Abstract
Objective: Skin damage induced by ischemia/reperfusion (I/R) is a multifactorial process that often occurs in plastic surgery. The mechanisms of I/R injury include hypoxia, inflammation, and oxidative damage. Hydrogen gas has been reported to alleviate cerebral I/R injury by acting as a free radical scavenger. Here, we assessed the protective effect of hydrogen-rich saline (HRS) on skin flap I/R injury. Methods: Abdominal skin flaps of rats were elevated and ischemia was induced for 3 h; subsequently, HRS or physiological saline was administered intraperitoneally 10 min before reperfusion. On postoperative Day 5, flap survival, blood perfusion, the accumulation of reactive oxygen species (ROS), and levels of cytokines were evaluated. Histological examinations were performed to assess inflammatory cell infiltration. Results: Skin flap survival and blood flow perfusion were improved by HRS relative to the controls. The production of malondialdehyde (MDA), an indicator of lipid peroxidation, was markedly reduced. A multiplex cytokine assay revealed that HRS reduced the elevation in the levels of inflammatory cytokines, chemokines and growth factors, with the exception of RANTES (regulated on activation, normal T-cell expressed and secreted) growth factor. HRS treatment also reduced inflammatory cell infiltration induced by I/R injury. Conclusions: Our findings suggest that HRS mitigates I/R injury by decreasing inflammation and, therefore, has the potential for application as a therapy for improving skin flap survival.
Keywords: Hydrogen-rich saline, Ischemia/reperfusion, Skin flaps, Oxidative damage, Inflammation
1. Introduction
Skin flap transfer is a routine practice in wound coverage and reconstruction in plastic surgery. Viability is the most important concern, but unfortunately occasional distal necrosis is a fact of life and good planning is not always sufficient to avoid such an outcome. Ischemia/reperfusion (I/R) injury is believed to be a major cause of flap loss. The formation of reactive oxygen species (ROS) during reperfusion triggers lipid peroxidation, protein and nucleic acid damage (Siemionow and Arslan, 2004), and initiates I/R damage (van den Heuvel et al., 2009). Also, inflammation is considered to be a critical element in the pathogenesis of I/R injury. The massive influx of neutrophils results in tissue injury beyond that caused by I/R alone. Numerous substances have been examined in an attempt to attenuate I/R injury in skin or muscle, such as hydrogen sulfide (Henderson et al., 2010), caffeic acid phenethyl ester (Aydogan et al., 2007), and vitamin E (Arato et al., 2010). Most of these studies have shown positive results but lacked independent large-scale follow-up reports.
Ohsawa et al. (2007) reported that inhalation of hydrogen (H2) gas could selectively mitigate ·OH damage in a rat model of middle cerebral artery occlusion, generating an antioxidant effect without affecting the signaling of other ROS. Further research demonstrated that the inhalation of H2 gas produced protective effects on I/R injury in various settings, such as myocardial infarction (Hayashida et al., 2008), intestinal injury (Buchholz et al., 2008), and hepatic injury (Fukuda et al., 2007). Furthermore, H2 has been reported to downregulate ConA-induced mouse liver inflammation (Kajiya et al., 2009) and to protect mice against multiple organ damage in a zymosan-induced generalized model of inflammation (Xie et al., 2010).
In contrast to other therapeutic but potentially toxic antioxidant gases (nitric oxide, carbon monoxide, hydrogen sulfide, and ozone), H2 is less expensive and safer for clinical application (Nakao et al., 2009). However, the clinical application of H2 gas is not convenient and may be dangerous because H2 gas is flammable and explosive. H2 gas-saturated physiological saline, or hydrogen-rich saline (HRS), is easy to use and safe to apply. To our knowledge, no published data exist on the protective effect of HRS on skin flap I/R injury. The primary objective of the present study was to evaluate the effect of HRS on the viability of abdominal skin flaps after I/R.
2. Materials and methods
2.1. Animals
All experiments were approved by the Committee on Animal Rights Protection of Peking Union Medical College Hospital and were performed in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Adult male Sprague-Dawley (SD) rats weighing 270–320 g were used in this study. The rats were housed under standard conditions at 22 °C to 25 °C with a 12-h light-dark cycle and were fed a normal diet with water provided ad lib pre- and postoperatively.
2.2. HRS production and distribution studies
HRS was made by dissolving H2 in normal saline (6 h) under high pressure (0.4 MPa) until supersaturated. The H2 content was confirmed by the method described by Ohsawa et al. (2007). Fresh HRS was produced weekly to ensure supersaturation. The H2 concentration was maintained above 0.6 mmol/L. Any remaining solution was discarded after the package was opened or damaged.
The rats were anesthetized with an intraperitoneal (i.p.) injection of pentobarbital at a dose of 40 mg/kg body weight (BW) and placed in a supine position. The rats were also i.p. injected with 5 ml/kg BW HRS, 10 ml/kg BW HRS, or the saline control. The H2 concentration in the skin flap tissue was measured using a needle-type H2 sensor (Unisense, Denmark).
2.3. Experimental protocol and group
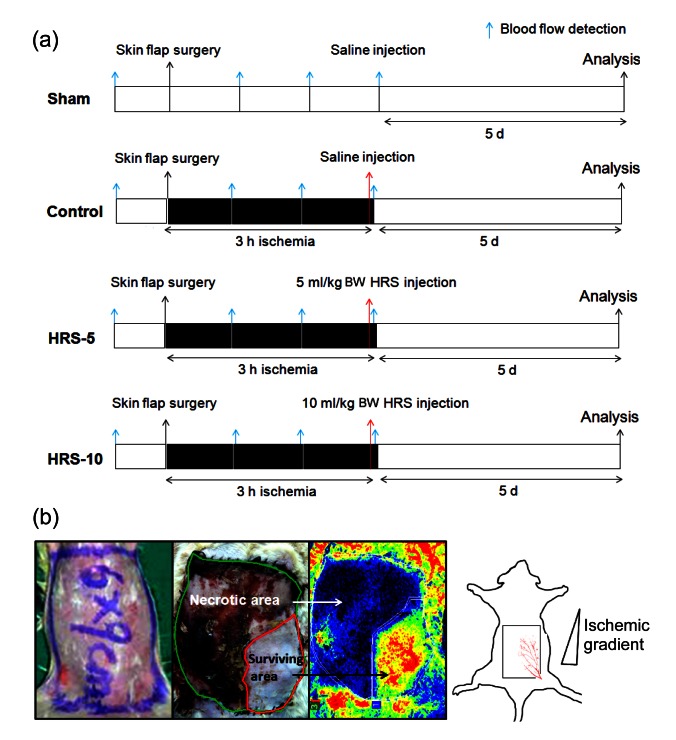
Sixty male SD rats were divided randomly into four groups consisting of 15 animals per group: (1) a sham-operated group: no I/R, HRS, or normal saline injection; (2) a control group: 3-h ischemia induced by clamping the right pedicle followed by an i.p. injection of 5 ml/kg BW normal saline 10 min before reperfusion; and (3) two HRS-treated groups: 3-h ischemia induced and followed by an i.p. injection of 5 ml/kg BW HRS (HRS-5 group) or 10 ml/kg BW HRS (HRS-10 group), 10 min prior to reperfusion (Fig. 1a).
Fig. 1.
Schematic illustration of the experimental protocol used to determine the effect of HRS on I/R skin flaps
(a) Experimental protocol: sixty SD rats were divided into four groups as described in the “Materials and methods” section. Occlusion (3 h) induced ischemia, and HRS or saline was injected. Blue arrows indicate blood flow detection, and red arrows indicate reperfusion time points. (b) A rectangular 6 cm×9 cm skin flap was raised in each animal and blood was supplied by the right superficial epigastric artery. Surgery led to ischemia and subsequent necrosis in the distal part of the skin flap. The percentage of tissue survival was quantified by planimetry, calculating the area of survival (red area) divided by the area of the skin flap (green area) (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
2.4. Epigastric skin flap preparation and survival
An extended epigastric adipocutaneous flap (6 cm×9 cm) was raised in each animal (Küntscher et al., 2002a). The left superficial epigastric artery and vein were ligated so that the blood was supplied only by the right pedicle. A microvascular clamp was used to occlude the artery and vein, inducing skin flap ischemia (3 h). The flap was resutured, and a gap was left for the removal of the clamp. To prevent neovascularization in the wound bed, a silicone sheet was placed between the flap and the abdominal muscle bed. At the end of the ischemic period, the clamp was removed, and heparin (50 000 U/L in 0.5 ml saline) was used to avoid thrombus formation.
Skin flap survival was evaluated by the general observation of survival and necrotic phenomena and subsequently confirmed by Laser Speckle Contrast Analysis (LASCA) cameras (Perimed AB, Sweden). The surviving and necrotic areas were measured using Acrobat 8.0 software. The percentage of flap survival was defined as the ratio of the surviving area to the original flap area (Fig. 1b).
2.5. Flap perfusion
Before scanning, the rats were secured onto the operative bed so that the whole flap, including part of the normal abdominal skin, was exposed. The PeriScan PSI system was positioned above the rats so that an 11 cm×7.5 cm area was imaged. The image acquisition rate was 3 s−1 and lasted for about 3 min. The ambient temperature was maintained between 22 and 25 °C during this process.
On the operative day, blood flow perfusion was detected before surgery and at 1, 2, and 3 h after surgery, denoted Hour 0, Hour 1, Hour 2, and Hour 3, respectively. Perfusion of the necrotic and survival areas was analyzed on the fifth postoperative day. Vascular flow was measured using perfusion units (PUs).
2.6. Hematoxylin and eosin (H&E) staining
On the fifth postoperative day, three pieces of the flap tissue (each about 1 cm2 in size) were taken from the surviving areas of the flap. The harvesting locations were proximal, middle, and distal along the vascular axis. Samples were sectioned into pieces and fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer. They were then embedded in paraffin, mounted onto slides, and H&E stained.
2.7. Malondialdehyde (MDA) measurement
Briefly, 25 mg of frozen flap tissues, cut into pieces on ice, were homogenized in RIPA buffer using a manual glass homogenizer, and centrifuged for 10 min. The supernatant was used for analysis. The MDA content of homogenates was determined spectrophotometrically by measuring the presence of thiobarbituric acid reactive substances (Cayman, USA). Results are expressed as c MDA in nmol/mg protein.
2.8. Multiplex cytokine assay
A rat 23-plex kit (Bio-Rad Laboratories, Hercules, CA, USA) was used to determine the concentrations of 23 cytokines simultaneously in the surviving flap tissues, including interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-17, IL-18, tumor necrotic factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, MIP-3α, interferon (IFN)-γ, erythropoietin (EPO), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), regulated on activation, normal T-cell expressed and secreted (RANTES) growth factor, and growth-regulated oncogene/KC (GRO/KC). The samples (100 mg per sample tissue) were homogenized using a cell lysis kit (Bio-Rad Laboratories), and the protein concentrations were measured by the Bradford assay (Tiangen, Beijing, China).
In brief, the lysates were diluted to 900 μg/ml, and 50 μl of each diluted sample was incubated with antibody-coupled beads. The complexes were washed, incubated with a biotinylated detection antibody, and then incubated with streptavidin-phycoerythrin prior to assessing the cytokine concentration titers. Assay standards resuspended in standard diluents were used to plot the standard curves. Cytokine concentrations were determined by comparison against a set of standards. The data were automatically processed and analyzed by Bio-Plex Manager Software 4.0.
2.9. Statistical analysis
All data are reported as the mean±standard error of the mean (SEM). The differences between the levels of cytokines in each group were determined via one-way analysis of variance (ANOVA), and the Student’s t-test was used to analyze the differences between the surviving and original flap areas. A 2-tailed probability level of P<0.05 was used to indicate statistical significance. Non-significant results are reported as ‘ns’.
3. Results
3.1. Intraperitoneal injection of HRS significantly increased the H2 concentration in the flap
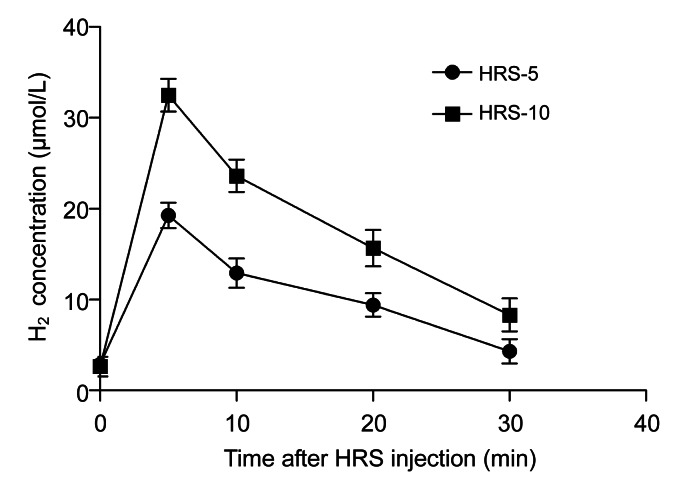
A linear correlation was found between the current value of the H2 microelectrode and the H2 concentration (0–50 μmol/L, R 2=0.9993; data not shown). H2 concentrations peaked at about 5 min after the HRS injection (Fig. 2). HRS treatment at 5 ml/kg BW increased the H2 concentration to (19.14±1.41) μmol/L, while 10 ml/kg BW HRS increased the H2 concentration to (32.18±0.56) μmol/L. These results suggest that i.p. administration of HRS may efficiently deliver H2 into skin flaps. Furthermore, we found no significant difference in the pH values between HRS and normal saline (HRS, 7.32±0.02; saline, 7.34±0.03).
Fig. 2.
Effect of HRS on H2 concentration in the skin flaps
HRS increases H2 concentration in the skin flaps. 5 or 10 ml/kg BW HRS was i.p. injected. The rats were anesthetized with pentobarbital (40 mg/kg BW). A H2 microelectrode (diam. 50 μm) was inserted into the skin flaps. H2 concentration peaked at about 5 min post-injection. The results are expressed as mean±SEM (n=15)
3.2. Flap survival was improved by HRS treatment
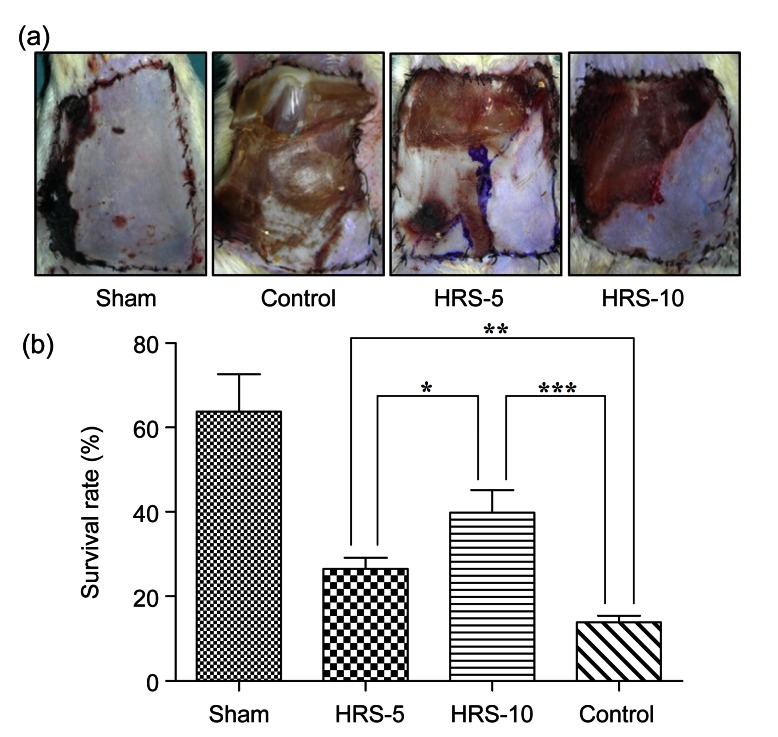
On the fifth postoperative day, necrotic tissues were observed and presented as grey, brown, or black areas with little elasticity. In contrast, the tissue in surviving areas maintained normal elasticity and the skin color was pink and white (Fig. 3a). The quantitative analysis showed that the survival in the control group accounted for (13.92±1.53)% of the total flap area, whereas it represented (26.52±2.60)% in the HRS-5 group (P<0.01 vs. control) and (39.85±5.32)% in the HRS-10 group (P<0.001 vs. control) (Fig. 3b). The control group flap showed a variable degree of full-thickness necrosis and petechiae. These parameters were significantly diminished in the HRS groups, suggesting a protective effect of HRS treatment on skin flap necrosis.
Fig. 3.
Effect of HRS treatment on the survival rate of skin flaps on postoperative Day 5
(a) Rats were anesthetized with i.p. pentobarbital injection and secured to the operative bed. The image was captured with a digital camera. Obvious necrosis was observed in the control group, whereas HRS significantly improved the survival rate in both the HRS-5 and HRS-10 groups. (b) Flap survival was assessed using the methods described in Fig. 1b. Survival area percentage was (13.92±1.53)% of the total flap area in the control group compared to (26.52±2.60)% in the HRS-5 group and (39.85±5.32)% in the HRS-10 group. The results are expressed as mean±SEM (n=15). * P<0.05, ** P<0.01, *** P<0.001
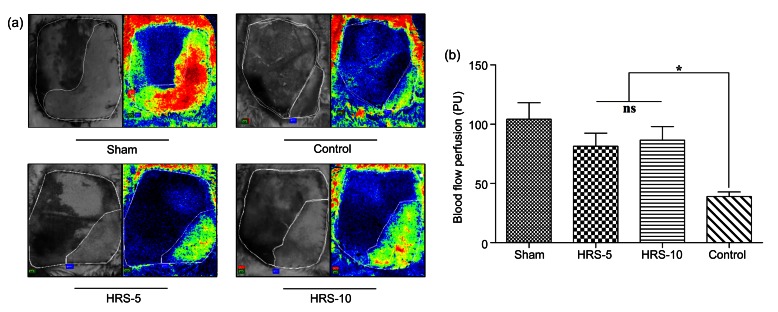
3.3. Flap perfusion was ameliorated by HRS treatment
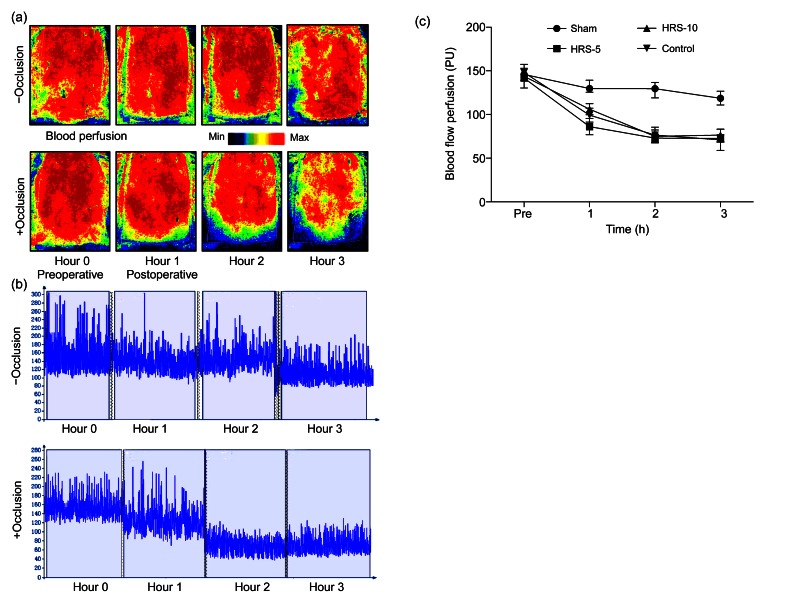
The evaluation of skin flap perfusion was accomplished as described in the “Materials and methods” section. As we expected, occlusion (3 h) significantly decreased blood flow (Figs. 4a and 4b). There were no significant differences in the decrease in blood perfusion among the three operative groups (HRS-5, HRS-10, and control). In contrast, the non-occluded sham group showed relatively stable and much better perfusion (Fig. 4c). These results indicate that a 3-h occlusion was enough to induce flap ischemia.
Fig. 4.
Laser speckle contrast imaging for measuring changes in blood flow perfusion during the perioperative period
(a) Laser speckle contrast imaging was used to estimate perfusion before the operation and at 1, 2 and 3 h after the operation. The region of interest (ROI) was the entire abdominal flap. Occlusion for 3 h was enough to cause skin flap ischemia. The color scale illustrates variation in blood flow from maximal (red) to minimal (dark) perfusion. (b) Flux patterns revealed that occlusion successfully caused the decrease in perfusion from 150 PU to 70 PU, whereas the sham group without occlusion had a relatively stable blood perfusion. The image acquisition rate was 3 s−1 and lasted for about 3 min at Hour 0, Hour 1, Hour 2, and Hour 3. (c) There was no significant difference in blood perfusion between the four groups before the operation. The sham group had a much higher perfusion rate at the 3 h time point. The results are expressed as mean±SEM (n=15) (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Because the flap was axial and centered on the right superficial epigastric artery and vein, the restoration of blood flow following severe ischemia began from the proximal portion of the flap. On postoperative Day 5, perfusion was only (38.98±3.81) PU in the control group. A significantly higher blood perfusion was measured in the HRS groups compared with the controls (Fig. 5a). The quantification of blood flow was (71.32±12.88) PU in the HRS-5 group and (81.79±13.56) PU in the HRS-10 group (P<0.05 vs. control). There was no significant difference in the blood flow levels between the HRS-5 and HRS-10 groups (t=3.17, P>0.05) (Fig. 5b). These data suggest that HRS treatment significantly improves blood flow in skin flaps during reperfusion.
Fig. 5.
Blood flow perfusion analysis using LACSA on postoperative Day 5
(a) The blood flow perfusion in the surviving and necrotic areas was detected. The images demonstrate that HRS ameliorated skin blood flow perfusion in both HRS treatment groups. (b) Quantitative analysis of blood flow on postoperative Day 5. HRS groups had higher blood perfusion compared with the controls. Blood perfusion is expressed as mean±SEM (n=15). * P<0.05
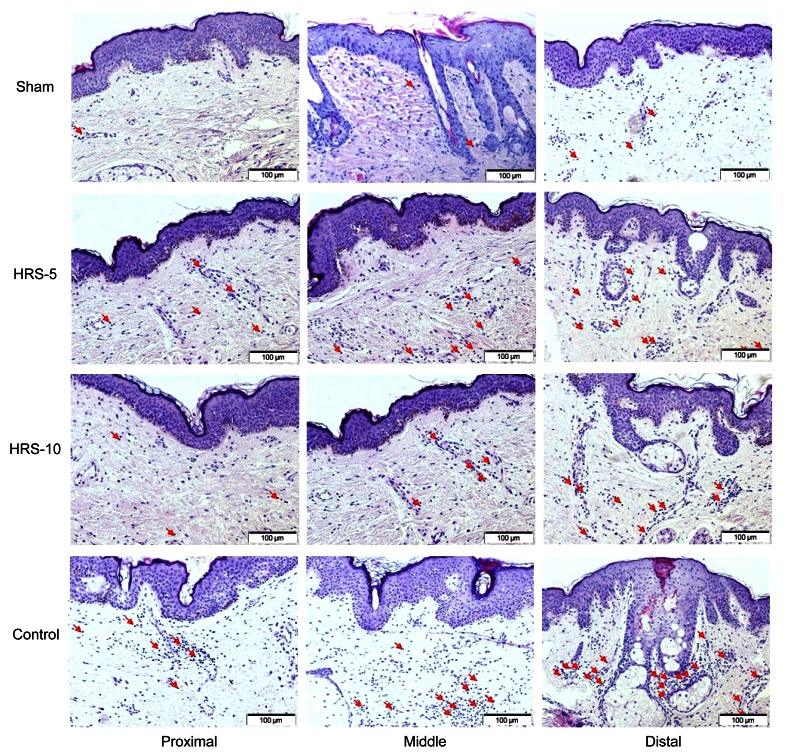
3.4. Histological analysis
Histological analysis of H&E stained tissues was performed to qualify ischemic lesions. As we expected, inflammation was progressively more severe moving from the proximal to distal portion of the tissue, as denoted by an increase in the number of inflammatory cells. No significant infiltration was observed in the sham-operated animals. In contrast, a significant number of infiltrated cells were observed in the skin flaps of the untreated rats in the control group. Thus, treatment with HRS decreased this infiltration, suggesting that HRS attenuates activation of the inflammatory response (Fig. 6).
Fig. 6.
Photomicrographs of the middle area of skin flaps in different groups
The number of infiltrated cells increased from the proximal to the distal portions of the skin flaps. Normal skin tissue was observed, and no obvious inflammation was observed in the sham group. We observed significant cellular infiltration in the control group, suggesting higher activation of the inflammatory process. In contrast, HRS-5 and HRS-10 treatments significantly decreased cellular infiltration. The red arrows indicate inflammatory cells (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
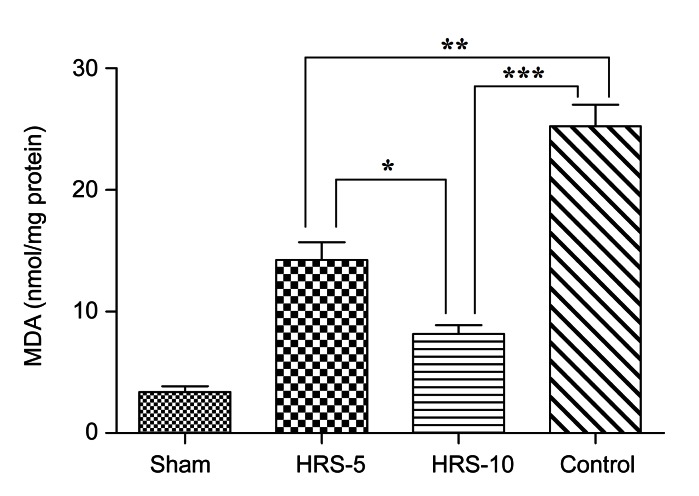
3.5. HRS treatment inhibited the elevation of MDA
As a measurement of lipid peroxidation, MDA levels in the proximal, middle and distal portions of the skin flap were measured. We found that the MDA levels increased from the proximal portion to the distal portion, but there were no significant differences between the three parts (data not shown). The results are expressed as the average of the three parts and correspond to samples obtained 5 d after surgery. The rats subjected to skin flap I/R exhibited an increase in MDA levels compared with the sham-operated rats on the fifth postoperative day (Fig. 7). MDA formation averaged (25.32±1.79) nmol/mg protein. Treatment with HRS resulted in a marked reduction in MDA levels in the HRS-5 group ((14.27±1.43) nmol/mg protein) and in the HRS-10 group ((8.17±0.72) nmol/mg protein). These results suggest that HRS treatment significantly attenuated lipid peroxidation in the I/R skin flaps.
Fig. 7.
Production of MDA in the skin flap
The control group exhibited a significantly higher MDA level, whereas HRS decreased the production of MDA. The results are expressed as mean±SEM (n=15). * P<0.05, ** P<0.01, *** P<0.001
3.6. Cytokine evaluation
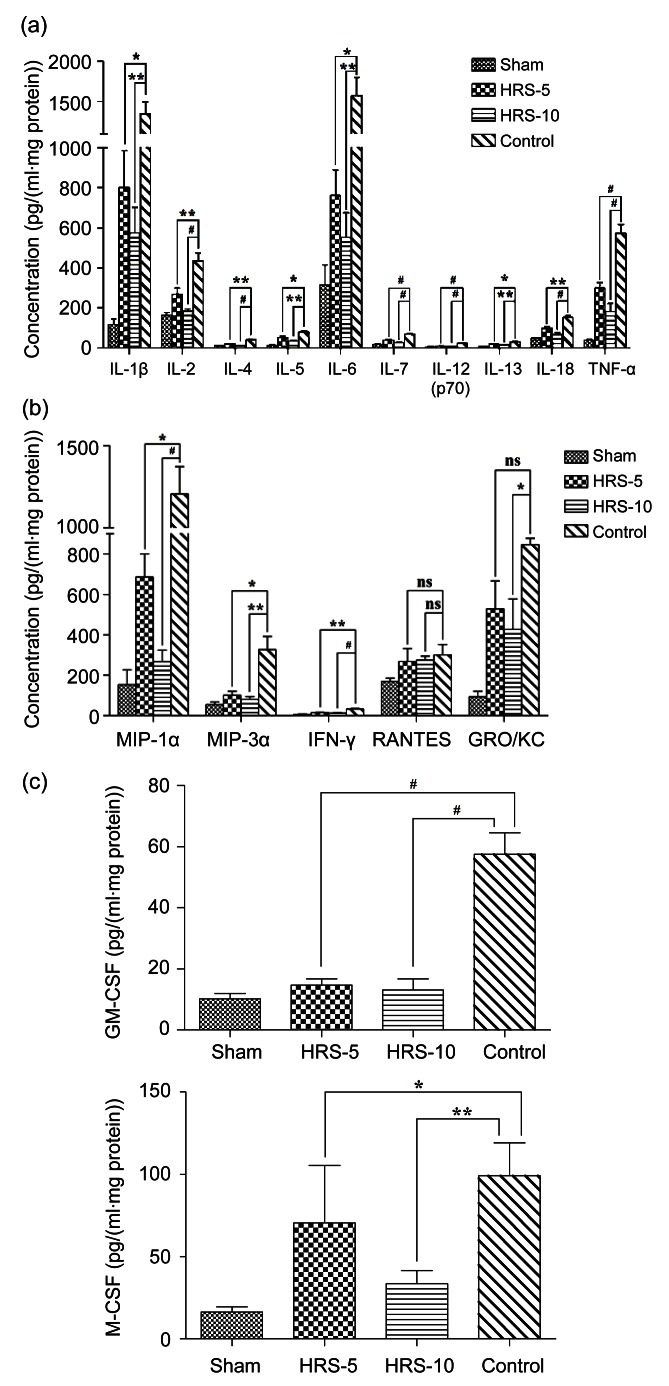
Cytokines in the proximal, middle, and distal portions of surviving tissue were detected, and the data are reported as the average of the three parts. We successfully detected 17 of all 23 cytokines. The levels of 17 cytokines were higher in the control group than in the sham-operated group. HRS treatment evidently prevented the upregulation of inflammatory mediators, including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-12, IL-13, IL-18, and TNF-α (Fig. 8a).
Fig. 8.
Cytokine profiles on skin flaps
Tissues were harvested at postoperative Day 5 from the proximal, middle, and distal points along the vessel axis. Cytokine production was assessed by multiplex analysis of tissue homogenates. (a) HRS administrated 10 min before reperfusion markedly reduced the up-regulation of all inflammatory cytokines. (b) Chemokines were reduced by HRS treatment, with the exception of RANTES. GRO/KC was markedly reduced in the HRS-10 group. (c) HRS treatments decreased the levels of GM-CSF and M-CSF in the tissues. Cytokine changes indicated the alleviation of inflammation. The results are expressed as mean±SEM of three parts (proximal, middle, and distal). * P<0.05, ** P<0.01, # P<0.001
I/R injury also induced the elevation of the concentrations of the detected chemokines (Fig. 8b). MIP-1α, MIP-3α, and IFN-γ levels were reduced by HRS in both the HRS-5 and HRS-10 groups. GRO/KC, a potent neutrophil chemoattractant, was reduced by higher-dose HRS administration. However, there was no significant difference in the RANTES level between the HRS group and the control group.
GM-CSF is a cytokine that functions as a white blood cell growth factor. M-CSF is a hematopoietic growth factor that is involved in the proliferation and differentiation of monocytes and macrophages. HRS treatment reduced the upregulations of GM-CSF and M-CSF compared to the controls (Fig. 8c).
4. Discussion
In this study, we report the protective effect of HRS on skin flaps after 3 h of ischemia. Our data demonstrate that i.p. HRS administration improved skin flap survival, ameliorated microvascular blood flow disturbances, and reduced MDA production. HRS also alleviated inflammation-related events by reducing leukocyte infiltration and pro-inflammatory cytokine production. Histology indicated that the recruitment of leukocytes was reduced, and sixteen measured inflammatory-relevant cytokines were downregulated by HRS treatment. Taken together, these results indicate that HRS may be a novel antioxidant and anti-inflammatory agent with a promising role in protecting skin flaps from I/R injuries.
Flap complications commonly occur in skin flap transfer procedures for wound coverage and reconstruction in plastic surgery. Although many strategies have been investigated to prevent flap morbidity, including pharmaceutical agents, preconditioning (Küntscher et al., 2002b; Dacho et al., 2009), and postconditioning (Moon et al., 2008; Yan et al., 2010), the total loss of microsurgically transferred flaps is about 1%–5% in experienced hands (Harder et al., 2008). The partial flap necrosis rate is 7%–20% of free flaps and 20%–30% of pedicled flaps. I/R injuries are an important reason for flap necrosis and failure.
During reperfusion many ROS evolve in a short period of time, thereby flooding the antioxidant system. ·OH is the strongest of the oxidant species, and mammalian species lack endogenous detoxification systems to neutralize it. Therefore, the therapeutic targeting of ·OH could be critical for the amelioration of oxidative injury. Free-radical scavengers can convert toxic ROS into common and relatively harmless end products. Many antioxidants, such as superoxide dismutase (Manson et al., 1983), grape seed proanthocyanidin extract (Karaaslan et al., 2010), and melatonin (Gurlek et al., 2006), have been used to limit tissue injury. However, strategies that decrease oxidative status intensively may produce unwanted side effects because at low levels ROS function as signaling molecules to regulate apoptosis, cell proliferation and differentiation.
H2 was reported as a new antioxidant and a selective scavenger of ·OH effective in various organs (Nagata et al., 2009; Zheng et al., 2009; Sun et al., 2011). H2 can convert hydroxyl into water (H2+·OH→H2O+·H). Because H2 is electronically neutral and a small molecule, it should easily penetrate the cellular and intracellular membranes that are normally barriers preventing water-soluble antioxidants from entering cells and organelles, such as the mitochondria, a major source of ROS production. We monitored the concentration of H2 in skin flaps using real-time dynamic methods and found that the skin flap H2 concentration reached a peak at about 5 min after HRS i.p. injection. MDA production during free radical attack on membrane lipoproteins and polyunsaturated fatty acids is an indicator of lipid peroxidation. In this study, the notable increase in MDA levels in the control group confirmed the oxidative damage in the skin flap. In contrast, HRS treatments markedly reduced MDA production; thus, we conclude that the i.p. administration of HRS may prevent damage associated with ROS.
Another important factor contributing to flap damage is the release of inflammatory mediators during reperfusion. ROS can activate TNF-α and IL-1β expression by upregulating the nuclear factor κB (NF-κB) signaling pathway. Subsequently, pro-inflammatory cytokines (TNF-α, IL-1, IL-6), chemokines (MIP-1α), and growth factors (GM-CSF, M-CSF) cause macrophage maturation, which further activates the immune/inflammatory cascade and results in the secretion of more inflammatory cytokines. On postoperative Day 5, inflammation remained severe in the controls as manifested by the higher expression of cytokines and increased inflammatory cell infiltration relative to the sham-operated group. HRS administration attenuated IR-induced expressions of pro-inflammatory cytokines, growth factors, and cytokines (IL-2, IL-3, IL-4, etc.). Histological examination also demonstrated clearly that HRS injection prevented structural skin damage with mild inflammatory accumulation. Given the complexity of cross-reactions between ROS and pro-inflammatory mediators, the H2-mediated suppression of inflammation induced by I/R injury may also involve antioxidant effects of H2.
Many materials are known to increase flap viability, but the majority of these agents with proven efficacy require systemic and relatively high dosages, which may cause system side effects. H2 can reach relatively high concentrations quickly, and excessive H2 can be eliminated from the body via breathing, causing no side effects (Chen et al., 2011). In addition, therapeutic H2 effects on humans have been reported for diabetes mellitus type 2 (Imai et al., 2008), hemodialysis (Nakayama et al., 2010), inflammatory myopathies (Ito et al., 2011), radiotherapy for liver cancer (Kang et al., 2011), and acute erythematous skin diseases (Ono et al., 2012). H2 can be administered through intravenous injection or drink. Potent antioxidant activity, easy application, lack of significant toxicological effects, and cost-effectiveness make HRS an ideal candidate for flap survival in the clinical setting.
5. Conclusions
HRS increased the surviving areas of rat epigastric adipocutaneous flaps and decreased oxidative stress and inflammation in these tissues. Thus, the results of the current study validate the therapeutic potential of HRS by demonstrating that HRS injection 10 min before reperfusion mitigates I/R injury.
Footnotes
Project (No. 7132169) supported by the Beijing Natural Science Foundation, China
Compliance with ethics guidelines: Ling ZHAO, You-bin WANG, Shi-rui QIN, Xue-mei MA, Xue-jun SUN, Ming-lian WANG, and Ru-gang ZHONG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Arato E, Kurthy M, Sinay L, Kasza G, Menyhei G, Hardi P, Masoud S, Ripp K, Szilagyi K, Takacs I, et al. Effect of vitamin E on reperfusion injuries during reconstructive vascular operations on lower limbs. Clin Hemorheol Microcirc. 2010;44(2):125–136. doi: 10.3233/CH-2010-1260. [DOI] [PubMed] [Google Scholar]
- 2.Aydogan H, Gurlek A, Parlakpinar H, Askar I, Bay-Karabulut A, Aydogan N, Fariz A, Acet A. Beneficial effects of caffeic acid phenethyl ester (CAPE) on the ischaemia-reperfusion injury in rat skin flaps. J Plast Reconstr Aesthet Surg. 2007;60(5):563–568. doi: 10.1016/j.bjps.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR, McCurry KR, Bauera AJ, Nakao A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8(10):2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Sun YP, Hu PF, Liu WW, Xiang HG, Li Y, Yan RL, Su N, Ruan CP, Sun XJ, et al. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J Surg Res. 2011;167(2):316–322. doi: 10.1016/j.jss.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Dacho A, Lyutenski S, Aust G, Dietz A. Ischemic preconditioning in a rat adipocutaneous flap model. HNO. 2009;57(8):829–834. doi: 10.1007/s00106-009-1901-8. (in German) [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361(3):670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 7.Gurlek A, Celik M, Parlakpinar H, Aydogan H, Bay-Karabulut A. The protective effect of melatonin on ischemia-reperfusion injury in the groin (inferior epigastric) flap model in rats. J Pineal Res. 2006;40(4):312–317. doi: 10.1111/j.1600-079X.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 8.Harder Y, Amon M, Laschke MW, Schramm R, Rucker M, Wettstein R, Bastiaanse J, Frick A, Machens HG, Küntscher M, et al. An old dream revitalised: preconditioning strategies to protect surgical flaps from critical ischemia and ischaemia-reperfusion injury. J Plast Reconstr Aesthet Surg. 2008;61(5):503–511. doi: 10.1016/j.bjps.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373(1):30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 10.Henderson PW, Singh SP, Weinstein AL, Nagineni V, Rafii DC, Kadouch D, Krijgh DD, Spector JA. Therapeutic metabolic inhibition: hydrogen sulfide significantly mitigates skeletal muscle ischemia reperfusion injury in vitro and in vivo. Plast Reconstr Surg. 2010;126(6):1890–1898. doi: 10.1097/PRS.0b013e3181f446bc. [DOI] [PubMed] [Google Scholar]
- 11.Imai S, Kozai H, Matsuda M, Hasegawa G, Obayashi H, Togawa C, Yamamura T, Watanabe K, Miyatani S, Yoshikawa T, et al. Intervention with delivery of diabetic meals improves glycemic control in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;42(1):59–63. doi: 10.3164/jcbn.2008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M, Ibi T, Sahashi K, Ichihara M, Ito M, Ohno K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med Gas Res. 2011;1(1):24. doi: 10.1186/2045-9912-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajiya M, Sato K, Silva MJ, Ouhara K, Do PM, Shanmugam KT, Kawai T. Hydrogen from intestinal bacteria is protective for concanavalin A-induced hepatitis. Biochem Biophys Res Commun. 2009;386(2):316–321. doi: 10.1016/j.bbrc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Kang KM, Kang YN, Choi IB, Gu Y, Kawamura T, Toyoda Y, Nakao A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res. 2011;1(1):11. doi: 10.1186/2045-9912-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaaslan O, Ulusoy MG, Kankaya Y, Tiftikcioglu YO, Kocer U, Kankaya D, Karaaslan GM, Tuncer S, Berktas M. Protective effect of grape seed extract against ischaemia/reperfusion injury in a rat epigastricflap model. J Plast Reconstr Aesthet Surg. 2010;63(4):705–710. doi: 10.1016/j.bjps.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Küntscher MV, Schirmbeck EU, Menke H, Klar E, Gebhard MM, Germann G. Ischemic preconditioning by brief extremity ischemia before flap ischemia in a rat model. Plast Reconstr Surg. 2002;109(7):2398–2404. doi: 10.1097/00006534-200206000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Küntscher MV, Juran S, Menke H, Gebhard MM, Erdmann D, Germann G. The role of pre-ischaemic application of the nitric oxide donor spermine/nitric oxide complex in enhancing flap survival in a rat model. Br J Plast Surg. 2002;55(5):430–433. doi: 10.1054/bjps.2002.3871. [DOI] [PubMed] [Google Scholar]
- 18.Manson PN, Anthenelli RM, Im MJ, Bulkley GB, Hoopes JE. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg. 1983;198(1):87–90. doi: 10.1097/00000658-198307000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon JG, Lim HC, Gye MR, Oh JS, Park JW. Postconditioning attenuates ischemia-reperfusion injury in rat skin flap. Microsurgery. 2008;28(7):531–537. doi: 10.1002/micr.20530. [DOI] [PubMed] [Google Scholar]
- 20.Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. 2009;34(2):501–508. doi: 10.1038/npp.2008.95. [DOI] [PubMed] [Google Scholar]
- 21.Nakao A, Sugimoto R, Billiar TR, McCurry KR. Therapeutic antioxidant medical gas. J Clin Biochem Nutr. 2009;44(1):1–13. doi: 10.3164/jcbn.08-193R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama M, Nakano H, Hamada H, Itami N, Nakazawa R, Ito S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: a clinical trial. Nephrol Dial Transplant. 2010;25(9):3026–3033. doi: 10.1093/ndt/gfq196. [DOI] [PubMed] [Google Scholar]
- 23.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 24.Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K, Nakao A. Hydrogen (H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med Gas Res. 2012;2(1):14. doi: 10.1186/2045-9912-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siemionow M, Arslan E. Ischemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery. 2004;24(6):468–475. doi: 10.1002/micr.20060. [DOI] [PubMed] [Google Scholar]
- 26.Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q, Chang Y, Liu Q, Sun X, Wu M, et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J Hepatol. 2011;54(3):471–480. doi: 10.1016/j.jhep.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 27.van den Heuvel MG, Buurman WA, Bast A, van der Hulst RR. Review: ischaemia-reperfusion injury in flap surgery. J Plast Reconstr Aesthet Surg. 2009;62(6):721–726. doi: 10.1016/j.bjps.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 28.Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong L, Hou L, Wang G. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock. 2010;34(5):495–501. doi: 10.1097/SHK.0b013e3181def9aa. [DOI] [PubMed] [Google Scholar]
- 29.Yan H, Zhang F, Kochevar AJ, Akdemir O, Gao W, Angel M. The effect of postconditioning on the muscle flap survival after ischemia-reperfusion injury in rats. J Invest Surg. 2010;23(5):249–256. doi: 10.3109/08941931003615529. [DOI] [PubMed] [Google Scholar]
- 30.Zheng X, Mao Y, Cai Y, Li Y, Liu W, Sun P, Zhang JH, Sun X, Yuan H. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res. 2009;43(5):478–484. doi: 10.1080/10715760902870603. [DOI] [PubMed] [Google Scholar]