Abstract
A highly enantioselective transformation catalyzed by chiral (acyclic diaminocarbene)gold(I) complexes is reported. The enantioselective synthesis of 2-substituted chromenyl pivalates from racemic phenol-substituted propargyl pivalates was developed. Rearrangement of the substrates in the presence of cationic gold gave allene intermediates, whose cyclization resulted in formation of enantioenriched product through a dynamic kinetic asymmetric transformation.
Gold(I)-catalyzed nucleophilic addition to allenes has been studied intensively in recent years as an atom-economical approach to the synthesis of heterocyclic compounds.1 While several examples of enantioselective reactions catalyzed by cationic gold(I) complexes bearing phosphorus ligands are now known,2–4 these complexes are not universally applicable in gold-catalyzed transformations. Notably, gold(I)–carbene complexes can catalyze reactions with reactivity and selectivity dramatically different from those observed when the phosphine-ligated complexes are employed as catalysts.5,6 However, transformations using chiral cationic gold(I)–carbene complexes as catalysts have generally proceeded with low to moderate levels of enantioselectivity.7 Herein, we report a highly enantioselective synthesis of substituted chromenyl pivalates catalyzed by a gold(I)–carbene complex.
 |
(1) |
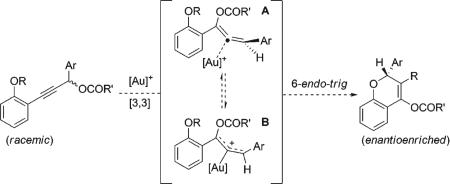
The gold-catalyzed rearrangement of propargylic esters to allenes through formal [3,3] sigmatropic rearrangement is well-known.8 We envisioned a scenario in which the intermediate gold(I) allene complex formed in such a rearrangement could be trapped by a pendant phenol nucleophile to give a 6-endo-trig cyclization intermediate (eq 1). In previously reported gold-catalyzed transformations involving allenes, both chirality transfer and loss of chirality have been observed, depending on both the substitution pattern of the allene and the nature of the gold catalyst.9 In the case of propargylic esters, the donating oxygen substituent of the product allene greatly stabilizes the planar allyl cation-like form B, resulting in relatively shallow barriers to loss of configuration.9b We hypothesized that chiral ligands for gold that provided access to this achiral intermediate could be employed in a dynamic kinetic asymmetric transformation.10
To test our hypotheses, we surveyed gold(I)–phosphine complexes that were previously used successfully in asymmetric allene additions. Encouragingly, when propargyl ester 1a was treated with (R)-DTBM-Segphos(AuCl)2 or (R)-DTBM-MeO-Biphep(AuCl)2 in the presence of sodium tetrakis[3,5-bis-(trifluoromethyl)phenyl]borate (NaBARF), the desired adduct 2a was formed with moderate enantioselectivity (Table 1, entries 1 and 2).
Table 1.
Au(I) Catalyst Screen
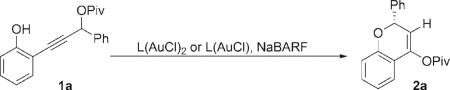
| |||
|---|---|---|---|
| entrya | precatalyste | R | % eef (% yieldh) |
| 1 | L1 · (AuCl)2 | – | 52 |
| 2 | L2 · (AuCl)2 | – | 60 |
| 3b | L3 · (AuCl) | – | 0 |
| 4b | L4 · (AuCl) | – | 7 |
| 5 | A1 · (AuCl)2 | H | 22 |
| 6 | A2 · (AuCl)2 | Ph | 56 |
| 7 | A3 · (AuCl)2 | 3,5-(MeO)2C6H3 | 7 |
| 8 | A4 · (AuCl)2 | 2,6-(MeO)2C6H3 | – g |
| 9 | A5 · (AuCl)2 | 2-Naph | 50 |
| 10 | A6 · (AuCl)2 | 4-tBuC6H4 | 63 |
| 11 | A7 · (AuCl)2 | 4-MeOC6H4 | 68 |
| 12 | A8 · (AuCl)2 | 4-CF3C6H4 | 73 (56) |
| 13c | A8 · (AuCl)2 | 4-CF3C6H4 | 85 (78) |
| 14d | A8 · (AuCl)2 | 4-CF3C6H4 | 91 |
Conditions: CH2Cl2 (0.05 M), 5 mol % precatalyst, 10 mol % NaBARF for 16 h at room temperature.
With 5 mol % NaBARF used.
With 10 mol % AgOTf used in place of NaBARF.
With 10 mol % AgOTf used in place of NaBARF, in CDCl3 (0.1 M) at 0 °C in 85% isolated yield.
L1 is (R)-DTBM-Segphos, and L2 is (R)-DTBM-MeO-Biphep.
Determined by chiral HPLC.
Low yield of 2a; enantioselectivity not determined.
Determined by 1H NMR with m-dinitrobenzene as the internal standard.
We posited that the modest enantioselectivity in these transformations might be the result of a slow gold-promoted equilibration of the allene enantiomers through B whose rate could be enhanced by electron-rich ligands for gold. In accord with this hypothesis, phosphoramidite catalyst L3 · (AuCl),3c which we have successfully applied in gold-catalyzed reactions of allenes, catalyzed the formation of 2a with poor enantioselectivity. Therefore, we hypothesized that electron-donating carbene ligands would be ideal for this transformation; however, chiral NHC complex L4 · (AuCl)7b afforded product 2a with poor enantioselectivity (entry 4).
Inspired by the success with 3,3′-modified BINOL-derived phosphoramidite ligands in gold catalysis,3 we considered acyclic carbene ligands11 based on 3,3′-functionalization of BINAM. Although initial experiments with the reported7a acyclic carbene A1 · (AuCl)2 as a catalyst resulted in disappointing enantioselectivities, we found that complex A2 · (AuCl)2 with phenyl substituents at the 3,3′-positions of the BINAM scaffold gave improved enantioselectivities (entry 6). We investigated the effect of 3,3′-substitution on reactivity and enantioselectivity in more detail. Of the substitution patterns surveyed, only 4-substitution of the 3,3′-aryl group resulted in improved enantioselectivities, while the electronics of the substituents appeared to be unimportant (entries 10–12). Further optimization of 4-tri-fluoromethylphenyl-substituted catalyst A8 (Figure 1) with respect to the counterion revealed that using AgOTf in place of NaBARF resulted in further improvements in yield and enantioselectivity (entry 13).12 Finally, lowering the reaction temperature to 0 °C afforded 2a in 85% yield and 91% enantiomeric excess (entry 14).
Figure 1.
X-ray structure of A8.
Under these conditions, we examined the scope of the gold-catalyzed kinetic dynamic asymmetric transformation (Table 2). Steric bulk at the ortho and para positions of the propargyl aryl ring was tolerated (entries 3–5). Moreover, electron-withdrawing substituents on the aryl moiety also furnished the desired adducts with good yields and enantioselectivities (entries 2 and 6). Good yields and enantioselectivities were also obtained from the gold-catalyzed rearrangement of substrates with further substitution on the phenol (entries 7 and 8).13
Table 2.
Enantioselective Synthesis of Chromenyl Pivalate from Phenols
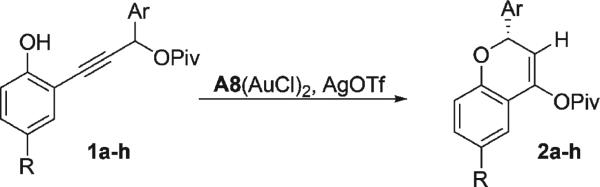
| |||||
|---|---|---|---|---|---|
| entrya,b | 1 | R | Ar | % yieldc | % eed |
| 1 | 1a | H | Ph | 85 | 91 |
| 2 | 1b | H | p-BrC6H4 | 86 | 86 |
| 3 | 1c | H | 2-Naph | 68 | 84 |
| 4 | 1d | H | o-MeC6H4 | 77 | 84 |
| 5 | 1e | H | p-iPrC6H4 | 73 | 89 |
| 6 | 1f | H | p-CF3C6H4 | 76 | 88 |
| 7 | 1g | Me | Ph | 85 | 90 |
| 8 | 1h | Br | Ph | 83 | 83 |
Conditions: CDCl3 (0.1 M), 5 mol % A8 · (AuCl)2, 10 mol % AgOTf for 4 h at 0 °C.
Absolute stereochemistry assigned by analogy to 4e (Table 3).
Isolated yield of product after column chromatography.
Determined by chiral HPLC.
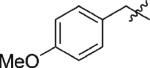
We next looked at alkylated phenols, to explore the possibility of generating 3-substituted chromenes. Carbodemetalation of the vinylgold intermediate by a stabilized carbocation lost from the oxonium moiety would result in formal 1,3-migration of an electron-rich alkyl group and formation of a carbon–carbon bond. Gratifyingly, p-methoxybenzylether 3a reacted smoothly under the standard conditions to afford 3-substituted product 4a in excellent yield and enantioselectivity (entry 1). A variety of other electron-rich arylmethyl phenyl ethers, including those derived from N-, O-, and S-heterocycles, similarly underwent cyclization and O-to-C transfer to provide the products in excellent enantioselectivity and yield (Table 3).14,15
Table 3.
Enantioselective Synthesis of Chromenyl Pivalate from Phenol Ethers
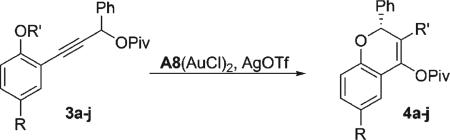
| |||||
|---|---|---|---|---|---|
| entrya,b | 3 | R | R' | % yieldb | % eed |
| 1 | 3a | H |
|
92 | >99 |
| 2 | 3b | Me | 75 | >99 | |
| 3c | 3c | Me |

|
62 | 99 |
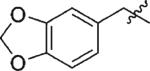
| 4 | 3d | H |
|
91 | >99 |
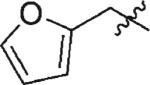
| 5 | 3e | H |
|
59 | 99 |
| 6c | 3f | H |

|
75 | >99 |
| 7 | 3g | H |
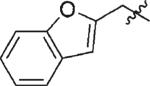
|
75 | 97 |
| 8 | 3h | H |
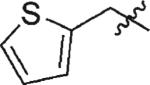
|
73 | 99 |
| 9c | 3i | H |

|
73 | 96 |
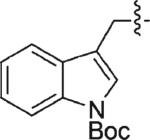
| 10 | 3j | H |
|
94 | 95 |
Conditions: CDCl3 (0.1 M), 5 mol % A8 · (AuCl)2, 10 mol % AgOTf for 4 h at 0 °C.
Isolated yield of product after column chromatography.
At room temperature.
Determined by chiral HPLC.
To gain a better mechanistic understanding of the reaction, the gold-catalyzed rearrangement of enantioenriched 1a (60% ee) was examined. When the reaction was halted at 70% conversion, starting material was reisolated in a diminished 17% ee and product 2a in 88% ee (eq 2). This observation suggests either racemization of starting material via B (eq 1) or selective reactivity of the enantiomers of 1a, resulting in kinetically resolved starting material. To distinguish between these possibilities, the A8 · (AuCl)2-catalyzed reaction of racemic 1a was conducted until 60% conversion had been reached (eq 2). Under these conditions, the recovered starting material 1a was isolated in 47% ee (enriched in the opposite enantiomer) and the product 2a in 90% ee. These results imply a kinetic resolution of the starting material and a relatively slow equilibration between the starting material and the intermediate gold-coordinated allene. Enantiodetermining cyclization onto the rapidly interconverting isomers of A and/or B then accounts for the observed dynamic kinetic resolution.
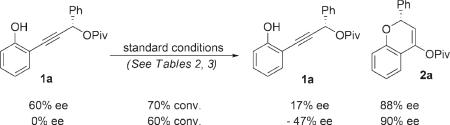 |
(2) |
 |
(3) |
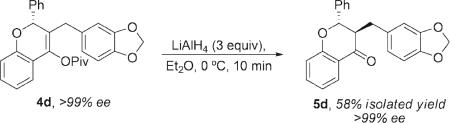
In summary, we report the application of modular 3,3′-substituted BINAM-derived acyclic diaminocarbene ligands to a highly enantioselective gold(I)-catalyzed reaction. The chiral acyclic carbenegold(I) catalyzed dynamic kinetic asymmetric transformation allows for the enantioselective synthesis of chromenyl pivalates from racemic propargyl pivalates. These adducts serve as useful precursors to enantioenriched chromanones. For example, treatment of 4d with LiAlH4 at 0 °C provided chromanone 5d as a single diastereomer in >99% ee (eq 3).16,17 The chiral carbene catalysts offer better enantioselectivity and reactivity than phosphine– and phosphoramidite–gold(I) catalysts that have previously been developed for enantioselective gold catalysis. Finally, we demonstrate that the generation of configurationally labile intermediates from propargyl esters is a promising strategy for asymmetric gold catalysis.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for National Institute of General Medical Sciences Grant RO1 GM073932 and to Amgen for financial support. C.N.K. and V.R. thank the Swiss National Science Foundation (SNF) and NSERC, respectively, for postdoctoral fellowships. We thank Takasago International Corp. and Solvias for the gift of chiral bisphosphine ligands and Johnson Matthey for a donation of AuCl3.
Footnotes
Supporting Information. Experimental procedures, compound characterization data, and CIF data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.For selected general reviews, see: Corma A, Leyva-Pérez A, Sabater MJ. Chem. Rev. 2011;111:1657. doi: 10.1021/cr100414u.Bandini M. Chem. Soc. Rev. 2011:1358. doi: 10.1039/c0cs00041h.Hashmi ASK, Bührle M. Aldrichimica Acta. 2010;43:27.Nevado C. Chimia. 2010;64:247. doi: 10.2533/chimia.2010.247.Shapiro ND, Toste FD. Synlett. 2010;5:675. doi: 10.1055/s-0029-1219369.Fürstner A. Chem. Soc. Rev. 2009;38:3208. doi: 10.1039/b816696j. For reviews of enantioselective gold catalysis, see: Pradal A, Toullec PY, Michelet V. Synthesis. 2011:1501.Sengupta S, Shi X. Chem-CatChem. 2010;2:609.
- 2.Recent examples using chiral bisphosphine ligands: Melhado AD, Amarante GW, Wang ZJ, Luparia M, Toste FD. J. Am. Chem. Soc. 2011;133:3517. doi: 10.1021/ja1095045.LaLonde RL, Wang ZJ, Mba M, Lackner AD, Toste FD. Angew. Chem., Int. Ed. 2010;49:598. doi: 10.1002/anie.200905000.Martínez A, Garciá-Garciá P, Fernández MA, Rodríguez F, Sanz R. Angew. Chem., Int. Ed. 2010;49:4633. doi: 10.1002/anie.201001089.Liu F, Qian DY, Zhao XL, Zhang JL. Angew. Chem., Int. Ed. 2010;49:6669. doi: 10.1002/anie.201003136.Kleinbeck F, Toste FD. J. Am. Chem. Soc. 2009;131:9178. doi: 10.1021/ja904055z.Zhang Z, Lee SD, Widenhoefer RA. J. Am. Chem. Soc. 2009;131:5372. doi: 10.1021/ja9001162.Uemura M, Watson IDG, Katsukawa M, Toste FD. J. Am. Chem. Soc. 2009;131:3464. doi: 10.1021/ja900155x.Watson IDG, Ritter S, Toste FD. J. Am. Chem. Soc. 2009;131:2056. doi: 10.1021/ja8085005.Chao C-M, Vitale MR, Toullec PY, Genêt J-P, Michelet V. Chem.-Eur. J. 2009;15:1319. doi: 10.1002/chem.200802341.Bandini M, Eichholzer A. Angew. Chem., Int. Ed. 2009;48:9533. doi: 10.1002/anie.200904388.
- 3.For examples using chiral phosphoramidite or phosphite ligands, see: González AZ, Benitez D, Tkatchouk E, Goddard WA, III, Toste FD. J. Am. Chem. Soc. 2011;133:5500. doi: 10.1021/ja200084a.Teller H, Flugge S, Goddard R, Fürstner A. Angew. Chem., Int. Ed. 2010;49:1949. doi: 10.1002/anie.200906550.González AZ, Toste FD. Org. Lett. 2010;12:200. doi: 10.1021/ol902622b.Alonso I, Trillo B, López F, Montserrat S, Ujaque G, Castedo L, Lledós A, Mascareňas JL. J. Am. Chem. Soc. 2009;131:13020. doi: 10.1021/ja905415r.
- 4.For an example using a chiral monodentate phosphine ligand, see: Campbell MJ, Toste FD. Chem. Sci. 2011;2:1369. doi: 10.1039/C1SC00160D. For a strategy involving achiral (phosphine)gold(I) complexes in the presence of chiral counterions: Hamilton GL, Kang EJ, Mba M, Toste FD. Science. 2007;317:496. doi: 10.1126/science.1145229.
- 5.For general discussions of effects of ligands in gold catalysis, see: Lopez S, Herrero-Gomez E, Perez-Galan P, Nieto-Oberhuber C, Echavarren AM. Angew. Chem., Int. Ed. 2006;45:6029. doi: 10.1002/anie.200602448.Shapiro ND, Toste FD. J. Am. Chem. Soc. 2007;129:4160. doi: 10.1021/ja070789e.Benitez D, Shapiro ND, Tkatchouk E, Wang Y, Goddard WA, III, Toste FD. Nat. Chem. 2009;1:482. doi: 10.1038/nchem.331. For reviews, see: Gorin DJ, Sherry BD, Toste FD. Chem. Rev. 2008;108:3351. doi: 10.1021/cr068430g.Klahn P, Kirsch SF. ChemCatChem. 2011;11:649.
- 6.For a review of (N-heterocyclic carbene)gold(I) complexes, see: Nolan SP. Acc. Chem. Res. 2011;44:91. doi: 10.1021/ar1000764.
- 7.Bartolomé C, García-Cuadrado D, Ramiro Z, Espinet P. Inorg. Chem. 2010;49:9758. doi: 10.1021/ic101059c.Matsumoto Y, Selim KB, Nakanishi H, Yamada K, Yamamoto Y, Tomioka K. Tetrahedron Lett. 2010;51:404.Wilckens K, Lentz D, Czekelius C. Organometallics. 2011;30:1287.Liu L-J, Wang F, Wang W, Zhao M-X, Shi M. Beilstein J. Org. Chem. 2011;7:555. doi: 10.3762/bjoc.7.64. For an example of a (chiral biscarbene)gold(I) complex catalyzed hydrogenation giving 95% ee, see: Arnanz A, González-Arellano C, Juan A, Villaverde G, Corma A, Iglesias M, Sánchez F. Chem. Commun. 2010;46:3001. doi: 10.1039/b922534j.
- 8.For reviews of gold-catalyzed reactions of propargyl esters, see: Wang S, Zhang G, Zhang L. Synlett. 2010:692.Marion N, Nolan SP. Angew. Chem., Int. Ed. 2007;46:2750. doi: 10.1002/anie.200604773.
- 9.a Gandon V, Lemière G, Hours A, Fensterbank L, Malacria M. Angew. Chem., Int. Ed. 2008;47:7534. doi: 10.1002/anie.200802332. [DOI] [PubMed] [Google Scholar]; b Mauleón P, Krinsky JL, Toste FD. J. Am. Chem. Soc. 2009;131:4513. doi: 10.1021/ja900456m. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Gonzalez AZ, Benitez D, Thatchouk E, Goddard WA, Toste FD. J. Am. Chem. Soc. 2011;133:5500. doi: 10.1021/ja200084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Bender CF, Widenhoefer RA. J. Am. Chem. Soc. 2007;129:14148. doi: 10.1021/ja0760731. [DOI] [PubMed] [Google Scholar]
- 11.a Parks JE, Balch AL. J. Organomet. Chem. 1973;57:C103. [Google Scholar]; b Bartolomé C, Ramiro Z, Pérez-Galán P, Bour C, Raducan M, Echavarren AM, Espinet P. Inorg. Chem. 2008;47:11391. doi: 10.1021/ic801446v. [DOI] [PubMed] [Google Scholar]; c Hashmi ASK, Hengst T, Lothschütz C, Rominger F. Adv. Synth. Catal. 2010;352:1315. [Google Scholar]
- 12.Replacing the NaBARF with silver salts in the (phosphine)gold(I)-catalyzed reactions (Table 1, entries 1 and 2) resulted in diminished yield and no improvement in enantioselectivity.
- 13.Substrates with electron-donating substituents on the propargyl aryl ring could not be prepared or gave poor yields because of extensive decomposition of starting material. When the propargyl aryl ring was replaced with a cyclohexyl group, the product mixture consisted of a 3:1 mixture of benzofuran product (from 5-endo-dig cyclization of the starting propargyl ester) and the desired chromenyl pivalate. Substrate 1a with an additional methyl group at the propargylic position gave desired product in only 11% ee.
- 14.Gold-catalyzed reactions terminated by a carbodeauration: Nakamura I, Sato T, Yamamoto Y. Angew. Chem., Int. Ed. 2006;45:4473. doi: 10.1002/anie.200601178.Dube P, Toste FD. J. Am. Chem. Soc. 2006;128:12062. doi: 10.1021/ja064209+.Nakamura I, Sato T, Terada M, Yamamoto Y. Org. Lett. 2008;10:2649. doi: 10.1021/ol8007556.Renault J, Qian Z, Uriac P, Gouault N. Tetrahedron Lett. 2011;52:2476. For a review of reactions of organogold compounds with electrophiles, see: Hashmi ASK, Ramamurthi TD, Toss MH, Tsang AS-K, Graf K. Aust. J. Chem. 2010;63:1619.
- 15.A crossover experiment performed using a 1:1 mixture of 3b and 3d gave no crossover products, as observed by 1H NMR and HRMS (ESI). This is consistent with direct intramolecular alkyl migration or migration via a contact ion pair and is consistent with previous reports.14
- 16.The structure and absolute configuration of 4e were determined by single-crystal X-ray diffraction (see the Supporting Information). The stereochemistry of the remaining products was assigned by analogy.
- 17.The relative configuration was assigned by analogy to trans-2-phenyl-3-propylchromanone: Saito A, Kasai J, Odaira Y, Fukaya H, Hanzawa Y. J. Org. Chem. 2009;74:5644. doi: 10.1021/jo900857c.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



