Background: FMR1 CGG expansion repeats in the premutation range have not been linked to astrocyte pathophysiology.
Results: Premutation cortical astrocytes display decreased Glu transporter expression/activity and enhanced asynchronous Ca2+ oscillations.
Conclusion: Glu transport and Ca2+ signaling defects in premutation astrocytes could contribute to FXTAS neuropathology.
Significance: Premutation astrocytes may have an etiological role in FXTAS neuropathology.
Keywords: Astrocytes, Calcium, Neurodegenerative Diseases, Neurological Diseases, Neurotransmitter Transport, Astrocytes, Ca2+ Oscillation, FXTAS, Glutamate Transporter, Premutation CGG Expansion
Abstract
Premutation CGG repeat expansions (55–200 CGG repeats; preCGG) within the fragile X mental retardation 1 (FMR1) gene can cause fragile X-associated tremor/ataxia syndrome. Defects in early neuronal migration and morphology, electrophysiological activity, and mitochondria trafficking have been described in a premutation mouse model, but whether preCGG mutations also affect astrocyte function remains unknown. PreCGG cortical astrocytes (∼170 CGG repeats) displayed 3-fold higher Fmr1 mRNA and 30% lower FMR1 protein (FMRP) when compared with WT. PreCGG astrocytes showed modest reductions in expression of glutamate (Glu) transporters GLT-1 and GLAST and attenuated Glu uptake (p < 0.01). Consistent with astrocyte cultures in vitro, aged preCGG mice cerebral cortex also displayed reduced GLAST and GLT-1 expression. Approximately 65% of the WT and preCGG cortical astrocytes displayed spontaneous asynchronous Ca2+ oscillations. PreCGG astrocytes exhibited nearly 50% higher frequency of asynchronous Ca2+ oscillations (p < 0.01) than WT, a difference mimicked by chronic exposure of WT astrocytes to l-trans-pyrrolidine-2,4-dicarboxylic acid (l-trans-PDC) or by partial suppression of GLAST using siRNA interference. Acute challenge with Glu augmented the frequency of Ca2+ oscillations in both genotypes. Additionally, 10 μm Glu elicited a sustained intracellular Ca2+ rise in a higher portion of preCGG astrocytes when compared with WT. Pharmacological studies showed that mGluR5, but not NMDA receptor, contributed to Glu hypersensitivity in preCGG astrocytes. These functional defects in preCGG astrocytes, especially in Glu signaling, may contribute to fragile X-associated tremor/ataxia syndrome neuropathology.
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS)3 is a late adult onset neurodegenerative disorder that occurs in individuals with a trinucleotide expansion (55–200 CGG repeats, premutation) within the fragile-X mental retardation 1 (FMR1) gene. Premutation alleles of the FMR1 gene have estimated frequencies of 1:130–250 females and 1:250–810 males (1, 2). These premutation carriers can display clinical features including behavioral and cognitive abnormalities in children (3–6), primary ovarian insufficiency in ∼20% of women (7), and FXTAS (7–9) in ∼40% of male carriers. The core clinical features of FXTAS include progressive gait ataxia and intention tremor, dysautonomia, peripheral neuropathy, and parkinsonism (7, 8, 10, 11). Larger CGG expansions (>200 repeats; full mutation) generally result in hypermethylation of the FMR1 gene with subsequent transcriptional silencing. The absence of the FMR1 protein (FMRP) leads to fragile X syndrome (FXS), the most common inherited form of cognitive impairment and a leading single-gene disorder associated with autism (12, 13). Consistent with observations in human premutation carriers (14), premutation CGG expansion (preCGG) mouse neurons display elevated Fmr1 mRNA and normal to variable reductions on the FMRP in brain lysate (15, 16). The absence of fragile X-associated primary ovarian insufficiency and FXTAS symptoms in full mutation patients (FXS) implies that FMRP deficiency per se is not responsible for premutation disorders and FXTAS. Excessive FMR1 mRNA is hypothesized to be toxic to neurons and glia (1, 17–20). However, whether the partial loss of FMRP in premutation carriers also contributes to the onset or progression of premutation disorders and FXTAS is not well established. Defects in neuronal morphology (21) and migration (22), aberrant spontaneous Ca2+ oscillations and clustered bursts of electrical firing (23), as well as abnormalities in the number, mobility, and metabolic function of mitochondria in the early stages of neuron differentiation in preCGG mouse model (24) have been reported.
Astrocytes actively modulate synaptic activity and synaptic network by releasing gliotransmitters such as glutamate (Glu), ATP, and d-serine in a Ca2+-dependent manner (25, 26). Defects in astrocytes and their impact on neuronal cell development have been reported in FXS (27, 28) and other neurodevelopmental disorders such as Rett syndrome (29–31). Normal astrocytes can prevent aberrant neuronal development in the Fmr1 knock-out (KO) mouse; conversely, fragile X astrocytes are capable of inducing developmental delays in dendrite maturation and synaptic protein expression (27, 28). Premutation mouse astrocytes display ubiquitin-positive intranuclear inclusions (15, 32), which are the neuropathological hallmark of FXTAS (19, 33). Moreover, the ubiquitin-positive intranuclear inclusions are present notably in a higher percentage in human cortical gray matter astrocytes (17%) than in neurons (4%) (18, 19, 33). These data indicate that the FMR1 CGG expansion repeats in the premutation range might also trigger functional abnormalities in astrocytes that promote neuropathology. However, little is known about astrocyte defects related to the presence of premutation CGG expansions.
Here, we report that cortical astrocytes cultured from hemizygous male pups with the Fmr1 allele expanded in the high premutation range (∼170 CGG repeats, designated preCGG throughout) display elevated Fmr1 mRNA and a moderate decrease of FMRP. When compared with the wild type (WT) controls, preCGG cortical astrocytes exhibit more frequent spontaneous Ca2+ oscillations and reduced expression of Glu transporters GLAST and GLT-1, consistent with attenuated Glu uptake. Pharmacological suppression of Glu transporters activity or decrease in the GLAST expression using GLAST siRNA enhances spontaneous asynchronous Ca2+ oscillations in WT astrocytes similar to those observed in preCGG astrocytes, suggesting that reduced Glu uptake may contribute to altered Ca2+ dynamics observed in preCGG astrocytes.
EXPERIMENTAL PROCEDURES
Antibodies and siRNA
GLAST antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). GLT-1 antibody was from Alpha Diagnostic International (San Antonio, TX). Anti-mGluR1/5 antibody was from University of California Davis/National Institutes of Health NeuroMab Facility (Davis, CA). FMRP antibody was produced as described previously (34). Predesigned GLAST siRNA pool (catalog number L-056182-01-0005) or nontargeting siRNA (catalog number D-001810-01-05) was from Thermo Scientific.
Animals and Genotyping
All preCGG KI and WT mice in the C57/B6 background were housed under standard vivarium conditions. PreCGG hemizygous male pups (range 155–200; average 170 CGG repeats) were obtained by breeding homozygous preCGG females with preCGG hemizygous males. Male WT pups delivered on the same day as male preCGG pups were used for paired cultures. The University of California, Davis Institutional Animal Care and Use Committee (IACUC) approved all animal use protocols. Genotypes were verified by PCR for each individual pup using Fmr1 forward and reverse specific primers as described previously (23).
Cortical Astrocyte Cultures and Transfection
Cortical astrocytes were obtained from postnatal day 0–1 (P0–1) WT or hemizygous preCGG KI male pups. The dissection and dissociation of cortical cells were performed as described previously (35). The dissociated cells were plated onto a poly-l-ornithine-coated T-75 culture flask at a density of 1.0–1.5 × 107 cells/flask and maintained in culture medium (DMEM + 10% FBS) in an incubator at 37 °C with 5% CO2 and 95% humidity. The culture medium was changed twice a week, and the cells were used between 2 and 4 weeks with no more than three passages. WT astrocytes were transfected with predesigned GLAST siRNA pool or nontargeting siRNA using DharmaFECT 1 transfection reagent (catalog number T-2001-01, Thermo Scientific) in serum-free culture medium. The final concentration of siRNA was 25 nm. The serum-free medium was replaced with culture medium after 24 h, and the astrocytes were cultured for another 48 h prior to harvest the samples for Western blot analysis.
Quantitative Measurements of Fmr1 mRNA
Total RNA from primary cortical astrocytes from both WT and preCGG KI mice was isolated by standard method (TRIzol, Ambion Inc., Austin, TX). Quantitative measurements of Fmr1 mRNA levels from total RNA were obtained by real time PCR as described previously (14).
Western Blots
Equal amounts (20 μg) of cell lysates were loaded onto a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane by electroblotting. After blocking with Odyssey blocking buffer (LI-COR Biotechnology, Lincoln, NE), the membranes were incubated with primary antibody overnight at 4 °C or 2 h at room temperature (anti-FMRP, 1:20,000; anti-tubulin, 1:10,000; anti-GLT-1, 1:1,000; anti-GLAST, 1:500; anti-GFAP, 1:10,000; and anti-mGluR1/5, 1:2000). After washing, the IRDye (800CW or 700CW)-labeled secondary antibody (LI-COR Biotechnology, 1:10,000 dilution) was added, and the blots were incubated for 1 h at room temperature. The membrane was imaged with the LI-COR Odyssey infrared imaging system (LI-COR Biotechnology). The densitometry analysis was performed using LI-COR Odyssey infrared imaging system application software 2.1.
Immunocytochemistry
After briefly being washed with PBS twice, astrocytes were fixed with 4% paraformaldehyde for 15 min. Cells were permeabilized with 0.1% Triton X-100 for 20 min. Following blocking with PBS containing 2% glycerol, 0.05 m NH4Cl, 5% PBS, and 2% goat serum for 2 h, cells were incubated with rabbit anti-GFAP (Dako, Glostrup, Denmark, 1:1,000) overnight at 4 °C. After washing with PBS for 5 min, cells were incubated with Alexa Fluor 568-conjugated goat anti-rabbit secondary antibody (1:500, Molecular Probes, Eugene, OR) for 1 h at room temperature. The cells were then washed and mounted in Prolong Gold antifade mounting medium with DAPI (Molecular Probes) and visualized in an IX-71 invert microscope (Olympus America, Center Valley, PA).
Ca2+ Imaging
After the medium was aspirated, astrocytes were incubated with dye loading buffer (100 μl/well) containing 4 μm Fluo-4 and 0.5 mg/ml BSA in Locke's buffer (35) for 30 min. The cells were excited at 488 nm using a DeltaRam illuminator (Photon Technologies International, Birmingham, NJ), and fluorescence emission was recorded at 525 nm. The images were captured with an Evolve® cooled charge-coupled device camera (Photometrics, Tucson, AZ) attached to an inverted Olympus IX71 microscope (Olympus America). The Fluo-4 fluorescent arbitrary signals were captured and analyzed for individual cells using EasyRatioPro software (Photon Technologies International, Birmingham, NJ). Data were presented as F/F0. To measure population responses to Glu challenge, the cells were plated and loaded with Fluo-4 as described above, and changes in intracellular Ca2+ concentration was determined as described previously using a fluorescent imaging plate reader (FLIPR® Tetra Station, Molecular Devices, Sunnyvale, CA) (36).
[3H]Glu Uptake Assay
The cortical astrocytes were plated onto poly-l-ornithine-coated 48-well plates at an initial density of 100,000 cells/well and cultured for 24–48 h until 90% confluent. The [3H]Glu (Moravek Biochemicals and Radiochemicals; 27 Ci/mmol) uptake assay was performed at room temperature. The astrocytes were incubated with 100 μm Glu for 30 min in Locke's buffer. After washing with ice-cold PBS, the cells were lysed by the addition of 300 μl of 0.5% SDS solution, and the lysates were subjected to liquid scintillation counting for determining the radioactivity. For each genotype, three adjacent wells were used to determine the protein concentration using the BCA method, and Glu uptake values were adjusted to protein content.
Data Analysis
Graphing and statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). Statistical significance between WT and preCGG was calculated using Student's t test, and p values below 0.05% were considered significant. The EC50 values and maximal response for Glu response were determined by nonlinear regression analysis using a logistic equation. Kinetic parameters (Km and Vmax) for Glu uptake were determined by nonlinear regression analysis of the saturation curves using the Michaelis-Menten equation.
RESULTS
PreCGG Cortical Astrocytes Display Elevated Fmr1 mRNA and Modestly Reduced FMRP
Given the ubiquitin-positive nuclear inclusions observed in astrocytes in the brains of preCGG KI mice (15, 32), we first examined the Fmr1 mRNA levels in both preCGG and WT cortical astrocyte cultures. The purity of cultured astrocytes was evaluated by labeling the cells with DAPI and the astrocyte-specific markers, glial fibrillary acidic protein (GFAP). As shown in Fig. 1, greater than 95% of the cells used in these experiments were GFAP-positive, indicating enrichment of astrocytes in our cultures. The Fmr1 levels in the cortical astrocytes cultured from the preCGG mice were 311 ± 50% (n = 6, p < 0.01) of WT control (Fig. 2a). Western blot analysis was used to measure FMRP expression using β-tubulin as a control for the protein loading. PreCGG cortical astrocytes displayed 70.1 ± 7.2% (n = 4, p < 0.01) of WT control on the FMRP expression (Fig. 2, b and c).
FIGURE 1.
Representative GFAP immunocytochemistry of astrocytes cultured from WT mice and knock-in FMR1 premutation (preCGG) mice. WT and preCGG cortical astrocytes exhibit similar morphology when stained with GFAP. Nearly all the cells were GFAP-positive in both WT and preCGG cortical astrocyte culture, indicating a high enrichment of astrocytes in our culture.
FIGURE 2.
PreCGG cortical astrocytes display higher Fmr1 mRNA level and reduced FMRP proteins when compared with WT controls. a, Fmr1 mRNA level comparison between WT and preCGG astrocytes. Quantitative RT-PCR was performed, and Fmr1 mRNA level was measured. PreCGG astrocytes showed 311 ± 50% (mean ± S.E.) of the WT control (p < 0.01, n = 6). b, representative Western blotting for FMRP expression in WT and preCGG cortical astrocytes. c, quantification of FMRP expression in WT and preCGG cortical cultures. PreCGG cortical astrocytes displayed 70.1 ± 7.2% of WT control (p < 0.01, n = 4).
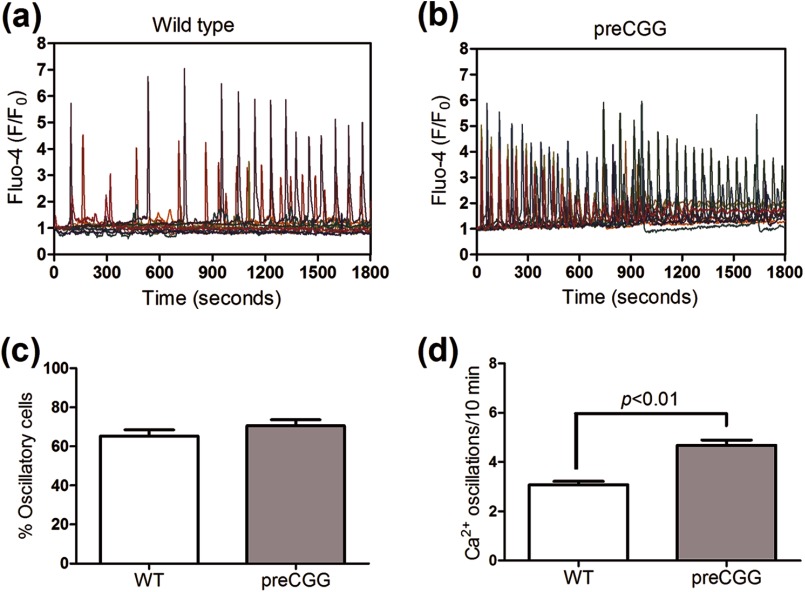
PreCGG Cortical Astrocytes Display Enhanced Spontaneous Asynchronous Ca2+ Oscillations
WT and preCGG astrocytes exhibited similar morphology when stained with GFAP (Fig. 1). When loaded with the Ca2+ indicator Fluo-4 and monitored by fluorescence imaging, astrocytes display spontaneous asynchronous Ca2+ oscillations previously shown to correlate with the release of gliotransmitters that regulate local neuronal cell networks (38). We examined the Ca2+ dynamics in WT and preCGG cortical astrocytes cultured on the same day. Consistent with previous studies, WT cortical astrocyte cultures display asynchronous Ca2+ oscillations (Fig. 3a) that are distinctly different from the synchronous Ca2+ oscillation observed in cortical neurons, which oscillate at much higher frequencies and shorter durations (35). The distinct temporal characteristics of asynchronous Ca2+ oscillations ensure that the signals recorded and analyzed were from astrocytes, not neurons in our culture system. The percentage of astrocytes that were oscillatory did not differ significantly between WT (65.3 ± 3.3%, n = 20 wells) and preCGG (70.6 ± 3.0%, n = 16 wells) (Fig. 3c). However, the preCGG astrocytes displayed more spontaneous Ca2+ oscillations when compared with WT control (Fig. 3d). Of the cells that were oscillatory, the average frequency of spontaneous Ca2+ oscillations for preCGG astrocyte was 4.68 ± 0.21/10 min (n = 431 cells), representing 148.6 ± 6.6% (p < 0.01) of the WT control (3.15 ± 0.14/10 min, n = 450 cells).
FIGURE 3.
PreCGG mutation mouse cortical astrocytes display more spontaneous Ca2+ oscillations. a and b, representative ten Ca2+ traces in WT (a) and preCGG (b) cortical astrocytes, respectively. Data are presented as F/F0. c, quantification of the percentage of oscillatory cells in WT and preCGG cortical astrocytes. The percentages of oscillatory astrocytes were 65.3 ± 3.3% (n = 20 wells) in preCGG cortical astrocytes and 70.6 ± 3.0% (n = 16 wells, p > 0.05) in WT controls. d, quantification of Ca2+ oscillation frequency in WT and preCGG oscillatory cortical astrocytes. PreCGG cortical astrocytes displayed more Ca2+ oscillations (4.68 ± 0.21/10 min, n = 431 cells) than WT control (3.15 ± 0.14/10 min, p < 0.01, n = 450 cells).
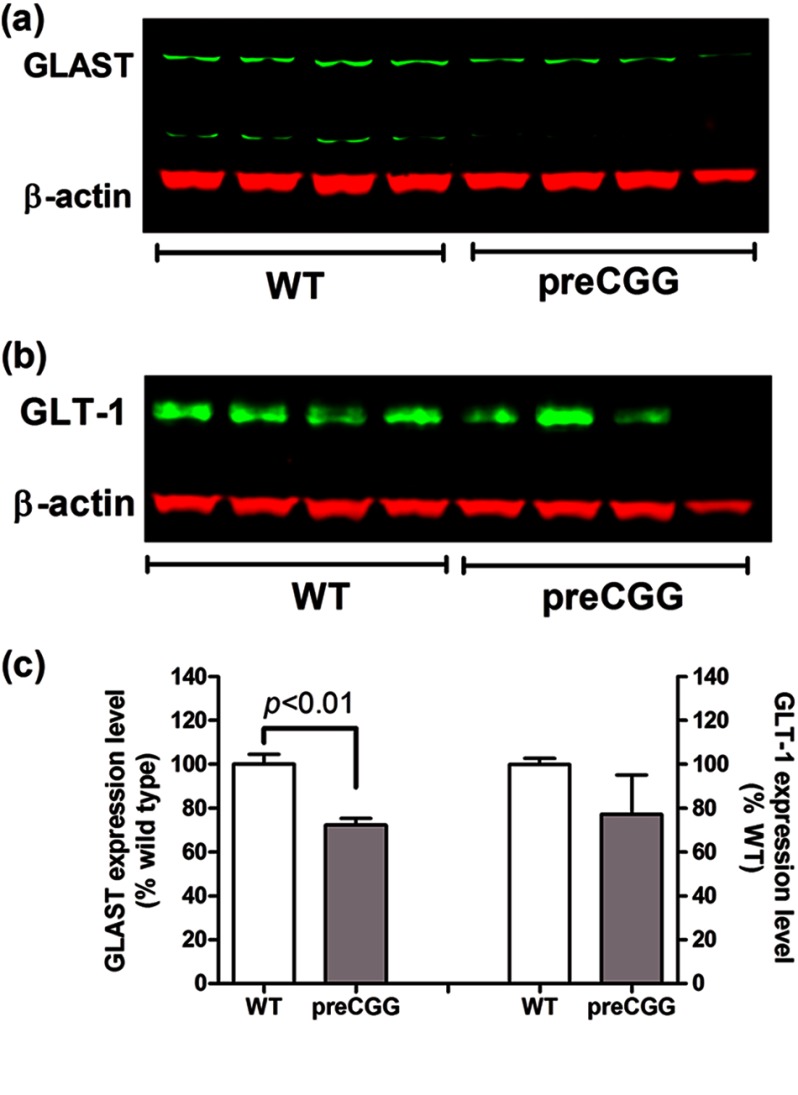
PreCGG Cortical Astrocytes and Brain Cortex Display Modest Decrease in the Expression of GLT-1 and GLAST
Glu is the most abundant excitatory neurotransmitter in the vertebrate CNS. The excitatory amino acid transporters (EAATs) are responsible for the rapid removal of Glu from the extracellular space and maintain Glu concentration at a subtoxic level. Within the EAATs family, Glu transporters GLAST (EAAT1 in human) and GLT-1 (EAAT2 in human) are highly expressed in glial cells, where they are the primary Glu uptake mechanism in the mammalian central nervous system (39). We examined the expression of GLT-1 and GLAST using Western blot analysis. As shown in Fig. 4, the expression levels for GLAST and GLT-1 in preCGG cortical astrocytes were slightly, although significantly decreased, representing 88.6 ± 2.4% (p < 0.05, n = 4) and 92.1 ± 1.8% (p < 0.01, n = 6) of WT controls, respectively. To test whether reduced expression of the major Glu transporters extends to the brain of aging (46–59-week-old) preCGG mutation mice, cortical tissue was dissected, and protein was extracted in lysis buffer. These aged mice used were 50 ± 3 weeks (WT, n = 4) and 53 ± 1.3 weeks (preCGG, n = 4). As shown in Fig. 5, the expression of GLAST was significantly decreased to 72.3 ± 3.1% (p < 0.01, n = 4) of WT control. The averaged expression of GLT-1 was 77.2 ± 17.8% of WT control (three out of four mice displayed decreased GLT-1 expression with one mouse having slightly increased GLT-1 expression level). However, due to the variation in the preCGG group, the decrease of GLT-1 expression could not reach statistical significance.
FIGURE 4.
PreCGG cortical astrocytes display lower expression of Glu transporters, GLT-1 and GLAST. a and b, representative Western blotting for GLAST (a) and GLT-1 (b) in WT and preCGG cortical astrocytes. The representative bands were from two independent paired cultures. c, quantification of GLAST and GLT-1 expression in WT and preCGG cortical astrocyte cultures, respectively. The expression for GLAST and GLT-1 was 88.6 ± 2.4% (p < 0.05, n = 4) and 92.1 ± 1.8% (p < 0.01, n = 6) of WT controls, respectively. These data were from at least three independent paired cultures.
FIGURE 5.
Reduced expression of Glu transporters, GLAST and GLT-1, in preCGG mouse cortex. a and b, representative Western blots for GLAST (a) and GLT-1 (b) in WT and preCGG brain cortex. Each genotype had four mice with an age range of 46–59 weeks (p > 0.05). c, quantification of GLAST and GLT-1 expression in WT and preCGG mice brain cortex, respectively. The expression for GLAST and GLT-1 was 72.3 ± 3.1% (p < 0.01, n = 4) and 77.2 ± 17.8% (p > 0.05, n = 4) of WT controls, respectively.
PreCGG Cortical Astrocytes Display Reduced Glu Uptake
We next evaluated the Glu uptake kinetics in both WT and preCGG cortical astrocyte cultures. The astrocytes were incubated with 100 μm Glu (a combination of [3H]Glu (50 nm) and unlabeled Glu) for 30 min in Locke's buffer. The Glu uptake was 99.1 ± 2.0 and 96.9 ± 1.4 nmol/mg of protein over 30 min in WT and preCGG cortical astrocytes, respectively (data not shown). To confirm that Glu transporters mediated Glu uptake, a nonselective Glu transporter inhibitor l-trans-PDC was applied 25 min prior to incubating the cells with Glu. l-trans-PDC (1 mm) completely inhibited Glu uptake in both WT and preCGG cortical astrocytes (data not shown). We then analyzed the kinetics of the Glu uptake by WT and preCGG cortical astrocytes. Preliminary data demonstrated that with a Glu concentration of 100 μm, the uptake rate was linear for at least 10 min with an r2 of 0.9799 (data not shown), and therefore, kinetic analyses were performed within 10 min. Uptake as a function of Glu concentration was fitted by the Michaelis-Menten equation. The half-maximal transport constant (Km) was similar between WT (33.38 (29.79–36.96) μm, mean and 95% confidence intervals (95% CI)), and preCGG astrocytes (31.39 (27.82–34.95) μm, mean and 95% CI). However, preCGG cortical astrocytes displayed reduced maximal Glu uptake rate (Vmax) (4.79 (4.62–4.97) nmol/mg of protein/min, mean and 95% CI) when compared with the WT controls (5.29 (5.10–5.47) nmol/mg of protein/min, mean and 95% CI) (Fig. 6). This modest yet statistically significant decrease on the Vmax in preCGG cortical astrocytes is consistent with decreased expression levels of Glu transporters.
FIGURE 6.
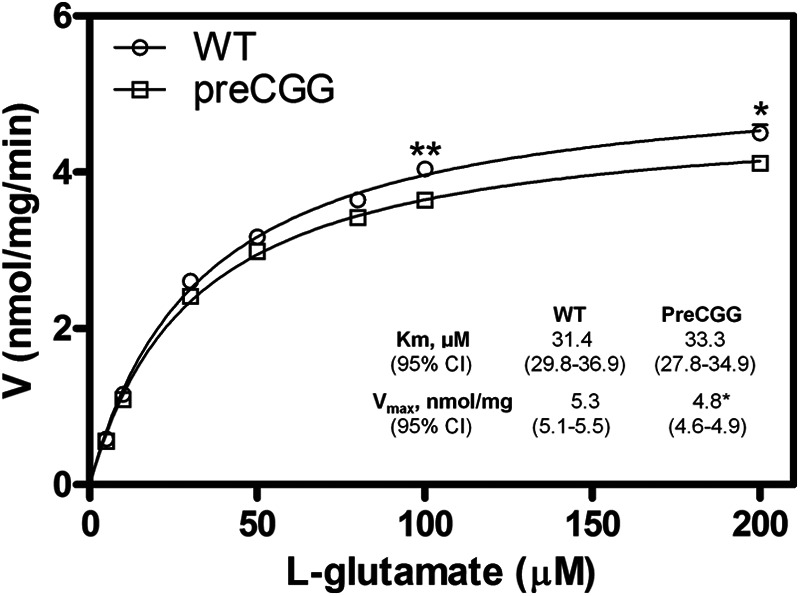
PreCGG astrocytes display decreased Glu uptake. Kinetic analysis for Glu uptake in WT and preCGG cortical astrocytes was performed. Each data point represents mean ± S.E. (n = 12) from four independent cultures performed in triplicates. Uptake as a function of Glu concentration was fitted by the Michaelis-Menten equation. The Km values for Glu uptake were similar between WT and preCGG cortical astrocytes. However, preCGG cortical astrocytes displayed reduced Vmax for Glu uptake, which was 5.29 (5.10–5.47 nmol/mg of protein/min, 95% CI) in WT cortical astrocytes and 4.79 (4.62–4.97 nmol/mg of protein/min, 95% CI) in preCGG cortical astrocytes. *, p < 0.05, **, p < 0.01.
l-trans-PDC Enhances Ca2+ Oscillation Frequency in WT Cortical Astrocytes
To examine the potential role for Glu transporters on the modulation of spontaneous Ca2+ oscillations, we pharmacologically suppressed Glu transporter activity by chronic exposure (48 h) of WT cortical astrocytes to low concentrations (1 and 10 μm) of l-trans-PDC. Based on the Ki value of l-trans-PDC (40), 10 μm l-trans-PDC would be predicted to inhibit 10–15% of the Glu transporters. Therefore, a concentration of 10 μm l-trans-PDC may mimic the cellular effect observed in the preCGG cortical astrocytes that displayed modest decrease (∼10%) in Glu uptake. As shown in Fig. 7, exposure of WT cortical astrocytes to 10 μm l-trans-PDC produced a statistically significant enhancement on the Ca2+ oscillations (139.1 ± 9.4%; p < 0.01, n = 94 cells) when compared with vehicle-treated WT astrocytes, which was comparable with that observed in preCGG cortical astrocytes. Cells exposed to the lower concentration of l-trans-PDC (1 μm) elicited a slight although statistically nonsignificant increase in the frequency of Ca2+ oscillations (109.1 ± 7.4% of WT, p > 0.05, n = 73 cells). Together these data demonstrate that suppression of Glu transporter activity can increase the frequency of spontaneous Ca2+ oscillations in WT cortical astrocytes that mimics those measured in preCGG, suggesting a potential role for Glu transporter impairment on the modulation of spontaneous Ca2+ oscillations in preCGG cortical astrocytes.
FIGURE 7.
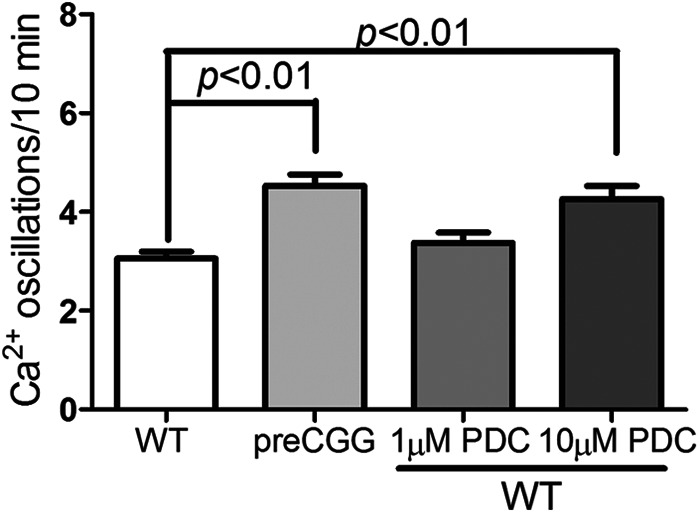
Chronic exposure to low concentrations of l-trans-PDC enhances the spontaneous Ca2+ oscillations in WT cortical astrocytes. WT cortical astrocytes chronically exposed to 10 μm l-trans-PDC displayed significantly increased Ca2+ oscillations when compared with vehicle-treated WT astrocytes. Each data point represents mean ± S.E. with at least 90 cells from two independent cultures.
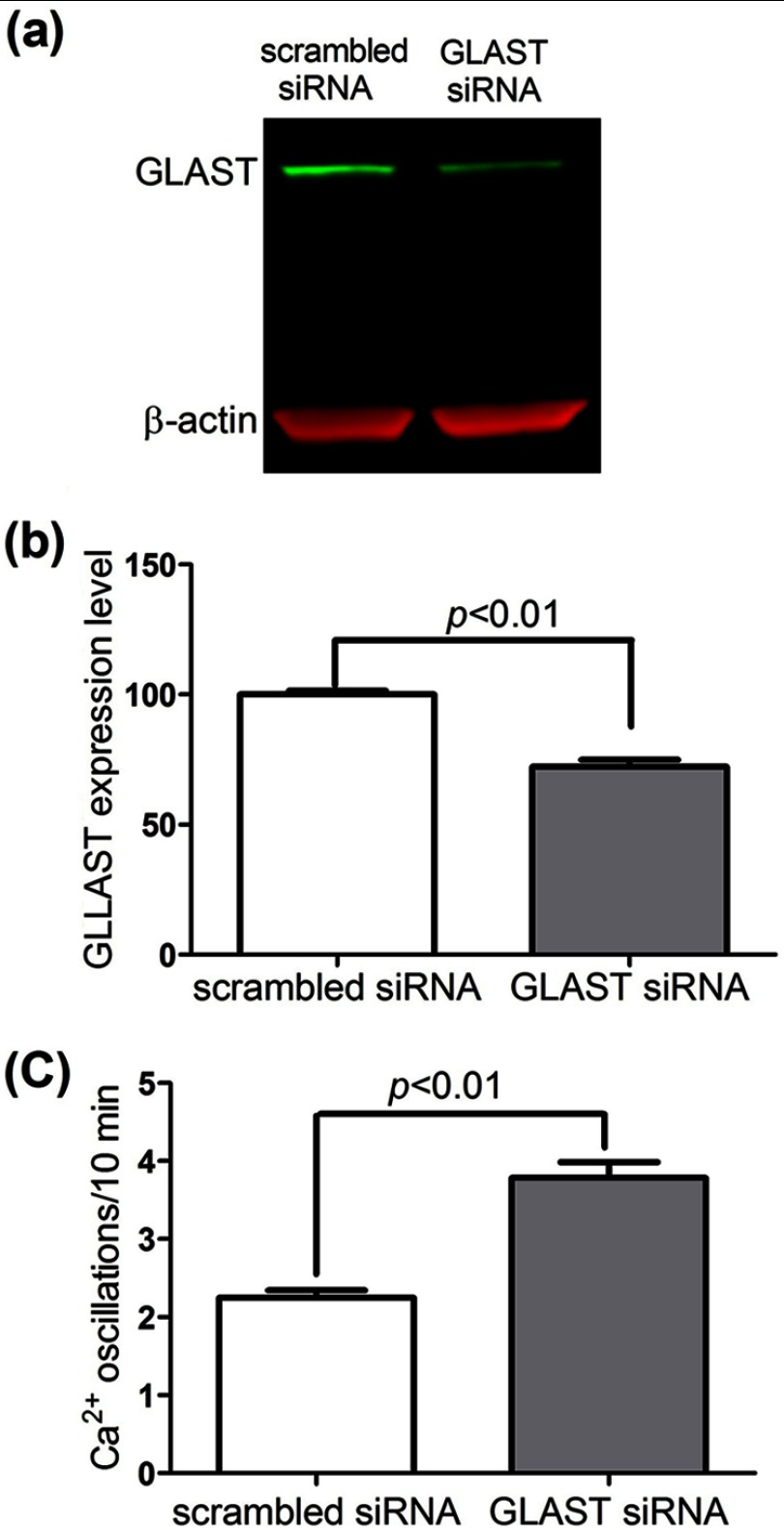
Decreased GLAST Expression Level Enhances Ca2+ Oscillation Frequency
To further assess the functional significance of Glu transporters on the regulation of spontaneous Ca2+ oscillation frequency in astrocytes, we evaluated the spontaneous Ca2+ oscillation frequency in the WT astrocytes transfected with GLAST siRNA. Transfection with GLAST siRNA decreased the GLAST expression to 72.1 ± 2.1% (n = 8, p < 0.01) of the control group (when compared with astrocytes transfected with the nontargeting siRNA) (Fig. 8, a and b). The astrocytes transfected with GLAST siRNA displayed significantly higher frequency of Ca2+ oscillations (3.79 ± 0.20/10 min, n = 183) than the astrocytes transfected with nontargeting siRNA (2.25 ± 0.09/10 min, n = 166) (Fig. 8c).
FIGURE 8.
Suppression of GLAST expression enhances the spontaneous Ca2+ oscillations in WT cortical astrocytes. WT cortical astrocytes were transfected with scrambled or GLAST siRNA. a, representative Western blot for GLAST expression after transfection with scrambled sequence or GLAST siRNA for 72 h. b, quantification of GLAST expression in the astrocytes transfected with scrambled sequence or GLAST siRNA. Transfection with GLAST siRNA decreased GLAST expression to 72.1 ± 2.8% (n = 8, p < 0.01) of the negative controls. c, astrocytes with GLAST siRNA transfection increased asynchronous spontaneous Ca2+ oscillations when compared with its negative control. Each data point represents mean ± S.E. with at least 160 cells from four independent cultures.
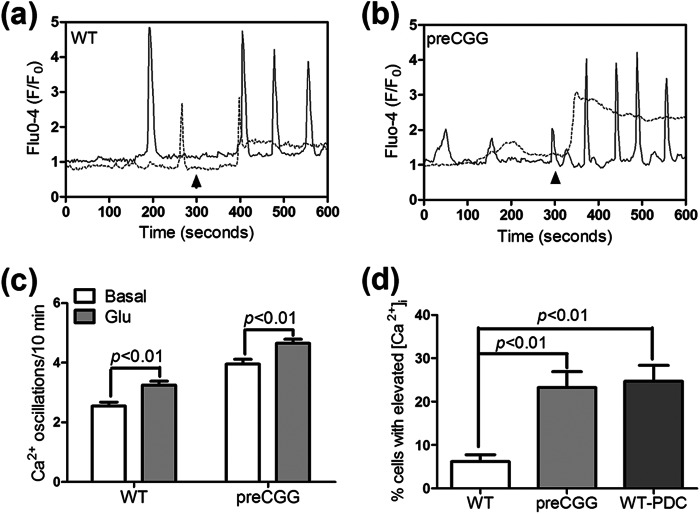
Glu Augments Spontaneous Ca2+ Oscillations in Both Wild Type and preCGG Cortical Astrocytes
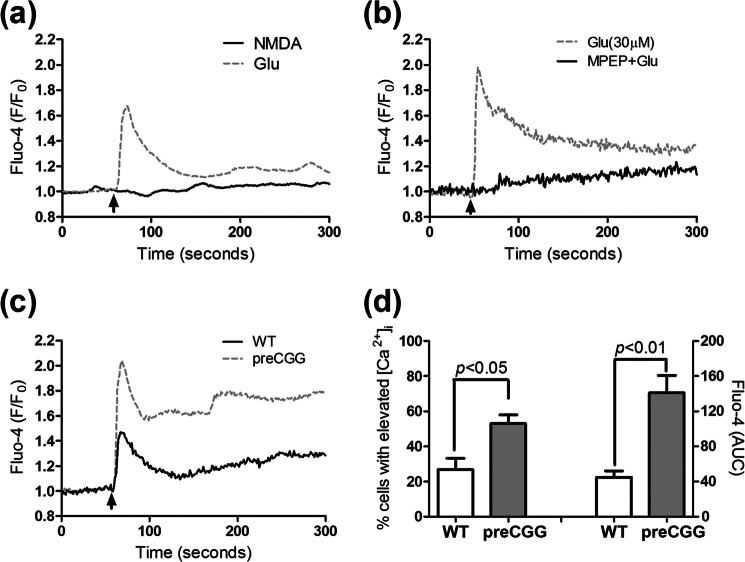
An acute challenge with Glu (10 μm) elicited a significant increase on the frequency of spontaneous Ca2+ in both WT and preCGG cortical astrocytes (Fig. 9, a–c), demonstrating the involvement of Glu receptors on the modulation of spontaneous Ca2+ oscillations. In addition to the increase of the frequency on the spontaneous Ca2+ oscillations, Glu (10 μm) induced a sustained intracellular Ca2+ increase in a fraction of responsive WT and preCGG astrocytes (Fig. 9, a, b, and d). A higher percentage of preCGG astrocytes (23.2 ± 3.7%, n = 4 wells) displayed sustained intracellular Ca2+ increase when compared with WT controls (6.2 ± 1.5%, n = 11 wells, p < 0.01, Fig. 9d). To examine the influence of Glu transporter activity on Glu-induced sustained intracellular Ca2+ increase. WT cortical astrocytes were chronically exposed to 10 μm l-trans-PDC for 48 h. Ca2+ imaging analysis revealed that a higher percentage (24.7 ± 3.6%, n = 9 wells, p < 0.01) of l-trans-PDC-treated astrocytes displayed sustained intracellular Ca2+ increase when compared with the vehicle controls (Fig. 9d).
FIGURE 9.
Influence of Glu on the Ca2+ dynamics in WT and preCGG cortical astrocytes. a and b, representative traces for Glu (10 μm) stimulation of Ca2+ response in WT (a) and preCGG (b) cortical astrocytes. Glu stimulated two distinct Ca2+ responses in both WT and preCGG cortical astrocytes. Some cells displayed more Ca2+ oscillations (solid trace), whereas the remaining responsive cells displayed sustained intracellular Ca2+ increase (dotted trace). Arrowheads indicate the addition of Glu (10 μm). c, quantification of the spontaneous Ca2+ oscillations before and after 10 μm Glu treatment. A concentration of 10 μm Glu increased the spontaneous Ca2+ oscillations in both WT and preCGG astrocytes. Each data point represents the mean ± S.E. from at least 150 cells for each group. d, quantification of the percentage of cells with sustained intracellular Ca2+ increase after treatment with 10 μm Glu in vehicle or 10 μm l-trans-PDC-exposed WT astrocytes and preCGG cortical astrocytes. Each data point represents the mean ± S.E. from at least 4 wells.
We next evaluated which type of Glu receptors could account for the differences in the Glu-triggered sustained Ca2+ response observed between WT and preCGG astrocytes. NMDA, a selective NMDA receptor agonist, had no detectable response on the intracellular Ca2+ increase in preCGG cortical astrocytes (Fig. 10a). However, the selective mGluR5 antagonist MPEP (10 μm) eliminated the Glu (30 μm)-induced sustained intracellular Ca2+ increase in preCGG cortical astrocytes. The mGluR5 agonist, DHPG, at an intermediate concentration of 2 μm, produced a sustained intracellular Ca2+ elevation in both WT and preCGG cortical astrocytes. Similar to the response induced by Glu in WT and preCGG astrocytes, a higher percentage of preCGG astrocytes (53.1 ± 4.8%, n = 4 wells) displayed sustained intracellular Ca2+ increase when compared with WT controls (26.8 ± 6.4%, n = 4 wells, p < 0.01, Fig. 10d). The DHPG-elicited Ca2+ response (area under the curve) is also significantly higher in preCGG astrocytes (70.5 ± 9.9, n = 4 wells) than in the WT controls (22.4 ± 3.6, n = 4 wells, Fig. 10d).
FIGURE 10.
Influence of Glu receptor agonist or antagonist on the Ca2+ dynamics in WT or preCGG cortical astrocytes. a, lack of Ca2+ response of NMDA in preCGG cortical astrocytes (solid black line). Glu (30 μm) was used as a positive control (gray dotted line). b, An mGluR5 antagonist, MPEP (10 μm) suppressed the Glu (30 μm)-induced intracellular Ca2+ increase in preCGG cortical astrocytes. The astrocytes were incubated with MPEP for 10 min before the addition of Glu (arrowhead). These data were repeated twice in triplicates with similar results. c, representative traces of type I mGluR receptor agonist DHPG (2 μm)-induced intracellular Ca2+ increase in both WT and preCGG cortical astrocytes. Black solid trace, WT; gray dotted trace, preCGG. These data were repeated twice in duplicates with similar results. d, quantification of the percentage of cells with sustained intracellular Ca2+ increase as well as Ca2+ response (area under the curve, AUC) after WT and preCGG cortical astrocytes were exposed to 2 μm DHPG. These data were from two independent cultures in duplicates.
PreCGG Cortical Astrocytes Are More Sensitive to Glu Exposure than Are WT Astrocytes
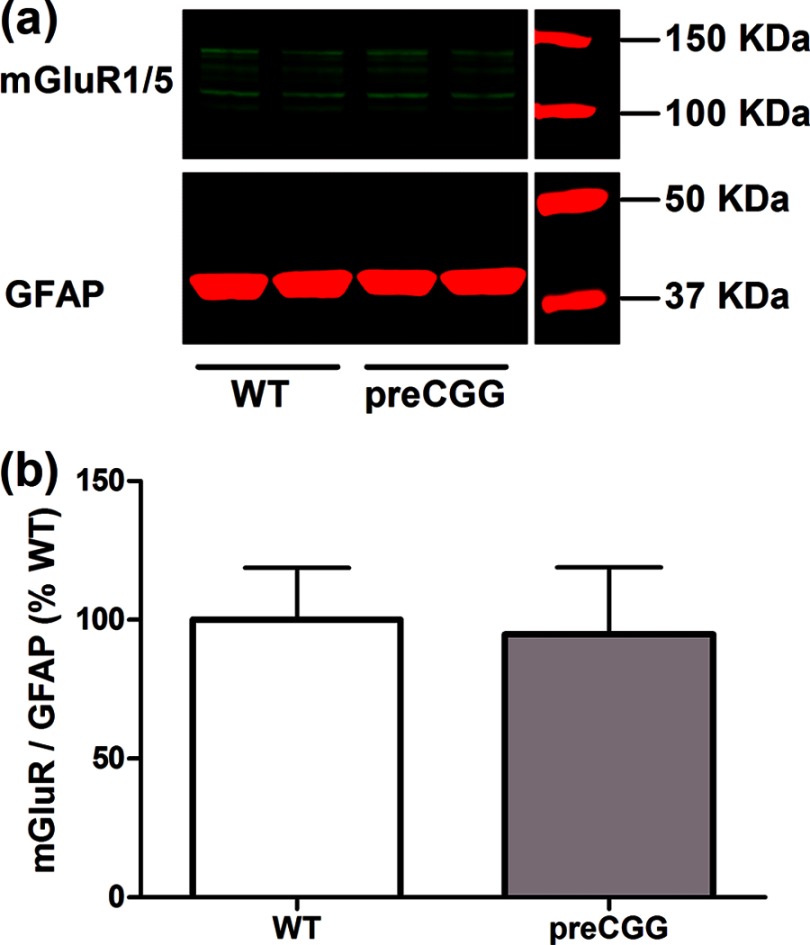
Given the observed differences in Glu-triggered intracellular Ca2+ responses between WT and preCGG cortical astrocytes, the concentration-response relationship for Glu was evaluated using FLIPR in a 96-well format. Because individual cells cannot be monitored using FLIPR, it is not possible to detect asynchronous Ca2+ oscillations. Nevertheless, this method permits detection of population-averaged Ca2+ signals in response to Glu challenge. After recording 3 min of base line, Glu (10–300 μm) or vehicle was introduced into each well simultaneously, and the Ca2+ response was recorded for an additional 5 min. Fig. 11 depicts the time- and concentration-response relationships for Glu-induced Ca2+ response in both WT and preCGG cortical astrocytes. The EC50 values were 35.23 ± 2.80 and 12.94 ± 3.89 μm (n = 3, p < 0.05) in WT and preCGG cortical astrocytes, respectively. Because FLIPR detects an average cell population response, this measurement likely reflected the increase in both Ca2+ oscillations and the sustained rise of intracellular Ca2+. In the Fmr1 KO mouse model of FXS, excessive protein synthesis downstream of mGluR5 activation following the loss of FMRP expression enhances the long term depression during synaptic transmission (41, 42). In the premutation CGG expansion hippocampal neurons, enhanced neuronal activity which was characterized by clustered burst firing stems, at least in part, from amplified signaling of type I mGluRs (23). Nevertheless, the two genotypes expressed similar levels of mGluR1/5 relative to GFAP (Fig. 12).
FIGURE 11.
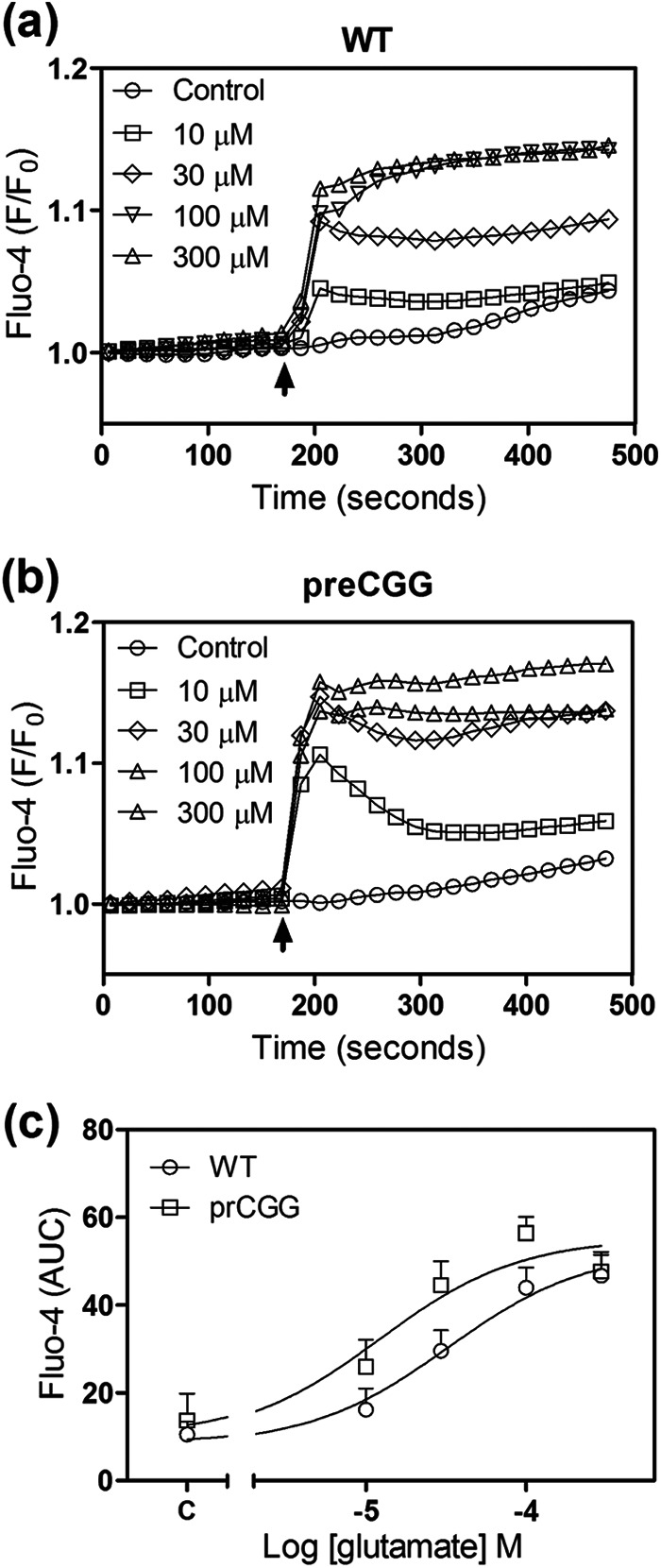
PreCGG cortical astrocytes are more sensitive to Glu exposure measured in a population level. a and b, Glu-induced Ca2+ response in WT (a) and preCGG (b) cortical astrocytes (error bars are omitted for clarity). c, concentration-response relationships for Glu-induced Ca2+ response (area under the curve, AUC) in WT and preCGG cortical astrocytes. Each data point represents mean ± S.E. (n = 8). The experiments were repeated three times, and the EC50 values were 35.23 ± 2.80 and 12.94 ± 3.89 μm (n = 3, p < 0.05) for Glu-induced Ca2+ response in WT and preCGG cortical astrocytes, respectively.
FIGURE 12.
PreCGG cortical astrocytes display similar expression levels of mGluR1/5. a, representative Western blots for mGluR1/5 in WT and preCGG cortical astrocytes. b, quantification of mGluR1/5 expression in WT and preCGG cortical astrocyte cultures relative to GFAP. The expression levels for mGluR1/5 were similar to WT controls (n = 8 from four independent cultures, p > 0.05).
DISCUSSION
In this study, we demonstrate that cortical astrocytes isolated from heterozygous KI mice with premutation CGG expansions (∼170 repeats) display ∼3-fold higher Fmr1 mRNA levels, yet 30% reduced FMRP expression when compared with paired astrocyte cultures from WT mice. The patterns of these two key markers are consistent with the observations in brain lysates from KI mice with preCGG expansions and from postmortem brain from human premutation carriers as well as those with clinical FXTAS (18, 23, 43–45). Neurons cultured from preCGG KI mice also maintain a pattern of elevated Fmr1 mRNA and modest to moderately reduced FMRP in vitro (21, 23). The aberrant Ca2+ dynamics, deficits in Glu uptake, and hypersensitivity to Glu receptor signaling reported here, coupled with previous studies of ubiquitin-positive inclusion bodies found in astrocytes in intact brain (15, 32), indicate that histopathological and functional abnormalities extend to preCGG astrocytes.
Cortical astrocytes cultured from preCGG KI mice are shown for the first time to display a higher frequency of asynchronous spontaneous Ca2+ oscillations. Alterations in Ca2+ dynamics have been demonstrated in neurons cultured from FMRP-null models of FXS (46, 47) as well as Fmr1 preCGG mouse neuronal cultures (23) and premutation human neurons derived from induced pluripotent stem cells (48). In the null Fmr1 Drosophila and Fmr1 KO mouse models, the Ca2+ impairments have been ascribed to the complete loss of FMRP expression. However, recent studies also demonstrated enhanced spontaneous Ca2+ oscillations in neurons derived from KI mice and human induced pluripotent stem cells bearing CGG expansion repeats in FMR1 in the premutation range that maintain >50% of the FMRP expression observed in corresponding WT cells. PreCGG neurons maintain significantly higher (>3-fold) Fmr1 mRNA levels when compared with neurons derived from their respective WT controls (23, 48), suggesting that an elevated Fmr1 RNA may also contribute to abnormal growth and Ca2+ dynamics observed in neuronal cell models of Fmr1 related disorders. However, we cannot exclude the potential contribution of reduced levels of FMRP and elevated Fmr1 mRNA in producing preCGG astrocyte pathophysiology.
Abnormal Ca2+ dynamics are associated with modest but consistent decreases in both Glu uptake and expression levels of GLAST and GLT-1 in preCGG astrocyte cultures. Attenuated Glu uptake and Glu transporter expression are likely contributing to the enhanced Ca2+ oscillatory behavior observed in preCGG cortical astrocytes because: 1) WT cortical astrocytes chronically exposed to Glu transporter inhibitor pharmacologically display enhanced spontaneous Ca2+ oscillations to an extent comparable with that found in preCGG cortical astrocytes; 2) WT astrocytes with reduced GLAST expression (∼30%) by siRNA interference exhibit increased spontaneous Ca2+ oscillations; and 3) Glu augments Ca2+ oscillations in both WT and preCGG cortical astrocyte. The latter is consistent with previous demonstrations for Glu receptor modulating Ca2+ oscillations in astrocytes where application of mGluR (group I and group II) antagonists suppressed spontaneous Ca2+ oscillations, whereas application of mGluR (group I and group II) agonists evoked Ca2+ oscillatory behavior at intermediate concentrations (38). Although how reduced Glu uptake activity influences astrocyte Ca2+ signaling remains unclear, reduced Glu uptake activity may transiently affect extracellular Glu concentration, which in turn can stimulate asynchronous Ca2+ oscillations (49).
Consistent with reduced expression of Glu transporters in preCGG astrocyte culture, we also demonstrated that cortical lysates from aged preCGG mice (50 ± 3 weeks) display significantly reduced GLAST expression when compared with age-matched WT mice. GLT-1 expression in preCGG brain cortex was also lower than that found in WT cortex, although the difference was not statistically significant due to the variation in the expression levels. A consistent trend of lower GLAST expression was also observed in younger (∼20 weeks) preCGG mice (∼155 CGG repeats) brain cortices relative to age-matched WT, but no differences in GLT-1 were detectable at younger ages (P0, P14, P20, and P35; data not shown). Collectively, these data suggest that the deficit in Glu transporters may progress with age in the preCGG KI mouse model.
Impaired Glu uptake has been reported in preCGG hippocampal astrocytes, which have lower affinity for Glu uptake than WT (23). This functional deficit is responsible, at least in part, for the aberrant neuronal network firing, which is likely directly pertinent to the reduced neuronal complexity identified in the same in vitro preCGG model (21). Embryonic premutation mice display early migration defects in the neocortex and altered expression of neuronal lineage markers (22). The abnormal asynchronous Ca2+ oscillatory behavior observed in preCGG astrocytes reported here offers more direct evidence of an inherent functional impairment that may indirectly contribute to abnormal neuronal cell growth because cellular Ca2+ dynamics are known to regulate the release of gliotransmitters, including Glu (50). Moreover, astrocytes can influence neuronal connectivity by improper clearance of Glu from synaptic sites (51). Reduction in Glu uptake can stimulate extrasynaptic NMDA and metabotropic glutamate receptor activation at hippocampal CA1 synapses of aged rats (52). Therefore, impaired Glu uptake as well as a higher frequency of spontaneous Ca2+ oscillations observed in preCGG astrocytes may contribute to neuropathological changes observed in premutation models and possibly to the etiology of FXTAS.
Altered Glu transport has been recently reported in Fmr1 KO astrocytes co-cultured with either WT or Fmr1 KO neurons (53). However, unlike our present finding in the preCGG KI model, Fmr1 KO astrocyte cultures showed similar levels of expression of both GLT-1 and GLAST when compared with WT controls. Another distinction between Fmr1 KO and FMR1 preCGG KI astrocytes is that the deficits of Glu transporters have been ascribed to reduced mGluR5 expression, which is regulated by FMRP (53), a difference not observed in the current study of preCGG cortical astrocytes, which maintain a moderate level of FMRP.
PreCGG astrocytes are functionally more sensitive than WT to Glu challenge. This is consistent with similar results obtained from induced pluripotent stem cell neurons derived from a patient with an FMR1 premutation expansion (48), as well as neuronal/astrocyte co-cultures and slices from preCGG mouse hippocampus that exhibit amplified type I mGluR-dependent clustered burst firing and enhanced mGluR-dependent long term depression, which is positively associated with the CGG repeat length (23, 54, 55).
The influence of abnormal astrocytes on neuronal development has also been demonstrated in other neurological disorders, such as Rett syndrome and FXS (28, 31). Restoration of MeCP2 in the astrocytes possessing mutant Mecp2 allele exerted a non-cell-autonomous positive effect on mutant neurons in vivo, restoring dendritic morphology and increasing the excitatory Glu transporter VGLUT1 level (31). Reactive astrocytes have been reported in an FXS mouse model (37). Co-culture with normal astrocytes prevented the development of abnormal dendrite morphology and precluded the reduction of presynaptic and postsynaptic protein clusters in neurons from a fragile X mouse (28). It is conceivable that correction of astrocyte pathology may be a potential therapeutic intervention to ameliorate the neuropathology of FXTAS.
In summary, we demonstrate alternations in Ca2+ dynamics and Glu uptake in mouse preCGG cortical astrocytes. It appears that the reduced Glu transporter activity leads to the enhanced spontaneous Ca2+ oscillations in preCGG cortical astrocytes. These deficits in astrocytes ultimately may contribute to the development of the neuronal pathology of FXTAS. Thus, the function of the rescue astrocytes may represent a potential therapeutic invention to ameliorate the neuropathology of FXTAS.
Acknowledgments
We thank Binh Ta for carrying out all of the genotyping and Lee Rognlie-Howes for coordinating the breeding of mice used in this study.
This work was supported, in whole or in part, by National Institutes of Health Grants RC1 AG036022 (to P. J. H. and I. N. P.), 1R01 HD064817 (to I. N. P.), and P01ES011269 (to I. N. P.). This work was also supported by the United States Environmental Protection Agency Grants R833292 and R829388 (to I. N. P.) and HD02274 (to F. T.).
- FXTAS
- fragile X-associated tremor/ataxia syndrome
- FMRP
- FMR1 protein
- FXS
- fragile X syndrome
- preCGG
- premutation CGG expansion
- l-trans-PDC
- l-trans-pyrrolidine-2,4-dicarboxylic acid
- GLAST
- glutamate aspartate transporter
- GFAP
- glial fibrillary acidic protein
- EAAT
- excitatory amino acid transporter
- MPEP
- 2-methyl-6-(phenylethynyl)pyridine
- DHPG
- dihydroxyphenylglycine
- mGluR
- metabotropic glutamate receptor
- KI
- knock-in
- P
- postnatal day
- CI
- confidence intervals.
REFERENCES
- 1. Hagerman P. J. (2008) The fragile X prevalence paradox. J. Med. Genet. 45, 498–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez-Revenga L., Madrigal I., Pagonabarraga J., Xunclà M., Badenas C., Kulisevsky J., Gomez B., Milà M. (2009) Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur. J. Hum. Genet. 17, 1359–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goodlin-Jones B. L., Tassone F., Gane L. W., Hagerman R. J. (2004) Autistic spectrum disorder and the fragile X premutation. J. Dev. Behav. Pediatr. 25, 392–398 [DOI] [PubMed] [Google Scholar]
- 4. Hessl D., Tassone F., Loesch D. Z., Berry-Kravis E., Leehey M. A., Gane L. W., Barbato I., Rice C., Gould E., Hall D. A., Grigsby J., Wegelin J. A., Harris S., Lewin F., Weinberg D., Hagerman P. J., Hagerman R. J. (2005) Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am. J. Med. Genet. B Neuropsychiatr. Genet. 139B, 115–121 [DOI] [PubMed] [Google Scholar]
- 5. Farzin F., Perry H., Hessl D., Loesch D., Cohen J., Bacalman S., Gane L., Tassone F., Hagerman P., Hagerman R. (2006) Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J. Dev. Behav. Pediatr. 27, S137–S144 [DOI] [PubMed] [Google Scholar]
- 6. Hagerman R. J. (2006) Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J. Dev. Behav. Pediatr. 27, 63–74 [DOI] [PubMed] [Google Scholar]
- 7. Amiri K., Hagerman R. J., Hagerman P. J. (2008) Fragile X-associated tremor/ataxia syndrome: an aging face of the fragile X gene. Arch. Neurol. 65, 19–25 [DOI] [PubMed] [Google Scholar]
- 8. Brouwer J. R., Willemsen R., Oostra B. A. (2009) The FMR1 gene and fragile X-associated tremor/ataxia syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 782–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tassone F., Adams J., Berry-Kravis E. M., Cohen S. S., Brusco A., Leehey M. A., Li L., Hagerman R. J., Hagerman P. J. (2007) CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 566–569 [DOI] [PubMed] [Google Scholar]
- 10. Berry-Kravis E., Abrams L., Coffey S. M., Hall D. A., Greco C., Gane L. W., Grigsby J., Bourgeois J. A., Finucane B., Jacquemont S., Brunberg J. A., Zhang L., Lin J., Tassone F., Hagerman P. J., Hagerman R. J., Leehey M. A. (2007) Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Movement Disorders 22, 2018–2030 [DOI] [PubMed] [Google Scholar]
- 11. Bourgeois J. A., Coffey S. M., Rivera S. M., Hessl D., Gane L. W., Tassone F., Greco C., Finucane B., Nelson L., Berry-Kravis E., Grigsby J., Hagerman P. J., Hagerman R. J. (2009) A review of fragile X premutation disorders: expanding the psychiatric perspective. J. Clin. Psychiatry 70, 852–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacquemont S., Hagerman R. J., Hagerman P. J., Leehey M. A. (2007) Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 6, 45–55 [DOI] [PubMed] [Google Scholar]
- 13. Hagerman R., Au J., Hagerman P. (2011) FMR1 premutation and full mutation molecular mechanisms related to autism. J. Neurodev. Disord. 3, 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tassone F., Hagerman R. J., Taylor A. K., Gane L. W., Godfrey T. E., Hagerman P. J. (2000) Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willemsen R., Hoogeveen-Westerveld M., Reis S., Holstege J., Severijnen L. A., Nieuwenhuizen I. M., Schrier M., van Unen L., Tassone F., Hoogeveen A. T., Hagerman P. J., Mientjes E. J., Oostra B. A. (2003) The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum. Mol. Genet. 12, 949–959 [DOI] [PubMed] [Google Scholar]
- 16. Brouwer J. R., Mientjes E. J., Bakker C. E., Nieuwenhuizen I. M., Severijnen L. A., Van der Linde H. C., Nelson D. L., Oostra B. A., Willemsen R. (2007) Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp. Cell Res. 313, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacquemont S., Farzin F., Hall D., Leehey M., Tassone F., Gane L., Zhang L., Grigsby J., Jardini T., Lewin F., Berry-Kravis E., Hagerman P. J., Hagerman R. J. (2004) Aging in individuals with the FMR1 mutation. Am. J. Ment. Retard. 109, 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tassone F., Iwahashi C., Hagerman P. J. (2004) FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS). RNA Biol. 1, 103–105 [DOI] [PubMed] [Google Scholar]
- 19. Greco C. M., Hagerman R. J., Tassone F., Chudley A. E., Del Bigio M. R., Jacquemont S., Leehey M., Hagerman P. J. (2002) Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125, 1760–1771 [DOI] [PubMed] [Google Scholar]
- 20. Arocena D. G., Iwahashi C. K., Won N., Beilina A., Ludwig A. L., Tassone F., Schwartz P. H., Hagerman P. J. (2005) Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum. Mol. Genet. 14, 3661–3671 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y., Tassone F., Berman R. F., Hagerman P. J., Hagerman R. J., Willemsen R., Pessah I. N. (2010) Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum. Mol. Genet. 19, 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham C. L., Martínez Cerdeño V., Navarro Porras E., Prakash A. N., Angelastro J. M., Willemsen R., Hagerman P. J., Pessah I. N., Berman R. F., Noctor S. C. (2011) Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum. Mol. Genet. 20, 64–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao Z., Hulsizer S., Tassone F., Tang H. T., Hagerman R. J., Rogawski M. A., Hagerman P. J., Pessah I. N. (2012) Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum. Mol. Genet. 21, 2923–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaplan E. S., Cao Z., Hulsizer S., Tassone F., Berman R. F., Hagerman P. J., Pessah I. N. (2012) Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J. Neurochem. 123, 613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haydon P. G. (2001) GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2, 185–193 [DOI] [PubMed] [Google Scholar]
- 26. Ben Achour S., Pont-Lezica L., Béchade C., Pascual O. (2010) Is astrocyte calcium signaling relevant for synaptic plasticity? Neuron Glia Biol. 6, 147–155 [DOI] [PubMed] [Google Scholar]
- 27. Jacobs S., Nathwani M., Doering L. C. (2010) Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci. 11, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobs S., Doering L. C. (2010) Astrocytes prevent abnormal neuronal development in the fragile X mouse. J. Neurosci. 30, 4508–4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maezawa I., Swanberg S., Harvey D., LaSalle J. M., Jin L. W. (2009) Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J. Neurosci. 29, 5051–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maezawa I., Jin L. W. (2010) Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J. Neurosci. 30, 5346–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lioy D. T., Garg S. K., Monaghan C. E., Raber J., Foust K. D., Kaspar B. K., Hirrlinger P. G., Kirchhoff F., Bissonnette J. M., Ballas N., Mandel G. (2011) A role for glia in the progression of Rett's syndrome. Nature 475, 497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wenzel H. J., Hunsaker M. R., Greco C. M., Willemsen R., Berman R. F. (2010) Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 1318, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greco C. M., Berman R. F., Martin R. M., Tassone F., Schwartz P. H., Chang A., Trapp B. D., Iwahashi C., Brunberg J., Grigsby J., Hessl D., Becker E. J., Papazian J., Leehey M. A., Hagerman R. J., Hagerman P. J. (2006) Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 129, 243–255 [DOI] [PubMed] [Google Scholar]
- 34. Iwahashi C., Tassone F., Hagerman R. J., Yasui D., Parrott G., Nguyen D., Mayeur G., Hagerman P. J. (2009) A quantitative ELISA assay for the fragile X mental retardation 1 protein. J. Mol. Diagn. 11, 281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao Z., LePage K. T., Frederick M. O., Nicolaou K. C., Murray T. F. (2010) Involvement of caspase activation in azaspiracid-induced neurotoxicity in neocortical neurons. Toxicol. Sci. 114, 323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao Z., Hammock B. D., McCoy M., Rogawski M. A., Lein P. J., Pessah I. N. (2012) Tetramethylenedisulfotetramine alters Ca2+ dynamics in cultured hippocampal neurons: mitigation by NMDA receptor blockade and GABAA receptor-positive modulation. Toxicol. Sci. 130, 362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuskaitis C. J., Beurel E., Jope R. S. (2010) Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome. Biochim. Biophys. Acta 1802, 1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zur Nieden R., Deitmer J. W. (2006) The role of metabotropic glutamate receptors for the generation of calcium oscillations in rat hippocampal astrocytes in situ. Cereb. Cortex. 16, 676–687 [DOI] [PubMed] [Google Scholar]
- 39. Rothstein J. D., Dykes-Hoberg M., Pardo C. A., Bristol L. A., Jin L., Kuncl R. W., Kanai Y., Hediger M. A., Wang Y., Schielke J. P., Welty D. F. (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 [DOI] [PubMed] [Google Scholar]
- 40. Arriza J. L., Fairman W. A., Wadiche J. I., Murdoch G. H., Kavanaugh M. P., Amara S. G. (1994) Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 14, 5559–5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huber K. M., Gallagher S. M., Warren S. T., Bear M. F. (2002) Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U.S.A. 99, 7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bear M. F., Huber K. M., Warren S. T. (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 [DOI] [PubMed] [Google Scholar]
- 43. Brouwer J. R., Severijnen E., de Jong F. H., Hessl D., Hagerman R. J., Oostra B. A., Willemsen R. (2008) Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology 33, 863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brouwer J. R., Huizer K., Severijnen L. A., Hukema R. K., Berman R. F., Oostra B. A., Willemsen R. (2008) CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J. Neurochem. 107, 1671–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allen E. G., He W., Yadav-Shah M., Sherman S. L. (2004) A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum. Genet. 114, 439–447 [DOI] [PubMed] [Google Scholar]
- 46. Tessier C. R., Broadie K. (2011) The fragile X mental retardation protein developmentally regulates the strength and fidelity of calcium signaling in Drosophila mushroom body neurons. Neurobiol. Dis. 41, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deng P. Y., Sojka D., Klyachko V. A. (2011) Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J. Neurosci. 31, 10971–10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu J., Koscielska K. A., Cao Z., Hulsizer S., Grace N., Mitchell G., Nacey C., Githinji J., McGee J., Garcia-Arocena D., Hagerman R. J., Nolta J., Pessah I. N., Hagerman P. J. (2012) Signaling defects in iPSC-derived fragile X premutation neurons. Hum. Mol. Genet. 21, 3795–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Padmashri R., Sikdar S. K. (2008) Glutamate transporter blockade affects Ca2+ responses in astrocytes. Neuroscience 151, 56–62 [DOI] [PubMed] [Google Scholar]
- 50. Koizumi S., Fujishita K., Tsuda M., Shigemoto-Mogami Y., Inoue K. (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. U.S.A. 100, 11023–11028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oliet S. H., Piet R., Poulain D. A. (2001) Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292, 923–926 [DOI] [PubMed] [Google Scholar]
- 52. Potier B., Billard J. M., Rivière S., Sinet P. M., Denis I., Champeil-Potokar G., Grintal B., Jouvenceau A., Kollen M., Dutar P. (2010) Reduction in glutamate uptake is associated with extrasynaptic NMDA and metabotropic glutamate receptor activation at the hippocampal CA1 synapse of aged rats. Aging Cell 9, 722–735 [DOI] [PubMed] [Google Scholar]
- 53. Higashimori H., Morel L., Huth J., Lindemann L., Dulla C., Taylor A., Freeman M., Yang Y. (2013) Astroglial FMRP-dependent translational down-regulation of mGluR5 underlies glutamate transporter GLT1 dysregulation in the fragile X mouse. Hum Mol Genet., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iliff A. J., Renoux A. J., Krans A., Usdin K., Sutton M. A., Todd P. K. (2013) Impaired activity-dependent FMRP translation and enhanced mGluR-dependent LTD in Fragile X premutation mice. Hum. Mol. Genet. 22, 1180–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hunsaker M. R., Kim K., Willemsen R., Berman R. F. (2012) CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus 22, 2260–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]