Summary
Bile acids (BAs) are amphipatic molecules that facilitate the uptake of lipids, and their levels fluctuate in the intestine as well as in the blood circulation depending on food intake. Besides their role in dietary lipid absorption, bile acids function as signaling molecules capable to activate specific receptors. These BA receptors are not only important in the regulation of bile acid synthesis and their metabolism, but also regulate glucose homeostasis, lipid metabolism and energy expenditure. These processes are important in diabetes and other facets of the metabolic syndrome, which represents a considerable increasing health burden. In addition to the function of the nuclear receptor FXRα in regulating local effects in the organs of the enterohepatic axis, increasing evidence points to a crucial role of the G-protein coupled receptor (GPCR) TGR5 in mediating systemic actions of BAs. Here we discuss the current knowledge on BA receptors, with a strong focus on the cell membrane receptor TGR5, which emerges as a valuable target for intervention in metabolic diseases.
Introduction
BAs are multifunctional in metabolism
Bile acids (BAs) are a component of bile, which also contains phosphatidylcholine, bilirubin, and cholesterol as main constituents. An important physiological role of BAs is to facilitate the uptake of lipids together with the fat-soluble vitamins A, D, E and K from the intestine. BAs facilitate these absorptive processes through their detergent properties, which allow the emulsification of lipids [1]. BAs also play a major role in influencing the intestinal microbial flora, as well as in the elimination of cholesterol from the body [2, 3]. More recently, BAs are increasingly being appreciated for their signaling properties to transmit information to cells and organs concerning the fasting/feeding state thereby regulating processes ranging from bile acid and lipid metabolism to glucose and energy homeostasis [4, 5]. This is in fact not too surprising given the central role BAs play in dietary lipid absorption. The scope of this review is to provide an update on the most recent developments in the field of BA signaling, and their pharmaceutical implications to treat metabolic diseases, such as diabetes and other risk factors of the metabolic syndrome.
Signaling pathways activated by BAs
Nuclear receptor signaling pathways
FXR is a nuclear receptor that controls BA homeostasis and transport as outlined (See Textbox 1-3). The FXR cDNA was first cloned from both mouse and rat in 1995 [34, 35]. Rodents have two FXR family members, FXRα and FXRβ. In humans only FXRα is expressed, although FXRβ is still present in the human genome as non-expressed pseudogene. FXRα is most potently activated by conjugated or unconjugated forms of CDCA with an EC50 in the range of 4.5-10μM [36-38], while FXRβ in mice is activated by lanosterol, an intermediate of bile acid synthesis [39].
Text box 1. BA synthesis and function.
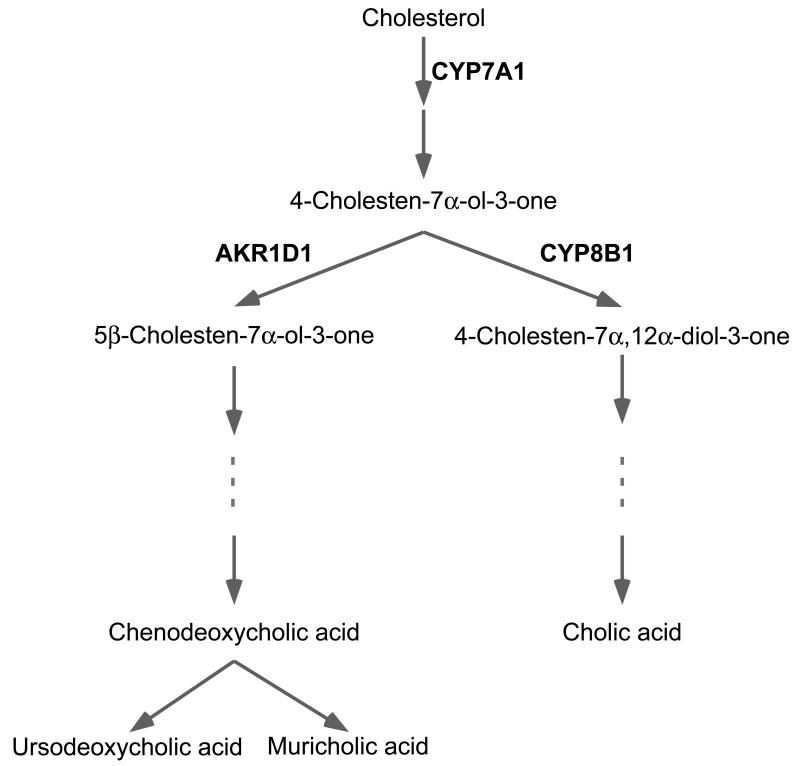
BAs are synthesized in hepatocytes by oxidation of cholesterol mainly via the so-called classical pathway, also referred to as the neutral pathway (see Figure 1). A large difference exist between the bile acid pool of mice and humans, as the predominant hydrophilic bile acid pool of mice consist of muricholic acid (MCA) and CA, which is different from the more hydrophobic bile acid pool of humans composed mainly of CDCA, CA and DCA. This difference arises amongst others from different hydroxylation reactions between mouse and humans which result in structural different primary BAs (Reviewed in [6]). The initial modification of cholesterol in this pathway is catalyzed by the microsomal cytochrome P-450 enzyme, cholesterol 7α hydroxylase (CYP7A1), leading to the generation of 7α-hydroxycholesterol, which is subsequently further converted to the primary BAs, chenodeoxycholic acid (CDCA) and cholic acid (CA) [2]. This classical pathway is responsible for at least 75% of all newly synthesized BAs in mice, as a disruption of the CYP7A1 gene decreases the BA pool to one fourth of the normal pool [7, 8]. The relative ratio CA/CDCA in humans and CA/MCA in mice (triols vs diols) is determined by the activity of another microsomal cytochrome P-450 enzyme, sterol 12α-hydroxylase (CYP8B1), which catalyzes the synthesis of CA. The final step in BA synthesis is the conversion of BAs into bile salts by glycine or taurine conjugation, which involves bile acid-CoA synthetase (BACS) and BA coenzyme A amino acid N-acyltransferase (BAAT) [9, 10]. In addition to BACS and BAAT, fatty acid transport protein 5 (FATP5) has been demonstrated to be involved in the conjugation of secondary bile acids as evidenced in mouse deficient for the latter protein [11]. The conjugated BAs produced in the hepatocytes are, together with the other constituents of bile, continually secreted into the bile canaliculi and further collected and stored in the gallbladder. Release of bile into the duodenum is controlled by the hormone cholecystokinin (CCK), which is secreted from specialized cells in the duodenum and triggers the contraction of the gallbladder. Once mixed with the nutrients, bile salts will reduce the surface tension on fat droplets and facilitate the enzymatic action of lipases. Because of their negative charge, bile salts are not readily absorbed by the upper part of the intestine, but reside in the intestinal lumen until most fat is digested and fatty acids absorbed. The primary bile salts that are not absorbed by the terminal ileum will be deconjugated and converted into secondary bile acids by the intestinal gut flora, of which lithocholic acid (LCA; derived from CDCA) and deoxycholic acid (DCA: derived from CA) are the most abundant ones [2, 3].
Text box 2. BA synthesis and its regulation.
BA synthesis is the prime pathway to eliminate excess cholesterol from the body. When cholesterol returns to the liver in a process termed reverse cholesterol transport, BA synthesis is induced [12, 13]. Part of the cholesterol that arrives in the liver, is then converted into oxysterols, potent agonist ligands for liver X receptor (LXR) [14], a nuclear receptor that in mice, but not in humans, controls the expression of CYP7A1, the rate limiting enzyme of the classic BA synthesis pathway [15]. BAs are potent repressors of Cyp7a1 gene expression and activity and ensure adequate feedback inhibition of the pathway [2]. In fact, when BAs reach a critical level in the liver, the nuclear receptor farnesoid X receptor-α (FXRα), induces the transcription of the atypical nuclear receptor small heterodimer partner (SHP). SHP subsequently inhibits a subset of nuclear receptors that are necessary for the activation of CYP7A1, including the liver receptor homolog (LRH-1), liver X receptor (LXR), and hepatocyte nuclear factor 4α (HNF-4α). The repression of the transcriptional activity of those nuclear receptors via SHP interaction will ultimately result in the repression of BA synthesis and prevention of further BA accumulation [16-20]. Both LRH-1 and HNF-4α are also critical modulators of bile acid composition via regulation of CYP8B1 [21-24]. Furthermore, activation of FXRα in the intestine results in the expression and secretion of fibroblast growth factor-19 (FGF19; designated Fgf15 in mouse). FGF19 is secreted into the circulation and signals to liver cells to inhibit CYP7A1-mediated BA synthesis via induction of tyrosine kinase and c-Jun N-terminal kinase (JNK) activation [25] downstream of the receptor complex of the FGF receptor 4 (FGFR4) and β-klotho [26]. This pathway allows the liver to anticipate an influx of BAs and together with the FXR-SHP-mediated suppression of CYP7A1, avoids accumulation of excessive levels of BA that are hepatotoxic. Besides its role in the suppression of hepatic BA synthesis, FGF19 also indirectly stimulates gallbladder filling by relaxing the contraction state, as gallbladders are almost empty in mice lacking FGF19, a phenotype rescued by recombinant FGF19. The effect of FGF19 is mediated via FGFR3 and antagonizes CCK signaling [27].
Text box #3. Recycling of BAs.
Most BAs secreted by the liver are derived from BAs that are recycled from the intestine, and only a small portion consists of de novo synthesized BAs. The conservation of BAs is very efficient, as about 95% of the secreted BAs are transported from the intestine back to the liver via the enterohepatic circulation, allowing BAs to undergo up to 12 cycles between the liver and the intestine per day (reviewed in [28]). The retrieval of BAs by the intestine is achieved both by passive diffusion as well as by active transport involving BA transporters [29]. The apical sodium/bile salt transporter (ASBT; also known as SLC10A2) is a BA transporter that mediates BA transport from the intestinal lumen. Mutations in ASBT are described to lead to primary BA malabsorption (PBMA). This condition not only causes a marked drop in plasma cholesterol levels, it also leads to severe diarrhea, illustrating hence the relevance of intestinal BA absorption [30]. FXRα plays an important role in the recycling of BAs. Activation of FXRα by BAs in the intestine occurs mainly in the ileum [31], and results amongst others in the induction of the organic solute transporter (OST)-α and OST-β at the basolateral membrane, which are important for the transport of BAs into the portal vein back to the liver. In addition to the BA transporters mentioned above, others transporters equally important for BA transport exist along the enterohepatic axis. Those BA transporters as well as their regulation by FXRα have been reviewed extensively [32, 33].
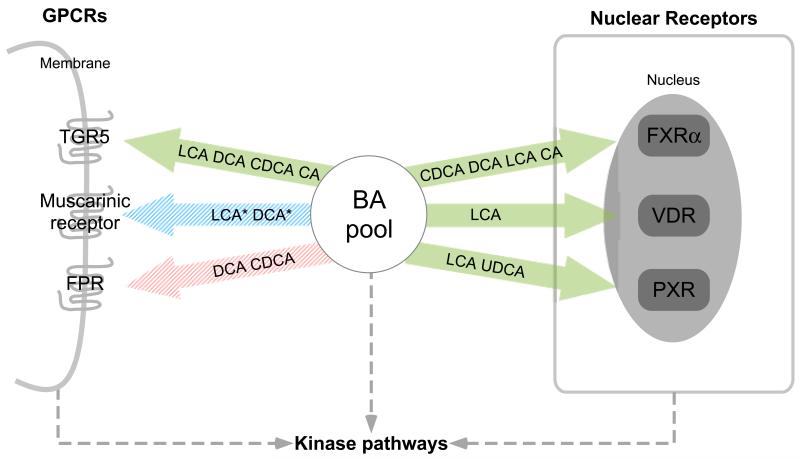
In addition to FXRα, other nuclear receptors directly activated by LCA are the pregnane X receptor (PXR), and the vitamin D receptor (VDR) [40-42] (see Figure 2). PXR is not only activated by LCA, but also by certain bile acid precursors, such as 7α-OH-4-cholesten-3-one [43]. PXR is like FXRα expressed in the liver and intestine [40, 41]. One of the physiological functions of PXR is to induce phase I detoxification metabolism through induction of CYP3A expression, which explains the finding that PXR transgenic mice are protected against LCA-induced liver toxicity in a model of cholestasis [40]. Like PXR, VDR plays a role in the detoxification of BAs through upregulation of CYP3A [42]. In addition to their roles in detoxifying BAs, both nuclear receptors inhibit BA synthesis. VDR is not expressed in hepatocytes [44]. However, activation of VDR in the intestine by vitamin D widely inhibits BA synthesis via the FGF19-CYP7A1 pathway [45], and activation of PXR with rifampicin, an agonist of human PXR, inhibits BA synthesis through a pathway involving HNF4α [46]. It should be noted that relative high concentrations of LCA (ranging from 30-100 μM) are needed to induce activation of VDR and PXR, which could raise questions about the physiological relevance of the direct regulation of their activity by BAs.
Figure 1.
Simplified overview of classical bile acid synthesis pathway in mice. CYP7A1 and CYP8B1 catalyze the first and third reaction respectively. Dotted lines represent several steps in the synthesis of BAs that involve multiple enzymes. AKR1D1 is an abbreviation for Δ4-3-oxosteroid 5β-reductase.
Figure 2.
Representation of signaling pathways that are modulated by BAs. Genomic and non-genomic actions of BAs are mediated by nuclear receptors and G-protein coupled receptors. The BAs that modulate the activity of the receptor are indicated in the arrow, and are sorted by decreasing potency (left:high to right:low). Arrows in green indicate agonistic actions, red indicates antagonistic/inhibitory actions and blue indicates a modulatory effect, either agonistic or antagonistic. Receptors for which only a limited number of BAs were tested are indicated with dashed arrows. *Only conjugated forms of LCA and DCA are reported to activate the muscarinic receptors.
Kinase-signaling pathways regulated by BAs
BAs also modulate kinase signaling pathways, such as the JNK pathway, which has been demonstrated to be involved in the downregulation of CYP7A1 [47]. Another study has coupled JNK activation indirectly to BAs as the mechanism of the FGF19-FGFR4 signaling pathway to suppress CYP7A1 [48]. In addition to JNK, also the p38 mitogen activated protein kinases (p38MAPK), the extracellular signal-regulated kinase (ERK) pathway, as well as Akt are activated by BAs [49, 50]. Although the precise mechanisms involved are not fully clear, a study has indicated that phosphorylation of ERK1, ERK2 and Akt by BAs is induced through both radical oxygen species (ROS)-dependent, as well as through GPCR-dependent pathways [50]. The activation of these phosphorylation cascades by BAs is, besides the suppression of CYP7A1, also involved in the regulation of apoptosis and cytoprotective effects [49, 51]. It is also tempting to speculate that some of these signaling pathways may be involved in the enhanced lifespan, observed in yeast exposed to BAs, as yeast do not express either the nuclear or membrane BA receptors [52].
GPCR signaling pathways modulated by BAs
Most relevant to this review is that BAs also bind and modulate the activity of specific G-protein coupled receptors (GPCRs) (See Figure 2). The total GPCR family comprises over 800 receptors, which are divided into 3 subgroups [53]. There are currently three GPCRs known of which the activity is modulated by BAs. Based on their sequence homology, these BA-modulated GPCRs are classified in the class A or rhodopsin-like receptor class, which represents the largest subgroup of GPCRs. BAs are able to modulate the activity of the muscarinic receptors (also designated as acetylcholine receptors) [54, 55], and inhibit the activity of the formyl-peptide receptors (FPRs) [56, 57]. FPR receptors have been described to be expressed in neutrophils and monocytes, and are activated by N-formyl groups, present on bacterial and mitochondrial proteins. This induces cell chemotaxis, believed to direct phagocytes to sites of tissue damage or infections. It is possible that the inhibition of activity of these receptors by BAs contributes to anti-inflammatory properties of BAs, as CDCA inhibited FPR-induced human leukocyte chemotaxis. [58]. The five muscarinic receptors (designated M1 to M5) regulate numerous physiological processes by mediating parasympathetic innervation via acetylcholine sensing. A dysfunction in their activity may contribute to several diseases, including metabolic diseases [59]. Muscarinic receptors are expressed in the central nervous system as well as in peripheral organs. For example, the M3 muscarinic receptors, which are predominantly expressed in gastrointestinal tissues, control smooth-muscle contractility and glandular secretion. Also the pancreatic β-islet cells express M3 muscarinic receptors, and induce insulin release during the pre-absorptive phase of feeding [60]. These data suggest that these receptors could potentially contribute to a broad range of effects observed by BAs, although it should be mentioned that high millimolar concentrations of BAs are necessary to induce activation of muscarinic receptors. The relevance has not been analyzed so far in humans.
The GPCR that has been most studied in relation to BAs is TGR5, also known as M-BAR, GPBAR or GPR131. This cell-surface BA receptor was discovered in 2002 [61], and first characterized in 2003 [62]. TGR5 is encoded by a single-exon gene, and its conservation among several vertebrate species underlines the relevance of this GPCR in vertebrate physiology [61]. Human TGR5 is activated by multiple BAs, with LCA being the most potent natural agonist with an EC50 of 0.53 μM [61, 62]. Next to LCA, other bile acids that activate TGR5 include conjugated and unconjugated forms of DCA, CDCA, and CA with an EC50 of 1.0, 4.4 and 7.7 μM, respectively [62]. Human and mouse TGR5 show basically the same affinity for the different BAs, although certain synthetic TGR5 agonist reveal a large difference between mouse and human TGR5 with regard to their efficacy to activate the receptor [63].
The bile acid receptor TGR5
Expression and signaling of TGR5
TGR5 is expressed in many different organs and cells with varying degree of expression. For example, high expression levels of TGR5 have been detected in gallbladder epithelium [64, 65] and in the intestine, mainly in the ileum and colon [61, 66]. TGR5 was also highly expressed in human monocytes as compared to other human leukocyte subsets examined [62]. In line with these findings, also rabbit spleen and rabbit alveolar macrophages were found to relatively express high levels of TGR5 versus other tissues [62]. TGR5 is detected in several liver cells, including rat liver sinusoidal endothelial cells, as well as in rat Kupffer cells, resident macrophages of the liver [67, 68]. TGR5 is also expressed in BAT, skeletal muscle, and selected areas of the central nervous system [69, 70]. Furthermore, TGR5 is ubiquitously expressed in many more different organs and cells with varying degree of expression [62, 64].
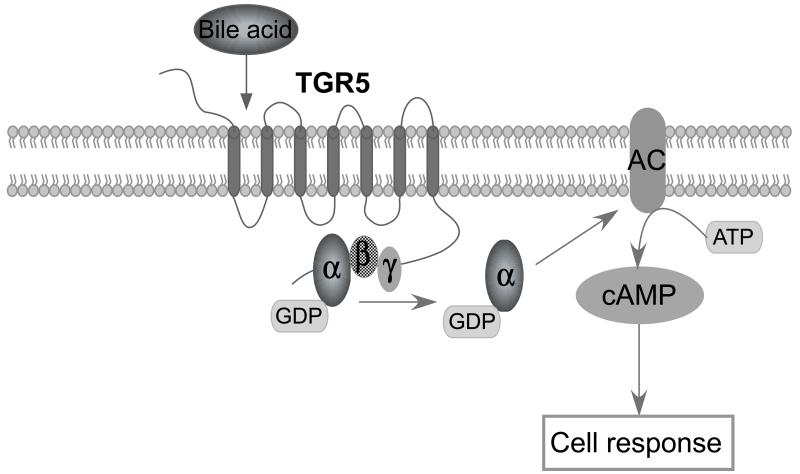
In resting conditions, GPCRs, including TGR5, are in a so-called low-affinity state. In response to binding of BAs to the ligand-binding pocket of the receptor, a complex is released from TGR5 consisting of G-protein-αs, β and γ [53, 62]. GDP is subsequently released from the G-protein and replaced by GTP, leading to dissociation of the G-protein complexes into G-protein-αs and βγ dimers. G-protein-αs subsequently activates adenylyl cyclase resulting in the induction of cAMP and activation of protein kinase A (PKA), which in turn induces further downstream signaling [62] (See Figure 3). Whether TGR5 may also bind to other G-proteins, which have distinct downstream effector molecules, is still an open question [53].
Figure 3.
Simplified overview of the TGR5-signaling pathway leading to downstream signaling via cAMP induction.
TGR5 in BA homeostasis and metabolism
To explore the biological role of TGR5, several groups have independently generated TGR5-/- mice [64, 66, 71]. Interestingly, the total bile acid pool size in TGR5-/- mice was decreased as compared to that of wild-type mice [66], which was also observed by our lab (JA & KS, unpublished data). The cause of the decreased BA pool in TGR5-/- mice is currently unknown, but appears not to be caused by changed fecal excretion of BAs, which was similar between TGR5-/- and control mice [66].
In addition to changes in the BA pool size, TGR5-/- mice fed a lithogenic diet were protected against cholesterol gallstone formation [72]. Hydrophobic bile salts decrease gallbladder smooth muscle function potentially via stimulation of TGR5, which could be a contributing factor in the manifestation of gallstone disease [73]. Additionally, TGR5 is expressed in human gallbladder epithelial cells and cholangiocytes and was shown to play a role in bile composition via the induction of chloride secretion [65, 74]. Taken together, the physiological changes observed in mice lacking TGR5 with regard to BA homeostasis combined with expression of TGR5 in human tissues relevant to BA homeostasis hint towards a role of TGR5 in bile formation and homeostasis in man [65, 74].
Effect of TGR5 on body weight
TGR5 activation induces a significant reduction of the body weight of mice fed a high fat diet. We first demonstrated that dietary supplementation of BAs significantly reduces body weight gain in C57Bl/6J mice fed a high fat diet [69]. CA administration completely prevented high fat diet-induced changes in adipose mass and morphology, and reversed 120 days of diet-induced weight gain within 30 days, without toxicity [69]. The weight reducing effects were not due to reduced caloric intake, but were the consequence of enhanced energy expenditure. Using deiodinase 2 (D2)-deficient mice, the effect of CA on energy expenditure was shown to require the induction of deiodinase 2 (D2) through a TGR5-cAMP-mediated pathway that was active in murine BAT and in human skeletal muscle myoblasts. Importantly, this effect of BAs on energy expenditure was independent of FXR, as the FXR agonist GW4064, did not increase cAMP levels in BAT and increased diet-induced obesity in mice. D2 is able to increase mitochondrial oxidative phosphorylation and energy expenditure in brown adipose tissue and muscle via the conversion of inactive thyroxine (T4) into active 3,5,3′-tri-iodothyronine (T3), which subsequently binds and activates the thyroid hormone receptor, thereby inducing energy expenditure [69]. It should be mentioned that postprandial plasma BA levels of 15 μM are capable of activating TGR5 [69]. The latter effects were subsequently confirmed in a study using the semi-synthetic BA, 6-ethyl-23(S)methyl-cholic acid (6EMCA or INT-777), which acts as a specific TGR5, but not FXR, agonist [71].
In agreement with our data, Maruyama et al. observed that female TGR5-/- mice have increased body weight [66]. The latter effect of TGR5 was already observed in heterozygous female mice. In agreement with this observation, female TGR5-/- mice had a higher fat content, while the lean body weight was unaffected as compared to wildtype mice. Also the body composition of the male TGR5-/- mice showed a tendency towards increased fat content [66]. Interestingly, in the TGR5-/- mice line that we have generated in our laboratory, we also observed a significant increase in body weight in males ([71] and KS&JA unpublished data). These two studies are, however, in contrast to the data of Vassileva et al. [72], which did not observe an effect on body weight in the absence of TGR5. This difference may very well be explained by different diets used, or by other differences in animal experimental conditions applied in this specific study. Furthermore, it needs to be underscored, that the mere absence of a receptor, as in the TGR5-/- mice, should not necessary translate in the opposite phenotype as observed after receptor activation by exogeneous ligands.
A recent study assessed BA levels in humans with respect to energy expenditure [75]. In this study, patients suffering from cirrhosis displaying elevated plasma BA levels and healthy controls were compared with respect to energy expenditure. It was concluded that no correlations between BA levels and energy expenditure exist. It should, however, be underlined that the many symptoms observed in cirrhosis are not controlled, and more than likely confound the study outcome. A better understanding of the impact of BA on energy expenditure in humans will require further studies specifically designed to address this question.
Effects of TGR5 on glucose metabolism and insulin sensitivity
High circulating levels of BAs have been linked with beneficial effects on glucose metabolism, such as improved insulin sensitivity and better postprandial glycemic control [76, 77]. We have demonstrated that BAs regulate glucose homeostasis through activation of TGR5 [78]. In agreement with the report that TGR5 induces GLP-1 secretion in cultured mouse enteroendocrine STC-1 cells [79], the semisynthetic BA, 6-ethyl-23(S)methyl-cholic acid (6EMCA or INT-777), which is a specific TGR5 agonist, induces GLP-1 secretion in both STC-1 cells as well as in human intestinal NCI-H716 cells, and contributes as such to the effects of TGR5 in glucose homeostasis. Silencing of TGR5 in STC-1 cells using short hairpin RNA prevented the secretion of GLP-1, illustrating the involvement of TGR5 in this response. Although the mechanism underlying TGR5-induced GLP-1 secretion is not yet completely established, stimulation of oxidative phosphorylation may be involved in triggering this process. The resulting increase in ATP/ADP ratio can subsequently induce membrane depolarization and Ca2+ mobilization in a way reminiscent to the cascade of events that precedes insulin release in pancreatic β-cells [71]. Using obese and insulin resistant mouse models, we have shown that mice with a gain-of-function of TGR5 became more glucose tolerant, whereas TGR5-/- mice showed a delayed glucose clearance compared to their wild-type littermates. This effect was correlated with a healthier pancreatic islet phenotype, and is at least partly explained by the tonic increase of GLP-1 secretion by TGR5 [78]. It was recently reported by Poole et al. that TGR5 is also expressed in inhibitory motor neurons and modulates intestinal motility [70]. This effect of TGR5 could be related to GLP-1 induction, which is also known to inhibit intestinal motility [80]. In apparent contrast to these observations are the findings in a recent study with independently generated TGR5-/- mice, demonstrating that female and male chow fed TGR5-/- mice show improved insulin sensitivity [72]. However, it was also shown in this study that male TGR5-/- mice fed with a high fat diet display impaired insulin sensitivity, which is in agreement with our findings. Other evidence that TGR5 activation is beneficial with regard to diabetes comes from the observation that the triterpenoid, oleanolic acid, a natural TGR5 agonist, also improves glucose homeostasis [81].
TGR5 modulates the immune response
One of the initial studies on TGR5 concerns its role in immune cells and has linked TGR5 to the immunomodulatory properties of BAs [62]. This action of TGR5 is very relevant, as low-grade inflammation is suggested to contribute to the development of the metabolic syndrome [82]. TGR5 is highly expressed in monocytes and macrophages, an observation derived from the finding that TGR5 expression is present in human spleen and human CD14+ monocytes, as well as in rabbit alveolar macrophages [62, 68]. In accordance with a report that cAMP inhibits LPS-induced cytokine secretion [83], BAs capable of activating TGR5 were found to increase cAMP production in alveolar macrophages [62]. In addition, BAs reduce the phagocytic activity of these cells and inhibit LPS-induced production of several pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), Interleukin (IL)-1α, IL-1β, IL-6 and IL-8 [62]. Human monocytic leukemia THP-1 cells transfected with TGR5 exhibit increased cAMP production and a reduction in LPS-induced TNF-α expression. These effects were not observed in untransfected THP-1 cells, which express low levels of TGR5, demonstrating that these effects of bile acids are mediated through activation of TGR5 [62]. Furthermore, stimulation of isolated rat Kuppfer cells with taurolithocholic acid (TLC) or other TGR5 agonists, such as oleanolic acid, as well as cAMP stimulation resulted in reduced expression of IL-1α, IL-1β, IL-6 and TNF-α following LPS treatment [68]. Although the inhibition of inflammation through TGR5 activation can be considered beneficial for atherosclerosis, steatosis and obesity, the anti-inflammatory action of TGR5 in macrophages might perhaps also explain observations that obstructive cholangitis is associated with enhanced susceptiblility for infections due to decreased clearance of intrabiliary bacteria [84]. Recently, association of 22 single nucleotide polymorphisms (SNPs) within the TGR5 genes were assessed with regard to pimary sclerosing cholangitis (PSC). A weak association of one of these SNPs, rs11554825, was observed with PSC as well as with ulcerative colitis, although it could not be excluded that SNPs in neighboring genes that are in linkage disequilibrium with rs11554825 are responsible for this effect [85].
TGR5 in liver function
Administration of the specific TGR5 agonist, INT-777 to high fat diet-fed mice reduces liver steatosis and associated hepatocyte damage, as measured by plasma liver enzymes LDH, ASAT and ALAT [71]. This is correlated with decreased plasma triglyceride and nonesterified fatty acid levels. The latter is consistent with the report by Vassileva et al. showing a pronounced hepatosteatosis in male TGR5-/- mice fed a high fat diet for 8 weeks [72]. These data suggest that activation of TGR5 may prevent non-alcoholic fatty liver disease (NAFLD), although the precise regulatory mechanisms by which TGR5 induces this effect on liver triglycerides remains to be dissected. Although TGR5 seems not expressed in hepatocytes, it is detected in many cell types of the liver where it could directly or indirectly modulate liver function and triglyceride metabolism. TGR5 is for instance highly expressed in Kupffer cells, which are resident liver macrophages [68]. It has become clear that these cells, together with their pro-inflammatory cytokine secretion, are critically involved in the progression of NAFLD [86]. The anti-inflammatory action of TGR5 in these cells could therefore contribute to the protective effects of TGR5 on steatosis. TGR5 has also been shown to modulate microcirculation and fluid secretion in the endothelial and biliary epithelial cells of the liver [67, 74]. Although the increased energy expenditure and GLP-1 secretion following TGR5 activation may very well explain the significant improvement in liver steatosis, it will be challenging to examine whether any of these other cell types in the liver contribute to the protective effects of TGR5 activation against steatosis. Cell-type specific TGR5-/- mouse models will be extremely valuable tools to address these questions.
BA signaling as a target for intervention in the metabolic syndrome
BA signaling as target for intervention
The significance of BAs in human triglyceride metabolism is underlined by findings that bile-acid binding resins induce the production of VLDL and that treatment of cholesterol gallstones in humans with CDCA reduces hypertriglyceridemia [87-89]. In addition to the effects of BAs on human triglyceride metabolism, BAs are correlated to increased insulin sensitivity in humans [76]. Furthermore, patients after bariatric surgery to correct for obesity have higher circulating levels of BAs, which are positively correlated to peak GLP-1 levels [77]. The latter observation was recently confirmed in obese patients, who have a decreased postprandial BA response and suboptimal GLP-1 secretion in comparison to normal weight subjects [90].
A few studies report on the positive effects of bile acid binding resins on glucose homeostasis [91-93]. The positive effects of bile acid sequestrants on glucose homeostasis could be the consequence of improved general metabolism subsequent to the elimination of cholesterol. It might, however, also raise the intriguing possibilities that bile acid binding resins through still unknown mechanisms trigger TGR5 activation and GLP-1 secretion, hence contributing to the beneficial effects on glucose homeostasis.
The biological properties of TGR5 described in this review (See Table 1), mostly observed in animal models, strongly indicate that TGR5 is linked to the beneficial properties of BAs in humans. This is underlined by the findings that the improvements of BAs on metabolic homeostasis are amongst others linked to GLP-1, for which its regulation by TGR5 is well documented [71, 79]. Furthermore, a recent human genetic study indicates a potential association of TGR5 with some aspects of the metabolic syndrome. This study addressed the link between the single nucleotide polymorphism (SNP) rs3731859 within the human TGR5 gene and the risk for type 2 diabetes [94]. Although no correlation was found between the onset of diabetes and the investigated SNP, nominal associations with body mass index, waist circumference, intramyocellular lipids and fasting GLP-1 levels were identified. This interesting result warrants further studies to explore the effect of this (and other) SNPs in the TGR5 gene in different populations and calls for further mechanistic studies that address whether this SNP, located at the 5′ untranslated region, influences TGR5 expression levels.
Table 1.
Cellular actions described for TGR5 in different cell types.
| Celltype | Species | Cellular action | References |
|---|---|---|---|
| Macrophages* | Human Rabbit Rat |
Inhibition of cytokine production | [62, 68] |
| Enteroendocrine cells |
Human Mouse |
Secretion of GLP-1 | [71, 79] |
| Brown adipocytes | Mouse | Increase in energy expenditure | [69] |
| Sketelal muscle cells | Human | Increase in energy expenditure | [69] |
| Sinusoidal endothelial cells |
Rat | Regulation of endothelial NO synthase | [67] |
| Biliary epithelial cells | Mouse | Promotion of chloride secretion | [65] |
| Astrocytes | Rat | Generation of ROS | [101] |
| Enteric neurons | Mouse | Release of NO and suppression of intestinal motility |
[70] |
| Gallbladder smooth muscle cells |
Mouse | Decrease of gallbladder smooth muscle cell function |
[73] |
Macrophages include alveolar macrophages, Kupffer cells and THP-1 cells
Pharmacological targeting of TGR5
BAs bind and activate both TGR5, as well as other (nuclear) receptors, among which FXR. The binding pocket for the membrane BA receptor TGR5 and the nuclear BA receptor FXRα is only partially conserved and minor structural modifications on the steroid side chain of BAs can dictate selectivity of the ligand towards TGR5 [95, 96]. This unique ligand binding pocket of TGR5 therefore allows the design of receptor selective ligands, leading to drugs that are able to target TGR5 exclusively.
TGR5 may be targeted by naturally compounds as well as by synthetic agonists. Such TGR5 agonists include natural BAs, semi synthetic BAs, such as 6-ethyl-23(S)methyl-cholic acid [97], bile alcohols, and triterpenoid compounds of plant origin, such as oleanolic acid and betulinic acid [81, 98-100]. Also certain steroid hormones potently activate TGR5 [98], an observation that has been recently confirmed [101]. Significant progress has also been made with the search for synthetic TGR5 agonists, as 3-aryl-4-isoxazolecarboxamides were recently identified to activate TGR5, and found to induce GLP-1 secretion in canines [102]. Also specific 2-aryl-3-aminomethylquinones were recently identified as TGR5 agonists and observed to induce GLP-1 secretion and to improve glucose homeostasis in diet-induced obese mice [63]. Furthermore, many drug companies have active TGR5 programs, which already resulted in the publication of several patents that describe additional TGR5 compounds. We await with impatience further biological studies using these novel selective and potent synthetic TGR5 agonists. Studies in animal models with tissue-specific deficiencies in TGR5 and this steadily increasing battery of TGR5 agonists will result in a better understanding of TGR5 biology and pharmacology, and perhaps new treatment options for different aspects of the metabolic syndrome.
Not all that shines is gold - issues with TGR5
Recently several properties of TGR5 have been described, that require further investigation as they could potentially be at the basis of side effects. For example, TGR5 has also been linked to epidermal growth factor receptor (EGFR) and c-Jun N-terminal kinase (JNK) signaling pathways in cell culture models, which modulates cell proliferation and apoptosis [103, 104]. This may suggest that TGR5 has a potential role in cancer development, but unfortunately evidence of such an effect was only ascertained in cultured cells and further studies in vivo are definitely required [103, 104]. TGR5 agonists, including certain steroids [98], were also reported to stimulate the generation of reactive oxygen species in cultured astrocytes [101]. The impact of this potential liability also requires further investigation in vivo. TGR5 activation has been suggested to influence cardiomyocytes as the TGR5-active bile acids tauro-CDCA and LCA activated AKT and inhibited glycogen synthase kinase-3β in these cells [105]. In view of the pleiotropic effects of BAs, it will be of interest to assess these effects in TGR5-/- mice. In addition to these cellular observations, it has been reported that TGR5-/- mice have reduced pancreatitis upon direct exposure of the pancreas to high concentrations of taurolithocholic acid 3-sulfate sodium salt (TLCS) [106]. The fact that the pancreas in this study is exposed to very high concentrations of TLCS, which are normally never reached, even under pathological conditions, may raise questions about its physiological relevance. Finally, the fact that TGR5-/- mice were protected against cholelithiasis [64], could imply that TGR5 agonism predisposes to this condition; there are, however, no data to support a negative effect of TGR5 agonists on cholelithiasis. It needs to be stressed that many of these potential unwanted side effects were either observed in cultured cells or in animal models where extremely high, non-physiological concentrations of BAs were used. Further detailed studies using conditions closer to physiology are therefore necessary to evaluate whether such effects are also relevant for the clinical setting.
Conclusions and future perspectives
The metabolic studies described above suggest that targeting TGR5 could provide an exciting new therapeutic approach to improve several aspects of the metabolic syndrome. Multiple studies reveal that TGR5 has beneficial effects on body weight in high-fat diet fed mice. In addition, TGR5 activation improves glucose homeostasis and reduces hepatic steatosis. Also beneficial effects of TGR5 on macrophage-driven inflammation, as evidenced by the reduction of pro-inflammatory cytokines, may contribute to a potential positive effect of TGR5 with regard to the metabolic syndrome. These properties of TGR5 clearly suggest that activation of this membrane receptor is valuable to combat multiple aspects of the metabolic syndrome in humans. In view of the established role of BA or BA like molecules (dafachronic acids) to promote longevity in yeast [52] and in the worm C. Elegans [107, 108], it is furthermore plausible that the various strategies to modulate BA-signaling described in this review could increase lifespan through their potent hormonal activities that improve metabolism and reduce inflammation, two important contributors that determine healthspan [109].
The development of several novel natural, semi-synthetic, and synthetic TGR5 agonists is likely to further advance this receptor as a target for the metabolic syndrome. In addition, localized or tissue-specific gene targeting will shed light on ways to increase the efficacy and specificity of drugs that target this BA receptor. Despite the fact that we are convinced that targeting BA signaling pathways through TGR5 holds great promise for the intervention in metabolic diseases, still much work needs to be done, especially to make sure that such compounds are safe.
Acknowledgements
We thank Swiss National Science Foundation, the ERC, the NIH, Nestlé and the Ecole Polytechnique Fédérale de Lausanne for funding. T.W.H.P. is supported by a long-term fellowship from FEBS, L.G.N. is supported by a CONACYT fellowship, and M.N. is supported by a fellowship from AXA. We acknowledge Charles Thomas for instructive discussions.
Footnotes
Disclosures Dr. Auwerx consults for Intercept pharmaceuticals, a company that develops bile acid based therapeutics”.
References
- [1].Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31(20):4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- [3].Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25(7):1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7(8):678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- [6].Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- [7].Ishibashi S, Schwarz M, Frykman PK, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. I. Postnatal lethality reversed by bile acid and vitamin supplementation. J Biol Chem. 1996;271(30):18017–18023. doi: 10.1074/jbc.271.30.18017. [DOI] [PubMed] [Google Scholar]
- [8].Schwarz M, Lund EG, Setchell KD, Kayden HJ, Zerwekh JE, Bjorkhem I, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem. 1996;271(30):18024–18031. doi: 10.1074/jbc.271.30.18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kwakye JB, Barnes S, Diasio RB. Identification of bile acid coenzyme A synthetase in rat kidney. J Lipid Res. 1993;34(1):95–99. [PubMed] [Google Scholar]
- [10].Falany CN, Johnson MR, Barnes S, Diasio RB. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994;269(30):19375–19379. [PubMed] [Google Scholar]
- [11].Hubbard B, Doege H, Punreddy S, Wu H, Huang X, Kaushik VK, Mozell RL, Byrnes JJ, Stricker-Krongrad A, Chou CJ, Tartaglia LA, Lodish HF, Stahl A, Gimeno RE. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterology. 2006;130(4):1259–1269. doi: 10.1053/j.gastro.2006.02.012. [DOI] [PubMed] [Google Scholar]
- [12].Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- [13].Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- [14].Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5(1):73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- [16].Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- [17].Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6(3):507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- [18].Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol Endocrinol. 2002;16(9):2065–2076. doi: 10.1210/me.2001-0194. [DOI] [PubMed] [Google Scholar]
- [19].Stroup D, Crestani M, Chiang JY. Identification of a bile acid response element in the cholesterol 7 alpha-hydroxylase gene CYP7A. Am J Physiol. 1997;273(2 Pt 1):G508–517. doi: 10.1152/ajpgi.1997.273.2.G508. [DOI] [PubMed] [Google Scholar]
- [20].De Fabiani E, Mitro N, Anzulovich AC, Pinelli A, Galli G, Crestani M. The negative effects of bile acids and tumor necrosis factor-alpha on the transcription of cholesterol 7alpha-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: a novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J Biol Chem. 2001;276(33):30708–30716. doi: 10.1074/jbc.M103270200. [DOI] [PubMed] [Google Scholar]
- [21].Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276(45):41690–41699. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- [22].del Castillo-Olivares A, Gil G. Alpha 1-fetoprotein transcription factor is required for the expression of sterol 12alpha -hydroxylase, the specific enzyme for cholic acid synthesis. Potential role in the bile acid-mediated regulation of gene transcription. J Biol Chem. 2000;275(23):17793–17799. doi: 10.1074/jbc.M000996200. [DOI] [PubMed] [Google Scholar]
- [23].Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C, Overmars H, Kulik W, Metzger D, Auwerx J, Schoonjans K. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27(23):8330–8339. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee YK, Schmidt DR, Cummins CL, Choi M, Peng L, Zhang Y, Goodwin B, Hammer RE, Mangelsdorf DJ, Kliewer SA. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22(6):1345–1356. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [26].Lin BC, Wang M, Blackmore C, Desnoyers LR. Liver-specific activities of FGF19 require Klotho beta. J Biol Chem. 2007;282(37):27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- [27].Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12(11):1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- [28].Houten SM, Auwerx J. The enterohepatic nuclear receptors are major regulators of the enterohepatic circulation of bile salts. Ann Med. 2004;36(7):482–491. doi: 10.1080/07853890410018790. [DOI] [PubMed] [Google Scholar]
- [29].Schiff ER, Small NC, Dietschy JM. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972;51(6):1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99(8):1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21(6):1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- [32].Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126(1):322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- [33].Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51(3):565–580. doi: 10.1016/j.jhep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- [34].Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9(1):72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- [35].Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- [36].Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- [37].Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- [38].Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- [39].Otte K, Kranz H, Kober I, Thompson P, Hoefer M, Haubold B, Remmel B, Voss H, Kaiser C, Albers M, Cheruvallath Z, Jackson D, Casari G, Koegl M, Paabo S, Mous J, Kremoser C, Deuschle U. Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003;23(3):864–872. doi: 10.1128/MCB.23.3.864-872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98(6):3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98(6):3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- [43].Goodwin B, Gauthier KC, Umetani M, Watson MA, Lochansky MI, Collins JL, Leitersdorf E, Mangelsdorf DJ, Kliewer SA, Repa JJ. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc Natl Acad Sci U S A. 2003;100(1):223–228. doi: 10.1073/pnas.0237082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gascon-Barre M, Demers C, Mirshahi A, Neron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37(5):1034–1042. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
- [45].Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010 doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7 alpha-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol. 2005;288(1):G74–84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- [47].Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem. 2001;276(19):15816–15822. doi: 10.1074/jbc.M010878200. [DOI] [PubMed] [Google Scholar]
- [48].Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17(13):1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Qiao D, Stratagouleas ED, Martinez JD. Activation and role of mitogen-activated protein kinases in deoxycholic acid-induced apoptosis. Carcinogenesis. 2001;22(1):35–41. doi: 10.1093/carcin/22.1.35. [DOI] [PubMed] [Google Scholar]
- [50].Dent P, Fang Y, Gupta S, Studer E, Mitchell C, Spiegel S, Hylemon PB. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 2005;42(6):1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- [51].Qiao L, Han SI, Fang Y, Park JS, Gupta S, Gilfor D, Amorino G, Valerie K, Sealy L, Engelhardt JF, Grant S, Hylemon PB, Dent P. Bile acid regulation of C/EBPbeta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol. 2003;23(9):3052–3066. doi: 10.1128/MCB.23.9.3052-3066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Goldberg AA, Richard VR, Kyryakov P, Bourque SD, Beach A, Burstein MT, Glebov A, Koupaki O, Boukh-Viner T, Gregg C, Juneau M, English AM, Thomas DY, Titorenko VI. Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes. Aging (Albany NY) 2010;2(7):393–414. doi: 10.18632/aging.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- [54].Raufman JP, Zimniak P, Bartoszko-Malik A. Lithocholyltaurine interacts with cholinergic receptors on dispersed chief cells from guinea pig stomach. Am J Physiol. 1998;274(6 Pt 1):G997–1004. doi: 10.1152/ajpgi.1998.274.6.G997. [DOI] [PubMed] [Google Scholar]
- [55].Raufman JP, Chen Y, Zimniak P, Cheng K. Deoxycholic acid conjugates are muscarinic cholinergic receptor antagonists. Pharmacology. 2002;65(4):215–221. doi: 10.1159/000064347. [DOI] [PubMed] [Google Scholar]
- [56].Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- [57].Ferrari C, Macchiarulo A, Costantino G, Pellicciari R. Pharmacophore model for bile acids recognition by the FPR receptor. J Comput Aided Mol Des. 2006;20(5):295–303. doi: 10.1007/s10822-006-9055-1. [DOI] [PubMed] [Google Scholar]
- [58].Chen X, Yang D, Shen W, Dong HF, Wang JM, Oppenheim JJ, Howard MZ. Characterization of chenodeoxycholic acid as an endogenous antagonist of the G-coupled formyl peptide receptors. Inflamm Res. 2000;49(12):744–755. doi: 10.1007/s000110050656. [DOI] [PubMed] [Google Scholar]
- [59].Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6(9):721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- [60].Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3(6):449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- [61].Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298(5):714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- [62].Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- [63].Herbert MR, Siegel DL, Staszewski L, Cayanan C, Banerjee U, Dhamija S, Anderson J, Fan A, Wang L, Rix P, Shiau AK, Rao TS, Noble SA, Heyman RA, Bischoff E, Guha M, Kabakibi A, Pinkerton AB. Synthesis and SAR of 2-aryl-3-aminomethylquinolines as agonists of the bile acid receptor TGR5. Bioorg Med Chem Lett. 2010;20(19):5718–5721. doi: 10.1016/j.bmcl.2010.08.014. [DOI] [PubMed] [Google Scholar]
- [64].Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, Keane K, Davis HR, Jr., Hedrick J, Gustafson EL. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398(3):423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50(3):861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- [66].Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191(1):197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- [67].Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, Haussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45(3):695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- [68].Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372(1):78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- [69].Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- [70].Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010 doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vassileva G, Hu W, Hoos L, Tetzloff G, Yang S, Liu L, Kang L, Davis H, Hedrick J, Lan H, Kowalski T, Gustafson E. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J Endocrinol. 2010 doi: 10.1677/JOE-10-0009. [DOI] [PubMed] [Google Scholar]
- [73].Lavoie B, Balemba OB, Godfrey C, Watson CA, Vassileva G, Corvera CU, Nelson MT, Mawe GM. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol. 2010 doi: 10.1113/jphysiol.2010.192146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391(7):785–789. doi: 10.1515/BC.2010.077. [DOI] [PubMed] [Google Scholar]
- [75].Brufau G, Bahr MJ, Staels B, Claudel T, Ockenga J, Boker KH, Murphy EJ, Prado K, Stellaard F, Manns MP, Kuipers F, Tietge UJ. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab (Lond) 2010;7:73. doi: 10.1186/1743-7075-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17(9):1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5--connecting nutrition and metabolism. Thyroid. 2008;18(2):167–174. doi: 10.1089/thy.2007.0255. [DOI] [PubMed] [Google Scholar]
- [79].Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- [80].Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellstrom PM. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. J Clin Invest. 1998;102(4):764–774. doi: 10.1172/JCI942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362(4):793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- [82].Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- [83].Yoshimura T, Kurita C, Nagao T, Usami E, Nakao T, Watanabe S, Kobayashi J, Yamazaki F, Tanaka H, Inagaki N, Nagai H. Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology. 1997;54(3):144–152. doi: 10.1159/000139481. [DOI] [PubMed] [Google Scholar]
- [84].Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res. 1994;57(2):316–336. doi: 10.1006/jsre.1994.1151. [DOI] [PubMed] [Google Scholar]
- [85].Hov JR, Keitel V, Laerdahl JK, Spomer L, Ellinghaus E, ElSharawy A, Melum E, Boberg KM, Manke T, Balschun T, Schramm C, Bergquist A, Weismuller T, Gotthardt D, Rust C, Henckaerts L, Onnie CM, Weersma RK, Sterneck M, Teufel A, Runz H, Stiehl A, Ponsioen CY, Wijmenga C, Vatn MH, Stokkers PC, Vermeire S, Mathew CG, Lie BA, Beuers U, Manns MP, Schreiber S, Schrumpf E, Haussinger D, Franke A, Karlsen TH. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5(8):e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51(1):212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Grundy SM, Ahrens EH, Jr., Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971;78(1):94–121. [PubMed] [Google Scholar]
- [88].Nestel PJ, Poyser A. Changes in cholesterol synthesis and excretion when cholesterol intake is increased. Metabolism. 1976;25(12):1591–1599. doi: 10.1016/0026-0495(76)90112-8. [DOI] [PubMed] [Google Scholar]
- [89].Angelin B, Einarsson K, Hellstrom K. Effect of cholestyramine on bile acid kinetics in patients with portal cirrhosis of the liver. Evidence of a selective defect in the formation of cholic acid. Am J Dig Dis. 1978;23(12):1115–1120. doi: 10.1007/BF01072887. [DOI] [PubMed] [Google Scholar]
- [90].Glicksman C, Pournaras DJ, Wright M, Roberts R, Mahon D, Welbourn R, Sherwood R, Alaghband-Zadeh J, le Roux CW. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem. 2010 doi: 10.1258/acb.2010.010040. [DOI] [PubMed] [Google Scholar]
- [91].Kobayashi M, Ikegami H, Fujisawa T, Nojima K, Kawabata Y, Noso S, Babaya N, Itoi-Babaya M, Yamaji K, Hiromine Y, Shibata M, Ogihara T. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56(1):239–247. doi: 10.2337/db06-0353. [DOI] [PubMed] [Google Scholar]
- [92].Yamakawa T, Takano T, Utsunomiya H, Kadonosono K, Okamura A. Effect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemia. Endocr J. 2007;54(1):53–58. doi: 10.1507/endocrj.k05-098. [DOI] [PubMed] [Google Scholar]
- [93].Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31(8):1479–1484. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mussig K, Staiger H, Machicao F, Machann J, Schick F, Schafer SA, Claussen CD, Holst JJ, Gallwitz B, Stefan N, Fritsche A, Haring HU. Preliminary report: genetic variation within the GPBAR1 gene is not associated with metabolic traits in white subjects at an increased risk for type 2 diabetes mellitus. Metabolism. 2009;58(12):1809–1811. doi: 10.1016/j.metabol.2009.06.012. [DOI] [PubMed] [Google Scholar]
- [95].Pellicciari R, Sato H, Gioiello A, Costantino G, Macchiarulo A, Sadeghpour BM, Giorgi G, Schoonjans K, Auwerx J. Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J Med Chem. 2007;50(18):4265–4268. doi: 10.1021/jm070633p. [DOI] [PubMed] [Google Scholar]
- [96].Macchiarulo A, Gioiello A, Thomas C, Massarotti A, Nuti R, Rosatelli E, Sabbatini P, Schoonjans K, Auwerx J, Pellicciari R. Molecular field analysis and 3D-quantitative structure-activity relationship study (MFA 3D-QSAR) unveil novel features of bile acid recognition at TGR5. J Chem Inf Model. 2008;48(9):1792–1801. doi: 10.1021/ci800196h. [DOI] [PubMed] [Google Scholar]
- [97].Pellicciari R, Gioiello A, Macchiarulo A, Thomas C, Rosatelli E, Natalini B, Sardella R, Pruzanski M, Roda A, Pastorini E, Schoonjans K, Auwerx J. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J Med Chem. 2009;52(24):7958–7961. doi: 10.1021/jm901390p. [DOI] [PubMed] [Google Scholar]
- [98].Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, Schoonjans K, Pellicciari R, Auwerx J. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51(6):1831–1841. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- [99].Genet C, Strehle A, Schmidt C, Boudjelal G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R, Wagner A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem. 2010;53(1):178–190. doi: 10.1021/jm900872z. [DOI] [PubMed] [Google Scholar]
- [100].Genet C, Schmidt C, Strehle A, Schoonjans K, Auwerx J, Saladin R, Wagner A. Redefining the TGR5 Triterpenoid Binding Pocket at the C-3 Position. ChemMedChem. 2010 doi: 10.1002/cmdc.201000329. [DOI] [PubMed] [Google Scholar]
- [101].Keitel V, Gorg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Haussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010 doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- [102].Evans KA, Budzik BW, Ross SA, Wisnoski DD, Jin J, Rivero RA, Vimal M, Szewczyk GR, Jayawickreme C, Moncol DL, Rimele TJ, Armour SL, Weaver SP, Griffin RJ, Tadepalli SM, Jeune MR, Shearer TW, Chen ZB, Chen L, Anderson DL, Becherer JD, De Los Frailes M, Colilla FJ. Discovery of 3-aryl-4-isoxazolecarboxamides as TGR5 receptor agonists. J Med Chem. 2009;52(24):7962–7965. doi: 10.1021/jm901434t. [DOI] [PubMed] [Google Scholar]
- [103].Yang JI, Yoon JH, Myung SJ, Gwak GY, Kim W, Chung GE, Lee SH, Lee SM, Kim CY, Lee HS. Bile acid-induced TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced caspase 8 activation in hepatocytes. Biochem Biophys Res Commun. 2007;361(1):156–161. doi: 10.1016/j.bbrc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- [104].Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354(1):154–159. doi: 10.1016/j.bbrc.2006.12.168. [DOI] [PubMed] [Google Scholar]
- [105].Desai MS, Shabier Z, Taylor M, Lam F, Thevananther S, Kosters A, Karpen SJ. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. 2010;51(6):2097–2107. doi: 10.1002/hep.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138(2):715–725. doi: 10.1053/j.gastro.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124(6):1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- [108].Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci U S A. 2007;104(12):5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142(1):9–14. doi: 10.1016/j.cell.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]