Abstract
Objective: To investigate the association of serum lipids and other risk factors with diabetic retinopathy (DR) in Chinese type 2 diabetic patients. Methods: Five hundred and twenty-three type 2 diabetic patients underwent ophthalmic examination by experienced retinal specialists to assess their DR. Serum lipids, including triglycerides, total cholesterol, high density lipoprotein cholesterol (HDLC), and low density lipoprotein cholesterol (LDLC), were measured using Roche automated clinical chemistry analyzers. The concentration of very low density lipoprotein cholesterol (VLDLC) was calculated based on total cholesterol, HDLC and LDLC. Hyperlipidemia was defined as a total cholesterol concentration of 6.2 mmol/L or higher or the use of lipid-lowering medications. The association of risk factors with any DR or proliferative diabetic retinopathy (PDR) was assessed using the odds ratio (OR) and its 95% confidence interval (CI), calculated from logistic regression models. Results: In multivariate logistic regression models, hyperlipidemia (OR=2.39, 95% CI: 1.02–5.66), higher VLDLC (OR=1.59, 95% CI: 1.14–2.23), and higher triglyceride (OR=1.18, 95% CI: 1.03–1.37) were associated with increased risk of DR. A longer diabetic duration was associated with increased risk of DR (P<0.0001) and PDR (P=0.002) in a dose-response manner. Higher systolic blood pressure (P=0.02) and higher serum creatinine (P=0.01) were independently associated with increased risk of DR, and female gender was associated with increased risk of PDR (P=0.03). Conclusions: Among Chinese type 2 diabetic patients, hyperlipidemia, higher VLDLC, and higher triglyceride were independently associated with increased risk of DR, suggesting control of serum lipids may decrease the risk of DR.
Keywords: Diabetic retinopathy, Serum lipids, Risk factors
1. Introduction
As a major public health problem in China, diabetes has reached an epidemic level, affecting 92.4 million adults with diabetes and 148.2 million with prediabetes (Yang et al., 2010). China has become the country with the largest number of diabetic patients. The chronic nature of the diabetes poses a significant public health burden due to its associated complications including macrovascular and microvascular diseases, such as cardiovascular diseases and diabetic retinopathy (DR). Assessing the risk factors of DR, particularly modified risk factors, is important for early intervention to reduce the risk or slow the progression of DR. Several population-based epidemiological studies (Yau et al., 2012), conducted mostly in Western populations, have investigated the risk factors of DR. These studies consistently established that a longer diabetic duration, hyperglycemia and hypertension were associated with increased risk of DR. Epidemiological studies and clinical trials also have investigated the association between serum lipids and DR; however, a conclusive association remains elusive (Lim and Wong, 2012; Yau et al., 2012).
Although China has the largest diabetic population, epidemiological data on the risk factors of DR in Chinese populations are relatively scarce. The generalizations of findings from Western populations to Chinese populations are uncertain, due to their differences in genetic susceptibility, socioeconomic status, environmental background, and lifestyle. Susceptibility to DR and its risk factors may vary among ethnic groups, as reported by a number of multiethnic studies (Haffner et al., 1993; Wong et al., 2006; Raymond et al., 2009; Yau et al., 2012).
The purpose of this study was to assess the risk factors of DR in Chinese type 2 diabetic patients, with particular interest in investigating the association between serum lipids and DR.
2. Materials and methods
2.1. Study participants
This was a cross-sectional study of patients with type 2 diabetes who were admitted between January 2009 and December 2011 to the Diabetic Clinic in the Department of Endocrinology of the First Affiliated Hospital of Zhejiang University in Hangzhou, Zhejiang Province of China. To be eligible for the study, patients had to have an eye examination for DR and have type 2 diabetes according to the criteria of the World Health Organization (Alberti et al., 1998), i.e., positive findings from any two of the following tests on different days: symptoms of diabetes mellitus plus a casual plasma glucose concentration of 200 mg/dl (11.1 mmol/L) or a fasting plasma glucose concentration of 126 mg/dl (7.0 mmol/L) or a 2-h post-test plasma glucose concentration of 200 mg/dl (11.1 mmol/L) after a 75-g oral glucose load.
The institutional review board approved the study protocol and a written consent form was obtained from each patient.
2.2. Risk factors and serum lipid assessment
After consenting for the study, patients were interviewed to obtain information on their age, duration of diabetes (in years), current use of diabetic, antihypertensive, and lipid-lower medications. Blood pressure was measured while the participant was seated.
A blood sample was drawn from each patient after 10 h of overnight fasting. Roche automated clinical chemistry analyzers (Roche Diagnostics, Indianapolis, IN, USA) were used to measure the concentrations of serum glucose and lipids including triglycerides, total cholesterol, high density lipoprotein cholesterol (HDLC), and low density lipoprotein cholesterol (LDLC). The concentration of very low density lipoprotein cholesterol (VLDLC) was calculated using the formula: c VLDLC=c TC−c HDLC−c LDLC, where c VLDLC, c TC, c HDLC, and c LDLC are concentrations of VLDLC, total cholesterol, HDLC, and LDLC, respectively.
2.3. Diabetic retinopathy determination
As a part of a comprehensive assessment of diabetic complications, the diabetic patients underwent ophthalmic examination for DR by two experienced retinal specialists, masked to the serum lipid findings. For ophthalmic examination, 0.01 g/ml tropicamide was administered to both eyes until the best possible mydriasis was achieved. Dilated ophthalmoscopy was used to assess the presence and severity of DR in each eye using standard clinical criteria (NHMRC, 1997), and ophthalmic examination findings on DR were recorded as: (1) normal, no apparent sign of DR; (2) non-proliferative DR (NPDR), including micro-aneurysms, hard exudates, intraretinal hemorrhages, venous beading or prominent intraretinal micro-vascular abnormality; (3) proliferative DR (PDR), including retinal or optic disc neovascularization, vitreous hemorrhage or preretinal hemorrhage.
2.4. Statistical analysis
The presence and severity of DR in a patient were determined based on the eye showing the worst retinopathy. Hypertension was defined as systolic blood pressure of 140 mmHg or more, or diastolic blood pressure of 90 mmHg or more, or the current use of antihypertensive medications. Hyperlipidemia was defined as total cholesterol of 6.2 mmol/L or higher or the use of lipid-lowering drugs.
The mean and standard deviation (SD) were used to summarize the continuous measures, and proportions were calculated for categorical measures.
The associations of each serum lipid measure and other risk factors with DR were assessed first by univariate logistic regression analyses, followed by multivariate logistic regression analyses. Risk factors with a P<0.20 from the univariate analyses were included in the multivariate analysis to assess the independent effect of each risk factor. The final multivariate models were created by applying a backward selection procedure that retained only risk factors with P≤0.10, plus each lipid measure included in the final multivariate models, regardless of its statistical significance, because our primary interest was to assess the association of each lipid measure with DR with adjustment for other risk factors. The association of each lipid measure with DR was summarized using the odds ratio (OR) and its 95% confidence interval (CI) calculated from the logistic regression models. Because total cholesterol, triglyceride, HDLC, LDLC, and VLDLC concentrations were highly correlated, separate logistic models were evaluated for each serum lipid measure. These analyses were performed for both outcomes of any DR or PDR.
All statistical analyses were performed in SAS V9.2 (SAS Institute Inc., Cary, NC, USA), and a two-tailed P<0.05 was considered to be statistically significant.
3. Results
The characteristics of the 523 study participants are summarized in Table 1. Their mean age was 58 years (ranging from 19 to 89 years), 60% were male, 64% had hypertension, and 44% were using antihypertensive medications. Their mean diastolic blood pressure was 80 mmHg, and their mean systolic blood pressure 133 mmHg. Eighty-three percent of patients were using diabetic medications. The mean diabetic duration was 8.5 years, with 18% less than 1 year, and 16% more than 15 years. Their mean hemoglobin A1c (HbA1c) concentration was 9.3%, and mean serum creatinine concentration was 6.2 μmol/L.
Table 1.
Characteristics of study participants (N=523)
| Characteristics | Value* |
| Gender | |
| Male | 312 (59.7%) |
| Female | 211 (40.3%) |
| Diabetic duration | |
| ≤1 year | 90 (17.6%) |
| >1 year, ≤5 years | 120 (23.5%) |
| >5 years, ≤10 years | 105 (20.6%) |
| >10 years, ≤15 years | 115 (22.5%) |
| >15 years | 81 (15.9%) |
| Unknown | 12 (2.3%) |
| Current use of diabetic medication | |
| No | 90 (17.2%) |
| Yes | 433 (82.8%) |
| Hypertension | |
| No | 185 (35.7%) |
| Yes | 334 (64.4%) |
| Unknown | 5 (0.9%) |
| Hyperlipidemia | |
| No | 488 (93.3%) |
| Yes | 35 (6.7%) |
| Diabetic retinopathy | |
| Normal | 317 (60.6%) |
| Non-proliferative diabetic retinopathy | 152 (29.1%) |
| Proliferative diabetic retinopathy | 54 (10.3%) |
| Age (year) | 58 (13.0) |
| Diabetic duration (year) | 8.5 (7.3) |
| HbA1c (%) | 9.3 (2.6) |
| Fasting insulin (mIU/L) | 15.8 (17.8) |
| Fasting plasma glucose (mIU/L) | 8.1 (3.4) |
| Diastolic blood pressure (mmHg) | 80 (12.7) |
| Systolic blood pressure (mmHg) | 133 (18.9) |
| Serum creatinine (μmol/L) | 6.2 (2.4) |
| Total cholesterol (mmol/L) | 4.6 (1.1) |
| Triglycerides (mmol/L) | 1.8 (1.6) |
| HDLC (mmol/L) | 1.2 (0.4) |
| LDLC (mmol/L) | 2.5 (0.7) |
| VLDLC (mmol/L) | 1.0 (0.7) |
Values are expressed as number (percent) or mean (standard deviation)
HDLC: high density lipoprotein cholesterol; LDLC: low density lipoprotein cholesterol; VLDLC: very low density lipoprotein cholesterol
The mean (SD) of each serum lipid measure was 4.6 (1.1) mmol/L for total cholesterol, 1.8 (1.6) mmol/L for triglyceride, 1.2 (0.4) mmol/L for HDLC, 2.5 (0.7) mmol/L for LDLC, and 1.0 (0.7) mmol/L for VLDLC. Thirty-five (6.7%) patients had hyperlipidemia, defined as a total cholesterol concentration of 6.2 mmol/L or higher, or the use of lipid-lowering drugs.
Among 523 participants undertaking an eye examination by retinal specialists, 206 (39.4%) had some form of DR, and 54 (10.3%) had PDR.
3.1. Risk factors associated with any diabetic retinopathy
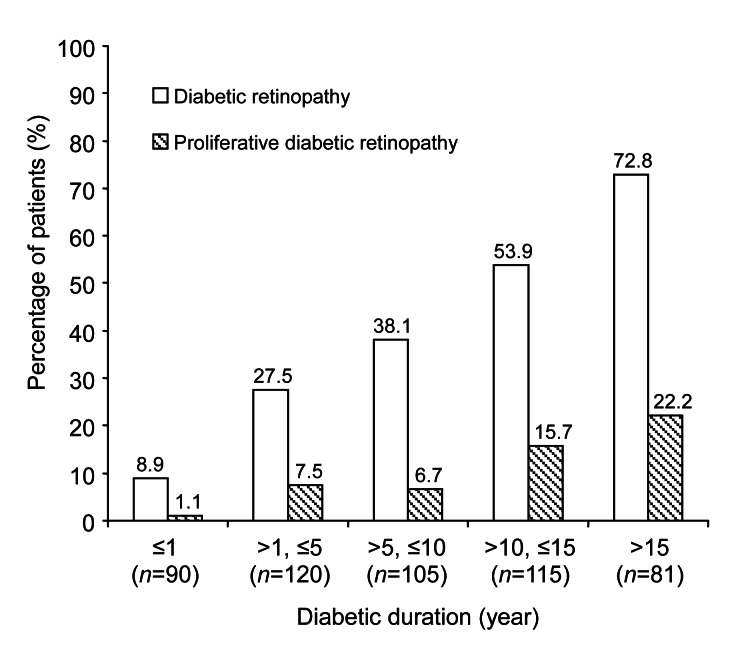
Univariate analysis (Table 2) showed that the statistically significant risk factors associated with increased risk of DR were: older age (OR=1.40 for each 10-year increase, P<0.0001), female gender (OR=1.59, P=0.01), longer duration of diabetes (P<0.0001, Fig. 1), current use of diabetic medication (OR=7.51, P<0.0001), higher fasting insulin level (OR=1.02 per mIU/L increase, P=0.01), hypertension (OR=2.11, P<0.0001), higher systolic blood pressure (OR=1.29 per 10 mmHg increase, P=0.0002), and higher serum creatinine (OR=1.18 per μmol/L increase, P=0.0001). Of all the serum lipid measures evaluated, only higher VLDLC was marginally associated with increased risk of DR (OR=1.27 per mmol/L increase, P=0.08). Hyperlipidemia was associated with increased risk of DR (OR=2.19, 95% CI 1.09–4.38, P=0.03).
Table 2.
Univariate analysis for the association of risk factors with diabetic retinopathy and proliferative diabetic retinopathy
| Risk factor | Diabetic retinopathy |
Proliferative diabetic retinopathy |
||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (per 10 years) | 1.40 (1.21–1.62) | <0.0001 | 1.11 (0.89–1.38) | 0.36 |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.59 (1.11–2.27) | 0.01 | 1.99 (1.13–3.51) | 0.02 |
| Years of diabetes | <0.0001 | 0.0005 | ||
| ≤1 | 1.00 | 1.00 | ||
| >1, ≤5 | 3.89 (1.70–8.91) | 7.22 (0.90–58.02) | ||
| >5, ≤10 | 6.31 (2.76–14.40) | 6.36 (0.77–52.72) | ||
| >10, ≤15 | 12.02 (5.32–27.01) | 16.51 (2.16–126) | ||
| >15 | 27.50 (11.51–66.02) | 25.40 (3.31–195) | ||
| HbA1C | 0.96 (0.90–1.03) | 0.27 | 1.06 (0.95–1.18) | 0.29 |
| Fasting insulin (per mIU/L increase) | 1.02 (1.01–1.03) | 0.01 | 1.00 (0.99–1.02) | 0.56 |
| Fasting plasma glucose (per mmol/L increase) | 0.96 (0.91–1.02) | 0.18 | 1.02 (0.94–1.11) | 0.70 |
| Hypertension | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.11 (1.43–3.11) | <0.0001 | 0.99 (0.55–1.79) | 0.97 |
| Current use of diabetic medication | ||||
| No | 1.00 | 1.00 | ||
| Yes | 7.51 (3.68–15.31) | <0.0001 | 6.00 (1.44–25.14) | 0.01 |
| Diastolic blood pressure (per 10 mmHg increase) | 1.00 (0.86–1.15) | 0.94 | 0.93 (0.74–1.17) | 0.53 |
| Systolic blood pressure (per 10 mmHg increase) | 1.29 (1.15–1.41) | 0.0002 | 1.13 (0.98–1.31) | 0.10 |
| Serum creatinine (per μmol/L increase) | 1.18 (1.09–1.29) | 0.0001 | 1.02 (0.91–1.14) | 0.71 |
| Total cholesterol (per mmol/L increase) | 1.14 (0.97–1.33) | 0.12 | 1.15 (0.90–1.46) | 0.28 |
| Triglycerides (per mmol/L increase) | 1.08 (0.97–1.20) | 0.17 | 0.87 (0.68–1.11) | 0.27 |
| HDLC (per mmol/L increase) | 1.02 (0.61–1.69) | 0.96 | 1.34 (0.60–3.00) | 0.48 |
| LDLC (per mmol/L increase) | 1.05 (0.83–1.34) | 0.68 | 1.33 (0.92–1.92) | 0.13 |
| VLDLC (per mmol/L increase) | 1.27 (0.97–1.65) | 0.08 | 0.92 (0.58–1.46) | 0.73 |
| Hyperlipidemia | 2.19 (1.09–4.38) | 0.03 | 1.97 (0.78–4.98) | 0.15 |
OR: odds ratio; CI: confidence interval; HDLC: high density lipoprotein cholesterol; LDLC: low density lipoprotein cholesterol; VLDLC: very low density lipoprotein cholesterol
Fig. 1.
Percent of patients with diabetic retinopathy and proliferative diabetic retinopathy by years of diabetic duration
Longer diabetic duration is association with higher percent of diabetic retinopathy (P<0.0001) and higher percent of proliferative diabetic retinopathy (P=0.0005)
Multivariate analysis (Table 3) showed that longer diabetic duration was independently associated with increased risk of DR in a dose-response manner (P<0.0001). Compared to patients with a diabetic duration of less than one year, the OR was 2.4 for a duration of from 1 to 5 years, 3.2 for from 5 to 10 years, 6.1 for from 10 to 15 years, and 12.3 for over 15 years. Systolic blood pressure was independently associated with DR (OR=1.15 for every 10 mmHg increase, P=0.02). A higher serum creatinine concentration was independently associated with increased risk of DR (OR=1.13 for per μmol/L increase, P=0.01). When each lipid measure was evaluated separately and adjusted for both diabetic duration and systolic blood pressure in multivariate analysis, higher triglyceride (OR=1.18, P=0.02), higher VLDLC (OR=1.59, 95% CI 1.14–2.23, P=0.006), and hyperlipidemia (OR=2.39, 95% CI 1.02–5.66, P=0.04) were independently associated with increased risk of DR.
Table 3.
Multivariate analysis for the association of risk factors with diabetic retinopathy and proliferative diabetic retinopathy
| Risk factor | Diabetic retinopathy |
Proliferative diabetic retinopathy |
||
| OR (95% CI)* | P | OR (95% CI)† | P | |
| Triglyceride (per mmol/L increase) | 1.18 (1.03–1.37) | 0.02 | 0.87 (0.67–1.12) | 0.28 |
| Total cholesterol (per mmol/L increase) | 1.17 (0.96–1.42) | 0.13 | 1.13 (0.88–1.46) | 0.33 |
| Hyperlipidemia | 2.39 (1.02–5.66) | 0.04§ | 1.83 (0.67–4.96) | 0.23‡ |
| HDLC (per mmol/L increase) | 0.85 (0.47–1.55) | 0.60 | 1.23 (0.54–2.85) | 0.62 |
| LDLC (per mmol/L increase) | 0.96 (0.72–1.29) | 0.80 | 1.36 (0.92–2.02) | 0.13 |
| VLDLC (per mmol/L increase) | 1.59 (1.14–2.23) | 0.006 | 0.92 (0.58–1.45) | 0.71 |
| Diabetic duration (year) | <0.0001§ | 0.002‡ | ||
| ≤1 | 1.00 | 1.00 | ||
| >1, ≤5 | 2.39 (0.86–6.65) | 7.62 (0.94–61.61) | ||
| >5, ≤10 | 3.15 (1.11–9.00) | 5.80 (0.70–48.30) | ||
| >10, ≤15 | 6.06 (2.15–17.12) | 16.01 (2.09–122) | ||
| >15 | 12.30 (4.09–37.20) | 21.74 (2.79–168) | ||
| Current use of diabetic medication | ||||
| No | 1.00 | |||
| Yes | 2.32 (0.86–6.26) | 0.096§ | ||
| Systolic blood pressure (per 10 mmHg increase) | 1.15 (1.03–1.28) | 0.02§ | ||
| Serum creatinine (per μmol/L increase) | 1.13 (1.03–1.24) | 0.01§ | ||
| Female | 1.97 (1.07–3.60) | 0.03‡ | ||
OR: odds ratio; CI: confidence interval; HDLC: high density lipoprotein cholesterol; LDLC: low density lipoprotein cholesterol; VLDLC: very low density lipoprotein cholesterol
Adjusted for duration of diabetes, current use of diabetic medication, systolic blood pressure, serum creatinine
Adjusted for duration and gender
From the multivariate model with duration of diabetics, current use of diabetic medication, systolic blood pressure, serum creatinine, and hyperlipidemia
From the multivariate model with duration of diabetics, female gender, and hyperlipidemia
3.2. Risk factors associated with proliferative diabetic retinopathy
Univariate analysis (Table 2, last two columns) showed that female gender (OR=1.99, P=0.02), longer diabetic duration (P=0.0005, Fig. 1), and current use of diabetic medication (OR=6.00, P=0.01) were associated with increased risk of PDR. None of the lipid measures was significantly associated with increased risk of PDR (all P>0.13). Hyperlipidemia was not associated with PDR (OR=1.97, P=0.15).
Multivariate analysis (Table 3, last two columns) showed that a longer diabetic duration was independently associated with increased risk of PDR (P=0.002). Compared to patients with a diabetic duration of less than one year, the OR of PDR was 7.6 for a duration of from 1 to 5 years, 5.8 for from 5 to 10 years, 16.0 for from 10 to 15 years, and 21.7 for over 15 years. Female gender was also significantly associated with increased risk of PDR (OR=1.97, P=0.03). When each serum lipid measure was adjusted for both diabetic duration and female gender in multivariate analysis, none were significantly associated with PDR (Table 3).
4. Discussion
This study evaluated the risk factors of DR, particularly the serum lipids, in Chinese type 2 diabetic patients. The study found that hyperlipidemia, higher triglyceride, and higher VLDLC were independently associated with increased risk of DR. Also, the study confirmed that a longer diabetic duration, higher systolic blood pressure, and reduced renal function were associated with increased risk of DR in Chinese type 2 diabetic patients.
Associations between serum lipid measures and DR have been investigated extensively in many epidemiological studies and clinical trials, conducted mostly in Western countries (Lim and Wong, 2012). However, these studies have not found a consistent association. Studies designed specifically to evaluate the association of serum lipid measures with DR in Chinese diabetic populations are scarce. This study evaluated the association of a full profile of serum lipids with any form of DR and PDR. To our knowledge, this is the first study that has evaluated the association between VLDLC and DR and found a statistically significant association. The significant association of hyperlipidemia with DR in our study is consistent with the findings from the recent Beijing Communities Diabetes Study (Xu et al., 2012), which reported that higher total cholesterol (P=0.003) and higher LDLC (P=0.007) were significantly associated with increased risk of DR. Our findings are also consistent with a few other studies of Chinese populations (Table 4), which reported significant or marginally significant associations between total cholesterol levels and DR (Liu et al., 2002; Cai et al., 2006; Wang F.H. et al., 2011; Wang S. et al., 2012). In the Handan eye study, the analysis by quartiles of lipid levels found that the third quartile of total cholesterol (5.2 to 5.9 mmol/L) was significantly associated with increased risk of DR (OR=2.36, 95% CI 1.29–4.33 from age and gender adjusted analysis). The third quartile LDLC (3.1 to 3.6 mmol/L) was also associated with increased risk of DR (OR=1.93, 95% CI 1.07–3.48), compared with the lowest quartile (Wang F.H. et al., 2011). The Beijing 301 Hospital study also found a marginally significant association between total cholesterol levels and DR (P=0.054) (Liu et al., 2002). The Beijing eye study reported a marginally significant association between HDLC levels and DR (OR=1.96, 95% CI 0.92–4.21, P=0.08 from multivariate analysis) and a higher risk of DR in patients with dyslipidemia (OR=1.34, 95% CI 0.93–1.93) although that association was not statistically significant (P=0.12) (Wang S. et al., 2012). However, our findings were not consistent with a few other studies of Chinese diabetic populations (Table 4). These studies tended to have smaller sample sizes or lower rates of DR, and thus may not have had sufficient statistical power to detect statistically significant associations.
Table 4.
Association of serum lipids with diabetic retinopathy from various studies in Chinese diabetic population
| Study/reference | Number of diabetic patients | Outcome | Serum lipid |
||||
| TC | TG | HDLC | LDLC | VLDLC | |||
| Beijing eye study (Wang S. et al., 2012) | 235 | DR | − | − | +/− | − | NA |
| Handan eye study (Wang F.H. et al., 2011) | 368 | DR | +/− | − | − | +/− | NA |
| Beijing community diabetes study (Xu et al., 2012) | 2 007 | DR PDR | + | − | − | + | NA |
| PDR | − | − | − | − | NA | ||
| Beijing 301 Hospital study (Liu et al., 2002) | 2 131 | DR | +/− | − | NA | NA | NA |
| Peking University People’s Hospital study (Cai et al., 2006) | 746 | DR | + | − | + | − | NA |
| PDR | − | − | − | − | NA | ||
| Shanghai diabetic complications study (Pang et al., 2012) | 799 | DR | − | − | − | − | NA |
| Current study | 523 | DR | + | + | − | − | + |
| PDR | − | − | − | − | − | ||
−: no statistically significant association; +: statistically significant association; +/−: marginally significance was dependent on how data were analyzed and presented. DR: diabetic retinopathy; PDR: proliferative diabetic retinopathy; TC: total cholesterol; TG: triglyceride; HDLC: high density lipoprotein cholesterol; LDLC: low density lipoprotein cholesterol; VLDLC: very low density lipoprotein cholesterol; NA: not available
Besides the serum lipids, our study also confirmed that a longer diabetic duration, higher systemic blood pressure, and reduced renal function were associated with increased risk of DR in Chinese diabetic patients. Epidemiological studies on Western populations have established that diabetic duration, hypertension, hyperglycemia, and renal disease are major risk factors for DR (Yau et al., 2012). This study extended these strong risk factors from Western populations (diabetic duration and hypertension) to a Chinese population, and confirmed the findings from other studies of Chinese populations (Liu et al., 2002; Cai et al., 2006).
Although HbA1c has been established as a risk factor for DR, our study found no statistically significant association between HbA1c and either DR or PDR. The lack of association may be due to the fact that our study subjects were in-patients. These patients may have better control of their HbA1c than subjects in other population-based studies. We found that patients who had a longer duration of diabetes had better control of HbA1c, because our data suggest that there is a negative correlation between HbA1c and diabetic duration (Pearson correlation coefficient −0.14, P=0.003).
Our study had several limitations. First, the study determined the DR from clinical examination by senior retinal specialists using ophthalmoscopy rather than from the standard grading of fundus photographs. Ophthalmoscopy may misclassify DR status. However, because DR was determined by senior retinal specialists who were masked to the lipid levels, we believe the misclassification of DR from ophthalmoscopy was not dependent on the serum lipid levels, and thus was unlikely to bias the association between serum lipids and DR. Second, the evaluation of risk factors for DR was not very comprehensive. We did not collect data on some risk factors that may be potentially associated with DR, such as cigarette smoking and body mass index. Thus, in our evaluation of the association between serum lipid levels and DR, although we adjusted for strong risk factors of DR (diabetic duration and hypertension), we did not account for some other risk factors. Lack of adjustment for other risk factors can potentially bias the association of lipid levels with DR in either direction. Third, this was a cross-sectional study of in-patients, and suffered from the limitations of such studies. It did not allow us to evaluate causal associations, and it may not be valid to extrapolate the results from in-hospital diabetic patients to all Chinese diabetic patients. Finally, the number of cases of PDR in this study was small and thus may not have provided sufficient power to evaluate the risk factors of PDR.
In summary, our study suggested that hyperlipidemia, higher triglyceride, and higher VLDLC were significantly associated with increased risk of DR. We also confirmed the dose-response relationship of diabetic duration with both any form of DR and PDR, and the significant associations of systolic blood pressure and renal function with DR. Given the increasing prevalence of diabetes mellitus in Chinese populations, and that most of these risk factors are modifiable, these findings could have a substantial public health impact on decreasing the prevalence and severity of vision-threatening DR, and on reducing the health burden from the complications of diabetes.
Footnotes
Project (No. 2011C33029) supported by the Zhejiang Provincial Technology Application Research Planning Grant, China
Compliance with ethics guidelines: Hui-yan ZHANG, Jian-yong WANG, Gui-shuang YING, Li-ping SHEN, and Zhe ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Alberti KGMM, Zimmet PZ, Consultation W. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus—provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Cai XL, Wang F, Ji LN. Risk factors of diabetic retinopathy in type 2 diabetic patients. Chin Med J. 2006;119(10):822–826. [PubMed] [Google Scholar]
- 3.Haffner SM, Mitchell BD, Moss SE, Stern MP, Hazuda HP, Patterson J, van Heuven WA, Klein R. Is there an ethnic difference in the effect of risk factors for diabetic retinopathy? Ann Epidemiol. 1993;3(1):2–8. doi: 10.1016/1047-2797(93)90003-m. [DOI] [PubMed] [Google Scholar]
- 4.Lim LS, Wong TY. Lipids and diabetic retinopathy. Exp Opin Biol Ther. 2012;12(1):93–105. doi: 10.1517/14712598.2012.641531. [DOI] [PubMed] [Google Scholar]
- 5.Liu DP, Molyneaux L, Chua E, Wang YZ, Wu CR, Jing H, Hu LN, Liu YJ, Xu ZR, Yue DK. Retinopathy in a Chinese population with type 2 diabetes: factors affecting the presence of this complication at diagnosis of diabetes. Diabetes Res Clin Pract. 2002;56(2):125–131. doi: 10.1016/S0168-8227(01)00349-7. [DOI] [PubMed] [Google Scholar]
- 6.NHMRC (National Health and Medical Research Council of Australia) Clinical Practice Guidelines: Management of Diabetic Retinopathy. Commonwealth of Australia. 1997:1–94.
- 7.Pang C, Jia LL, Jiang SF, Liu W, Hou XH, Zuo YH, Gu HL, Bao YQ, Wu Q, Xiang KS, et al. Determination of diabetic retinopathy prevalence and associated risk factors in Chinese diabetic and pre-diabetic subjects: Shanghai diabetic complications study. Diabetes Metab Res Rev. 2012;28(3):276–283. doi: 10.1002/dmrr.1307. [DOI] [PubMed] [Google Scholar]
- 8.Raymond NT, Varadhan L, Reynold DR, Bush K, Sankaranarayanan S, Bellary S, Barnett AH, Kumar S, O′Hare JP, Retinopath UADS. Higher prevalence of retinopathy in diabetic patients of south Asian ethnicity compared with White Europeans in the community a cross-sectional study. Diabetes Care. 2009;32(3):410–415. doi: 10.2337/dc08-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang FH, Liang YB, Peng XY, Wang JJ, Zhang F, Wei WB, Sun LP, Friedman DS, Wang NL, Wong TY, et al. Risk factors for diabetic retinopathy in a rural Chinese population with type 2 diabetes: the Handan eye study. Acta Ophthalmol. 2011;89(4):E336–E343. doi: 10.1111/j.1755-3768.2010.02062.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Xu L, Jonas JB, Wang YX, You QS, Yang H. Dyslipidemia and eye diseases in the adult Chinese population: the Beijing eye study. PLoS One. 2012;7(3):e26871. doi: 10.1371/journal.pone.0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong TY, Klein R, Islam A, Frances M, Folsom AR, Klein BE, Sharrett AR, Shea S, Athersclerosis MES. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Wei WB, Yuan MX, Yuan SY, Wan G, Zheng YY, Li YB, Wang S, Xu L, Fu HJ, et al. Prevalence and risk factors for diabetic retinopathy: the Beijing Communities Diabetes Study 6. Retina. 2012;32(2):322–329. doi: 10.1097/Iae.0b013e31821c4252. [DOI] [PubMed] [Google Scholar]
- 13.Yang WY, Lu JM, Weng JP, Jia WP, Ji LN, Xiao JZ, Shan ZY, Liu J, Tian HM, Ji QH, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 14.Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]