Background: Signaling pathways underlying BV8-mediated oncogenesis remain unknown.
Results: BV8-STAT3 forms a feed-forward loop in both normal and malignant myeloid cells and promotes tumor growth.
Conclusion: JAK2/STAT3 signaling plays critical roles in BV8-mediated myeloid cell-dependent oncogenesis.
Significance: This study identifies a novel role of BV8-STAT3 signaling in mediating cross-talk between tumor microenvironment and tumor cells.
Keywords: Myeloid Cell, shRNA, Signal Transduction, STAT3, Tumor, BV8
Abstract
An important role of BV8 in mobilization of myeloid cells and myeloid cell-dependent angiogenesis has been established. Recently, it has also been shown that granulocyte colony-stimulating factor (G-CSF)-induced BV8 expression is STAT3 dependent in CD11b+Gr1+ myeloid cells. However, the BV8 downstream signaling pathway(s) intrinsic to myeloid cells crucial for angiogenesis, and potentially also for development of cancers of myeloid origin, remains largely unknown. Here we show that BV8 activates STAT3, which is critical for regulating genes important for both tumor cell proliferation/survival and tumor angiogenesis, in both normal and malignant myeloid cells. Further, BV8-induced STAT3 activation requires Janus-activated kinase 2 (JAK2) activity as shown by both genetic and pharmacologic inhibition. Knocking down BV8 in human myeloid leukemia cells inhibits STAT3 activity and expression of STAT3 downstream angiogenic and pro-proliferation/survival genes, leading to a decrease in tumor cell viability. BV8 shRNA expressing leukemia cells exhibit reduced STAT3 activity and tumor growth in vivo. Taken together, we have delineated a signaling pathway downstream of BV8 that plays critical roles in both the tumor microenvironment and malignant myeloid cells for angiogenesis and tumor cell proliferation/survival.
Introduction
BV8/prokineticin-2 is a small and secreted protein, which belongs to a family of peptides with a five-disulfide bridge motif (1–3). BV8 and its homologous protein, endocrine gland-derived VEGF, bind to two highly related G-protein-coupled receptors, prokineticin receptor (PKR)3 1 and 2 (4, 5). BV8 has been shown to participate in a wide range of biological activities, including angiogenesis (6), neurogenesis (7), inflammatory pain (8), and circadian rhythm control (9). Intensive research over the past several years has revealed an important role of BV8 in promoting myeloid cell mobilization (10), as well as myeloid cell-mediated tumor angiogenesis (11). However, the underlying mechanisms mediating these functions remain largely unknown. In myeloid cells, BV8 expression can be strongly induced by G-CSF in vitro and in vivo (11). Moreover, a recent study showed that such induction in normal mouse myeloid cells is STAT3-dependent (12, 13).
STAT3 is a well known transcription factor that is important for up-regulation of many genes critical for tumor cell invasion/mobilization and tumor angiogenesis (14–18). Meanwhile, STAT3 regulates numerous genes underlying tumor cell survival and proliferation (14, 15, 19, 20). In addition to being a point of convergence for numerous oncogenic tyrosine kinase signaling pathways, recent studies have demonstrated that STAT3 can also be activated by G-protein-coupled receptor(s), specifically, sphingosine-1-phosphate receptor 1 (S1PR1), via JAK2 (17). The receptors for BV8, PKR1 and PKR2, are also G-protein-coupled receptors. How BV8, through its receptors, might stimulate myeloid cell motility and tumor angiogenesis remains undefined.
In the current study, we extend the previous finding in mouse myeloid cells (13) into human leukemia cells that STAT3 is a direct transcription factor for the BV8 gene. We have also identified that the JAK2/STAT3 axis underlies BV8/its receptor(s) signaling. This feed-forward loop between BV8-STAT3 sheds new light on how BV8 promotes myeloid cell-mediated angiogenesis and identifies a novel role of BV8 in promoting oncogenesis intrinsic to malignant cells of myeloid origin.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant human BV8 and G-CSF were obtained from PeproTech (Rocky Hill, NJ) and R&D Systems (Minneapolis, MN), respectively. JAK2 inhibitor AZD1480 was provided by AstraZeneca (Waltham, MA) and dissolved in dimethyl sulfoxide (DMSO) for in vitro studies. For in vivo experiments, AZD1480 was dissolved in water supplemented with 0.5% hypromellose and 0.1% Tween® 80. Antibodies recognizing phospho-STAT3 (Tyr-705), phospho-JAK2 (Tyr-1007/1008), and JAK2 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies recognizing STAT3 (C-20), Bcl-xL (B cell lymphoma-extra large) (H-50), VEGF (A-20), poly(ADP-ribose) polymerase-2 (PARP) (H-250), and BV8 (H-51), as well as human BV8 shRNA lentiviral particles (sc-61409-V), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FLAG-M2 and anti-β-actin were from Sigma. Human STAT3 and control shRNA lentiviral particles were also purchased from Sigma.
Cell Lines
Acute human myelogenous leukemia cell line, KG1, was kindly provided by Dr. Carlotta Glackin (Beckman Research Institute, City of Hope National Medical Center, Duarte, CA). Human U937 monocytic leukemia cell line and mouse B16 melanoma cell line were purchased from the American Type Culture Collection. Mouse renal cell carcinoma cell line, Renca, was provided as a generous gift by Dr. Alfred Chang (University of Michigan Medical Center, Ann Arbor, MI). Mouse endothelial cell lines derived from prostate were kindly provided by S. Huang and J. Fidler (M.D. Anderson Cancer Center, Houston, TX). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS).
Transduction of shRNA Lentiviral Particles and Transfection of Plasmids
Transduction of lentiviral particles into KG1 and U937 cells to generate stable cell lines that expressed human BV8, STAT3, or control shRNA was performed according to the manufacturer's instructions (Sigma). BV8 or STAT3 expression in pooled puromycin-resistant cells was examined by real-time PCR and Western blotting. Stable cell lines were maintained in RPMI 1640 with 10% FBS containing 5 ng/ml puromycin (Sigma). pRC/CMV/STAT3C-FLAG (active form of STAT3) and pRC/CMV were transfected into KG1 cells using the Amaxa cell line nucleofector kit R according to the manufacturer's instructions (Lonza).
Mice and in Vivo Experiments
Animal use procedures were approved by the Institutional Animal Care and Use Committees of the Beckman Research Institute at City of Hope. Athymic nude (NCR− nu/nu), BALB/c, and C57BL/6 mice were purchased from NCI, National Institutes of Health (Bethesda, MD). Jak2flox/flox and Stat3flox/flox mice were kindly provided by Drs. Kay-Uwe Wagner (University of Nebraska Medical Center, Omaha, NE) (21) and S. Akira (Osaka University, Japan), respectively. Both Jak2flox/flox and Stat3flox/flox mice were crossed with Mx1-Cre mice, which were obtained from The Jackson Laboratory. Mice with Jak2−/− or Stat3−/− hematopoietic cells were generated by treating Mx1-Cre/Jak2flox/flox or Mx1-Cre/Stat3flox/flox mice with poly(I-C) as described previously (22). Deletion of Stat3 and Jak2 was verified by real-time RT-PCR. For in vivo KG1 tumor challenge, 1 × 106 of KG1 cells expressing either control or BV8 shRNA were injected intraperitoneally into 7–8-week-old nude mice, which were euthanized at day 60. Tumor volumes were determined at the end of the study, and tumor tissues were collected for further analysis. For Renca tumor challenge, 2.5 × 106 of Renca cells were subcutaneously injected into the flank of 7–8-week-old BALB/c mice. When the average tumor volume reached ∼150 mm3, AZD1480 or vehicle was administered daily by oral gavage at the dose of 50 mg/kg of body weight for 6 consecutive days. For B16 tumor challenge, 1 × 105 of B16 cells were subcutaneously injected into the flank of mice with Stat3+/+, Stat3−/−, Jak2+/+, or Jak2−/− hematopoietic cells. Mice were euthanized 14 days after tumor inoculation. The preparation of single-cell suspension from mouse spleen and the enrichment of splenic CD11b+ myeloid cells were performed as described previously (22).
BV8, G-CSF Stimulation, and AZD1480 Treatment in Vitro
To determine STAT3 activation in KG1 and U937 cells, tumor cells were first starved with serum-free medium for 24 h and subsequently treated with 200 ng/ml human BV8 for the indicated time. Alternatively, freshly isolated mouse splenic CD11b+ myeloid cells were treated with 100 ng/ml human BV8 in RPMI 1640 medium with 1% FBS. Recombinant human Bv8 proteins show cross-reactivity to mouse and rat proteins (PeproTech, Rocky Hill, NJ). For G-CSF stimulation, KG1 cells expressing either control or STAT3 shRNA cultured in Hanks' balanced salt solution with 0.02% bovine serum albumin (BSA) were stimulated with 50 ng/ml G-CSF for 6 h. To examine the effects of AZD1480 in vitro, splenic CD11b+ cells from naive C57BL/6 mice were treated with AZD1480 (1 μm) or DMSO in RPMI 1640 medium with 1% FBS for 1 h and subsequently stimulated with BV8 (100 ng/ml) for various times. To test cell viability, KG1 or U937 cells were cultured in the presence of 200 ng/ml BV8 in RPMI 1640 with 1% FBS for 48 h.
Cell Viability, Cell Cycle, Apoptosis, and Western Blotting Analyses
Viable cell number was determined by counting cell number manually after 0.04% trypan blue staining. Each of the viability tests was repeated at least six times. For cell cycle analysis, KG1 cells in the culture medium (1 × 106/ml) were stained with propidium iodide according to the manufacturer's instructions (BD Biosciences). For apoptosis assays, KG1 cells expressing control or BV8 shRNA were grown in serum-free RPMI 1640 medium for 48 h. Cells were stained with annexin V, Allophycocyanin Conjugate (eBioscience, San Diego, CA) and assessed for the percentage of annexin V-positive population using an Accuri C6 flow cytometer. For Western blotting assay, harvested cells were lysed in the lysis buffer (25 mm Tris-HCl, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 5% glycerol, and 1 mm EDTA). Tumor tissues collected from KG1 xenograft were first homogenized using a homogenizer (Fisher Scientific) and then lysed in the lysis buffer. An equal amount of protein was subjected to SDS-PAGE and blotted with indicated antibodies.
Immunofluorescence
Immunofluorescent staining of tumor sections was described previously (16). Anti-mouse CD31 (BD Biosciences) was used to detect blood vessels in xenograft tumors. The representative images were captured at 200× magnification. Quantification data were obtained by counting CD31-positive vessels manually on three random fields (200×) per slide, three slides per tumor, with four tumors per group.
Real-time RT-PCR
Total RNA was extracted using an RNeasy kit (Qiagen). cDNA was synthesized by using an iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was conducted using iQ SYBR Green supermix (Bio-Rad) and performed by using a Chromo-4 real-time detector (Bio-Rad). Gene-specific primer sets for human/mouse BV8, human HIF1A (hypoxia inducible factor-1α), CDC25A, BCL2, VEGF, and IL1B were purchased from Qiagen. Either GAPDH or 18S rRNA housekeeping gene was used as internal control.
Immunoprecipitation
KG1 cells (10 × 106) expressing control, STAT3, or BV8 shRNA were seeded and cultured in 25 ml of RPMI 1640 medium without FBS for 48 h. Conditioned medium was collected and then concentrated by Amicon Ultra-15 centrifugal filter devices (Millipore Corp., Billerica, MA) according to the manufacturer's instructions. Concentrated medium was mixed with an equal volume of lysis buffer and incubated with 4 μg of anti-BV8 rabbit polyclonal antibody or control rabbit IgG for overnight at 4 °C.
Chromatin Immunoprecipitation (ChIP)
The chromatins were prepared from KG1 cells (5 × 106) expressing control or STAT3 shRNA and subjected to ChIP assay as described previously (18). 4 μg of anti-STAT3 rabbit polyclonal antibody or control rabbit IgG were used for immunoprecipitations. The following primers, 5′-tcatttttgttgagggaaacaggc-3′ and 5′-aacgcctggtttcgagtgccagcc-3′, were used to amplify the human BV8 promoter fragment (−1053 to −783, from the transcription start site) that contained the putative STAT-binding site as suggested by TRANSFAC software.
Statistics
Two-tailed Student's t test was used for statistical analysis.
RESULTS
BV8 Induces STAT3 Activation in Both Normal and Malignant Myeloid Cells
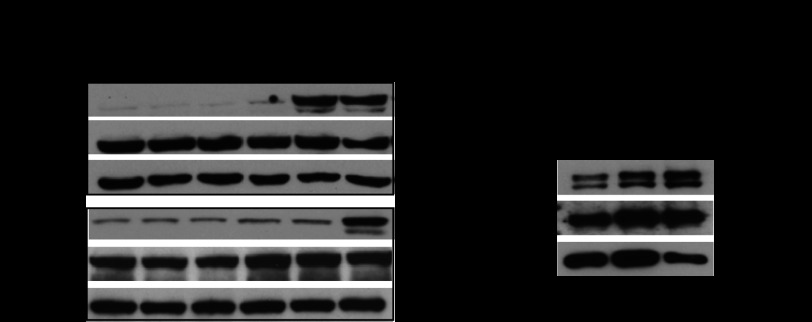
BV8 plays a critical role in promoting tumor growth through angiogenesis (11), but the downstream signaling pathways mediating such effects are largely unknown. Recently, STAT3 has been shown to induce BV8 transcription in myeloid cells (12). However, whether BV8 also activates STAT3 to promote tumor growth has never been studied. To determine whether BV8 could activate STAT3, we stimulated human myeloid leukemia cells KG1 and U937 with BV8 and tested STAT3 activity at different time points. Western blotting analysis showed that BV8 induced significant STAT3 activation after 6 or 16 h of treatment in both KG1 and U937 cells (Fig. 1A). Because Bv8 is majorly produced by CD11b+ myeloid cells and is able to promote the mobilization of myeloid cells to tumor sites (11), we further studied whether BV8 could activate STAT3 in splenic CD11b+ myeloid cells from B16 tumor-bearing mice. Western blotting analysis showed robust STAT3 activation in these CD11b+ myeloid cells upon 6 or 16 h of treatment with Bv8 (Fig. 1B).
FIGURE 1.
BV8 induces STAT3 activation in both malignant and normal myeloid cells. A, Western blotting showing phospho-STAT3 (p-STAT3) and STAT3 protein amounts in KG1 and U937 cells treated with human BV8 (200 ng/ml) at the indicated time points. B, Western blotting showing increased expression of phospho-STAT3 (p-STAT3) proteins in tumor-primed, splenic CD11b+ myeloid cells upon treatment with BV8 (100 ng/ml) for 6 or 16 h. Cells were enriched from splenocytes of B16 tumor-bearing mice; mean ± S.E., n = 3.
STAT3 Regulates BV8 Expression in Both Normal and Malignant Myeloid Cells
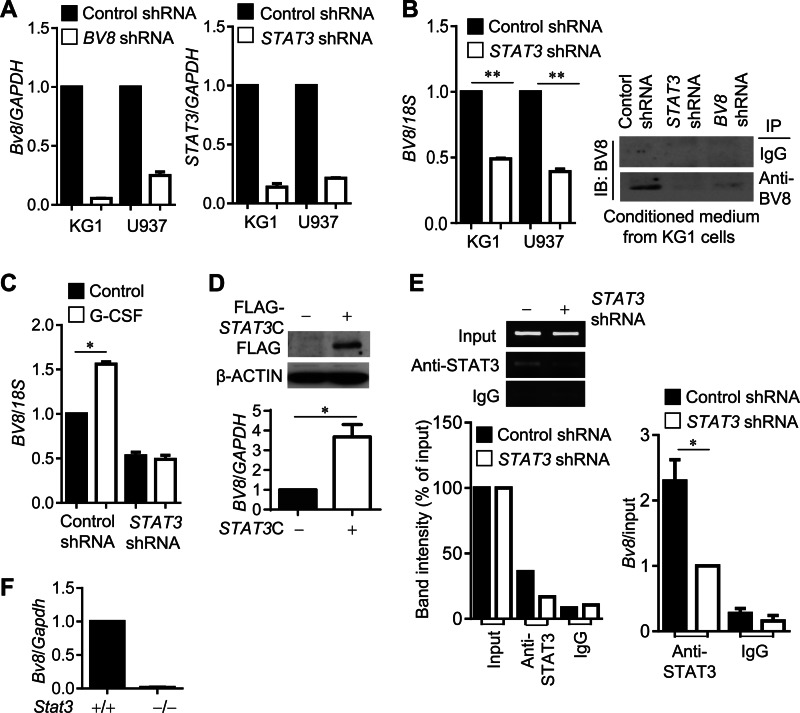
A recent study indicated that STAT3 is a key transcription factor for G-CSF-induced BV8 expression in mouse CD11b+Gr1+ myeloid cells (12). However, whether STAT3 also regulates BV8 in cancer cells of myeloid origin has not been characterized. We therefore generated stable KG1 and U937 cell lines expressing BV8 or STAT3 shRNAs to address whether STAT3 is required for BV8 expression in the malignant cells of myeloid origin. Real-time RT-PCR showed that knockdown efficiency of BV8 or STAT3 gene in both cell lines reached 85–90% (Fig. 2A). BV8 mRNA expression level was drastically reduced in both KG1 and U937 cells expressing STAT3 shRNA (Fig. 2B, left). Importantly, levels of BV8 protein secreted to the culture medium of BV8 or STAT3 shRNA-expressing cells were also significantly reduced when compared with the control shRNA-expressing cells (Fig. 2B, right). To confirm whether G-CSF-induced BV8 expression required STAT3 in malignant cells of myeloid origin, we treated KG1 cells expressing STAT3 or control shRNA with G-CSF and checked their BV8 expression. Real-time PCR showed that BV8 expression was not induced by G-CSF in STAT3 shRNA-expressing cells (Fig. 2C). Furthermore, BV8 expression was significantly up-regulated by overexpression of STAT3C, a constitutively active mutant form of STAT3 (23) in KG-1 cells (Fig. 2D). In addition, we evaluated STAT3 binding activity to the human BV8 promoter in both control and STAT3 shRNA-expressing KG1 cells using ChIP analysis. The data indicated that the binding of STAT3 to the BV8 promoter was reduced in STAT3 knockdown cells (Fig. 2E). Consistently, Bv8 expression was abolished in Stat3-deficient mouse myeloid cells (Fig. 2F). Taken together, we show that BV8 induces STAT3 activation and STAT3 regulates BV8 expression, thereby forming a feed-forward loop in both normal myeloid cells and human malignant cells of myeloid origin.
FIGURE 2.
STAT3 regulates BV8 expression in both malignant and normal myeloid cells. A, BV8 and STAT3 gene expression levels in stable cell lines (KG1 and U937) expressing indicated shRNA were analyzed by real-time RT-PCR. B, left, real-time RT-PCR showing BV8 gene expression in KG1 or U937 cells expressing control or STAT3 shRNA; **, p < 0.01. Right, immunoprecipitation (IP) by BV8 antibodies followed by Western blotting (IB) showing expression levels of KG1 cell-derived BV8 protein. C, BV8 mRNA levels in G-CSF-treated KG1 cells expressing control or STAT3 shRNA shown by real-time RT-PCR; *, p < 0.05. D, upper, BV8 mRNA levels in KG1 cells transfected with control plasmid or plasmid encoding STAT3C; *, p < 0.05. Lower, Western blotting to detect FLAG-tagged STAT3C protein in cells transfected with the indicated plasmids. E, ChIP assays showing STAT3 binding to the BV8 promoter in KG1 cells stably expressing control or STAT3 shRNA. Immunoprecipitated DNA fragments were detected by PCR (left) or quantitative real-time RT-PCR (right). Bottom left, a graph showing the relative intensity of DNA bands in the PCR gel (top), which were obtained by ImageJ software and then plotted as a percentage of the band intensity in each sample to the corresponding input (100%) (bottom left). Bottom right, data from real-time PCR assay were first calculated as (DNA amount in anti-STAT3 immunoprecipitation minus DNA in IgG immunoprecipitation)/(DNA in input), and then compared with the data from the anti-STAT3 antibody immunoprecipitation sample of STAT3 shRNA-expressing cells, which is designated as 1; mean ± S.E., n = 3; *, p < 0.05. F, real-time RT-PCR measuring Bv8 mRNA levels in splenic CD11b+ cells freshly isolated from tumor-bearing mice with Stat3+/+ or Stat3−/− hematopoietic cells (mean ± S.E., n = 3).
BV8-induced STAT3 Activation Requires JAK2
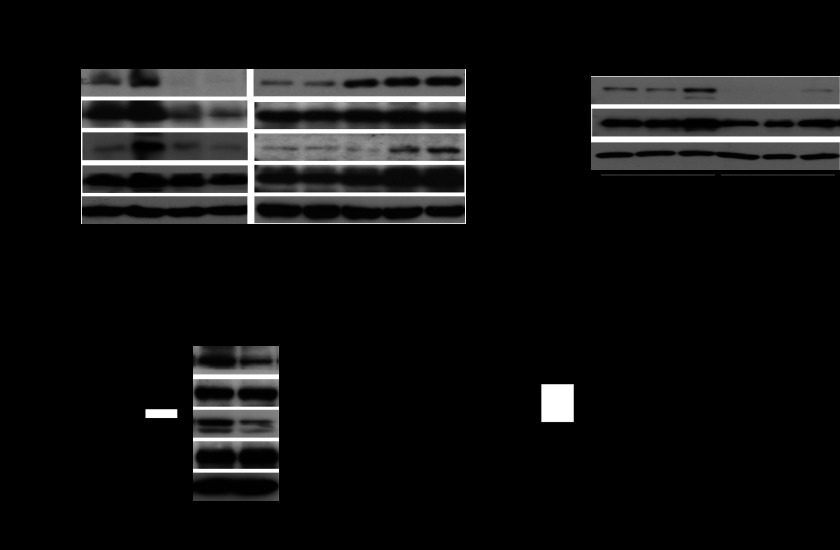
BV8 binds to G-protein-coupled receptors, PKR1 and PKR2, and our previous studies have shown that sphingosine-1-phosphate (S1P), which binds to another G-protein-coupled receptor S1PR1, activates STAT3 through JAK2 (17). Therefore, we investigated whether JAK2 is involved in and necessary for BV8-indcued STAT3 activation. Western blotting analysis showed that phosphorylation of JAK2 was triggered after BV8 stimulation in myeloid cells isolated from B16 tumor-bearing mice or mouse endothelial cells, which correlated with STAT3 activity (Fig. 3A). In addition, BV8 was not able to induce Stat3 activity in Jak2-deficient myeloid cells (Fig. 3A). Moreover, our results showed that AZD1480, a JAK2 inhibitor (24, 25), blocked BV8-induced STAT3 activation in myeloid cells (Fig. 3B). These data indicate that JAK2 is required for BV8-induced STAT3 activation. We further investigated whether JAK2 is required for STAT3 up-regulation of BV8 expression. Treatment with JAK2 inhibitor AZD1480 in Renca mouse model reduced phospho-JAK2 and phospho-STAT3 levels and also led to a reduction in Bv8 expression in myeloid cells (Fig. 3C). In addition, we found that Bv8 expression was significantly reduced in Jak2-deficient myeloid cells (Fig. 3D).
FIGURE 3.
BV8-induced STAT3 activation requires JAK2. A, Western blotting showing expression levels of indicated proteins in splenic CD11b+ cells enriched from B16 tumor-bearing mice with Jak2+/+ or Jak2−/− hematopoietic cells or in mouse endothelial cells. Cells were treated with BV8 for 6 h. p-JAK2, phospho-JAK2; p-STAT3, phospho-STAT3. B, Western blotting to determine expression levels of indicated proteins in splenic CD11b+ cells, which were enriched from naive C57BL/6 mice, treated with AZD1480 (1 μm) or DMSO for 1 h, and subsequently stimulated with BV8 (100 ng/ml) for the indicated times. C, real-time RT-PCR to measure Bv8 mRNA levels in splenic CD11b+ cells freshly isolated from Renca tumor-bearing mice treated with AZD1480 or vehicle for 6 days (left); **, p < 0.01 (mean ± S.E., n = 3). Western blotting showing expression levels of indicated proteins (right). D, Bv8 mRNA levels in splenic CD11b+ cells isolated from B16 tumor-bearing mice with Jak2+/+ and Jak2−/− hematopoietic cells *, p < 0.05 (mean ± S.E., n = 3).
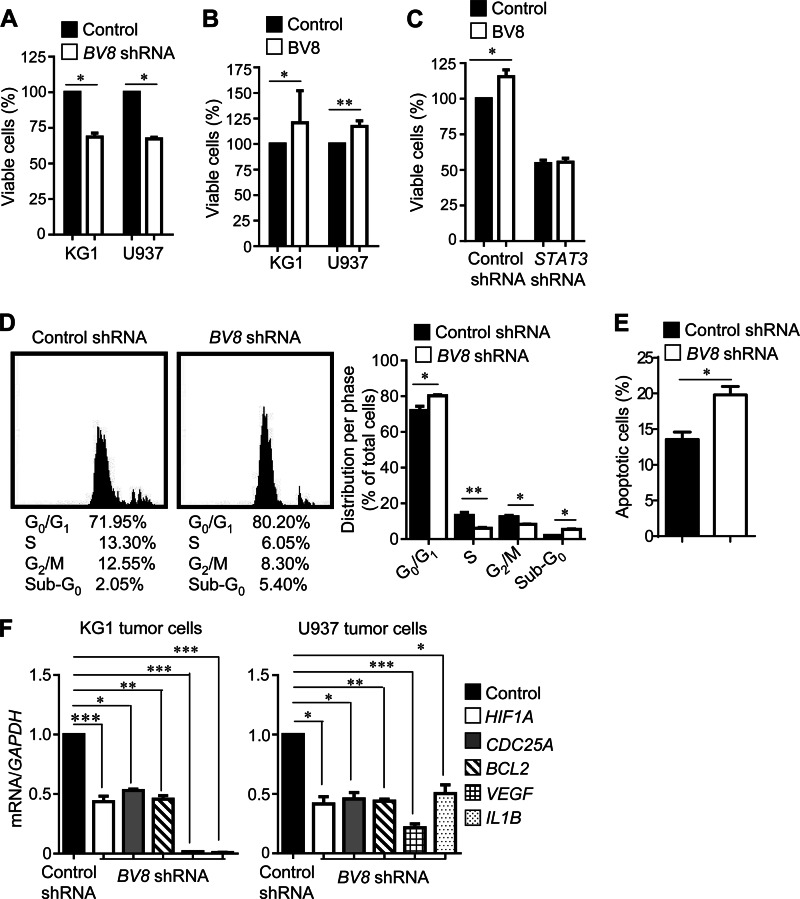
Silencing BV8 Expression Reduces Myeloid Leukemia Cell Viability and Expression of Proliferation/Survival and Angiogenic Genes
To address whether BV8 could also promote tumor growth through increasing tumor cell survival, proliferation, and angiogenic potential, we knocked down BV8 in human myeloid leukemia cells by shRNA and subsequently tested the viability of these cells. Results from these experiments indicated that silencing BV8 expression in the leukemia cells significantly reduced their viability (Fig. 4A). Consistently, treatment with BV8 considerably enhanced the viability of leukemia cells (Fig. 4B). To address whether BV8 promotes the viability of myeloid leukemia cells through STAT3, we knocked down STAT3 by shRNA in KG1 cells and treated them with BV8. In this case, BV8 was unable to increase the viability of leukemia cells (Fig. 4C). These data demonstrated that BV8 promotes myeloid leukemia cell viability in a STAT3-dependent manner. We next carried out cell cycle analysis using flow cytometry to determine whether the modulation of tumor cell growth by BV8 was the result of cell cycle arrest or induction of apoptosis or the simultaneous activation of these two modes. The results revealed that the percentage of KG1 cells expressing BV8 shRNA was increased in sub-G0 and G0/G1 phase, which was accompanied by a corresponding reduction in the percentage of cells in S and G2/M phase (Fig. 4D). Relevant to this, the apoptotic cell percentage was increased in KG1 cells expressing BV8-shRNA when compared with KG1 cells expressing control shRNA (Fig. 4E). Furthermore, expression of genes crucial for proliferation, survival, and angiogenesis in the tumor cells, such as CDC25A, BCL2, HIF1A, VEGF, and IL1B, was significantly reduced in myeloid leukemia cells expressing BV8 shRNA (Fig. 4F).
FIGURE 4.
Silencing BV8 expression reduces myeloid leukemia cell viability and expression of proliferation/survival and angiogenic genes. A–C, BV8-STAT3 regulates leukemia cell proliferation/survival. Cell viability assay was performed in KG1 or U937 cells expressing either control or BV8 shRNA cultured in RPMI 1640 medium with 5% FBS for 48 h (A); KG1 or U937 cells cultured in RPMI 1640 medium (200 ng/ml BV8, 1% FBS) for 48 h (B); or KG1 cells expressing either control or STAT3 shRNA cultured in RPMI 1640 medium (200 ng/ml BV8, 1% FBS) for 48 h (C). Data are generated from six independent experiments with triplicates for each experiment; *, p < 0.05; **, p < 0.01. D, flow cytometric analysis of cell cycle phase in KG1 cells expressing control or BV8 shRNA. Cells in the culture medium were stained with propidium iodide solution. Representative histograms show the cell cycle distribution in KG1 cells. Mean percentages of cell counts in each cycle are shown under the histogram as well as in the graph; mean ± S.D.; n = 2, *, p < 0.05; **, p < 0.01. E, a graph showing the percentage of apoptotic KG1 cells. KG1 cells were serum-starved for 48 h before staining with annexin V solution. Data are representative of three independent experiments; *, p < 0.05. F, real-time RT-PCR showing mRNA levels of STAT3 target genes in KG1 or U937 cells stably expressing control or BV8 shRNA; mean ± S.E., n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Inhibiting BV8 Expression in Myeloid Leukemia Cells Reduces STAT3 Activity and Tumor Growth
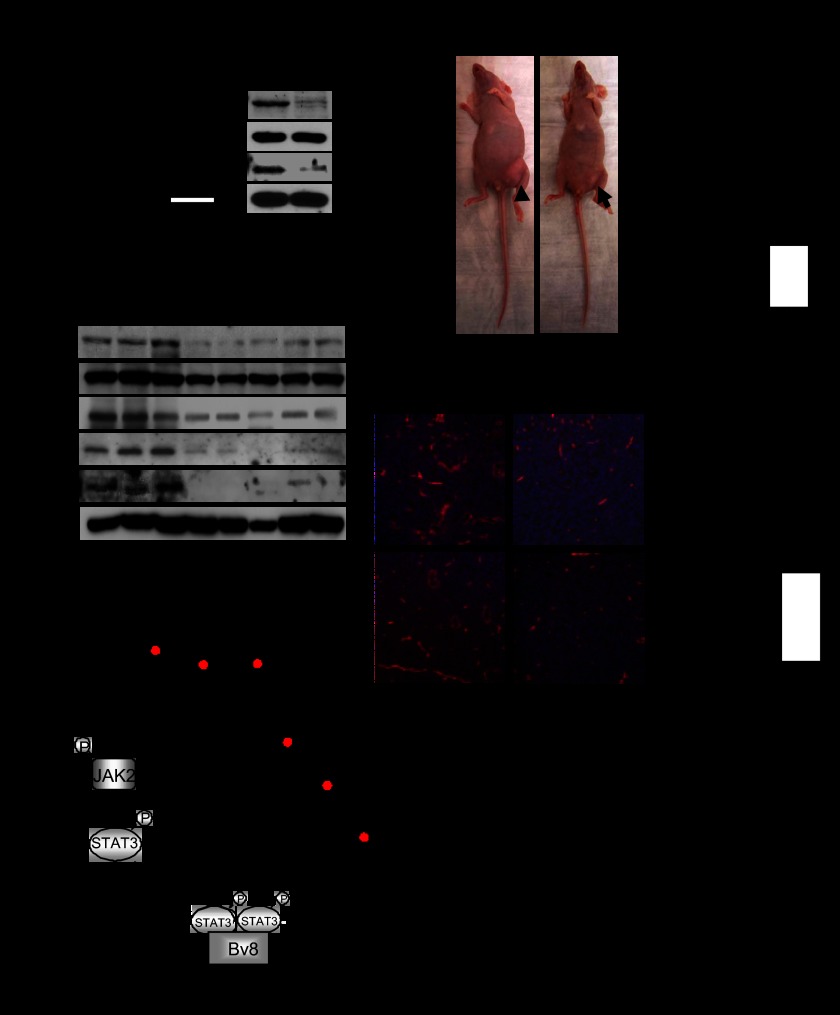
BV8 has emerged as a therapeutic target to inhibit tumor angiogenesis. Anti-BV8 antibody reduced tumor growth and angiogenesis (11). Thus, we knocked down BV8 in human myeloid leukemia cells by shRNA and studied its effect in tumor growth. First, STAT3 activity was significantly reduced in BV8 shRNA-transfected human myeloid leukemia cells (Fig. 5A). Next, we implanted these tumor cells in nude mice. Tumor volume was significantly reduced in mice implanted with BV8 knockdown leukemia cells when compared with mice implanted with control leukemia cells (Fig. 5B). In addition, inhibiting BV8 also attenuated tumor expression of VEGF and Bcl-xL (Fig. 5C). Furthermore, we demonstrated that blood vessels were also decreased in the xenograft KG1 tumors transfected with BV8 shRNA (Fig. 5D).
FIGURE 5.
Inhibiting BV8 expression in myeloid leukemia cells reduces STAT3 activity and tumor growth. A, BV8 gene expression (left) and phospho-STAT3 (p-STAT3) protein expression levels (right) in KG1 cells transduced with control or BV8 shRNA were analyzed by real-time RT-PCR and Western blotting, respectively (mean ± S.E., n = 3). B, representative photo images (left) show the differential growth of abdominal tumors in mice implanted with control or BV8 shRNA-expressing KG1 cells. Right, bar graph showing tumor volumes in each group at the end of studies; for control shRNA group, and for Bv8 shRNA group, *, p < 0.05 (mean ± S.E., n = 3). C, Western blotting showing expression levels of indicated proteins in tumors. Each lane represents a sample from an individual mouse. D, left, representative images of immunofluorescent staining showing CD31+ blood vessels in xenograft tumors. Right, quantifications of CD31+ blood vessels per field (200×) are also shown *, p < 0.05 (mean ± S.E., n = 3). E, a proposed model for a BV8-STAT3 feed-forward loop in both normal and malignant myeloid cells.
DISCUSSION
Our current study has identified a novel JAK2-dependent BV8-STAT3 feed-forward signaling loop intrinsic to myeloid-derived leukemia cells, which contributes to tumor cell proliferation/survival (Fig. 5E). Although STAT3 activation has been traditionally characterized as rapid in the context of certain cytokines and growth factors (14, 15), our previous studies have demonstrated the distinct STAT3 activation mode by G-protein-coupled receptor signaling S1P/S1PR1 that elicits persistent STAT3 activation in tumors (17). BV8 and its cognate receptor signaling, to our knowledge, would be the second example of G-protein-coupled receptor signaling that activates STAT3, through JAK2. Similar to S1P/S1PR1, activation of STAT3 by BV8/BV8 receptor is slower and long lasting relative to IL-6, the quintessential STAT3 activator.
In tumor cells, it takes 6 h to see high levels of STAT3 activation by BV8, which may indicate an indirect effect. However, in endothelial cells, the activation of JAK2 is within an hour. Because the time frame could still be within the limit of direct activation, i.e. in the absence of de novo protein synthesis that causes secondary effects/activation, the activation by BV8 can be direct. At the same time, our data do not rule out completely the involvement of expression of some immediate early genes that might contribute to JAK2 activation, thereby leading to STAT3 activation.
In addition, STAT3 not only up-regulates BV8 transcriptional activation as reported previously (12) but also release of functional BV8 proteins from tumor cells to the tumor milieu to further activate STAT3. Although a role of BV8 in promoting myeloid cell-facilitated tumor angiogenesis has been demonstrated (11), our study unravels a novel role of tumor cell-derived BV8 in providing direct growth advantage to tumor cells. Therefore, a JAK2-dependent BV8-STAT3 signaling in tumors can be an efficient therapeutic target for the treatment of myeloid-derived human cancers.
Although a crucial role of STAT3 for diverse human cancer has been established, being a transcription factor, STAT3 remains a challenging target for current therapeutic interventions. STAT3 is well known to be the signal transducer for many cytokines and growth factors, which activate STAT3 through their tyrosine kinase activity. For that reason, several frontline drugs for cancer therapies, such as Sunitinib and Sorafenib, are tyrosine kinase inhibitors that can inhibit STAT3 at proper doses (26, 27). Unlike the conventional tyrosine kinase inhibitors, the receptors for BV8 are G-protein-coupled receptors, which are among the most druggable targets. Our studies thus open up new opportunities to target STAT3 with inhibitors of BV8/receptors, including both antibody and small molecule inhibitors, for the treatment of myeloid leukemia. Because other signaling pathways, such as ERK/MAPK, PI3K/AKT, Wnt/β-catenin, and NF-κB pathways, are also critical for proliferation/survival of the leukemia cells (28–31), combining inhibitors against BV8/receptors, and these pathways may be necessary to effectively treat myeloid cell leukemia. Our studies also show that JAK2 is critical for BV8/receptor-mediated STAT3 activation, suggesting that the use of JAK2 inhibitor(s) may enhance the efficacy of BV8/receptor inhibitors. Ultimately, when an effective direct STAT3 inhibitor(s) is available, the combination use of drugs against STAT3 and its upstream activators, which include BV8/receptor, may be an optimal option for treating myeloid leukemia. Further studies focusing on combinational therapy are therefore warranted.
Acknowledgments
We thank staff members of the DNA Synthesis Core and the Animal Facility of Beckman Research Institute at City of Hope for their excellent technical support.
This study was supported by the Tim Nesvig Fund at the City of Hope Comprehensive Cancer Center, the National Institutes of Health (Grants R01 CA122976, U54 CA163117, P50 CA107399, and R01CA146092 to H. Y. and P30 CA033572 to the City of Hope Cancer Center, the V Foundation Translational Research Grant, and the HEADstrong Foundation, in memory of Nicholas E. Colleluori.
- PKR
- prokineticin receptor
- DMSO
- dimethyl sulfoxide
- S1PR1
- sphingosine-1-phosphate receptor 1
- S1P
- sphingosine-1-phosphate
- Bcl
- B cell lymphoma.
REFERENCES
- 1. Li M., Bullock C. M., Knauer D. J., Ehlert F. J., Zhou Q. Y. (2001) Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol. 59, 692–698 [DOI] [PubMed] [Google Scholar]
- 2. LeCouter J., Kowalski J., Foster J., Hass P., Zhang Z., Dillard-Telm L., Frantz G., Rangell L., DeGuzman L., Keller G. A., Peale F., Gurney A., Hillan K. J., Ferrara N. (2001) Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412, 877–884 [DOI] [PubMed] [Google Scholar]
- 3. Mollay C., Wechselberger C., Mignogna G., Negri L., Melchiorri P., Barra D., Kreil G. (1999) Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur. J. Pharmacol. 374, 189–196 [DOI] [PubMed] [Google Scholar]
- 4. Lin D. C., Bullock C. M., Ehlert F. J., Chen J. L., Tian H., Zhou Q. Y. (2002) Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J. Biol. Chem. 277, 19276–19280 [DOI] [PubMed] [Google Scholar]
- 5. Masuda Y., Takatsu Y., Terao Y., Kumano S., Ishibashi Y., Suenaga M., Abe M., Fukusumi S., Watanabe T., Shintani Y., Yamada T., Hinuma S., Inatomi N., Ohtaki T., Onda H., Fujino M. (2002) Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem. Biophys. Res. Commun. 293, 396–402 [DOI] [PubMed] [Google Scholar]
- 6. LeCouter J., Lin R., Tejada M., Frantz G., Peale F., Hillan K. J., Ferrara N. (2003) The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 100, 2685–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melchiorri D., Bruno V., Besong G., Ngomba R. T., Cuomo L., De Blasi A., Copani A., Moschella C., Storto M., Nicoletti F., Lepperdinger G., Passarelli F. (2001) The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur. J. Neurosci. 13, 1694–1702 [DOI] [PubMed] [Google Scholar]
- 8. Giannini E., Lattanzi R., Nicotra A., Campese A. F., Grazioli P., Screpanti I., Balboni G., Salvadori S., Sacerdote P., Negri L. (2009) The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc. Natl. Acad. Sci. U.S.A. 106, 14646–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng M. Y., Bullock C. M., Li C., Lee A. G., Bermak J. C., Belluzzi J., Weaver D. R., Leslie F. M., Zhou Q. Y. (2002) Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410 [DOI] [PubMed] [Google Scholar]
- 10. LeCouter J., Zlot C., Tejada M., Peale F., Ferrara N. (2004) Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc. Natl. Acad. Sci. U.S.A. 101, 16813–16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shojaei F., Wu X., Zhong C., Yu L., Liang X. H., Yao J., Blanchard D., Bais C., Peale F. V., van Bruggen N., Ho C., Ross J., Tan M., Carano R. A., Meng Y. G., Ferrara N. (2007) Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 [DOI] [PubMed] [Google Scholar]
- 12. Qu X., Zhuang G., Yu L., Meng G., Ferrara N. (2012) Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: key role of Stat3 signaling. J. Biol. Chem. 287, 19574–19584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu R., Kujawski M., Pan H., Shively J. E. (2012) Tumor angiogenesis mediated by myeloid cells is negatively regulated by CEACAM1. Cancer Res. 72, 2239–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu H., Jove R. (2004) The STATs of cancer – new molecular targets come of age. Nat. Rev. Cancer 4, 97–105 [DOI] [PubMed] [Google Scholar]
- 15. Yu H., Kortylewski M., Pardoll D. (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7, 41–51 [DOI] [PubMed] [Google Scholar]
- 16. Kujawski M., Kortylewski M., Lee H., Herrmann A., Kay H., Yu H. (2008) Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Invest. 118, 3367–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee H., Deng J., Kujawski M., Yang C., Liu Y., Herrmann A., Kortylewski M., Horne D., Somlo G., Forman S., Jove R., Yu H. (2010) STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat. Med. 16, 1421–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kortylewski M., Xin H., Kujawski M., Lee H., Liu Y., Harris T., Drake C., Pardoll D., Yu H. (2009) Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 15, 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowman T., Broome M. A., Sinibaldi D., Wharton W., Pledger W. J., Sedivy J. M., Irby R., Yeatman T., Courtneidge S. A., Jove R. (2001) Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 7319–7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grandis J. R., Drenning S. D., Zeng Q., Watkins S. C., Melhem M. F., Endo S., Johnson D. E., Huang L., He Y., Kim J. D. (2000) Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 97, 4227–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krempler A., Qi Y., Triplett A. A., Zhu J., Rui H., Wagner K. U. (2004) Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis 40, 52–57 [DOI] [PubMed] [Google Scholar]
- 22. Kortylewski M., Kujawski M., Wang T., Wei S., Zhang S., Pilon-Thomas S., Niu G., Kay H., Mulé J., Kerr W. G., Jove R., Pardoll D., Yu H. (2005) Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 11, 1314–1321 [DOI] [PubMed] [Google Scholar]
- 23. Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E., Jr. (1999) Stat3 as an oncogene. Cell 98, 295–303 [DOI] [PubMed] [Google Scholar]
- 24. Hedvat M., Huszar D., Herrmann A., Gozgit J. M., Schroeder A., Sheehy A., Buettner R., Proia D., Kowolik C. M., Xin H., Armstrong B., Bebernitz G., Weng S., Wang L., Ye M., McEachern K., Chen H., Morosini D., Bell K., Alimzhanov M., Ioannidis S., McCoon P., Cao Z. A., Yu H., Jove R., Zinda M. (2009) The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 16, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xin H., Herrmann A., Reckamp K., Zhang W., Pal S., Hedvat M., Zhang C., Liang W., Scuto A., Weng S., Morosini D., Cao Z. A., Zinda M., Figlin R., Huszar D., Jove R., Yu H. (2011) Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res. 71, 6601–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xin H., Zhang C., Herrmann A., Du Y., Figlin R., Yu H. (2009) Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 69, 2506–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang F., Jove V., Buettner R., Xin H., Wu J., Wang Y., Nam S., Xu Y., Ara T., DeClerck Y. A., Seeger R., Yu H., Jove R. (2012) Sorafenib inhibits endogenous and IL-6/S1P induced JAK2-STAT3 signaling in human neuroblastoma, associated with growth suppression and apoptosis. Cancer Biol. Ther. 13, 534–541 [DOI] [PubMed] [Google Scholar]
- 28. Wu J., Wong W. W., Khosravi F., Minden M. D., Penn L. Z. (2004) Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 64, 6461–6468 [DOI] [PubMed] [Google Scholar]
- 29. Tamburini J., Elie C., Bardet V., Chapuis N., Park S., Broët P., Cornillet-Lefebvre P., Lioure B., Ugo V., Blanchet O., Ifrah N., Witz F., Dreyfus F., Mayeux P., Lacombe C., Bouscary D. (2007) Constitutive phosphoinositide 3-kinase/Akt activation represents a favorable prognostic factor in de novo acute myelogenous leukemia patients. Blood 110, 1025–1028 [DOI] [PubMed] [Google Scholar]
- 30. De Toni F., Racaud-Sultan C., Chicanne G., Mas V. M., Cariven C., Mesange F., Salles J. P., Demur C., Allouche M., Payrastre B., Manenti S., Ysebaert L. (2006) A crosstalk between the Wnt and the adhesion-dependent signaling pathways governs the chemosensitivity of acute myeloid leukemia. Oncogene 25, 3113–3122 [DOI] [PubMed] [Google Scholar]
- 31. Guzman M. L., Neering S. J., Upchurch D., Grimes B., Howard D. S., Rizzieri D. A., Luger S. M., Jordan C. T. (2001) Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 98, 2301–2307 [DOI] [PubMed] [Google Scholar]