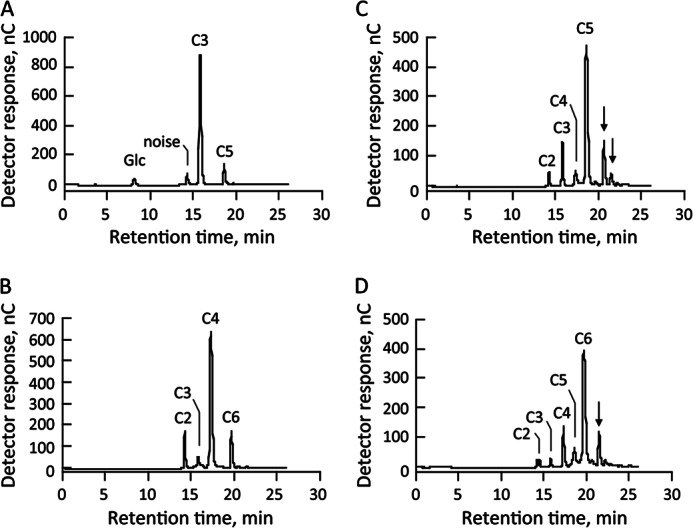
Transglucosylation of cellooligosaccharides by recombinant enzyme. Cellooligosaccharides (A, cellotriose; B, cellotetraose; C, cellopentaose; D, cellohexaose) at final concentrations of 5 mm were independently incubated with recombinant enzyme (0.02 μg) in 50 mm sodium phosphate buffer (pH 6.0) at 40 °C for 20 min. A portion of the reaction mixture was subjected to HPLC analysis. Arrows indicate unidentified reaction products.
Production of cellopentaose and cellobiose from cellotriose provided at concentrations ranging from 0.312 to 10 mm was investigated to determine the primary reaction products in detail. Cellopentaose was generated at all substrate concentrations, and the amount of cellopentaose produced increased as the substrate concentration increased up to 5 mm (Fig. 3A). These results suggest that cellotriose is cleaved into cellobiose and glucose, and then the generated cellobiose is transferred to cellotriose, resulting in the production of cellopentaose. In contrast, little cellobiose was produced in any of the reaction conditions (Fig. 3B). If cellotriose was hydrolyzed by the enzyme, more cellobiose should be produced. Therefore, these results indicate the occurrence of a transglucosylation reaction by recombinant enzyme encoded by Mocel7B that cleaves cellooligosaccharide as a donor substrate and transfers the newly generated cellobiose unit to another molecule as an acceptor substrate.
FIGURE 3.
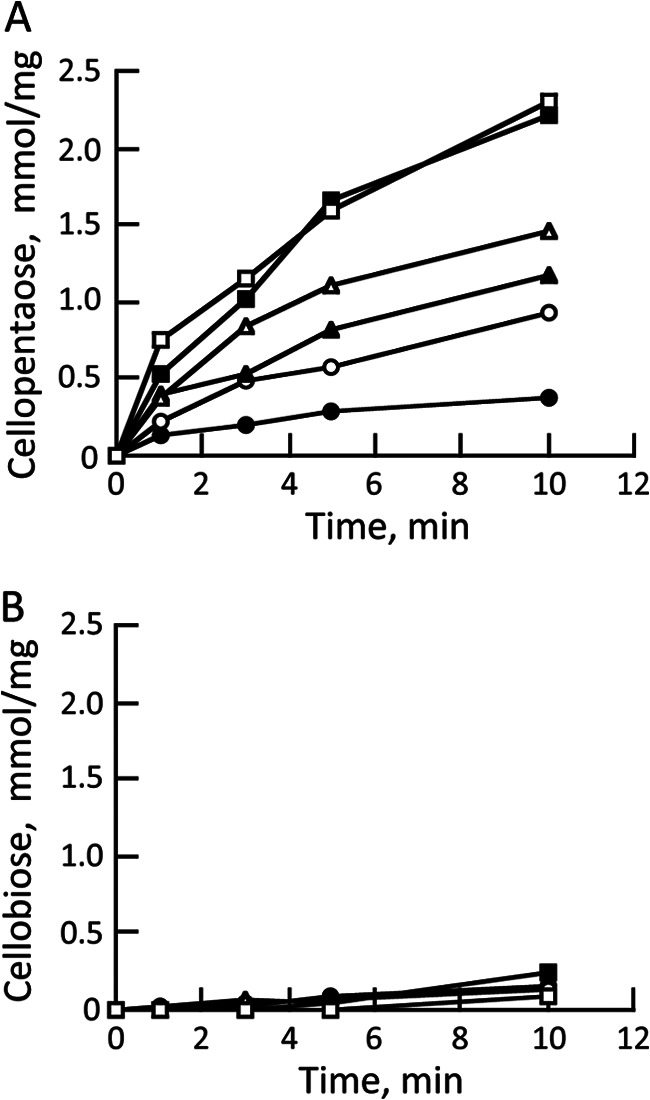
Accumulation of cellopentaose and cellobiose from cellotriose. Cellotriose at a final concentration of 0.312 mm (closed circles), 0.625 mm (open circles), 1.25 mm (closed triangles), 2.5 mm (open triangles), 5 mm (closed squares), 10 mm (open squares) was incubated with recombinant enzyme (0.02 μg) in 50 mm sodium phosphate buffer (pH 6.0) at 40 °C for 10 min. Reaction products from cellotriose were detected by HPLC, and the amounts of cellopentaose (A) and cellobiose (B) were calculated based on calibration curves of the corresponding cellooligosaccharides.
Acceptor Substrate Specificity
Disaccharides and oligosaccharides were tested as acceptor substrates for recombinant enzyme encoded by Mocel7B in reactions where cellotriose was used as the donor substrate; when testing glucose as an acceptor substrate, cellotetraose was used as the donor substrate. When cellotriose only was incubated with the enzyme, cellopentaose and glucose were synthesized by a transglucosylation reaction. The addition of cellobiose, laminaribiose, laminaritriose gentiobiose, and xyloglucan nonasaccharide to the reaction mixture containing cellotriose generated additional product spots on TLC plates (Fig. 4). Furthermore, a faint spot was also observed when xylobiose or mannobiose was added; however, neither xylose nor mannose served as an acceptor substrate (data not shown). These results indicate that the enzyme could transfer the generated cellobiose unit to not only β-1,4-linked glucose but also to β-1,3- and β-1,6-linked glucose and disaccharides containing xylose and mannose residues. When cellotetraose only was incubated with the enzymes, cellohexaose and cellobiose were synthesized. The addition of glucose to the reaction mixture containing cellotetraose generated an additional spot corresponding to cellotriose was detected. This implies that cellobiose generated from cellotetraose is transferred to glucose by the transglycosylation reaction. Thus, a variety of oligosaccharides and glucose were recognized as acceptor substrates for the enzyme encoded by Mocel7B.
FIGURE 4.

Acceptor substrate specificity of recombinant enzyme. Transfer to oligosaccharides (cellobiose (C2), laminaribiose (L2), laminaritriose (L3), gentiobiose (G2), xylobiose (X2), mannobiose (M2), and xyloglucan nonasaccharide (XLLG) was examined using cellotriose as the donor substrate. To assay the transfer to glucose, cellotetraose was used as the donor substrate. The products were separated on TLC plates and stained with thymol in H2SO4:EtOH.
Enhanced Depolymerization by Endotransglucosylation
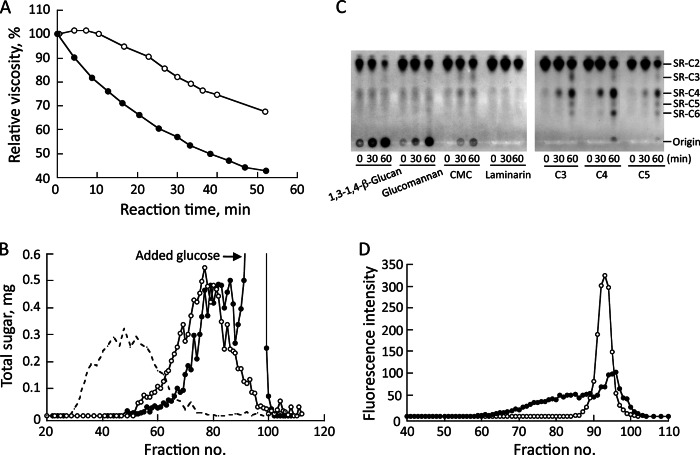
Changes in the viscosity of 1,3–1,4-β-glucan by the enzyme encoded by Mocel7B were examined in the presence or absence of glucose. The viscosity of 1,3–1,4-β-glucan decreased slightly after incubation with recombinant enzyme in the absence of glucose, probably because of low hydrolytic activity as shown in Fig. 3, but rapidly decreased in the presence of glucose (Fig. 5A). The molecular weight distribution of 1,3–1,4-β-glucan in the absence of glucose after incubation with the enzyme was shifted to a lower molecular weight by gel permeation chromatography on Sepharose CL-6B (Fig. 5B). Furthermore, the addition of glucose shifted the distribution lower than that without glucose, leading to enhancement of depolymerization of 1,3–1,4-β-glucan. When SR-C2 and cellooligosaccharide (cellotriose (C3), cellotetraose (C4), or cellopentaose (C5)) were used as substrates, SR-C4 was observed after the first 30-min incubation, and then products with larger molecular weights were detected after a 60-min incubation, indicating transfer of generated cellobiose from cellooligosaccharides to SR-C2. When SR-C2 and 1,3–1,4-β-glucan, glucomannan, or CMC were used as the substrates, SR-labeled products were detected at the origin of the TLC plate (Fig. 5C). However, no product was detected when laminarin was used as the substrate, which is identical to the result shown in Table 1. If disaccharides including cellobiose were generated from polysaccharides and transferred to SR-C2 by the action of the enzyme, SR-C4 or reaction products immobilized at the origin would be observed. However, significant SR-labeled products from polysaccharides were only observed at the origin of the TLC plate. These results suggest that the enzyme cleaves polysaccharides randomly and transfers glucans to an acceptor substrate, unlike the case of cellooligosaccharides that are cleaved and the generated cellobiose is transferred. These results indicate that the enzyme encoded by Mocel7B endo-typically cleaves polysaccharides and transfers glucans to another molecules, and the reaction is accelerated by the presence of glucose that can be utilized as an acceptor substrate.
FIGURE 5.
Enhanced degradation of 1,3–1,4-β-glucan by the addition of glucose. The effect of glucose on 1,3–1,4-β-glucan degradation by recombinant enzyme was assayed by measuring viscometric changes (A) and by the molecular weight distribution of 1,3–1,4-β-glucan on Sepharose CL-6B (B) in the presence (closed circles) or absence (open circles) of glucose. The molecular weight distribution of 1,3–1,4-β-glucan used as the substrate is indicated with a broken line. To analyze transglucosylation products, reaction mixtures containing SR-C2, 100 mm sodium phosphate (pH 6.0), 5 mm cellooligosaccharide (cellotriose (C3), cellotetraose (C4), and cellopentaose (C5)), or 0.5% (w/v) polysaccharide and recombinant enzyme (0.1 μg) were incubated for 0, 30, and 60 min at 40 °C and developed by TLC in butan-1-ol:acetic acid:water (2:1:1, v/v) (C). SR-C2 (open circles) and SR-labeled 1,3–1,4-β-glucan produced by the transglycosylation reaction (closed circles) were fractionated on Sepharose CL-6B equilibrated with 50 mm sodium phosphate buffer (pH 6.0) containing 0.1 m NaCl (D).
Furthermore, SR-labeled substances generated from 1,3–1,4-β-glucan were confirmed to be larger than SR-labeled celooligosaccharides by gel permeation chromatography on Sepharose CL-6B (Fig. 5D). These results indicate the incorporation of SR-C2 into 1,3–1,4-β-glucan and glucomannan by the enzyme. From this evidence, the enzyme encoded by Mocel7B is considered to be an ETG, hereafter referred to as ETG, that catalyzes endo-acting cleavage for β-1,4-glucan polymers as donor substrates and transfers the cleaved glucan to another molecule as an acceptor substrate, thereby reducing the molecular size of the β-glucan substrates.
Extension Measurement of Wheat Coleoptiles Treated with ETG
Wheat coleoptile specimens were loaded at a linear increasing tension of 500 mN/min for 36 s in an extensometer after incubation with ETG in the presence of glucose. The length was measured automatically per second and strain values were calculated (Fig. 6). Strain measurements of coleoptiles treated with ETG and the control increased similarly until the force attained was at about 100 mN; thereafter, differences in strain between coleoptiles treated with ETG and the control were observed. At 300 mN force, the strain of coleoptiles treated with ETG was about 1.5-fold higher than that of the control. Thus, pre-treatment with ETG increased the strain, suggesting that cleavage of wheat coleoptile cell wall components by ETG caused a decrease in wall strength followed by an increase in coleoptile extension.
FIGURE 6.
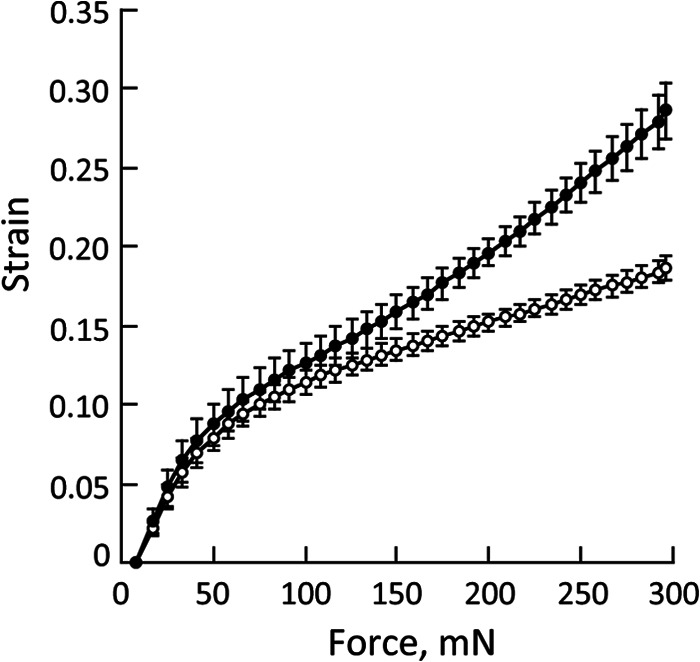
Extension measurement of inactivated wheat coleoptiles after treatment with recombinant enzyme in the presence of glucose (closed circles) or with BSA as a control (open circles). The length of specimens was measured until a force reached at 300 mN. Strain was calculated by (Lt − L0)/L0 (Lt; length of coleoptiles at each time points, L0; length of coleoptiles at time 0). Data are the mean ± S.E. of independent 5 determinations.
Analyses of Transcript Levels
Rice leaves at 4 days after inoculation with M. oryzae were used to analyze the expressed genes. About 72,000 26-mer oligonucleotide sequences derived from M. oryzae were detected, in which 32 genes composed of 919 tag sequences were proposed to be involved in plant cell wall polysaccharide degradation (Table 2). Xylanases, endo-β-glucanase, galactanase, and mannanase hydrolyze hemicellulosic and pectic polysaccharides of plant cell walls. Proteins responsible for producing monosaccharides such as β-glucosidases and β-xylosidases were also expressed highly. Notably, β-glucosidases may supply glucose as an acceptor substrate for ETG. ETG encoded by the Mocel7B gene, initially identified from the extracellular proteins of liquid culture filtrates of M. oryzae mycelia, also was found to be expressed highly during M. oryzae infection of rice leaves. These results suggest that a variety of enzymes might act cooperatively to degrade wall polysaccharides that would, therefore, enhance invasion and acquisition of carbon sources.
TABLE 2.
Gene expression of the M. oryzae glucoside hydrolases
Rice leaves at 4 days postinoculation with M. oryzae were used for analyzing the expressed genes.
| Accession No. | Classification | Annotation | Tag count |
|---|---|---|---|
| XM_003719371 | 53 | Endo-1,4-β-galactanase | 628 |
| XM_00371016 | 61 | Copper-dependent polysaccharide monooxygenase | 142 |
| XM_003716363 | 3 | β-Glucosidase | 26 |
| XM_003717744 | 16 | Endo-1,3 (4)-β-glucanase/endo-1,4-β-galactosidase | 21 |
| Endo-1,3-β-galactosidase | |||
| XM_003714958 | 18 | Chitinase | 20 |
| XM_003711188 | 43 | β-Xylosidase/α-arabinofuranosidase/arabinanase xylanase | 17 |
| XM_370166 | 7 | Endo-1,4-β-glucanase | 11 |
| XM_003709059 | 88 | Glycoside hydrolase | 9 |
| XM_003721226 | 7 | Endo-1,4-β-glucanase (Mocel7B) | 6 |
| XM_003710858 | 2 | β-galactosidase | 5 |
| XM_370079 | 18 | Chitinase | 4 |
| XM_003713152 | 11 | Endo-xylanase | 4 |
| XM_003717827 | 97 | α-Glucosidase | 3 |
| XM_003720297 | Not classified | 3 | |
| XM_003718423 | 43 | β-Xylosidase | 3 |
| XM_003713152 | 11 | Endo-xylanase | 2 |
| XM_003712107 | 32 | Invertase | 2 |
| XM_003708980 | Not classified | 2 | |
| XM_003720117 | 61 | Copper-dependent polysaccharide monooxygenase | 2 |
| XM_001411258 | 61 | Copper-dependent polysaccharide monooxygenase | 2 |
| XM_003715772 | 18 | Chitinase | 2 |
| XM_003708976 | 61 | Copper-dependent polysaccharide monooxygenase | 1 |
| XM_003710734 | 76 | α-1,6-Mannanase | 1 |
| XM_003714468 | 10 | Endo-xylanase | 1 |
| XM_003716053 | Not classified | 1 | |
| XM_003715686 | 11 | Endo-xylanase | 1 |
Cellulose Synthesis from Cellohexaose by the ETG Reaction
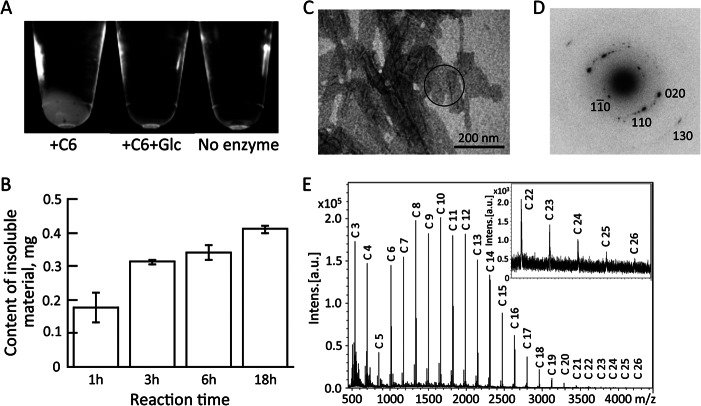
We have documented that cellooligosaccharides, 1,3–1,4-β-glucan, and glucomannan were cleaved and transferred to another molecule by the action of ETG; however, when cellohexaose was incubated with ETG, a water-insoluble substance appeared (Fig. 7A). Occurrence of the insoluble substance was also observed when cellotetraose or cellopentaose was used as substrate (data not shown). The addition of glucose to the reaction mixture prevented the generation of the insoluble substance, suggesting transfer to glucose as an acceptor substrate interferes with production of the insoluble substance. The amount of the insoluble substance increased as the incubation time increased and did not disappear after long incubation (Fig. 7B). Electron microscopic observation of the insoluble substance showed lamellar crystals (Fig. 7C). The electron diffraction diagram of the lamellar crystal revealed four pairs of diffractions with 0.722, 0.445, 0.404, and 0.221 nm d-spacings, which are indexed as 1 [overbar]1 0, 110, 020, 130 of cellulose II crystal, respectively (Fig. 7D). This pattern is interpreted as the hk0 pattern of the cellulose II crystal, indicating that the lamellar crystal is cellulose II in which cellulose chains are packed perpendicularly to the basal plane of the lamellae. The degree of polymerization of this synthesized cellulose II was analyzed by MALDI-TOF MS (Fig. 7E). The mass spectra revealed a dehydrated glucose series with an interval of m/z 162 as sodium ion adducts. The majority of the signals represented glucans with degree of polymerizations of 8–14 that could be synthesized by one to four repeated transfers of cellobiose. In addition, signals were detected maximally at m/z 4255.58, corresponding to a glucan with a degree of polymerization of 26 with a sodium ion.
FIGURE 7.
Cellulose synthesis by recombinant enzyme. Cellohexaose was incubated with recombinant enzyme (0.5 μg) in 50 mm sodium phosphate buffer (pH 6.0) in the presence or absence of glucose at 40 °C. The mixtures were centrifuged and photographed (A). Insoluble material was dissolved in 72% (w/v) H2SO4, and the sugar content was determined using anthrone in H2SO4 (B). Water-insoluble material was observed by electron microscopy with negative staining (C) and the lamellar structure was subjected to electron diffraction analysis (D). Molecular mass of the sodium ion adducts of the water-insoluble substance was determined by MALDI-TOF MS (E).
DISCUSSION
Enzymatic degradation of plant cell wall polysaccharides by CWDEs of plant pathogens simultaneously enhances pathogen invasion into host plants and produces glucose as a carbon source to promote the infection. The M. oryzae ETG encoded by the Mocel7B gene was identified as a protein involved in 1,3–1,4-β-glucan degradation and transglycosylation by partial purification followed by LC/MS/MS analysis. In addition, the Mocel7B gene was found to be expressed during M. oryzae infection of rice (Table 2). Thus, ETG appears to function significantly in 1,3–1,4-β-glucan degradation during M. oryzae growth.
The deduced amino acid sequence of M. oryzae ETG is identical to the Magnaporthe grisea GH7 enzyme (MgEGL). MgEGL is proposed to be an endo-acting hydrolytic enzyme toward CMC (30). A phylogenetic tree generated by ClustalW shows that ETG is a member of the endohydrolase class of GH7 enzymes (supplemental Fig. S5). However, detailed enzymatic properties and the effects of this enzyme on plant cell walls were unknown. ETG, produced by homologous overexpression and purified by histidine-tag affinity chromatography, exhibited high degrading activity toward 1,3–1,4-β-glucan, glucomannan, and CMC, but low activity toward water-insoluble β-glucans such as crystalline cellulose and phosphoric acid-swollen cellulose (Table 1). When cellooligosaccharides were used as substrates for ETG, reaction products with both lower and higher molecular weights than the starting substrates were detected. These findings imply that ETG catalyzes exo-acting cleavage of oligosaccharides and transfer of the newly generated reducing end of cellobiose to the non-reducing end of another molecule (Fig. 8A).
FIGURE 8.
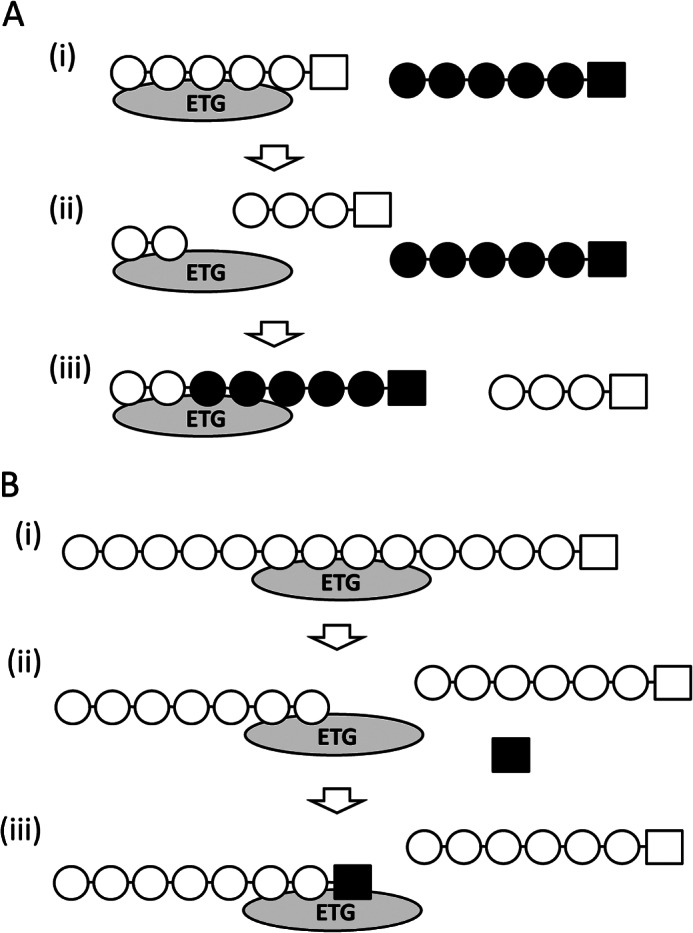
Proposed action of ETG in depolymerization and synthesis of β-glucan. Schematic diagrams of the ETG reaction causing polymerization of oligosaccharides (A) and depolymerization of a polysaccharide (B).
Cellooligosaccharides with a degree of polymerization greater than 3 can be recognized as donor and acceptor substrates, and glucose and cellobiose serve only as acceptor substrates. If a substrate greater than 3 glucose units was supplied, a reaction product theoretically forms a longer chain than the supplied substrate. However, transfer to glucose does not cause an increase in molecular size of the reaction product. Interestingly, laminaribiose, laminaritriose, and gentiobiose that consist of glucose molecules joined by β-1,3 and β-1,6 linkages, respectively, also are good acceptor substrates (Fig. 4). Xylobiose and mannobiose, disaccharides composed of xylose and mannose, respectively, can be acceptor substrates for ETG although neither xylose nor mannose residues can serve as acceptor substrates (data not shown). This action facilitates the production of a hetero-polymer that might share the individual properties.
The transglycosylation reaction of celloligosaccharides has been studied for endoglucanases belonging to the GH7 family from T. reesei, Trichoderma viride, and Humicola insolense (12, 31, 32). In plants, xyloglucan endotransglucosylases, members of the GH16 family, catalyze the cleavage of xyloglucan and the transfer to another xyloglucan molecule (33, 34). Subsequently, hetero-transglucosylation reactions by xyloglucan endotransglucosylases from barley and horsetails were documented in which polymers such as hydroxyethyl cellulose, acid-swollen cellulose, and 1,3–1,4-β-glucan were cleaved and transferred to xyloglucan oligosaccharide derivatives (35, 36). To our knowledge, the recognition of glucose, laminarioligosaccharides, xylobiose, and mannobiose as acceptor substrates by ETG, and the use of 1,3–1,4-β-glucan and glucomannan as donor substrates is a novel hetero-transglucosylation reaction catalyzed by a fungal enzyme and resulting in the production of hetero-carbohydrates.
Degradation of 1,3–1,4-β-glucan was enhanced by the addition of glucose as judged by the viscometric assay and the 1,3–1,4-β-glucan molecular weight distribution after incubation with ETG (Fig. 5, A and B). Therefore, the enhancement of 1,3–1,4-β-glucan degradation by the addition of glucose is proposed to accelerate the enzymatic reaction cycle in which ETG completes one reaction by cleavage of a donor substrate and then transfer of the newly generated reducing end of the glucan to an acceptor substrate as shown in Fig. 8B. Enzymes such as β-glucosidases produce glucose from oligosaccharides and the generated glucose could be utilized as an acceptor substrate for ETG, which could accelerate the endotransglucosylation reaction by ETG. This hypothesis is supported by the fact that gene expression of β-glucosidase was observed when the transcript level of ETG was high during M. oryzae infection. Furthermore, ETG cleaves randomly to generate longer chains from polysaccharides and transfers the glucan to acceptor substrates because SR-labeled products were detected at the origin of TLC plates, whereas SR-C4 was generated by ETG when cellooligosaccharides and SR-C2 are used as substrates (Fig. 5C). Thus, ETG cleaves β-glucans to generate cellobiose from cellooligosaccharides, but does cleave longer chains that are produced by endo-typical cleavage of polysaccharides, and transfers the glucans to other molecules.
The mixed-linkage glucan, 1,3–1,4-β-glucan, is widespread in Poaceous plants and is thought to function as a structural and storage polysaccharide. Most 1,3–1,4-β-glucan is found in expanding cells of organs such as maize and barley coleoptiles where the polymer is thought to act as inter-fibrillar tethers between cellulose microfibrils (37, 38). Cleavage of the tethers by plant-derived enzymes will loosen the wall during cell enlargement, a process supported by the evidence that endoglucanase inhibitor and antibodies against 1,3–1,4-β-glucan inhibited the decrease in 1,3–1,4-β-glucan content as well as auxin-induced elongation of Avena and Zea coleoptiles (39, 40). Rheological analysis of wheat coleoptiles demonstrated increased strain after treatment with ETG in the presence of glucose (Fig. 6). This result can be interpreted by the decrease in physical properties caused by cleavage of 1,3–1,4-β-glucan tethers. An M. oryzae knockdown mutant in genes encoding GH6 and GH7 family enzymes produced fewer lesions and was less able to penetrate and infect fewer cells compared with the parental strain (17). Hence, we propose that M. oryzae secrets ETG to degrade 1,3–1,4-β-glucan in the rice cell wall that could enhance invasion by loosening the wall.
In our experiments, ETG produced a polymerized, water-insoluble substance when, not only cellohexaose, but also cellopentaose and cellotetraose were used as substrates, but not when cellotriose was used (data not shown). Electron microscopic observations showed that the insoluble substance formed lamellar crystals, similar in appearance to the cellulose II produced by cellodextrin phosphorylase (41). The d-spacing values obtained by electron diffraction diagrams were almost identical to the theoretical values for cellulose II. These results let us conclude that the insoluble substance was cellulose II, an anti-parallel oriented cellulose. Furthermore, the maximum chain length detected by MALDI-TOF MS was 26 glucose units. To the contrary, glucose is a good acceptor substrate for ETG whose participation in the reaction enhances depolymerization of 1,3–1,4-β-glucan as shown in Fig. 5. Thus, whether the reaction results in polymerization or depolymerization depends on the acceptor substrates. In vivo, cellooligosaccharides are present but the concentrations are at the nanomolar level in auxin-treated pea stems, as reported previously (42). Furthermore, the content of cellooligosaccharides in the apoplastic fraction of rice leaves was much lower than that of glucose (supplemental Fig. S6). If cellooligosaccharides were accumulated locally in the apoplast, it may be possible to synthesize higher molecular mass molecules by ETG as shown in Fig. 7. However, a much higher concentration of glucose than cellooligosaccharides present in apoplast generally would promote ETG to depolymerize plant cell wall polymers. This is supported by the fact that addition of glucose inhibited cellulose II synthesis from cellohexaose by ETG (Fig. 7A). That is, polysaccharide synthesis could occasionally occur locally, but depolymerization of polysaccharides would occur more frequently.
In vitro cellulose synthesis has been achieved by the transglycosylation reaction of partially purified cellulases using chemically modified β-cellobiosyl and β-lactosyl fluorides (10, 11). The ability of ETG to synthesize cellulose from cellooligosaccharides is a significant benefit, removing the need to chemically modify the substrates. Furthermore, the T. reesei GH7 family enzyme, endoglucanase I, was shown to synthesize cellulose with a maximum length of 16 glucose units in the presence of about 1 m cellotriose that likely disappeared after long incubation (16). In our study, water-insoluble cellulose was generated by ETG in the presence of only 5 mm cellohexaose as a substrate. Synthesizing cellulose using ETG is an effective method even though cellooligosaccharides with more than 4 glucose units are needed as substrates. One more important difference is that the amount of cellulose synthesized by ETG increased by continuing the incubation and did not disappear (Fig. 7B). As ETG barely cleaves crystalline cellulose and phosphoric acid-swollen cellulose as shown in Table 1, newly synthesized cellulose could accumulate unlike T. reesei endoglucanase I. This finding is due to the crystallinity of ETG-generated cellulose being higher than what ETG can degrade, a characteristic that could be predicted from the high average molecular mass of the synthesized cellulose. Thus, the endotransglucosylation reaction by ETG is significantly involved in 1,3–1,4-β-glucan degradation that affects the physical property of plant cell walls and may also be utilized in in vitro cellulose synthesis.
Acknowledgments
We thank Takahisa Hayashi for valuable discussions. We also thank R. Oba, M. Kikuchi, and M. Iwai for technical assistance in growing and transforming M. oryzae and for assaying hydrolytic activity.
This work was supported in part by the Iwate prefecture and Grant 21612009 from the Japanese Society for the Promotion of Science.

This article contains supplemental Figs. S1–S6 and Methods.
- CWDE
- cell wall-degrading enzyme
- GH
- glycoside hydrolase
- ETG
- endotransglucosylase
- N
- newton
- CMC
- carboxymethyl cellulose
- SR
- sulforhodamine.
REFERENCES
- 1. Wood T. M. (1992) Fungal cellulases. Biochem. Soc. Trans. 20, 46–53 [DOI] [PubMed] [Google Scholar]
- 2. Nidetzky B., Steiner W., Hayn M., Claeyssens M. (1994) Cellulose hydrolysis by the cellulases from Trichoderma reesei. A new model for synergistic interaction. Biochem. J. 298, 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henrissat B., Driguez H., Viet C., Schülein M. (1985) Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Nat. Biotech. 3, 722–726 [Google Scholar]
- 4. Takeda T., Takahashi M., Nakanishi-Masuno T., Nakano Y., Saitoh H., Hirabuchi A., Fujisawa S., Terauchi R. (2010) Characterization of endo-1,3–1,4-β-glucanases in GH family 12 from Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 88, 1113–1123 [DOI] [PubMed] [Google Scholar]
- 5. Qin Q., Bergmann C. W., Rose J. K., Saladie M., Kolli V. S., Albersheim P., Darvill A. G., York W. S. (2003) Characterization of a tomato protein that inhibits a xyloglucan-specific endoglucanase. Plant J. 34, 327–338 [DOI] [PubMed] [Google Scholar]
- 6. Kim H., Ahn J. H., Görlach J. M., Caprari C., Scott-Craig J. S., Walton J. D. (2001) Mutational analysis of β-glucanase genes from the plant-pathogenic fungus Cochliobolus carbonum. Mol. Plant Microbe Interact. 14, 1436–1443 [DOI] [PubMed] [Google Scholar]
- 7. Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr., Warren R. A. (1991) Domains in microbial β-1,4-glycanases. Sequence conservation, function and enzyme families. Microbiol. Rev. 55, 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang S., Jiang Z., Yan Q., Zhu H. (2008) Characterization of a thermostable extracellular β-glucosidase with activities of exoglucanase and transglycosylation from Paecilomyces thermophila. J. Agric. Food Chem. 56, 602–608 [DOI] [PubMed] [Google Scholar]
- 9. Pal S., Banik S. P., Ghorai S., Chowdhury S., Khowala S. (2010) Purification and characterization of a thermostable intra-cellular β-glucosidase with transglycosylation properties from filamentous fungus Termitomyces clypeatus. Bioresour. Technol. 101, 2412–2420 [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi S., Kashiwa K., Kawasaki T., Shoda S. (1991) Novel method for polysaccharide synthesis using an enzyme. The first in vitro synthesis of cellulose via a nonbiosynthetic path utilizing cellulase as catalyst. J. Am. Chem. Soc. 113, 3079–3084 [Google Scholar]
- 11. Shoda S., Kawasaki T., Obata K., Kobayashi S. (1993) A facile enzymatic synthesis of cellooligosaccharide derivatives using β-lactosyl fluoride. Carbohydr. Res. 249, 127–137 [DOI] [PubMed] [Google Scholar]
- 12. Kwon I., Ekino K., Oka T., Goto M., Furukawa K. (2002) Effects of amino acid alterations on the transglycosylation reaction of endoglucanase I from Trichoderma viride HK-75. Biosci. Biotechnol. Biochem. 66, 110–116 [DOI] [PubMed] [Google Scholar]
- 13. Fort S., Boyer V., Greffe L., Davies GJ., Moroz O., Christiansen L., Schülein M., Cottaz S., Driguez H. (2000) Highly efficient synthesis of β(1→4)-oligo- and polysaccharides using a mutant cellulase. J. Am. Chem. Soc. 122, 5429–5437 [Google Scholar]
- 14. Hreggvidsson G. O., Kaiste E., Holst O., Eggertsson G., Palsdottir A., Kristjansson J. K. (1996) An extremely thermostable cellulase from the thermophilic eubacterium Rhodothermus marinus. Appl. Environ. Microbiol. 62, 3047–3049 [Google Scholar]
- 15. Bauer M. W., Driskill L. E., Callen W., Snead M. A., Mathur E. J., Kelly R. M. (1999) An endoglucanase, EglA, from the hyperthermophilic archaeon Pyrococcus furiousus hydrolyzes β-1,4 bonds in mixed-linkage (1→3),(1→4)-β-glucans and cellulose. J. Bacteriol. 181, 284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hattori T., Ogata M., Kameshima Y., Totani K., Nikaido M., Nakamura T., Koshino H., Usui T. (2012) Enzymatic synthesis of cellulose II-like substance via cellulolytic enzyme-mediated transglycosylation in an aqueous medium. Carbohydr. Res. 353, 22–26 [DOI] [PubMed] [Google Scholar]
- 17. Kars I., Krooshof G. H., Wagemakers L., Joosten R., Benen J. A., van Kan J. A. (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J. 43, 213–225 [DOI] [PubMed] [Google Scholar]
- 18. Federici L., Di Matteo A., Fernandez-Recio J., Tsernoglou D., Cervone F. (2006) Polygalacturonase inhibiting proteins. Players in plant innate immunity? Trends Plant Sci. 11, 65–70 [DOI] [PubMed] [Google Scholar]
- 19. Van Vu B., Itoh K., Nguyen Q. B., Tosa Y., Nakayashiki H. (2012) Cellulases belonging to glycoside hydrolase families 6 and 7 contribute to the virulence of Magnaporthe oryzae. Mol. Plant-Microbe Interact. 25, 1135–1141 [DOI] [PubMed] [Google Scholar]
- 20. Cosgrove D. J. (2000) Loosening of plant cell walls by expansins. Nature 407, 321–326 [DOI] [PubMed] [Google Scholar]
- 21. Takeda T., Furuta Y., Awano T., Mizuno K., Mitsuishi Y., Hayashi T. (2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc. Natl. Acad. Sci. U.S.A. 99, 9055–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park Y. W., Baba K., Furuta Y., Iida I., Sameshima K., Arai M., Hayashi T. (2004) Enhancement of growth and cellulose accumulation by overexpression of xyloglucanase in poplar. FEBS Lett. 564, 183–187 [DOI] [PubMed] [Google Scholar]
- 23. Burton R. A., Fincher G. B. (2009) (1,3;1,4)-β-d-Glucans in cell walls of the Poaceae, lower plants, and fungi. A tale of two linkages. Mol. Plant 2, 873–882 [DOI] [PubMed] [Google Scholar]
- 24. Kimpara T., Aohara T., Soga K., Wakabayashi K., Hoson T., Tsumuraya Y., Kotake T. (2008) β-1,3:1,4-Glucan synthase activity in rice seedlings under water. Ann. Bot. 102, 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumura H., Nirasawa S., Terauchi R. (1999) Technical advance. Transcript profiling in rice (Oryza sativa) seedlings using serial analysis of gene expression (SAGE). Plant J. 20, 719–726 [DOI] [PubMed] [Google Scholar]
- 26. Lever M. (1972) A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47, 273–279 [DOI] [PubMed] [Google Scholar]
- 27. Dische Z. (1958) in Methods in Carbohydrate Chemistry (Whistler R. L., Wolfrom M. K., eds) Vol. 1, pp. 475–514, Academic Press, New York [Google Scholar]
- 28. Fry S. C. (1997) Novel “dot-blot” assays for glycosyltransferases and glycosylhydrolases. Optimization for xyloglucan endotransglycosylase (XET) activity. Plant J. 11, 1141–1150 [Google Scholar]
- 29. Hashimoto A., Shimono K., Horikawa Y., Ichikawa T., Wada M., Imai T., Sugiyama J. (2011) Extraction of cellulose-synthesizing activity of Gluconacetobacter xylinus by alkylmaltoside. Carbohydr. Res. 346, 2760–2768 [DOI] [PubMed] [Google Scholar]
- 30. Zhou J., Zheng X. Z., Lan L., Lin C. Z., Wu Y. B., Lin X. J., Ebbole D., Lu G. D., Wang Z. H. (2008) Biochemical and molecular characterization of a putative endoglucanase in Magnaporthe grisea. Curr. Genet. 53, 217–224 [DOI] [PubMed] [Google Scholar]
- 31. Claeyssens M., van Tilbeurgh H., Kamerling J.P., Berg J., Vrsanska M., Biely P. (1990) Studies of cellulolytic system of the filamentous fungus Trichoderma reesei QM9414. Substrate specificity and transfer activity of endoglucanase I. Biochem. J. 270, 251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schou C., Rasmussen G., Kaltoft M. B., Henrissat B., Schülein M. (1993) Stereochemistry, specificity and kinetics of the hydrolysis of reduced cellodextrins by nine cellulases. Eur. J. Biochem. 217, 947–953 [DOI] [PubMed] [Google Scholar]
- 33. Fry S. C., Smith R. C., Renwick K. F., Martin D. J., Hodge S. K., Matthews K. J. (1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem. J. 282, 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishitani K., Tominaga R. (1992) Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J. Biol. Chem. 267, 21058–21064 [PubMed] [Google Scholar]
- 35. Hrmova M., Farkas V., Lahnstein J., Fincher G. B. (2007) A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-β-d-glucans. J. Biol. Chem. 282, 12951–12962 [DOI] [PubMed] [Google Scholar]
- 36. Fry S. C., Mohler K. E., Nesselrode B. H., Franková L. (2008) Mixed-linkage β-glucan:xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant J. 55, 240–252 [DOI] [PubMed] [Google Scholar]
- 37. Gibeaut D. M., Carpita N. C. (1993) Synthesis of (1,3;1,4)-β-d-glucan in the Golgi apparatus of maize coleoptiles. Proc. Natl. Acad. Sci. U.S.A. 90, 3850–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gibeaut D. M., Pauly M., Bacic A., Fincher G. B. (2005) Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta. 221, 729–738 [DOI] [PubMed] [Google Scholar]
- 39. Sakurai N., Nevins N. J., Masuda Y. (1977) Auxin- and hydrogen ion-induced cell wall loosening and cell extension in Avena coleoptile segments. Plant Cell Physiol. 18, 371–380 [Google Scholar]
- 40. Hoson T., Nevins D. J. (1989) β-d-Glucan antibodies inhibit auxin-induced cell elongation and change in the cell wall of Zea coleoptile segments. Plant Physiol. 90, 1353–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hiraishi M., Igarashi K., Kimura S., Wada M., Kitaoka M., Samejima M. (2009) Synthesis of highly ordered cellulose II in vitro using cellodextrin phophorylase. Carbohydr. Res. 344, 2468–2473 [DOI] [PubMed] [Google Scholar]
- 42. Tominaga R., Samejima M., Sakai F., Hayashi T. (1999) Occurrence of cello-oligosaccharides in the apoplast of auxin-treated pea stems. Plant Physiol. 119, 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]