Abstract
The aim of this study is to investigate the effects and possible mechanisms of sodium ferulate (SF) on anti-apoptosis in steroid-induced femoral head osteonecrosis in rabbits. Japanese white rabbits were randomly divided into three groups (control group, treatment group, and model group), each with 24 rabbits. The model and treatment groups were first injected with an intravenous dose of horse serum, 10 ml/kg, three weeks later with an intravenous dose of 7.5 ml/kg, and two weeks later with an intramuscular dose of methylprednisolone, 45 mg/kg, three times in order to establish rabbit models of osteonecrosis. Concurrently, the treatment group was injected with intravenous doses of SF 20 mg/kg for two weeks, once per day. Three time points, Weeks 2, 4, and 8, were selected after modeling was completed. Osteonecrosis was verified by histopathology with haematoxylin-eosin (HE) staining. The apoptosis rate of osteonecrosis was observed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The apoptosis expressions of caspase-3 and Bcl-2 were analyzed by immunohistochemistry and Western blot. The rabbit models of osteonecrosis were successfully established and observed by HE staining. SF was effective in intervening in apoptosis and decreasing the apoptosis rate in femoral head necrosis by the immunohistochemistry and TUNEL assay (P<0.01). Western blot analysis indicated that there were statistical significances in the protein levels of caspase-3 and Bcl-2 (P<0.01). SF has a protective effect by reducing the incidence of early steroid-induced femoral head necrosis in rabbits, effectively intervening in apoptosis through decreasing caspase-3 expression and up-regulating Bcl-2 expression.
Keywords: Femoral head necrosis, Glucocorticoid, Sodium ferulate, Apoptosis, Rabbit
1. Introduction
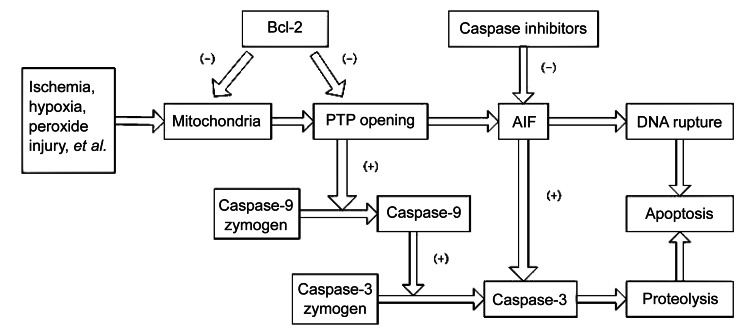
Steroid-induced femoral head osteonecrosis is characterized by trabecular bone necrosis and bone marrow necrosis because of the long-term glucocorticoid used. Glucocorticoid is one of the most important factors in nontraumatic osteonecrosis, and the proportion could reach anywhere between 35% and 45% (Mont and Hungerford, 1995; Lavernia et al., 1999; Jones and Hungerford, 2004). Generally, when the cumulative dosage of prednisone gets to be more than 2 000 mg, osteonecrosis may occur (Felson and Anderson, 1987; Koo et al., 2002; Aaron and Gray, 2007). Currently, the complex pathogenesis of osteonecrosis has not yet been clarified, and the possible mechanisms include fat embolism, intravascular coagulation, intraosseous pressure, osteoporosis, immune factors, etc., which may also be caused by several factors working together. Caspase-3 and Bcl-2 play key roles in the apoptotic pathway. These cytokines have been studied in depth and many studies have demonstrated that caspase-3 can stimulate apoptosis, and Bcl-2 is an apoptosis inhibitor (Tsujimoto and Shimizu, 2000; Kroemer and Martin, 2005; Mazumder et al., 2008) (Fig. 1). Eberhardt et al. (2001) suggested that ischemia results from coagulation were difficult to reproduce experimentally, and early changes occur at times that the vasculature is normal. Recently, some studies have shown that support for bone vasculature in regions with active metabolism, which requires vascular endothelial growth factor, is reduced secondarily to the decline in adrenocorticotropic-hormone (to nearly zero) that is also caused by high-dose glucocorticoids. This suggested that earlier changes in cellular response mediate the later changes seen in bone after 4–8 weeks (Zaidi et al., 2010). Therefore, it is necessary to find a reasonable application that not only allows glucocorticoid administration, but also prevents the occurrence of steroid-induced femoral head osteonecrosis.
Fig. 1.
Diagram of apoptotic mechanism for Bcl-2 and caspase-3
Sodium ferulate (SF) is water-soluble and is a new type of non-peptide antagonist of the endothelin receptor 3-methoxy-4-hydroxycinnamate sodium (Fig. 2). SF, which is stable and has low toxicity, can be easily synthesized. At present, it is widely used in scientific researches and clinical treatment (Graf, 1992; Balasubashini et al., 2004; Wenk et al., 2004; Khanduja et al., 2006; Perluigi et al., 2006; Srinivasan et al., 2006; Yogeeta et al., 2006). SF has a variety of physiological functions and beneficial effects, such as preventing DNA from being damaged, having anti-apoptotic activity, protecting endothelial cells, promoting the vascular smooth muscle cell proliferation, and improving the local blood supply. SF was also reported to be effective in preventing bone loss in ovariectomized rats (Sassa et al., 2003), promoting bone remodeling, and leading to a predominantly osteoblastic phase. It was also found to inhibit radiation-induced DNA strand breaks and enhance the survival of mice (Maurya et al., 2005; Maurya and Devasagayam, 2013). Therefore, it is interesting to investigate the potential advantages of SF on the femoral head osteonecrosis. Currently, the effects of SF on osteonecrosis have been investigated in vitro, but rarely studied in vivo.
Fig. 2.
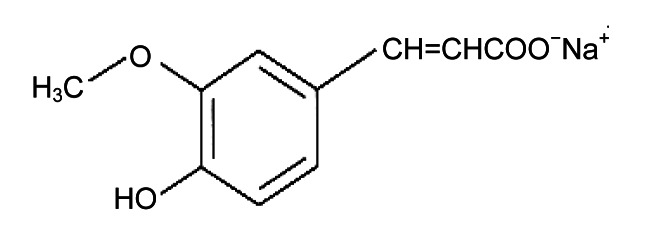
Chemical structure of sodium ferulate (SF)
The rabbit model was established using a modified method of injecting horse serum intravenously, and then injecting methylprednisolone intramuscularly. This model of osteonecrosis was first successfully copied by Matsui et al. (1992). SF had been widely used in clinics, and the clinical application of the optimal dose had basically been clarified and used in accordance with the drug manual. Therefore, we did not create a group of high or low doses or a positive control group to perform further comparisons. Particularly, according to the adult clinical regular dose and the dose conversion formula of man to rabbit derived by the body surface area, we calculated the rabbit dose of 20 mg/kg. The aim of present study was to demonstrate the effect of SF as an anti-apoptotic agent on bone cells in osteonecrosis, and to further investigate the possible pathogenesis of steroid-induced femoral head osteonecrosis.
2. Materials and methods
2.1. Animals, reagents, drugs, and instruments
The animals used were 72 adult male Japanese white rabbits (mean age: 20 months; mean body weight: 3.2 kg; provided by the Experimental Animal Center of Xi’an Jiaotong University, China). The rabbits were randomly divided into three groups: control group, treatment group (methylprednisolone plus SF), and model group (methylprednisolone only), each consisting of 24 rabbits. The rabbits of model and treatment groups first received intravenous injection of horse serum at a dose of 10 ml/kg, and three weeks later an intravenous injection of a dose of 7.5 ml/kg. Two weeks later methylprednisolone was injected intramuscularly at a dose of 45 mg/kg three times, once per day. Concurrently, the treatment group was injected SF intravenously at a dose of 20 mg/kg, once per day, for a total of two weeks. Each rabbit was daily injected with penicillin by one hundred thousand units in a week for preventing infection. The control group was not given any handling and housed and fed under identical conditions. Eight rabbits of each group were randomly sacrificed by an overdose of anesthesia in turn by Weeks 2, 4, and 8.
2.2. Histopathology
The bilateral femoral heads harvested from the animals were immediately fixed with 10% neutral buffered formalin, decalcified with 10% ethylenediaminetetraacetic acid (EDTA) neutralized with sodium sulphate buffer for about six weeks, then embedded in paraffin, and cut along the coronal plane into 4 μm thick sections. The sections were processed by haematoxylin-eosin (HE) staining. The tissues of bone and marrow were observed by optical microscopy at 200 times magnification. Osteonecrosis was identified by the necrosis of the medullary haematopoietic cells, empty lacunae, or condensed nuclei in osteocytes. As previously reported, a few empty lacunae and lesions in the normal trabecular bone and/or just only fat cell necrosis were excluded from the diagnosis of osteonecrosis. In counting the empty lacunae, the observer was blinded as to the groups to assure that all the work was honestly reported.
2.3. Immunohistochemistry
The sections were processed by immunohistochemical staining to detect the expression of caspase-3 and Bcl-2, respectively. After being immersed in phosphate-buffered saline (PBS) with 0.1% Tween 20 (pH 7.4) for 30 min and 0.2% H2O2 for 1 h for blocking endogenous peroxidase, the sections were incubated with mouse anti-rabbit monoclonal primary antibodies against caspase-3 and Bcl-2 separately (Santa Cruz Biotechnology, USA). The antibodies were dissolved in a solution consisting of 0.5% (5 g/L) bovine serum albumin (BSA) and 0.1% (1 g/L) sodium azide in 0.1 mol/L PBS for 24 h at 4 °C. After three washings with PBS, the sections were exposed to biotinylated goat anti-mouse IgG diluted 1:300 for 2 h at room temperature. The sections were washed three times with PBS after being processed by the avidin-biotin-peroxidase complex diluted 1:100 for 2 h at room temperature. Then, the peroxidase reaction was developed for 15 min in 0.1 mol/L Tris buffer (pH 7.6) consisting of 0.01% 3,3-diaminobenzidine tetrahydrochloride and 0.01% H2O2. The sections were observed and photographed by the eclipse 50i optical microscope imaging system (Nikon Co., Ltd., Tokyo, Japan). Positive brown staining for caspase-3 and Bcl-2 in subchondral bone of the femoral heads was visible, and also distributed in the bone marrow and trabecular bone. Samples without primary antibodies processing were used as the negative control.
The staining intensities of immunohistochemistry for caspase-3 and Bcl-2 on images from all groups were quantitatively analyzed. It is crucial to emphasize that all sections were analyzed simultaneously for the quantitative study. The positive staining images were analyzed at the 200 times magnification by the analysis software Image Pro Plus (Media Cybernetics, Baltimore, MD, USA). Each analyzed area was approximately the same in size. In the given region, we evaluated the sum of all the pixel intensity or optical density values (Mendonça et al., 2005). We obtained the numerical values from twenty different regions in each tissue, and then calculated the average, which represented the specified marker in the given tissue. In counting the average positive staining areas of caspase-3 and Bcl-2, the observer was blinded as to the groups to assure that all the work was honestly reported.
2.4. TUNEL apoptosis detection
The sections were processed to observe the presence and location of apoptosis using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) detection kit (Promega Co., Ltd., Beijing, China). The detecting procedures were strictly followed by the kit protocols. The sections were photographed with the eclipse 50i optical microscope imaging system (Nikon Co., Ltd., Tokyo, Japan). TUNEL-positive staining was visibly located in the bone nucleus in subchondral bones of the femoral heads, and the color set was vividly tan or brown, and a few positive granules appeared in cytoplasm. In addition, the brown puncta and bundles were also distributed in the trabecular bone and bone marrow. Samples without TUNEL assay were the negative control. Twenty high magnified views (200 times magnification) were randomly selected in every section. Fifty bone cells were counted in each region, and the positive staining area and apoptosis index (apoptosis cells/all cells) were calculated. The apoptosis cells in osteocytes and marrow were all calculated by blinded observers. In counting the positive level of apoptotic cells, the observer was blinded as to the groups to assure that all the work was honestly reported.
2.5. Western blot analysis
The specimens were washed repeatedly with cold PBS and then immersed in RIPA buffer, with a proportion of protein cleavage in 0.2 ml/g. Subsequently, the specimens were ground in liquid nitrogen for 20 min, and the supernatant solution was extracted into centrifuge tubes. The insoluble materials were removed by microcentrifugation at 12 000 r/min for 10 min at 4 °C. Cell lysates were mixed analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to polyvinylidene difluoride membranes. After being blocked with a solution of Tris buffered saline (TBS) and 5% (50 g/L) skim milk, membranes were incubated with mouse anti-rabbit monoclonal antibodies against caspase-3 and Bcl-2 (Santa Cruz Biotechnology, USA). For the detection of chemiluminescence, membranes were incubated in the enhanced chemiluminescence substrate for horseradish peroxidase (HRP) in the dark room. The luminescent signal was detected and gathered by the CCD camera, and transmitted to the computer controller unit and transformed to fixed quantity by the analysis equipment linked to computer. Then the data were handled for analysis and documentation. HRP-conjugated mouse anti-rabbit β-actin antibody was used as an internal control. In counting the expressions of caspase-3 and Bcl-2, the observer was blinded as to the groups to assure that all the work was honestly reported.
2.6. Statistical analysis
The numerical values were expressed as mean±standard deviation (SD). The SPSS 17.0 software was used for analysis. One-way analysis of variance (ANOVA) was performed to analyze the statistical data, while Chi-square test was performed for the count data. In all tests, a P value of less than 0.05 was considered as statistically significant.
3. Results
3.1. Histopathology
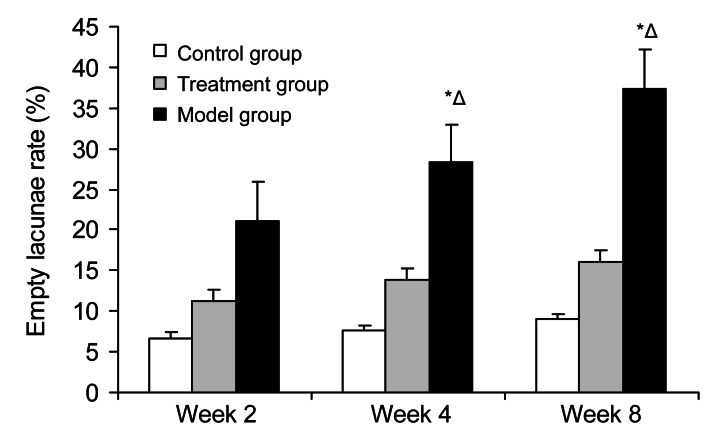
In the control group, there was scant osteonecrosis in trabecular bone and bone marrow; conversely, osteonecrosis was observed in 13 rabbits in the model group (54%) and in 4 rabbits in the treatment group (17%). Osteonecrosis appeared at Week 2 after steroid administration and was obvious at Week 8 in the model group. Except the 4 bone necrotic rabbits in the treatment group, there was no statistical significance between the control group and the treatment group (Fisher’s exact test, P=0.458). The rate of empty lacunae in the model group was significantly different compared with that in the control group at Weeks 2, 4, and 8 (P<0.05); compared with the treatment group, there was statistical significance at Weeks 4 and 8 (P<0.05), but no significance at Week 2 (P=0.0612).
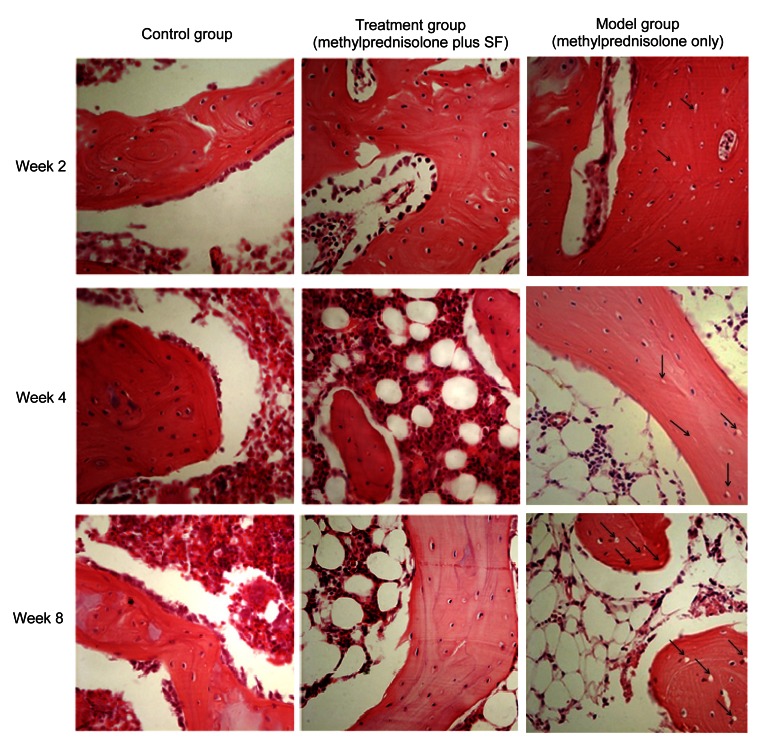
Necrotic areas in the model group were clearly distinguished from the surrounding normal tissue. The marrow tissue also had necrotic changes of hematopoietic and fat cells. Trabecular bone had also shown necrotic changes, such as condensed nuclei and empty lacunae in osteocytes. In the edge of trabecular bone, many fibroblasts and fibrous tissues also existed. In addition, many activated osteoclasts were observed in the necrotic trabecular bone and marrow. Necrotic areas in the treatment group were observed as slight degeneration or necrosis of medullary hematopoietic cells and fat cells, and trabecular bone showed mild bone necrosis. Although the degeneration or osteonecrosis had existed, the architecture of trabecular bone did not collapse. Few fibroblasts and fibrous tissues were observed in the edge of trabecular bone (Figs. 3 and 4)
Fig. 3.
Effects of methylprednisolone and methylprednisolone plus sodium ferulate (SF) on osteocytes in HE-stained sections at Weeks 2, 4, and 8
Week 2: The empty lacunae in trabecular bone were visible and the fat cells were increased in the model group. In the treatment group, the osteonecrosis was not obvious. The arrows pointed few empty lacunae in the model group. Week 4: In the model group, the empty lacunae in trabecular bone and the fat cells in the marrow were increased, necrotic changes of hematopoietic cells and fat cells were also observed in the marrow tissue, and the trabecular bone became thin and partially ruptured. In the treatment group, there were only few empty lacunae in trabecular bone, and the fat cells slightly increased. The arrows pointed to more empty lacunae in the model group. Week 8: In the model group, the empty lacunae and the fat cells were increased significantly, the trabecular bone was widely sparse and fractured. A large number of necrotic fat cells were filled in the bone marrow. In the treatment group, it showed slight osteonecrosis, the empty lacunae and fat cells increased to some extent, but far less than those in the model group. The arrows pointed to a lot of empty lacunae in the model group. Magnification: ×200
Fig. 4.
Rate of empty lacunae in the three groups at Weeks 2, 4, and 8
There was no statistical significance between the control group and the treatment group (Fisher’s exact test, P=0.458). In the model group, the rate of empty lacunae showed significant differences compared with that in the control group at Weeks 2, 4, and 8 (P<0.05); compared with the treatment group, there was a statistical significance at Weeks 4 and 8 (P<0.05), but no significance at Week 2 (P=0.0612). Values are expressed as mean±SD (n=8). * P<0.05 versus control group; Δ P<0.05 versus treatment group
3.2. Immunohistochemistry
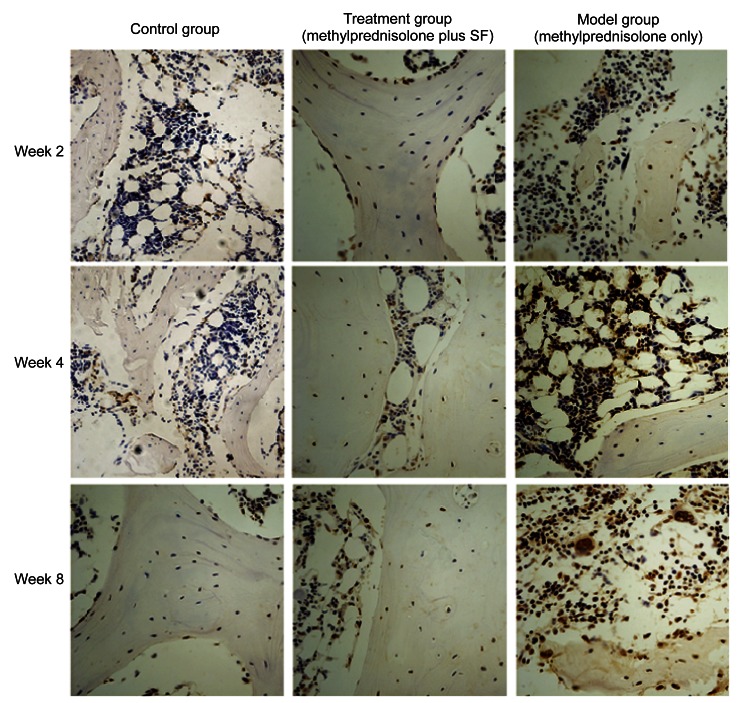
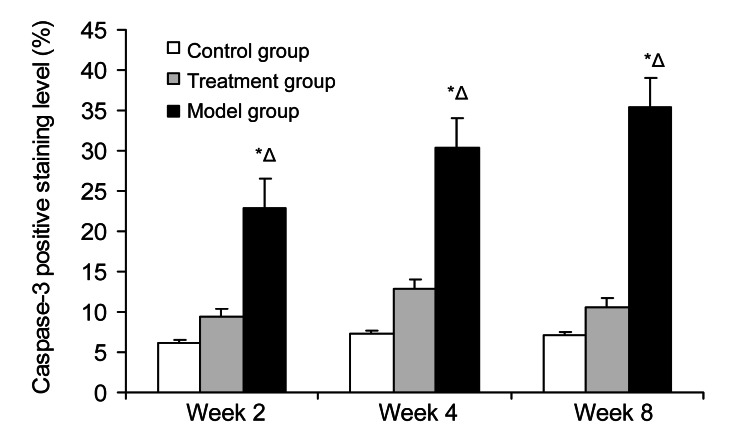
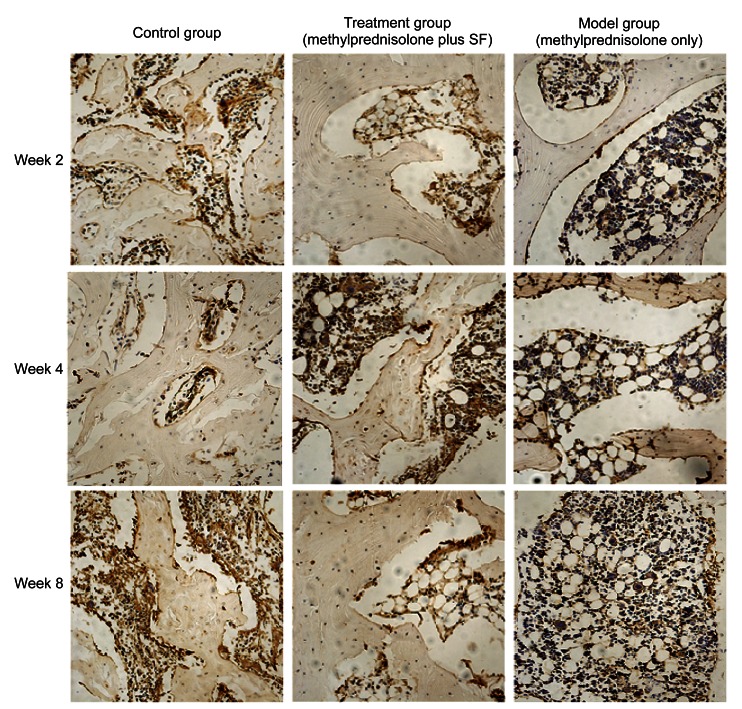
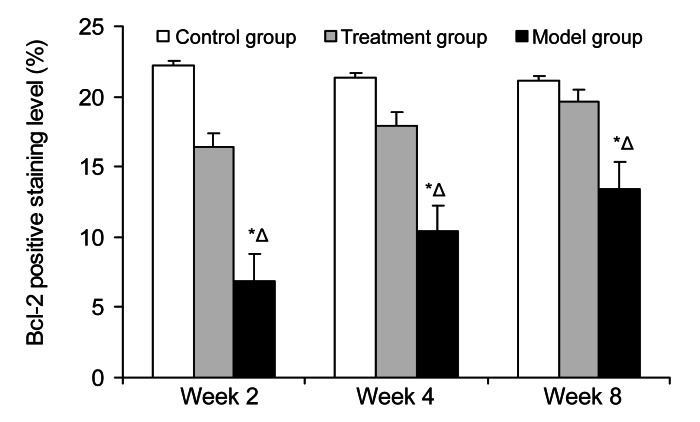
The subchondral section of the femoral head was the interested region. The immunostaining for caspase-3 was most intense in the model group and increased gradually (Fig. 5). Quantitative image analysis illustrated a meaningful increase in immunolabeling as a percentage of total bone volume in the model group compared with that in the control group (P=4.06×10−5) and the treatment group (P=0.0059) (Fig. 6). The immunostaining for Bcl-2 was lowest in the model group, whereas it increased gradually in the treatment group from Week 2 to Week 8 (Fig. 7). Quantitative imaging analysis demonstrated a significant decrease as a percentage of total bone volume in the model group compared with that in the control group (P=0.0038), then a significant increase in the treatment group compared with that in the model group (P=0.017) (Fig. 8).
Fig. 5.
Effects of methylprednisolone and methylprednisolone plus sodium ferulate (SF) on osteocytes in immunohistochemical staining of caspase-3 at Weeks 2, 4, and 8
Week 2: The positive staining is brown, and the staining in the model group was more intense than that in the control and treatment groups. The visible positive staining was hardly observed in the control and treatment groups. Week 4: The positive staining between the trabecular bone and marrow further deepened in the model group. The positive staining in the treatment group also increased, but it was far less than that in the model group. Week 8: The positive staining was richly intense between the trabecular bone and marrow in the model group. There was intense staining in some individual regions of the treatment group, but overall, the positive staining was slight. Magnification: ×200 (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Fig. 6.
Average staining area of caspase-3 in the three groups at Weeks 2, 4, and 8
The immunostaining for caspase-3 was most intense in the model group and increased gradually. It illustrated a meaningful increase in immunolabeling as a percentage of total bone volume in the model group compared with that in the control group (P=4.06×10−5) and the treatment group (P=0.0059). Values are expressed as mean±SD (n=8). * P<0.05 versus control group; Δ P<0.05 versus treatment group
Fig. 7.
Effect of methylprednisolone and methylprednisolone plus sodium ferulate (SF) on osteocytes in immunohistochemical staining of Bcl-2 at Weeks 2, 4, and 8
Week 2: The positive staining is brown, and the staining in the model group was less intense than that in the control and treatment groups. The visible positive staining was hardly observed in the model group. Week 4: The positive staining was most significant in the treatment group, and lowest in the model group. Week 8: The positive staining in the treatment group was intense and similar with that in the control group. The positive staining in the model group slightly increased but was far less than that in the other two groups. Magnification: ×200 (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Fig. 8.
Average staining area of Bcl-2 in the three groups at Weeks 2, 4, and 8
The immunostaining for Bcl-2 was lowest in the model group, whereas it increased gradually in the treatment group. It demonstrated a significant decrease as a percentage of total bone volume in the model group compared with that in the control group (P=0.0038), then a significant increase in the treatment group compared with that in the model group (P=0.017). Values are expressed as mean±SD (n=8). * P<0.05 versus control group; Δ P<0.05 versus treatment group
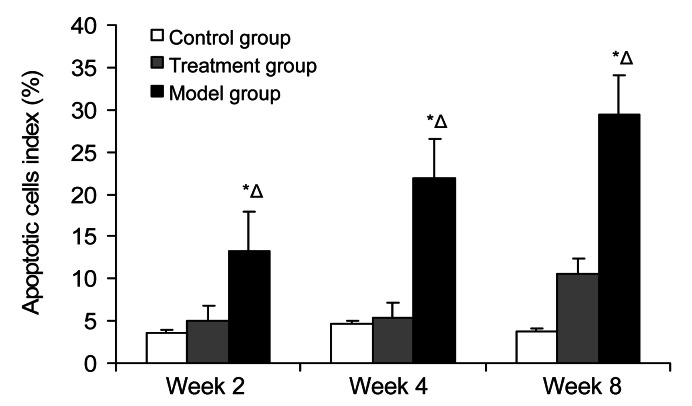
3.3. TUNEL apoptosis
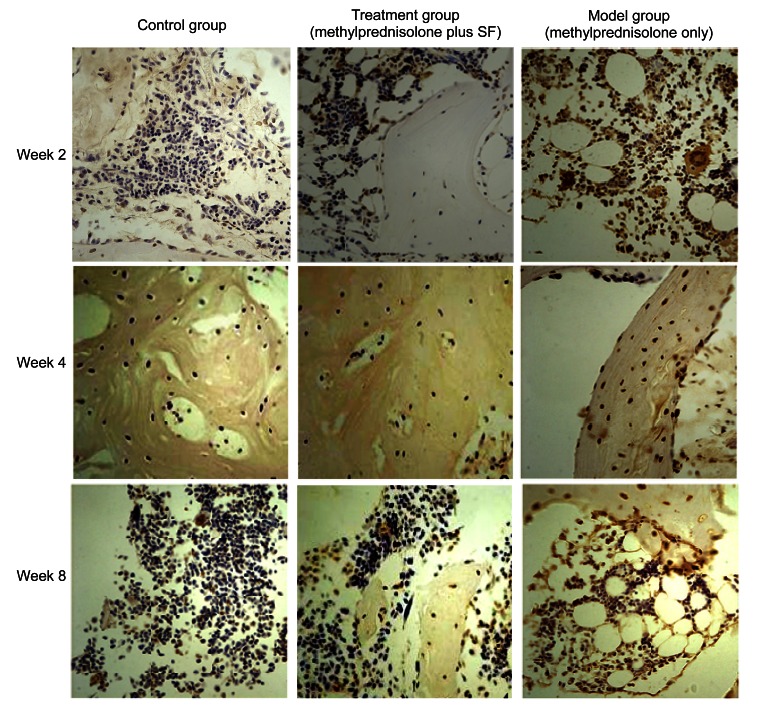
Positive cells were localized in the nucleus, and were brown or brown-yellow, or a few positive granules appeared in cytoplasm. There were individual apoptotic cells scattered in trabecular bone and marrow in the control group, and the mean apoptotic rate was 4.05%. There were brown particles of positive staining located in cell nucleus of trabecular bone and marrow in the model group. From Week 2 to Week 8, the expressions of apoptotic osteocytes and intramedullary apoptotic fat cells increased gradually. The rate of mean apoptosis in the model group was much higher (31.51%), and was statistically significant compared with that in the control group (P<0.01). There was a little apoptotic staining in bone marrow and bone nucleus in the treatment group, and the mean apoptotic rate was 10.96%, which showed no significance compared with that in the control group (P>0.05); conversely, there was a statistical significance compared with that in the model group (P<0.01). From Week 2 to Week 8, the expressing tendency of apoptotic cells increased a little, some trabecular bone became thinner, and the number of osteocytes reduced slightly (Figs. 9 and 10).
Fig. 9.
Effect of methylprednisolone and methylprednisolone plus sodium ferulate (SF) on osteocytes in TUNEL detected sections at Weeks 2, 4, and 8
Week 2: Positive cells were localized in the nucleus, and were brown or brown-yellow, or a few positive granules appeared in cytoplasm. There were individual apoptotic cells scattered in trabecular bone and marrow in the control group and few apoptotic staining in trabecular bone and marrow in the treatment group. A number of brown particles of positive staining were located in cell nucleus of trabecular bone and marrow in the model group. Week 4: The apoptotic cells had increased significantly in the model group, but the increase was not significant in the treatment group. Week 8: The apoptotic staining was most significant between the osteocytes and marrow cells in the model group. There were not significant changes of apoptotic cells in the treatment group. Magnification: ×200 (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Fig. 10.
Positive levels of apoptotic cells in the three groups at Weeks 2, 4, and 8
The mean apoptotic rate in the control group was 4.05%. From Week 2 to Week 8, the rate of mean apoptosis in the model group was much higher (31.51%), and it was statistically significant compared with that in the control group (P<0.01). There was a little apoptotic staining in bone marrow and bone nucleus in the treatment group, and the mean apoptotic rate was 10.96%, which showed no significance compared with that in the control group (P>0.05); conversely, there was a statistical significance compared with that in the model group (P<0.01). Values are expressed as mean±SD (n=8). * P<0.05 versus control group; Δ P<0.05 versus treatment group
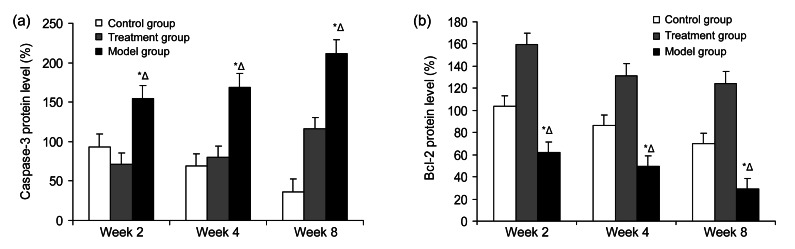
3.4. Protein expression of caspase-3 and Bcl-2
The caspase-3 expression was less in the treatment group (P<0.01), but the expression was promoted and highest in the model group (P<0.05). The protein level of Bcl-2 was highest in the treatment group (P<0.01), but the expression was least and was inhibited in the model group (P<0.05). Pretreatment with SF (20 mg/kg) markedly reversed the steroid-induced suppression of Bcl-2 protein level (P<0.01) and it also significantly suppressed the increase of caspase-3 expression (P<0.01). All results demonstrated the potential protective effect of SF on steroid-induced apoptosis in the femoral head osteonecrosis (Figs. 11 and 12).
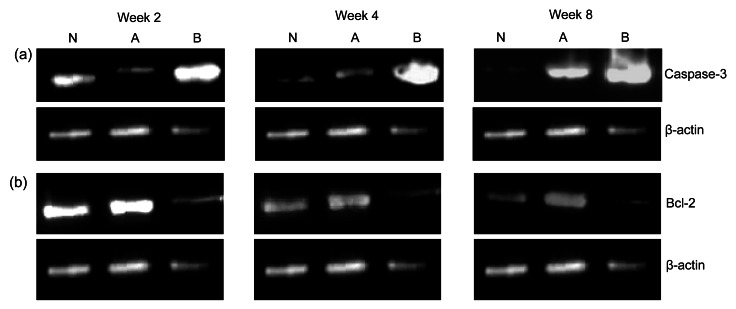
Fig. 11.
Effects of methylprednisolone and methylprednisolone plus sodium ferulate (SF) on the protein levels of caspase-3 (a) and Bcl-2 (b) in the three groups at Weeks 2, 4, and 8
(a) The caspase-3 expression significantly increased and was highest in the model group compared with that in the control and treatment groups. The increase of caspase-3 expression was not too obvious in the treatment group. (b) It was visible that the Bcl-2 expression in the model group was weakest and gradually declined. The expression in the treatment group was highest. N: control group; A: treatment group (methylprednisolone plus SF); B: model group (methylprednisolone only)
Fig. 12.
Protein expression of caspase-3 (a) and Bcl-2 (b) in the three groups at Weeks 2, 4, and 8
(a) Caspase-3 expression was less in the treatment group (P<0.01), but the expression was promoted and highest in the model group (P<0.05). Pretreatment with SF (20 mg/kg) significantly suppressed the increase of caspase-3 expression (P<0.01). (b) Protein level of Bcl-2 was highest in the treatment group (P<0.01), but the expression was least and inhibited in the model group (P<0.05). Pretreatment with SF (20 mg/kg) markedly reversed the steroid-induced suppression of Bcl-2 protein level (P<0.01). * P<0.05 versus control group; Δ P<0.05 versus treatment group
4. Discussion
Femoral head necrosis is a serious and common degenerative disease in orthopedics, which mostly occurs in the middle-aged, and finally leads to femoral head collapse and post-secondary degenerative arthritis. It seriously affects patients’ normal lives and has a high morbidity. Moreover, most patients eventually have to endure hip arthroplasty. Glucocorticoid is a strong angiogenesis inhibitor, which can destroy blood circulation in a series of ways, leading to the reduction in local blood perfusion (Hashimoto et al., 2002). It can break the dynamic equilibrium of osteocytes in the femoral head, inhibit their regeneration, and accelerate their degradation. Finally, it leads to ischemia and osteocyte apoptosis (Kerachian et al., 2009), inhibits repair and reconstruction of necrotic bone tissue, and intensifies bone necrosis (Bradway and Morrey, 1993; Mont et al., 2006).
The crucial treatment of steroid-induced femoral head osteonecrosis depends on the early diagnosis and intervention to delay the occurrence of bone necrosis, and ultimately, to postpone the treatment of hip arthroplasty. However, it has not clearly demonstrated the necrosis of osteocytes, for no feature of necrotic cells, such as swelling and inflammation, is observed. Eberhardt et al. (2001) studied rabbits using large doses of hormone and found that there was a large area of osteoblasts apoptosis. Rojas et al. (2003) found that the apoptosis of osteoblasts was significantly different before and after hormone therapy in transplant patients by the application of bone biopsy. After hormone therapy, the normal osteoblasts reduced, but the apoptotic osteoblasts increased. In addition, with the increase in the dose of hormones, more and more osteoblasts became inactive and underwent apoptosis. O′Brien et al. (2003) found that hormones could directly affect osteoblasts and stimulate apoptosis, but osteoclasts were not affected in mice. Hormones played a potential role in the osteocytes apoptosis. Weinstein et al. (2000) studied five human femoral head specimens caused by hormones, three cases of alcoholism, one by injury, and five cases of sickle cell disease, and found that hormones caused a large number of apoptosis cells by TUNEL technique, but there were rarely any or no apoptotic cells in other sections. Hormones, an important factor in osteonecrosis as an apoptosis inducer, could promote osteoblast apoptosis. Hormone-induced oxidative stress could also lead to cell damage and non-normal cell apoptosis (Wang et al., 2004).
SF is a sodium salt of ferulic acid with a variety of pharmacological functions extracted from traditional Chinese herbs, such as Angelica sinensis and Cimicifuga heracleifolia, some grain, roasted coffee, and tomato. It is stable, lower in toxicity, soluble in water and can be synthesized. At present, SF has been widely used and confirmed in cardiovascular medicine, renal medicine, endocrinology, and other fields. It has been proven that SF plays a role in lowering lipids, inhibiting thrombosis and apoptosis, and improving the microcirculation of blood supply. Some previous work showed effects of SF on mitogen-activated protein (MAP) kinases, cell adhesion molecules, and on vascular endothelial growth factor itself. It was likely that the beneficial effects of the SF are multifactorial, and could include effects on key pathways supporting adequate perfusion and cell differentiation as well as anti-apoptotic pathways (Cheng et al., 2008; Wang et al., 2011; Kim and Lee, 2012). Because of the structure of phenolic hydroxyl, SF could interfere in the conduct of apoptosis pathway. Recent reports showed that SF was used in treating osteoporosis, osteoarthritis, and cartilage degeneration, and showed good results. However, it was rarely studied on the early intervention and treatment of osteonecrosis.
Our results show that apoptosis indeed exists in osteocytes and bone marrow cells in the early stage of femoral head osteonecrosis. The changes of necrosis and apoptosis in the model group were more obvious than those in the other two groups. SF could interfere in apoptosis, prevent or delay the occurrence of steroid-induced osteonecrosis, and the probable mechanisms were: (1) SF could reduce the oxygen free radicals and cytotoxicity caused by excessive glucocorticoid, which could induce peroxidation and DNA damage (Rukkumani et al., 2004); (2) SF could reduce the protein level of caspase-3, increase the Bcl-2 expression, protect the DNA from being damaged, and inhibit the apoptosis pathway (Sudheer et al., 2007).
The process of apoptosis is regulated by Bcl-2 expression. Bcl-2 is an anti-apoptotic protein, mainly presented in the outer membrane of mitochondria, and known for its regulation of the apoptotic pathways. It plays a central role in the regulation of cell proliferation and apoptosis (Karlnoski et al., 2007). Caspase-3 is expressed downstream in the Bcl-2 pathway, and plays a crucial role in the execution and index of apoptosis in the caspase family (Park et al., 2008). The ways to induce apoptosis by caspase-3 are different, such as cleavage of DNA repair molecules, anti-apoptotic protein degradation, and cleavage of extracellular matrix proteins and skeleton proteins. It was confirmed that excessive glucocorticoid decreased Bcl-2 expression, increased protein level of caspase-3, and mediated apoptosis (Byun et al., 2005). However, SF altered or reversed these changes. The results show that SF reduced the apoptosis rate and protected bone marrow-derived mesenchymal stem cells (BMSCs) and osteocytes from apoptosis induced by steroid-induced oxidative stress. Our study in vivo has demonstrated the basis and further evaluated the clinical prophylactic treatment of steroid-induced osteonecrosis. It shows significant short-term effects in the preliminary experiments, but its long-term efficacy needs to be observed further and confirmed in more studies.
In short, our results provide experimental evidence that SF has protective and apoptosis-intervening effects on excessive steroid-induced osteonecrosis in femoral head of cultured rabbits to regulate the expressions of caspase-3 and Bcl-2. With the continuous development of science and technology, a variety of steroid-induced femoral head necrosis in-depth studies will be done, and more and more effective measures to prevent or reverse the occurrences of bone necrosis will be found.
Acknowledgments
The authors thank the teachers and professors of the Animal Center and Department of Pathology and Immunology of the Medical College of Xi’an Jiaotong University for technical assistance in the immunohistochemistry and the other help. This work was supported by the Department of Orthopedics, the Second Affiliated Hospital of Xi’an Jiaotong University, China.
Footnotes
Compliance with ethics guidelines: Lei TIAN, Xiao-qian DANG, Chun-sheng WANG, Pei YANG, Chen ZHANG, and Kun-zheng WANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Aaron RK, Gray RL. Osteonecrosis: Etiology, Natural History, Pathophysiology, and Diagnosis. In: Callaghan JJ, Rosenberg AG, Rubash JE, editors. The Adult Hip. 2nd Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 463–476. [Google Scholar]
- 2.Balasubashini MS, Rukkumani R, Viswanathan P, Menon VP. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother Res. 2004;18(4):310–314. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
- 3.Bradway J, Morrey B. The natural history of the silent hip in bilateral atraumatic osteonecrosis. J Arthropl. 1993;8(4):383–387. doi: 10.1016/S0883-5403(06)80036-7. [DOI] [PubMed] [Google Scholar]
- 4.Byun CH, Koh JM, Kim DK, Park SI, Lee KU, Kim GS. α-lipoic acid inhibits TNF-α-induced apoptosis in human bone marrow stromal cells. J Bone Miner Res. 2005;20(7):1125–1135. doi: 10.1359/JBMR.050302. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CY, Su SY, Tang NY. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 6.Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid-treated rabbits. Endocrinology. 2001;142(3):1333–1340. doi: 10.1210/en.142.3.1333. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;329(8538):902–906. doi: 10.1016/S0140-6736(87)92870-4. [DOI] [PubMed] [Google Scholar]
- 8.Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13(4):435–448. doi: 10.1016/0891-5849(92)90184-I. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto I, Nakanishi H, Shono Y, Toda M, Tsuda H, Arase S. Angiostatic effects of corticosteroid on wound healing of the rabbit ear. J Med Invest. 2002;49(1-2):61–66. [PubMed] [Google Scholar]
- 10.Jones LC, Hungerford DS. Osteonecrosis: etiology, diagnosis, and treatment. Curr Opin Rheumatol. 2004;16(4):443–449. doi: 10.1097/01.moo.0000127829.34643.fd. [DOI] [PubMed] [Google Scholar]
- 11.Karlnoski R, Wilcock D, Dickey C, Ronan V, Gordon MN, Zhang WR, Morgan D, Taglialatela G. Up-regulation of Bcl-2 in APP transgenic mice is associated with neuroprotection. Neurobiol Dis. 2007;25(1):179–188. doi: 10.1016/j.nbd.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114(3-5):121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanduja KL, Avti PK, Kumar S, Mittal N, Sohi KK, Pathak CM. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: a Bcl-2 independent mechanism. Biochim Biophys Acta. 2006;1760(2):283–289. doi: 10.1016/j.bbagen.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Kim HY, Lee SM. Ferulic acid attenuates ischemia/reperfusion-induced hepatocyte apoptosis via inhibition of JNK activation. Eur J Pharm Sci. 2012;45(5):708–715. doi: 10.1016/j.ejps.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Koo KH, Kim R, Kim YS, Ahn IO, Cho SH, Song HR, Park YS, Kim H, Wang GJ. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21(4):299–303. doi: 10.1007/s100670200078. [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 17.Lavernia CJ, Sierra RJ, Grieco FR. Osteonecrosis of the femoral head. J Am Acad Orthop Surg. 1999;7(4):250–261. doi: 10.5435/00124635-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Matsui M, Saito S, Ohzono K, Sugano N, Saito M, Takaoka K, Ono K. Experimental steroid-induced osteonecrosis in adult rabbits with hypersensitivity vasculitis. Clin Orthop Relat Res. 1992;277:61–72. [PubMed] [Google Scholar]
- 19.Maurya DK, Devasagayam TP. Ferulic acid inhibits gamma radiation-induced DNA strand breaks and enhances the survival of mice. Cancer Biother Radiopharm. 2013;28(1):51–57. doi: 10.1089/cbr.2012.1263. [DOI] [PubMed] [Google Scholar]
- 20.Maurya DK, Salvi VP, Nair CK. Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions. Mol Cell Biochem. 2005;280(1-2):209–217. doi: 10.1007/s11010-005-0170-4. [DOI] [PubMed] [Google Scholar]
- 21.Mazumder S, Plesca D, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2008;414:13–21. doi: 10.1007/978-1-59745-339-4_2. [DOI] [PubMed] [Google Scholar]
- 22.Mendonça DM, Chimelli L, Martinez AM. Quantitative evidence for neurofilament heavy subunit aggregation in motor neurons of spinal cords of patients with amyotrophic lateral sclerosis. Braz J Med Biol Res. 2005;38(6):925–933. doi: 10.1590/S0100-879X2005000600015. [DOI] [PubMed] [Google Scholar]
- 23.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg (Am) 1995;77A(3):459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg (Am) 2006;88(5):1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 25.O′Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2003;145(4):1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 26.Park SK, Kang H, Kwon CH. Caspase-dependent cell death mediates potent cytotoxicity of sulfide derivatives of 9-anilinoacridine. Anticancer Drugs. 2008;19(4):381–389. doi: 10.1097/CAD.0b013e3282f9adb2. [DOI] [PubMed] [Google Scholar]
- 27.Perluigi M, Joshi G, Sultana R, Calabrese V, de Marco C, Coccia R, Cini C, Butterfield DA. In vivo protective effects of ferulic acid ethyl ester against amyloid-β peptide 1-42-induced oxidative stress. J Neurosci Res. 2006;84(2):418–426. doi: 10.1002/jnr.20879. [DOI] [PubMed] [Google Scholar]
- 28.Rojas E, Carlini RG, Clesca P, Arminio A, Suniaga O, de Elguezabal K, Weisinger JR, Hruska KA, Bellorin-Font E. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003;63(5):1915–1923. doi: 10.1046/j.1523-1755.2003.00938.x. [DOI] [PubMed] [Google Scholar]
- 29.Rukkumani R, Aruna K, Varma PS, Menon VP. Influence of ferulic acid on circulatory prooxidant-antioxidant status during alcohol and PUFA induced toxicity. J Physiol Pharmacol. 2004;55(3):551–561. [PubMed] [Google Scholar]
- 30.Sassa S, Kikuchi T, Shinoda H, Suzuki S, Kudo H, Sakamoto S. Preventive effect of ferulic acid on bone loss in ovariectomized rats. In Vivo. 2003;17(3):277–280. [PubMed] [Google Scholar]
- 31.Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology. 2006;228(2-3):249–258. doi: 10.1016/j.tox.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Sudheer AR, Muthukumaran S, Kalpana C, Srinivasan M, Menon VP. Protective effect of ferulic acid on nicotine-induced DNA damage and cellular changes in cultured rat peripheral blood lymphocytes: a comparison with N-acetylcysteine. Toxicol in Vitro. 2007;21(4):576–585. doi: 10.1016/j.tiv.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS lett. 2000;466(1):6–10. doi: 10.1016/S0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Yuan Z, Zhao H. Ferulic acid promotes endothelial cells proliferation through up-regulating cyclin D1 and VEGF. J Ethnopharmacol. 2011;137(2):992–997. doi: 10.1016/j.jep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Wang JH, Yin QS, Huang QC, Wu YS. Effect of vascular endothelial growth factor on angiogenesis and its regulation. Acad J Sec Mil Med Univ. 2004;25(2):210–212. (in Chinese) [Google Scholar]
- 36.Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85(8):2907–2912. doi: 10.1210/jc.85.8.2907. [DOI] [PubMed] [Google Scholar]
- 37.Wenk GL, McGann GK, Hauss WB, Ronchetti D, Maucci R, Rosi S, Gasparini L, Ongini E. Attenuation of chronic neuroinflammation by a nitric oxide-releasing derivative of the antioxidant ferulic acid. J Neurochem. 2004;89(2):484–493. doi: 10.1111/j.1471-4159.2004.02359.x. [DOI] [PubMed] [Google Scholar]
- 38.Yogeeta SK, Gnanapragasam A, Senthilkumar S, Subhashini R, Devaki T. Synergistic salubrious effect of ferulic acid and ascorbic acid on membrane-bound phosphatases and lysosomal hydrolases during experimental myocardial infarction in rats. Life Sci. 2006;80(3):258–263. doi: 10.1016/j.lfs.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu L, Liu X, Li J, Peng Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. PNAS. 2010;107(19):8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]