Abstract
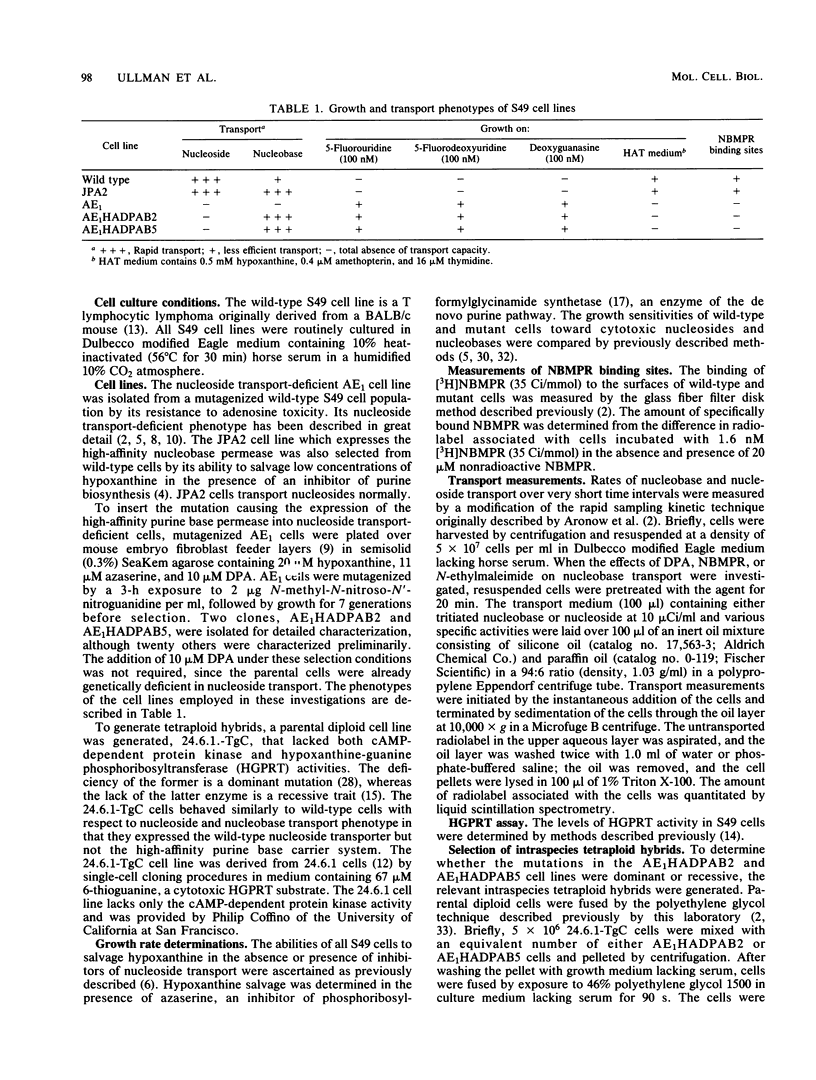
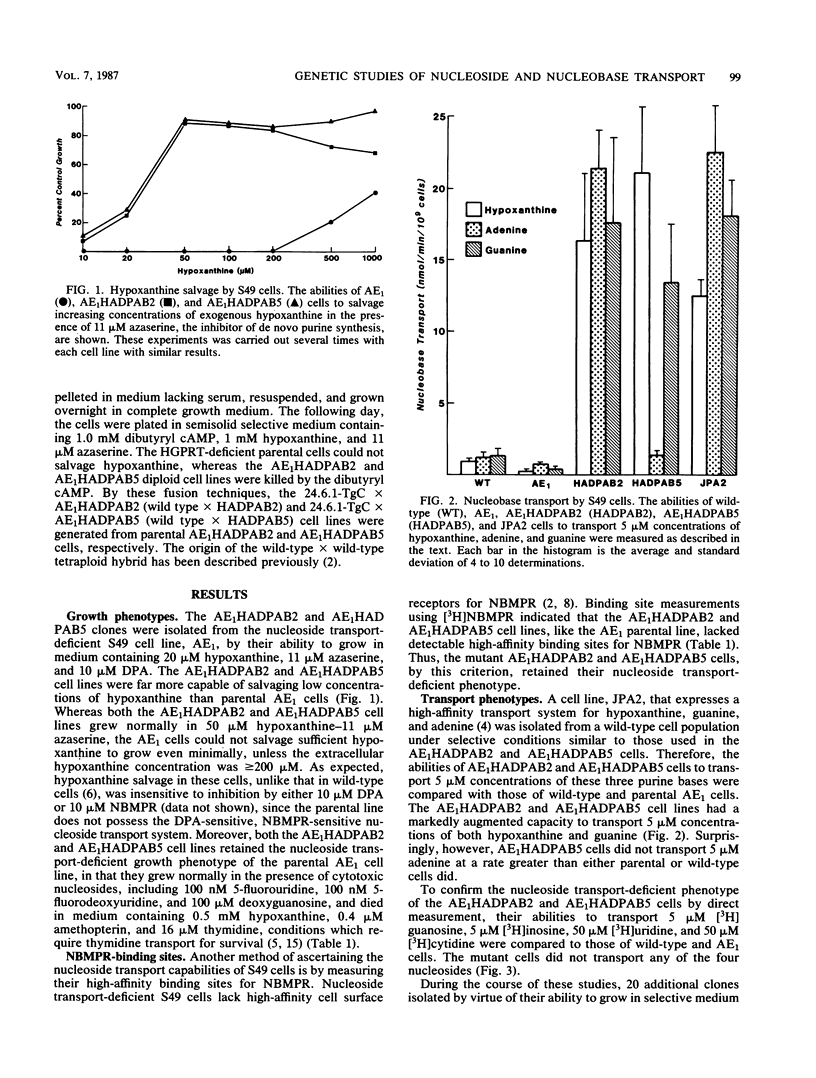
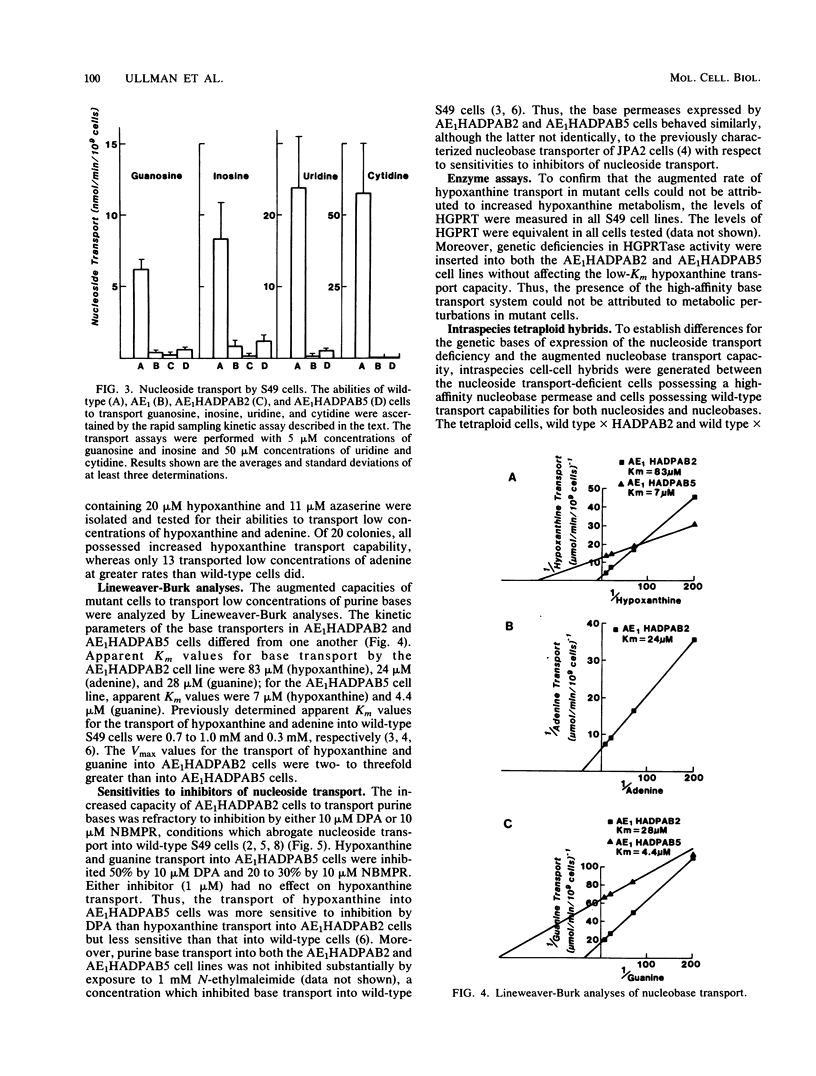
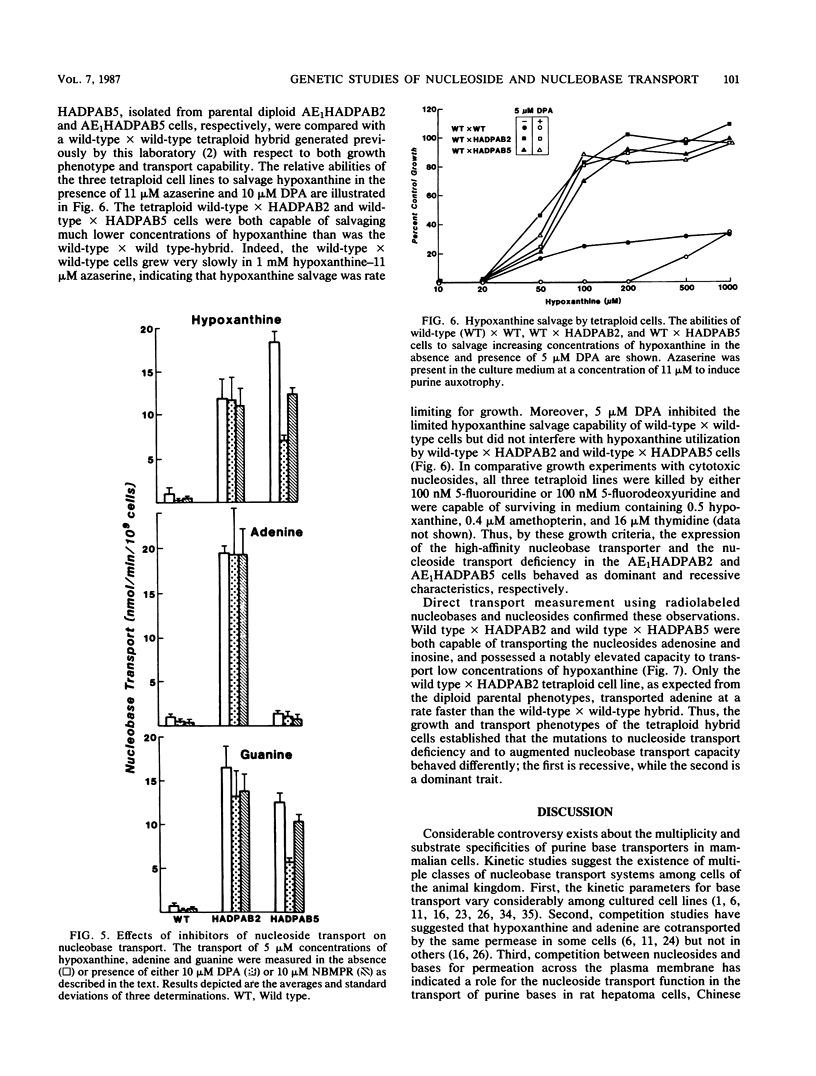
A novel type of somatic mutation that causes the expression of a high-affinity purine base permease (B. Aronow, D. Toll, J. Patrick, P. Hollingsworth, K. McCartan, and B. Ullmann, Mol. Cell Biol. 6:2957-2962, 1986) has been inserted into nucleoside transport-deficient S49 cells. Two classes of mutants expressing this nucleobase permease were generated. The first, as exemplified by the AE1HADPAB2 cell line, possessed an augmented capacity to transport low concentrations of the three purine bases, hypoxanthine, guanine, and adenine. The second class of mutants, as typified by the AE1HADPAB5 clone, possessed an augmented capability to translocate low levels of hypoxanthine and guanine, but not adenine. Neither the AE1HADPAB2 nor the AE1HADPAB5 cells could transport nucleosides, suggesting that the expression of the high-affinity base transporter did not reverse the mutation in the nucleoside transport system. The transport of purine bases by both AE1HADPAB2 and AE1HADPAB5 cells was much less sensitive than that by wild-type cells to inhibition by dipyridamole, 4-nitrobenzylthionosine, and N-ethylmaleimide, potent inhibitors of nucleoside and nucleobase transport in wild-type S49 cells. Fusion of the AE1HADPAB2 and AE1HADPAB5 cell lines with wild-type cells indicated that the expression of the high-affinity base transporter behaved in a dominant fashion, while the nucleoside transport deficiency was a recessive trait. These data suggest that the high-affinity purine base transporter of mutant cells and the nucleoside transport function of wild-type cells are products of different genes and that expression of the former probably requires the unmasking or alteration of a specific genetic locus that is silent or different in wild-type cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford B. L., Barnes E. M., Jr Hypoxanthine transport by cultured Chinese hamster lung fibroblasts. J Biol Chem. 1976 Aug 25;251(16):4823–4827. [PubMed] [Google Scholar]
- Aronow B., Allen K., Patrick J., Ullman B. Altered nucleoside transporters in mammalian cells selected for resistance to the physiological effects of inhibitors of nucleoside transport. J Biol Chem. 1985 May 25;260(10):6226–6233. [PubMed] [Google Scholar]
- Aronow B., Hollingsworth P., Patrick J., Ullman B. Incomplete nucleoside transport deficiency with increased hypoxanthine transport capability in mutant T-lymphoblastoid cells. Mol Cell Biol. 1986 Apr;6(4):1296–1303. doi: 10.1128/mcb.6.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow B., Toll D., Patrick J., Hollingsworth P., McCartan K., Ullman B. Expression of a novel high-affinity purine nucleobase transport function in mutant mammalian T lymphoblasts. Mol Cell Biol. 1986 Aug;6(8):2957–2962. doi: 10.1128/mcb.6.8.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow B., Ullman B. Role of the nucleoside transport function in the transport and salvage of purine nucleobases. J Biol Chem. 1986 Feb 15;261(5):2014–2019. [PubMed] [Google Scholar]
- Aronow B., Ullman B. Thymidine incorporation in nucleoside transport-deficient lymphoma cells. J Biol Chem. 1985 Dec 25;260(30):16274–16278. [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Membrane transport of purine and pyrimidine bases and nucleosides in animal cells. Int Rev Cytol. 1975;42:287–336. doi: 10.1016/s0074-7696(08)60983-3. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Kolassa N., Uehara Y., Dahlig-Harley E., Harley E. R., Paterson A. R. Absence of binding sites for the transport inhibitor nitrobenzylthioinosine on nucleoside transport-deficient mouse lymphoma cells. Biochim Biophys Acta. 1981 Dec 21;649(3):769–777. doi: 10.1016/0005-2736(81)90182-6. [DOI] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Cohen A., Ullman B., Martin D. W., Jr Characterization of a mutant mouse lymphoma cell with deficient transport of purine and pyrimidine nucleosides. J Biol Chem. 1979 Jan 10;254(1):112–116. [PubMed] [Google Scholar]
- Cybulski R. L., Fry D. W., Goldman I. D. Adenosine stimulation of uphill adenine transport in L1210 leukemia cells. Evidence for a novel countertransport mechanism. J Biol Chem. 1981 May 10;256(9):4455–4459. [PubMed] [Google Scholar]
- Daniel V., Litwack G., Tomkins G. M. Induction of cytolysis of cultured lymphoma cells by adenosine 3':5'-cyclic monophosphate and the isolation of resistant variants. Proc Natl Acad Sci U S A. 1973 Jan;70(1):76–79. doi: 10.1073/pnas.70.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Goebel D., Allen K., Kaur K., Ullman B. Genetic analysis of adenine metabolism in Leishmania donovani promastigotes. Evidence for diploidy at the adenine phosphoribosyltransferase locus. J Biol Chem. 1984 Dec 10;259(23):14617–14623. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- MELNICK I., BUCHANAN J. M. Biosynthesis of the purines. XIV. Conversion of (alpha-N-formyl) glycinamide ribotide to (alpha-N-formyl) glycinamidine ribotide; purification and requirements of the enzyme system. J Biol Chem. 1957 Mar;225(1):157–162. [PubMed] [Google Scholar]
- Marz R., Wohlhueter R. M., Plagemann P. G. Purine and pyrimidine transport and phosphoribosylation and their interaction in overall uptake by cultured mammalian cells. A re-evaluation. J Biol Chem. 1979 Apr 10;254(7):2329–2338. [PubMed] [Google Scholar]
- Oliver J. M., Paterson A. R. Nucleoside transport. I. A mediated process in human erythrocytes. Can J Biochem. 1971 Feb;49(2):262–270. doi: 10.1139/o71-038. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Oliver J. M. Nucleoside transport. II. Inhibition by p-nitrobenzylthioguanosine and related compounds. Can J Biochem. 1971 Feb;49(2):271–274. doi: 10.1139/o71-039. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Brown R. R., Paul B., Paterson A. R. Binding of the nucleoside transport inhibitor 4-nitrobenzylthioinosine to erythrocyte membranes. Can J Biochem. 1973 May;51(5):666–672. doi: 10.1139/o73-083. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Hypoxanthine transport in mammalian cells: cell type-specific differences in sensitivity to inhibition by dipyridamole and uridine. J Membr Biol. 1984;81(3):255–262. doi: 10.1007/BF01868718. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Nucleoside transport in cultured mammalian cells. Multiple forms with different sensitivity to inhibition by nitrobenzylthioinosine or hypoxanthine. Biochim Biophys Acta. 1984 Jun 13;773(1):39–52. doi: 10.1016/0005-2736(84)90548-0. [DOI] [PubMed] [Google Scholar]
- Puziss M. B., Wohlhueter R. M., Plagemann P. G. Adenine transport and binding in cultured mammalian cells deficient in adenine phosphoribosyltransferase. Mol Cell Biol. 1983 Jan;3(1):82–90. doi: 10.1128/mcb.3.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C. Studies on the uptake of nucleic acid precursors into cells in tissue culture. Biochim Biophys Acta. 1968 Jun 24;158(3):435–447. doi: 10.1016/0304-4165(68)90297-3. [DOI] [PubMed] [Google Scholar]
- Slaughter R. S., Barnes E. M., Jr Hypoxanthine transport by Chinese hamster lung fibroblasts: Kinetics and inhibition of nucleosides. Arch Biochem Biophys. 1979 Oct 1;197(1):349–355. doi: 10.1016/0003-9861(79)90255-8. [DOI] [PubMed] [Google Scholar]
- Slaughter R. S., Fenwick R. G., Jr, Barnes E. M., Jr Hypoxanthine and thymidine compete for transport in Chinese hamster fibroblasts. Arch Biochem Biophys. 1981 Oct 1;211(1):494–499. doi: 10.1016/0003-9861(81)90482-3. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., van Daalen Wetters T., Coffino P. Kinase-negative mutants of S49 mouse lymphoma cells carry a trans-dominant mutation affecting expression of cAMP-dependent protein kinase. Cell. 1978 Dec;15(4):1351–1361. doi: 10.1016/0092-8674(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Taube R. A., Berlin R. D. Membrane transport of nucleosides in rabbit polymorphonuclear leukocytes. Biochim Biophys Acta. 1972 Jan 17;255(1):6–18. doi: 10.1016/0005-2736(72)90003-x. [DOI] [PubMed] [Google Scholar]
- Ullman B. Characterization of mutant murine lymphoma cells with altered inosinate dehydrogenase activities. J Biol Chem. 1983 Jan 10;258(1):523–528. [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Clift S. M., Martin D. W., Jr Isolation and characterization of purine-nucleoside phosphorylase-deficient T-lymphoma cells and secondary mutants with altered ribonucleotide reductase: genetic model for immunodeficiency disease. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1074–1078. doi: 10.1073/pnas.76.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Ullman B., Kaur K., Watts T. Genetic studies on the role of the nucleoside transport function in nucleoside efflux, the inosine cycle, and purine biosynthesis. Mol Cell Biol. 1983 Jul;3(7):1187–1196. doi: 10.1128/mcb.3.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witney F. R., Taylor M. W. Role of adenine phosphoribosyltransferase in adenine uptake in wild-type and APRT- mutants of CHO. Biochem Genet. 1978 Oct;16(9-10):917–926. doi: 10.1007/BF00483743. [DOI] [PubMed] [Google Scholar]
- Zylka J. M., Plagemann P. G. Purine and pyrimidine transport by cultured Novikoff cells. Specificities and mechanism of transport and relationship to phosphoribosylation. J Biol Chem. 1975 Aug 10;250(15):5756–5767. [PubMed] [Google Scholar]



