Abstract
Nucleus is a complex and highly compartmentalized organelle, which organization undergoes major changes during cell differentiation allowing cells to become specialized and fulfill their functions.During terminal differentiation of the epidermal keratinocytes, nucleus undergoes programmed transformation from active status, associated with execution of the genetic programs of cornification and epidermal barrier formation, to fully inactive condition and becomes a part of the keratinized cells of the cornified layer. Tremendous progress achieved within the last two decades in understanding the biology of the nucleus and epigenetic mechanisms controlling gene expression allowed defining several levels in the regulation of cell differentiation-associated gene expression programs, including an accessibility of the gene regulatory regions to DNA-protein interactions, covalent DNA and histone modifications and ATP-dependent chromatin remodeling, as well as higher-order chromatin remodeling and nuclear compartmentalization of the genes and transcription machinery. Here, we integrate our current knowledge of the mechanisms controlling gene expression during terminal keratinocyte differentiation with distinct levels of chromatin organization and remodeling. We also propose the directions to further explore the role of epigenetic mechanisms and their interactions with other regulatory systems in the control of keratinocyte differentiation in normal and diseased skin.
Introduction
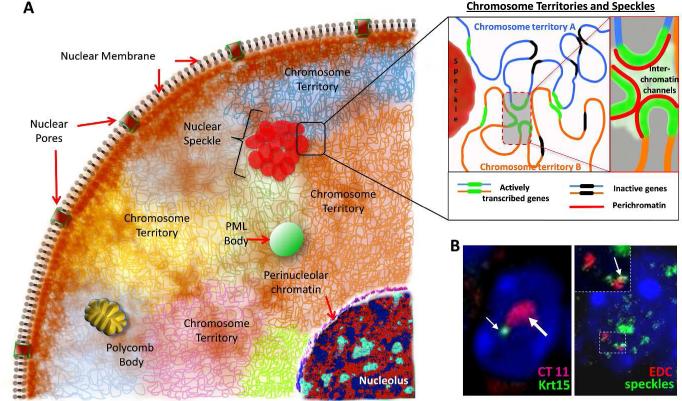
The cell nucleus is a complex organelle, in which genetic material (DNA) is compacted up to several thousand fold and organized into chromosomes in a form that allows the genome to be replicated, repaired and transcribed in a cell-type specific manner (Hemberger et al., 2009; Ho and Crabtree, 2010). In three-dimensional space, the interphase nucleus represents a highly compartmentalized organelle that consists of the nuclear membrane, individual chromosomes occupying the distinct territories, as well as a number of nuclear bodies (nucleoli, Cajal bodies, promyelocytic leukaemia (PML) bodies, nuclear speckles, Polycomb bodies, etc.) located in the inter-chromosomal compartments (Hubner and Spector, 2010; Lanctot et al., 2007) (Fig. 1 A). In contrast to the cytoplasmic organelles, distinct nuclear compartments are not separated by the membranes and are formed by the dynamically-changing protein-protein and/or nucleic acid-protein complexes (Dundr and Misteli, 2010).
Figure 1. Schematic structure of interphase nucleus.
A - Nucleus is surrounded by nuclear envelope that consists of outer and inner membranes, nuclear pore complexes and lamina located beneath the inner nuclear membrane. Chromosomes occupy distinct territories, in which distinct chromatin domains are permeated by interchromatin channels connected with a network of larger channels and lacunas separating distinct chromosomes and harboring a number of nuclear bodies including speckles (inset). Nucleus also contains a number of nuclear bodies including nucleolus, PML bodies, speckles and Polycomb bodies.
B: Images of nuclei of murine basal epidermal keratinocytes showing chromosome territory 11 (left image, large arrow) with keratin 15 gene (small arrow), as well as Epidermal Differentiation Complex locus (right image, red color) contacting SC-35-positive speckle (inset, green color, arrow).
In the mammalian epidermis, progenitor cells of the basal layer proliferate and differentiate, forming multi-layered epithelium, as well as give rise to a variety of skin appendages (hair follicles, nails, glands, etc.) (Blanpain and Fuchs, 2009; Fuchs, 2007; Koster and Roop, 2007). Keratinocyte differentiation in the epidermis and skin appendages is driven by coordinated changes in the expression of the lineage-specific genes including those that constitute several genomic loci (Keratin type I and II loci, Epidermal Differentiation Complex (EDC), keratin-associated protein locus, etc.), and encode the essential structural components of the epidermal barrier or appendage-specific cellular products (Bazzi et al., 2007; Segre, 2006). Gene expression in these loci is regulated in a cell type-specific and differentiation stage-specific manner by a number of signaling pathways and transcription factors (Blanpain and Fuchs, 2009). However, mechanisms that regulate the balance of expression between different genes in these loci and how cell type-specific and differentiation stage-specific patterns of gene expression are achieved at the levels of whole loci remain unclear.
During transition of keratinocytes from basal epidermal layer to granular layer, the nucleus undergoes programmed transformation from highly active status, associated with execution of the genetic program of epidermal barrier formation, to fully inactive condition and, through a process of DNA degradation, becomes a part of the keratinized cells of the cornified epidermal layer (Fischer et al., 2011). During last two decades, a tremendous progress has been achieved in understanding the genetic basis of skin disorders and identification of a large number of mutations underlying the development of many pathological skin conditions associated with alterations of the epidermal differentiation and integrity, hair loss and pigmentation [for review, see (Shimomura and Christiano, 2010; Tamai et al., 2009)]. However, despite the fact that the genome-wide association studies and comparative genome analyses brought invaluable insights into the physiological role of genetic information, the question how genome of keratinocytes is organized beyond its linear sequence and which epigenetic mechanisms control gene expression programs in healthy and diseased epidermis remain largely unknown.
Currently, epigenetics is defined as a discipline that, in broad terms, investigates the heritable changes in the genome functions occurring without alterations to the DNA sequence (Berger et al., 2009). Epigenetic inheritance is often based on the inheritance of relatively stable chromatin states in the distinct genomic regions (Berger et al., 2009). Despite the fact that not all chromatin structural states are epigenetically transmitted during cell cycle (Probst et al., 2009), such mechanisms play an important role in the control of cellular functions in living organisms and are currently considered as a driving force of the development, phenotypic plasticity and evolutionary adaptation (Feinberg, 2007).
During last few years, it was shown that epigenetic mechanisms are involved in the control of epidermal development and terminal keratinocyte differentiation. In particular, an involvement of the DNA methytransferase DNMT1, histone demethylase JMJD3 and histone methyltransferase Setd8, histone deacetylases 1/2, ATP-dependent chromatin modifying enzymes (Brg1, Mi-2β), the polycomb group proteins (Bmi1, Cbx4, Ezh2, Jarid2) and genome organizer Satb1 in establishing tissue-specific differentiation programs in the epidermis was demonstrated (Driskell et al., 2011; Eckert et al., 2011; Ezhkova et al., 2011; Ezhkova et al., 2009; Fessing et al., 2011; Indra et al., 2005; Kashiwagi et al., 2007; Landeira and Fisher, 2011; Lien et al., 2011; Luis et al., 2011; Sen et al., 2010). However, many aspects of the epigenetic control of gene expression programs in keratinocytes including an integration of the distinct levels of regulation in the context of three-dimensional nuclear organization and its dynamics associated with terminal keratinocyte differentiation remain to be elucidated.
Here, we overview data on epigenetic mechanisms that control tissue-specific gene expression programs in keratinocytes. We focused our attention on normal epidermal keratinocytes, whereas epigenetic mechanisms underlying the response of keratinocytes to ionizing radiation, as well as changes of keratinocyte epigenetic status in pathological skin conditions are outside of the scope of this review. Also, the roles of non-coding and microRNAs in the control of keratinocyte differentiation are not discussed here, because of several recent reviews published elsewhere (Botchkareva, 2012; Wang and Chang, 2011; Yi and Fuchs, 2011). However, we propose the directions on how to further explore the role of epigenetic mechanisms and their cross-talk with other regulatory systems in the control of keratinocyte growth and differentiation in normal and diseased skin.
Distinct levels of chromatin organization and the control of gene expression programs in keratinocytes
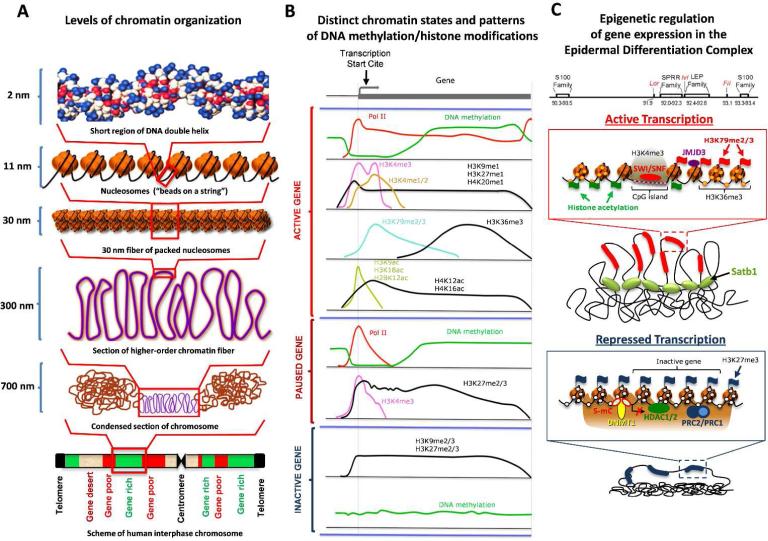
Eukaryotic genome is organized into distinct chromosomes that are formed by a nucleo-protein complex called chromatin. Chromatin consists of the nucleosomes, in which 147 base-pairs of genomic DNA form a loop around the histone octamer core represented by two molecules of each core histone (H2A, H2B, H3 and H4) (Andrews and Luger, 2011). Nucleosomes are connected by non-histone associated linker DNA of approximately 10-80 bp length resulting in a formation of the “beads on the string” structures, which are folded into 30 nm fiber (Felsenfeld and Groudine, 2003). Histone H1 binds to DNA as it exits the nucleosome, interacts with the linker DNA and plays an essential role for the condensation of nucleosomes and 30 nm fibers into higher order loop structures. These loop structures are less rigid compared to the nucleosomes and are folded via still poorly understood mechanisms into larger loops to accommodate the genome within the 3D nuclear space (Heermann, 2011) (Fig. 2 A).
Figure 2. Distinct levels of chromatin organization, characteristics of the active and inactive chromatin domains and epigenetic control of gene expression in the Epidermal Differentiation Complex locus.
A – Levels of chromatin folding and size of the corresponding chromatin domains of the interphase chromosome. The lowest unit of chromatin organization is nucleosome, in which DNA form 1 and ¾ loops around the histone octamer core. Nucleosomes are connected by linker DNA and form the “beads on the string” structures, which are folded into 30 nm fiber followed by further folding into higher-order loop structures (modified from Felsenfeld and Groudine, 2003).
B – Distribution of different epigenetic marks among the distinct regions of actively transcribed, paused and inactive genes. Actively transcribed genes are characterized by high levels of Polymerase II occupancy, low levels of DNA methylation, high levels of H3K4me3 and H3K9acetyl/H3K18acetyl at the transcription start site, as well as by high levels of H3K79me2/3 and H3K36me3 at the gene body region. Genes with paused transcription show high levels of Polymerase II occupancy, low levels of DNA methylation, high levels of H3K4me3 and H3K27me2/3 at the transcription start site. Inactive genes show lack of Polymerase II, high levels of DNA methylation and H3K9me2/3 at the transcription start site (modified from Wang et al., 2009).
C – Epigenetic control of expression of terminal differentiation-associated genes in the Epidermal Differentiation Complex locus occupying about 3 Mb in mouse chromosome 3. Scheme of the locus shows chromosomal localization of the distinct genes and gene families. Genes activated during terminal keratinocyte differentiation constitute a central domain of the locus. Active transcription in the central domain is maintained by coordinated involvement of the regulators of local chromatin structure (ATP-dependent chromatin remodelers Brg1 and Mi-2β, histone demethylase JMJD3) and genome organizers Satb1 that support formation of specific higher-order chromatin folding in this region. Inhibition of transcription is associated with presence of DNA methyltransferase 1, HDAC1/2 and components of the Polycomb complexes Cbx4, Ezh1/2 and Bmi1, which promote formation of the repressive chromatin structure, as well as with lack of specific 3D-organization of the locus.
Epigenetic mechanisms of the control of gene expression include several levels of regulation which are based on:
Distinct patterns of DNA methylation and recently discovered DNA hydroxymethylation [reviewed in (Feng et al., 2010)].
Variability in distribution of the core histone variants and histone covalent modifications in the nucleosomes at the specific genome regions [reviewed in (Campos and Reinberg, 2009)];
Changes in the nucleosome positioning relatively to specific DNA sequences (translational nucleosome positioning) and ATP-dependent modulation of the DNA-histone interactions within nucleosomes (ATP dependent chromatin remodeling) [reviewed in (Clapier and Cairns, 2009)];
Changes in higher-order chromatin folding and genome organization within 3D nuclear space (higher-order chromatin remodeling) [reviewed in (Cremer and Cremer, 2011; Eskiw et al., 2010)].
Execution of cell-type specific differentiation program in multi-potent progenitor cells requires a high degree of coordination between the distinct levels of chromatin organization (Joffe et al., 2010; Lanctot et al., 2007; Schoenfelder et al., 2010a). Below, we review the mechanisms that control chromatin remodeling at distinct levels of its organization with special emphasis on execution of keratinocyte-specific gene expression programs in developing and postnatal mammalian skin (data on the genetically-modified in vivo models illustrating the roles of chromatin remodeling factors in the control of skin development are shown in Table 1).
DNA methylation and hydroxymethylation
In plants and vertebrates, DNA methylation is a post-replication modification resulting in addition of the methyl group at C5 position of cytosine to form 5-methylthytocyne (5mC). In vertebrates, this modification occurs predominantly at CpG dinucleotides (Feng et al., 2010). Approximately 60-70% of CpG sites are methylated in mammalian genome, except the relatively short regions with high CpG density called CpG islands mainly located at the promoters and first exons of the majority of housekeeping genes (Reik, 2007) (Fig. 2 B). CpG sites are often differentially methylated in a cell type-specific fashion in gene regulatory regions beyond proximal promoters, particularly in recently discovered CpG island shores, located at approximately 2 kb from the transcription start sites or even further (Feng et al., 2010).
DNA methylation is usually associated with gene repression and is catalyzed by DNA methyltransferases (DNMTs) (Feng et al., 2010). In mammals, DNMT1 is the major methyltransferase that maintain the methylation status of the genome via methylation of cytosine within hemi-methylated CpG sites after DNA replication (Feng et al., 2010). DNMT3a and DNMT3b are the major vertebrate de novo DNA methylases, while DNMT3l, which lacks the catalytic domain, binds to DNMT3A/b and facilitates their chromatin targeting (Goll and Bestor, 2005). DNA methylation patterns are significantly changed during distinct developmental stages (in germ cells and pre-implantation embryos), as well as during adult somatic cell differentiation, thus representing important epigenetic mechanisms in establishing cell-type specific programs driving tissue development and homeostasis (Feng et al., 2010; Reik, 2007).
In general, DNA methylation represses gene expression either through inhibiting transcription factor binding to DNA, or via interaction with methyl-DNA binding proteins that target repressive chromatin remodeling complexes to the methylated genome regions (Feng et al., 2010; Klose and Bird, 2006; Reik, 2007). However, DNA methylation is also required for induction of the subset of C/EBPα target genes by creating new binding sites from half-CRE sequences for this transcription factor (Rishi et al., 2010), demonstrating that in some cases, DNA methylation contributes to gene activation.
An essential role of the DNMT1 in maintenance of the epidermal progenitor cells and the epidermal tissue renewal was recently demonstrated in human 3D culture and after grafting of DNMT1-deficient keratinocytes onto nude mice (Khavari et al., 2010; Sen et al., 2010). The lack of DNTM1 in epidermal progenitor cells results in the severe defects in cell proliferation and loss of the tissue self-renewal capacity (Sen et al., 2010). Importantly, analysis of changes in gene expression program after DNMT1 ablation in epidermal progenitor cells showed abnormal induction of the genes associated with cell cycle arrest (cycling-dependent kinase inhibitors p15INK4B and p16INK4A), while co-expression of Cdk4 and Cdk6 partially rescued the effect of DNMT1 inactivation on cell proliferation (Sen et al., 2010). Expressions of DNMT1, DNMT3A/B in keratinocytes are strongly elevated after UV irradiation, which contributes to gene silencing during UV-induced photocarcinogenesis (Nandakumar et al., 2011). These results clearly demonstrate the importance of maintenance of the DNA methylation patterns in preserving epidermal progenitor cell identity and the remodeling of this pattern during terminal differentiation and UV response.
However, some gene promoters remain active being methylated, and in undifferentiated primary mouse keratinocytes the promoters of about 20% actively transcribed genes are methylated (Rishi et al., 2010). Methylation of these promoters is important for binding of the transcription factor C/EBPα and subsequent activation of gene expression, while inhibition of DNA methylation by 5-aza-cytidine results in failure to induce the subset of these genes during calcium-induced differentiation in culture (Rishi et al., 2010). 5-aza-cytidine treatment also differentially affects gene expression within the EDC locus in normal human keratinocytes: while expressions of SPRR1/2 and involucrin were markedly increased, the expression of S100A2 was decreased compared to controls (Elder and Zhao, 2002). These data suggests that DNA methylation is capable of regulating both gene repression and activation and that the basal status of promoter methylation is important for the control of expression of individual genes in keratinocytes.
Interestingly, a novel DNA modification - the hydroxylation of the 5mC in DNA into 5-hydroxymethylcytocine (5hymC) regulated by TET enzymes, has recently been demonstrated both in vitro and in vivo (Williams et al., 2011). DNA hydroxymethylation leads to conversion of 5mC into non-modified C and result in changes of the DNA methylation patterns (Feng et al., 2010; Williams et al., 2011). It has been proposed that hydroxylation of the 5mC occurs at the regulatory regions of those genes that are programmed to be activated during development and this mechanism plays a role in orchestrating the balance between pluripotent and lineage committed states in embryonic stem cells (Wu and Zhang, 2011). However, the significance of this novel DNA modification in the control of lineage-specific gene expression programs in keratinocyte progenitor cells remains unclear.
Covalent histone modifications
Histone molecules are subject to numerous post-translational covalent modifications (PTM), particular in their N-terminal tails. Such modifications include acetylation, methylation, phosphorylation, ADP-ribosylation, ubiquitination, sumolyation, argenine deamination and proline isomerysation (Allis et al., 2007). Histone PTMs have profound effect on the chromatin structure and are actively involved in regulation of gene transcription via at least three major mechanisms that modulate: a) DNA-histone contacts within the core nucleosome particles; b) Inter-nucleosomal contacts and higher-order chromatin folding; c) Changes in interaction of the chromatin with non-histone regulatory proteins (Allis et al., 2007; Campos and Reinberg, 2009; Talbert and Henikoff, 2010).
Modification of histones is catalyzed by specific groups of enzymes, including lysine acetyl-transferases, lysine and argenine methyl-transferases, kinases etc. Some modificatons (for example lysine acetylation, methylation and ubiquitination) are reversible. The removal of the acetyl and/or methyl groups from the histone molecules is also catalyzed by specific enzymes (including lysine or histone deacetylases, lysine or histone demethylases, etc.) (Allis et al., 2007). These enzymes do not usually have absolute substrate specificity (for example, CBP/P300 acetylates several lysine residues in all core histones) and could also have non-histone substrates (Allis et al., 2007). We review here the data on two most studied histone modifications (methylation and acetylation), whereas the impact of other modifications in the control of gene expression remain to be defined.
Histone methylation is dynamically regulated by histone methyltransferases and demethylases (Wang et al., 2009). Analysis of the genome-wide patterns of histone PTMs, RNA polymerase II occupation and gene expression revealed the complex correlation between histone methylation and gene transcription status (Barski et al., 2007). These studies showed a strong correlation between the histone methylation patterns and the transcription activity in the distinct genomic regions (Fig. 2 B). Lysine methylation in the histone 3 molecule could be associated with either transcription activation or repression depending on the extent (mono-, di-, or tri-methylation) and position of this modification in the histone tails (Barski et al., 2007).
Actively transcribed genes are characterized by trimethylation of the histone 3 at lysine 4 position (H3K4me3) that marks gene transcription start sites (Fig. 2 B). H3K4me3 also enriched in the genes with the paused RNA polymerase II and some inactive genes without the polymerase II bound to the promoter, while H3K4Me2/1 occupies broader area around the transcription start site and 5’ region of active genes (Wang et al., 2009). In addition, H3K79me2/3 is enriched through the 5’ portion of actively transcribed genes, and H3K36me3 is associated with 3’ parts of such genes (Wang et al., 2009).
In contrast, chromatin of the inactive genes is enriched in H3K9me2/3, H3K27me2/3 and H4K20me3 modifications (Wang et al., 2009) (Fig. 2 B). The role of H3K27 trimethylation in human epidermal progenitor cells has been revealed by inactivation of H3K27me3 demethylase JMJD3 leading to block of the progenitor cell differentiation (Sen et al., 2008). The genes normally induced during epidermal differentiation (such as KRT1, KRT10, IVL and S100A8) were repressed in epidermal progenitor cells lacking JMJD3 protein and their induction during differentiation were associated with H3K27me3 de-methylation in the promoter regions (Sen et al., 2008).
Histone H4 mono-methylation at lysine 20 is regulated by Setd8 histone methytransferase, which ablation in the epidermis results in impairment of both cell proliferation and differentiation (Driskell et al., 2011). Interestingly, Setd8 is a transcriptional target of c-Myc and an essential mediator of Myc-induced epidermal differentiation. However, Setd8 also serve as upstream regulator of p63 expression, which is lost in Setd8-null epidermis (Driskell et al., 2011). Setd8 inhibits apoptosis in the skin and its activity is essential for epidermal stem cell survival, proliferation and differentiation, at least, in part, via regulation of p63 expression (Driskell et al., 2011).
The analysis of global histone modifications reveals that in addition to gene promoters enriched in either active (H3K4me3) or repressive (H3K27me3) marks, some gene promoters are simultaneously enriched by both types of histone modifications and called “bivalent domains” (Cui et al., 2009) (Fig. 2 B). Bivalent promoters are frequently seen in embryonic stem cells and depict the specific “paused” state for a number of genes. Since H3K4me3 is often associated with transcription initiation, while H3K27me3 is associated with gene repression (Wang et al., 2009), differentiation of stem cells towards distinct cell lineages is accompanied by either activation of selected “paused” genes and loss of H3K27me3 mark, or by complete repression of other genes and loss of the H3K4me3 active mark (Araki et al., 2009).
Although bivalent chromatin domains were identified in hematopoetic and neuronal stem cells and some T cells (Fisher and Fisher, 2011), recent studies identified only a small number of the bivalent chromatin domains in the hair follicle quiescent and activated stem cells compared to embryonic stem cells (Lien et al., 2011). These data suggest that bivalency is rather required for maintenance of broader differentiation potentials in pluripotent stem cells and is no longer required in adult tissue-specific stem cells with more restricted differentiation options (Lien et al., 2011).
In adult epithelial stem cells in the skin, actively transcribed genes that maintain stemness show both H3K4me3 and H3K79me2 histone marks, while repressed non-epidermal genes and genes activated during cell differentiation are enriched by H3K27me3 (Lien et al., 2011). Stem cell transition towards hair follicle-specific differentiation is accompanied by loss of H3K27me3 and appearance of H3K4me3 and H3K79me2 in the chromatin of those genes (Lef1, Bmp4, Wnt5a, Msx1, etc.) that become active, whereas the chromatin of key stemness genes (Cd34, Sox9, Nfatc1) that become repressed in transient-amplifying cells showed loss of H3K4me3/H3K79me2 and appearance of H3K27me3 (Lien et al., 2011). However, chromatin of the cell cycle-associated genes that are repressed in stem cells do not contain H3K27me3 and show instead H3K4me3, while their activation in transient amplifying cells is accompanied, in addition to H3K4me3, by the appearance of H3K79me2 (Lien et al., 2011). These data suggest that more complex regulatory mechanisms are involved in the control of repression of cell cycle-associated genes in stem cells, which may include the activity of anti-proliferative signaling pathways, lack of pro-proliferative signaling molecules, etc.
Histone acetylation
Acetylation state of lysine residues of histones affects gene expression through influence on chromatin conformation and is controlled by two counteracting enzyme families, histone acetyl transferases (HATs) and histone deacetylases (HDACs) (Suganuma and Workman, 2011). Histone hyper-acetylation has been correlated with transcription activation of numerous genes (Araki et al., 2009), and several transcription co-activators including CBP/P300 and PCAF/CGN5 are, in fact, histone acetyl-transferases (Suganuma and Workman, 2011). On the other hand, histone hypo-acetylation is usually correlated with gene repression and some transcription co-repressors possess histone deacetylase activity (Allis et al., 2007) (Fig. 2 B).
The promoter regions of actively transcribed genes are enriched in histone H3 acetylated at lysine 9 (H3K9ac), H3K18ac and H2B12ac, whereas H4K12ac and H4K16ac are broadly distributed through the whole body of the active genes (Wang et al., 2009). Quiescent stem cells in the interfollicular epidermis and the hair follicle bulge are hypo-acetylated at histone H4, whereas c-Myc-induced exit from stem cell niche correlated with increased histone H4 acetylation (Frye et al., 2007). The significance of global histone acetylation levels for keratinocyte-specific gene expression programs was also demonstrated in vitro using histone deacetylase inhibitors trichostatin A or sodium butyrate, which both induced growth arrest in primary human keratinocytes via inhibition of expression of Cdk1 and stimulation of expression of differentiation markers involucrin and transglutaminase 1 (Elder and Zhao, 2002; Saunders et al., 1999). Consistently with these data, trichostatin A treatment of cultured epidermal explants resulted in inhibition of cell proliferation and stimulation of terminal differentiation (Markova et al., 2007). However, the effects of trichostatin A on gene expression in the EDC locus were gene-specific and also resulted in the inhibition of the expression of profillagrin and loricrin genes, suggesting that more complex mechanisms are involved in the control of expression of each individual gene in the EDC locus (Markova et al., 2007; Robertson et al., 2012).
Histone deacetylases (HDACs) are part of a multiprotein family, in which each member has its specialized functions (Brunmeir et al., 2009). HDACs play pivotal roles in coordinating the interaction of signaling pathways with chromatin remodeling and transcription factor function to modulate gene expression (Brunmeir et al., 2009). Repressive action of HDAC1/3 complex is also achieved via a specific histone H2A monoubiquitination at lysine 119 by 2A-HUB ubiquitin ligase, which blocks RNA PolII release at the early stage of elongation (Zhou et al., 2008). Conditional deletion of HDAC1/2 in the mouse epidermis demonstrated their crucial role in the control of the gene expression program in the epidermal progenitor cells and epidermal differentiation (LeBoeuf et al., 2010). The HDAC1/2 -/- epidermis failed to differentiate and remained single-layered, similarly to p63-null mice (LeBoeuf et al., 2010). The analysis of gene expression program in HDAC1/2 deficient mouse epidermis indicated that expression of p63 gene and its positive gene targets were not affected, whereas selected genes normally repressed by dNp63 including p21 and p16INK4A were up-regulated in the absence of the HDAC1/2 activity (LeBoeuf et al., 2010). Correspondingly, upregulation of HDAC1/2/3 in ARNT-deficient human epidermal keratinocytes resulted in suppression of AREG/EGFR pathway and concomitant enhancement of terminal differentiation (Robertson et al., 2012).
Polycomb group (PcG) proteins
PcG proteins were originally identified in Drosophila as negative regulators of Hox gene expression essential for the fly body patterning (reviewed in (Simon and Kingston, 2009). In mammals, PcG proteins form two families of the polycomb repressive complexes (PRCs) called PRC1 and PRC2, which compact chromatin and promote transcriptional silencing (Eckert et al., 2011; Simon and Kingston, 2009). These complexes are crucial for the development of many organs including skin, as well as for maintenance and regulation of stem cell activity (Simon and Kingston, 2009). PRC1 complex contains Ring, Bmi1, Cbx and Phi core subunits and might include other proteins, while PRC2 consist of H3K27 methyltransferases Ezh1 or Ezh2, Suz12, one of the Eed variants, Rbap48 and might also contain additional subunits (Bantignies and Cavalli, 2011; Simon and Kingston, 2009).
Polycomb complexes exhibit their repressive effects on transcription via at least three mechanisms: 1) The component of PRC2 complex histone methylase Ezh2 promotes tri-methylation of H3K27 followed by targeting the Cbx proteins as a part of PRC1 complex to H3K27me3 and subsequent ubiquitinilation of H2A at lysine 119 catalyzed by the PRC1 component Ring1B (Lee et al., 2007). This compact the chromatin and inhibits transcription through preventing binding of transcription machinery to gene promoters; 2) PRC complexes also inhibit transcription elongation via binding to initiating RNA polymerase II that result in a production of the short, but not full length RNA, indicating that PRC complex inhibits their transcription at post-initiation steps (Fachinetti et al., 2010); 3) Polycomb complexes control higher-order chromatin remodelling and promote formation of the repressive hubs where repressed genes might be grouping (Bantignies and Cavalli, 2011).
It was recently shown that a component of the PRC1 complex Cbx4 plays an important role in the maintenance of the quiescent state in human epidermal progenitor cells (Luis et al., 2011). Cbx4 protects them from senescence through PRC-dependent mechanisms by repressing INK4a locus, as well as controls their activation and differentiation through PRC-independent mechanisms (Luis et al., 2011). Other component of the PRC1 complex Bmi1 is also implicated in the control of keratinocyte senescence and inhibits the expression of p16INK4A and TGF-beta signaling (Cordisco et al., 2010; Kim et al., 2010). Bmi-1 is expressed in the basal and suprabasal layers of human epidermis, stimulates keratinocyte proliferation, promotes their survival and protects from apoptosis (Eckert et al., 2011; Lee et al., 2008).
Recent studies also provided important insights in the role of PRC2 complexes in skin development. Conditional inactivation of Ezh2 in mouse epidermis led to premature epidermal differentiation during morphogenesis (Ezhkova et al., 2009). Ezh2 controls proliferative potentials of basal progenitors by repressing the INK4A-INK4B locus and prevents their premature differentiation by inhibiting recruitment of AP1 transcriptional activator to the promoters of terminal differentiation-associated genes including those located in the EDC (Ezhkova et al., 2009).
Ezh2 expression is restricted to basal keratinocytes in embryonic skin, and Ezh1 is expressed in supra-basal keratinocytes (Ezhkova et al., 2011). Whereas single inactivation of Ezh1 or Ezh2 in keratinocytes does not lead to any defects in postnatal skin development, their simultaneous inactivation results in the arrest of hair follicle morphogenesis and subsequent degeneration due to defective proliferation and increased apoptosis (Ezhkova et al., 2011). These effects were accompanied by complete lack of H3K27me3 in epithelial stem cells and resulted in up-regulation of expression of many non-skin genes, as well as of INK4a/INK4b and Mdm2 genes (Ezhkova et al., 2011). Surprisingly, Ezh1/2 knockout mice did not show any epidermal defects, thus suggesting that loss of their activities in epidermal keratinocytes might be compensated by other PRC2 components (Ezhkova et al., 2011).
Repressive effects of Ezh1/2 on transcription are potentiated by a member of Jumonji family of chromatin-remodelling factors Jarid2, which also serves as a component of PRC2 complex (Landeira and Fisher, 2011). Ablation of Jarid2 in mouse epidermis inhibits proliferation and promotes differentiation of progenitor cells postnatally, without affecting epidermal development. Jarid2 deficiency in keratinocytes reduces H3K27 trimethylation and results in delayed hair follicle cycling as a consequence of decreased proliferation of HF stem cells and their progeny (Mejetta et al., 2011). Thus, similarly to Ezh1/2, Jarid2 is required for the maintenance of cell proliferation and inhibition of differentiation in epidermal stem and progenitor cells (Mejetta et al., 2011).
ATP-dependent chromatin remodeling
ATP-dependent chromatin remodeling complexes alter DNA-histone interactions by using an energy of the ATP hydrolysis (Clapier and Cairns, 2009). Their action could lead to changes of the nucleosome translational positioning and/or nucleosome conformation and could also facilitate eviction and deposition of nucleosomes (Bowman, 2010). ATP-dependent chromatin remodeling activity resides in multi-subunit complexes, which always contain core ATPase. The additional subunits could modulate the activity of the core ATPase and facilitate targeting of the complexes to the specific genomic regions. Based on their presence, ATP-dependent chromatin remodeling complexes are divided into several (SWI/SNF, ISWI and CHD) groups (Clapier and Cairns, 2009).
ATPases of the SWI/SNF group contain bromo-domain which binds to acetylated histone tails. In mammals, SWI/SNF group ATPases are represented by two major subunits: Brm (Smarca 2 or Snf2α) and Brg1 (Smarca4 or Snf2β), which form complexes with other subunits called BAFs (Clapier and Cairns, 2009). The genetics studies demonstrated a crucial role of Brg1 in the development of different organs including skin, while Brm appears to be mostly dispensable (de la Serna et al., 2006; Indra et al., 2005; Muchardt et al., 1998). In the developing skin, Brg1 deficiency does not alter keratinocyte proliferation or early differentiation, but lead to the defects in terminal differentiation and formation of non-functional epidermal barrier (Indra et al., 2005). qRT-PCR analysis demonstrate decrease of expression of transglutaminase 1/3 genes, as well as of corneodesmosin gene in Brg1-deficient epidermis (Indra et al., 2005). Interestingly, inactivation of Brm gene in addition to Brg1 increased severity of epidermal defects in comparison to inactivation of Brg1 gene alone, thus suggesting about a partial redundancy of their functions in keratinocytes (Indra et al., 2005).
ATP-dependent chromatin remodelers of the CHD group contain 2 chromo-domains in their N-terminal part (Marfella and Imbalzano, 2007). These domains could directly bind to DNA, RNA and methylated histone H3. CHD group proteins are divided into several subgroups based on presence of additional domains together with the chromo-domain. One of such subfamilies includes Chd3 (or Mi-2α) and Chd4 (also known as Mi-2β) proteins that harbor paired PHD (plant homeo domain) domains in their N-terminus involving in chromatin remodeling and binding to methylated histones. Chd3 and Chd4 protein are found in multi-subunit complexes that posses both histone deacetylase and ATP-dependent chromatin remodeling activities. These complexes are called NURD (nucleosome remodeling and histone deacetylase) and contain several proteins usually associated with transcriptional repression: HDAC1 and HDAC2, Rba48 and Rba46, MTA1, MTA2 and MTA3, and MBD3 (Denslow and Wade, 2007).
Keratinocyte-specific inactivation of Chd4 demonstrated that its role in the control of epidermal development is stage-dependent: ablation of Chd4 during early steps of skin development (E10.5) results in the defects in the basal epidermal layer formation, while loss of Chd4 during later stages (E13.5) did not cause any significant epidermal defects, but, instead, led to impaired induction and development of the hair follicles (Kashiwagi et al., 2007). The expression of the hair follicle placode markers, including Edar, β-catenin, Lef1, Shh, Patched1 and Bmp2, were also consistently reduced in the Chd4 mutant skin (Kashiwagi et al., 2007). However, the targets of Chd4 and other ATP-dependent chromatin remodellers in keratinocytes remain to be identified.
Higher-order chromatin remodeling and 3D genome organization
The term “higher order chromatin folding or structure” is used to describe spatial organization of chromatin beyond “beads on the string” repeat [reviewed in (Ferrai et al., 2010)]. Numerous studies demonstrated that chromatin organization in 3D nuclear space is not random. Light microscopic analysis of the nucleus performed over hundred years ago revealed that some chromatin regions are stained with basic dyes brighter and called heterochromatin, while the others are stained scarcer and represent euchromatin. Heterochromatin is more condensed, gene-poor, replicates late during cell cycle, generally associates with low transcription activity and could be sub-divided into constitutive and facultative, while euchromatin is less condensed, gene-rich, replicates relatively early and more transcriptionally active (Dimitri et al., 2009). Constitutive heterochromatin incorporates centromeric, telomeric and other silenced repetitive regions of higher eukaryotic genomes, while facultative heterochromatin contains specific genes and gene loci that could be repressed in some cells and activated in the others in different contexts (Dimitri et al., 2009; Maison et al., 2010).
Recent progress in analyses of 3D genome organization has been driven by using two approaches: i) confocal microscopy after fluorescent in situ hybridization (FISH) or gene loci labeling with transgenic fluorescent chimeric proteins containing specific DNA binding domains (Joffe et al., 2010; Kumaran et al., 2008), and ii) chromatin conformation capture (3C and its variations) technologies based on the restriction of cross-linked chromatin followed by ligation at high dilution to facilitate the formation of intra-molecular but not inter-molecular products (Naumova and Dekker, 2010; Sanyal et al., 2011). Both approaches revealed that in interphase nucleus chromosomes occupy separate areas (chromosome territories) and genes within distinct chromosomes are non-randomly positioned relatively to each other and other nuclear sub-organelles (reviewed in (Cremer and Cremer, 2011; Naumova and Dekker, 2010; Sanyal et al., 2011)).
Chromosomes and chromosomal territories
Chromosomes are the largest units of genome organization occupying distinct territories in the interphase nucleus (Cremer and Cremer, 2001; Cremer and Cremer, 2011) (Fig. 1). Each chromosome contains a centromere, pericentromeric chromatin enriched in α-satellite repetitive sequences, chromosome arms containing the gene-rich and gene-poor domains enriched in the GC- and AT-sequences and visualized as the light and dark bands by Gimsa staining, respectively, as well as the telomeres (Fukui, 2009).
Although highly variable, the relative positioning of chromosomes in the nucleus is not random, and there is evidence suggested that positioning of the chromosomes could be cell-type specific and also dependent on their size (Cremer and Cremer, 2011). In cultured human keratinocytes, positions of the chromosomes 18 and 19 are changed during calcium-induced differentiation (Marella et al., 2009). However, data obtained from mouse epidermis show that position of the chromosome 3 in keratinocytes of the basal and suprabasal layers is more peripheral compared to the chromosomes 11 and 15 and does not show changes during embryonic and post-natal development (Fig. 1 B, our unpublished observations).
Chromosome positioning in post-mitotic nuclei is controlled either through tethering of specific chromatin regions to the nuclear lamina via specialized lamina-associated domains or chromatin-associated proteins (BAF, heterochromatin protein 1, etc.), or through association of the chromosomes bearing the nucleolar-organizing region domains with nucleoli [reviewed in (Joffe et al., 2010; McKeown and Shaw, 2009; Misteli, 2007)]. Several indications suggest that distinct chromosomes may form clusters in the nuclei of differentiated cells in a tissue-specific manner, thus explaining an increased frequency of translocations between the distinct chromosomal parts in the corresponding tumors (Brianna Caddle et al., 2007; Khalil et al., 2007; Parada et al., 2004; Roix et al., 2003). However, it is unclear whether non-random distribution of chromosomes may have any functional significance for execution of tissue-specific gene expression programs and whether genes from neighboring chromosomes may share common regulatory mechanisms required for their transcription.
Recently developed approach with using three-dimensional ultra-high resolution light microscopy revealed that each chromosome territory resembles a sponge-like structure and consists of the chromatin domains permeated by interchromatin channels connected with a network of larger channels and lacunas separating distinct chromosomes and harboring a number of nuclear bodies (Markaki et al., 2011). The network of interchromatin channels starts at nuclear pores and expands throughout the nuclear space, while chromatin domains in each territory are separated from the interchromatin channels by a 100-200-nm layer of decondensed chromatin, called the perichromatin and enriched by nascent DNA and RNA, RNA polymerase II (RNA Pol II), as well as by histone modifications specific for transcriptionally active chromatin (Markaki et al., 2011).
These observations provide a solid background for the chromosome territory-interchromatin compartment (CT-IC) model (Cremer and Cremer, 2011) and suggest a large degree of flexibility in the positioning of distinct chromatin domains inside of each chromosome territory. These data also help in better understanding the results published previously and demonstrating the intermingling between the chromatin domains of neighboring chromosome territories (Branco and Pombo, 2006), as well as the phenomenon of gene looping outside the chromosome territory and formation of associations in 3D-nuclear space between the genes from different chromosomes (Schoenfelder et al., 2010a). However, functional significance of such associations and mechanisms of their formation remain mostly unknown.
Transcription factories, nuclear speckles and transcriptionally-active chromatin domains
It was shown that actively transcribed genes tend to be associated closely to each other and form active domains within chromosomal territories, whereas inactive genes are also clustered to form silenced chromosomal domains (Lieberman-Aiden et al., 2009). Moreover, many indications suggest that gene activation or silencing are associated with changes of their positioning in the nucleus relatively to neighboring genes, distinct chromosomal domains and/or nuclear bodies (Egecioglu and Brickner, 2011; Fraser and Bickmore, 2007; Misteli, 2007; Schoenfelder et al., 2010a). The dynamic changes in higher-order chromatin structure associated with cell differentiation are quite consistent with data obtained with super-resolution confocal microscopy that revealed a network of channels and lacunas inside of both euchromatin and heterochromatin, thus implicating a large degree of flexibility in the positioning of genes and chromosomal domains relatively to each other (Markaki et al., 2011) (Fig. 1 A).
Many indications suggest that actively transcribed genes are associated with transcription factories visualized in the nucleus by immunostaining to phosphorylated at Serine-5 and/or Serine-2 RNA Polymerase II isoforms that depict the initiating or elongating forms of enzyme, respectively (Cook, 1999; Schoenfelder et al., 2010b). It was proposed that several actively transcribed genes might share single transcription factory, which is formed through the aggregation of RNA Pol II-containing pre-initiation complexes assembled next to each other in the nuclear space (Razin et al., 2011). However, super-resolution confocal microscopy revealed that single PolII sites are also frequently seen in the nucleus, indicating that transcription might also occur outside of such factories (Markaki et al., 2011).
There is evidence that distinct transcription factories might contain unique combinations of transcription factors and/or other molecules facilitating transcription of co-regulated genes from the same or different chromosomes (Eskiw et al., 2010). Data obtained from erythroid cells demonstrate that tissue-specific Hbα/β globin genes are co-localized with hundreds of other transcribed genes from different chromosomes in the transcription factories enriched by Klf1 transcription factor that promote erythroid differentiation (Schoenfelder et al., 2010b). This supports an idea that distinct transcription factories might serve as hubs for inter- and intra-chromosomal interactions between many genes to achieve an efficient and coordinated transcriptional control required for proper execution of cell type-specific differentiation programs (Chakalova and Fraser, 2010).
During development, many lineage-specific genes change their positioning and move away from the nuclear periphery towards nuclear interior [reviewed in (Egecioglu and Brickner, 2011)]. Several examples (selected Hox genes in embryonic stem cells, Shh gene in the developing limb bud, etc.) demonstrate that some genes and gene loci form loops and become localized outside of the corresponding chromosomal territories after activation or in highly active state (Amano et al., 2009; Bickmore et al., 2004; Ferrai et al., 2010). In human primary keratinocytes, EDC locus also shows looping outside of the chromosomal territory 1 (Williams et al., 2002). However, many active genes are also found inside or on the border of the chromosome territories, suggesting that repositioning might not be essential for maintenance of their expression (Kupper et al., 2007; Mahy et al., 2002).
Developmentally-regulated gene repositioning might be explained by the necessity for selected genes to reach a permissive nuclear environment required for maintenance or co-regulation of their expression (Lanctot et al., 2007). Nuclear speckles localized in the interchromatin compartments and enriched by the components of the RNA splicing machinery might provide such permissive environment, and, indeed, many highly-transcribed genes including the EDC locus in keratinocytes show a tendency to cluster around speckles (Brown et al., 2008; Hu et al., 2008; Spector and Lamond, 2011; Szczerbal and Bridger, 2010) (Fig. 1 A). Recent data demonstrate that relocation of the proliferation-associated genes from the repressive Polycomb bodies to nuclear speckles depends on methylation/demethylation of the PRC1 protein Cbx4: methylated form of Cbx4 binds to the non-coding RNA TUG1, while demethylated Cbx4 binds to non-coding RNA MALAT1/NEAT2, located in the Polycomb bodies or speckles, respectively (Yang et al., 2011). Repositioning of genes to speckles results in their activation through MALAT1/NEAT2-mediated interactions with multiple co-activators of transcription and/or splicing factors (Yang et al., 2011). Frequent associations between EDC and nuclear speckles are also seen in the epidermal keratinocytes (Fig. 1 B). However, functional significance of keratinocyte-specific gene loci co-localization with nuclear speckles remains to be determined.
Long-range chromatin interactions and establishing specific chromatin conformations in tissue-specific genomic loci
Increased evidence of data suggest that inter- and intra-chromosomal long-range associations between distinct genomic regions are essential for maintenance of cell type-specific transcriptional profiles and form “transcriptional interactome”, which is quite dynamic in its nature (Schoenfelder et al., 2010a). In a number of tissue-specific gene loci (beta-globin locus, TH2-cytokine locus, etc.), long-range interactions help to establish specific loop structures, in which distant locus control elements are brought in close proximity to gene promoters to regulate transcription, whereas alterations in spatial organization of such loop structures significantly impact gene expression programs in differentiating cells (Egecioglu and Brickner, 2011). In differentiating keratinocytes, calcium stimulation result in increased physical proximity of the enhancer and the promoter regions of the peptidylarginine deaminase 3 gene that control metabolism of the filaggrin (Chavanas et al., 2008).
Repositioning of the genes and long-range interactions between genomic loci are energy-dependent processes and are regulated, at least in part, by actin-myosin nuclear motor complexes (Chuang et al., 2006; Hu et al., 2008; Mehta et al., 2010). Formation of the chromatin loop-like structures inside of the chromosomes (in cis) and between different chromosomes (in trans) is also controlled by several proteins that function as topological genome organizers, such as CTCF, cohesin and SATB1 (Cai et al., 2006; Galande et al., 2007; Merkenschlager, 2010; Ohlsson et al., 2010). CTCF is DNA-binding zinc-finger protein implicated in the control of many important processes in the nucleus (Ohlsson et al., 2010). CTCF binding sites are spread all over the genome, thus providing the possibility to form thousands of inter-chromosomal loops of different size, as well as to bring genes located on different chromosomes into close proximity (Ohlsson et al., 2010; Zlatanova and Caiafa, 2009). Recent studies revealed that CTCF recruits the mitosis regulator cohesin, which is essential for proper chromosome segregation, to specific chromosomal locations in the interphase nucleus (Hadjur et al., 2009). In T-cell receptor locus, CTCF-cohesin complex is required for long-range promoter-enhancer interactions, maintenance of transcription and demarcation of the locus from interspersed elements and neighbouring housekeeping genes (Seitan et al., 2011). Thus, in addition to its global role in mitosis regulation, cohesin controls chromosome conformation and gene expression (Merkenschlager, 2010).
AT-rich binding protein Satb1 also controls establishing specific three-dimensional conformations in tissue-specific gene loci (Cai et al., 2003; Cai et al., 2006). Satb1 targets chromatin remodelling enzymes and transcription factors to specific genomic regions, establishes region-specific epigenomic modification status, and plays a fundamental role in the execution of tissue-specific gene expression programs (Cai et al., 2006). In epidermal keratinocytes, Satb1 regulates conformation of the central domain of the EDC locus containing genes activated during terminal keratinocyte differentiation (Fessing et al., 2011). Ablation of Satb1 results in alterations of 3D-chromatin structure and elongation of this domain, accompanied by marked alterations of gene expression and epidermal morphology (Fessing et al., 2011). This suggests that three-dimensional organization of multi-gene tissue-specific loci is indeed important for maintenance of the proper balance of gene expression and outcomes of the transcription programs (Fig. 2 C).
Heterochromatin, transcriptionally-repressive domains and gene silencing
In almost all mammalian somatic cells, heterochromatin is associated with nuclear membrane, nucleoli, and centromeric repeat clusters, whereas small regions of facultative heterochromatin are also incorporated into euchromatin (Dimitri et al., 2009). Key features of heterochromatin are its associations with repressive epigenetic marks including DNA methylation and H3K9 tri-methylation, as well as with Heterochromatin Protein 1 (HP1) that interacts with a number of other proteins involved in the control of chromatin remodeling (Kwon and Workman, 2011; Probst and Almouzni, 2011).
Currently, three distinct types of transcriptionally inactive heterochromatin domains are described: 1) Domains associated with centromeres and marked by DNA methylation, H3K9me3, H3K20me3 and α/β/γ HP1 ιsoforms; 2) Chromatin associated with nuclear lamina via Lamina-associated domains that enriched by H3K9me2 and HP1α/β; 3) Chromatin marked by H3K27me3 and Polycomb group proteins (Meister et al., 2011; Probst and Almouzni, 2011). Although all three types of heterochromatin domains contain actively transcribed genes, transcription of the majority of genes associated with these domains is repressed either constitutively (at the centromere regions) or temporarily in the developmentally-regulated or context-dependent manners (lamina-associated and Polycomb-repressed genes) (Meister et al., 2011).
Heterochromatin Protein 1 plays a critical role in organization and formation of heterochromatin (Kwon and Workman, 2011). In mammals, HP1 consists of three isoforms (α, β, γ), encoded by three different chromobox genes (Cbx5, Cbx1 and Cbx3, respectively), which are quite distinct in their functions: HP1α/β bind H3K9me3 and target HP1 to heterochromatin, whereas HP1γ via histone chaperone FACT binds to elongating form of PolII and interacts with euchromatin (Kwon and Workman, 2011). HP1α/β interactions with H3K9me3 is important for condensing pericentric chromatin regions to maintain transcriptional repression, as well as for binding Lamin B receptor and maintenance of lamina-associated heterochromatin (Black and Whetstine, 2011). In contrast to HP1α/β, HP1γ is concentrated in 3’-end of active genes and might positively regulate transcription (Kwon and Workman, 2011).
Polycomb proteins also play crucial role in maintenance of the chromatin repressed states via interaction with Polycomb response elements and gene promoters and formation of topologically complex structures (repressive chromatin hubs) that promotes gene silencing (Lanzuolo et al., 2007). Repressive chromatin loops might be organized in 3D nuclear space into Polycomb bodies that are considered as chromatin silencing factories and contain a number of transcriptional repressors including non-coding RNA TUG1 (Bantignies et al., 2011; Hodgson and Brock, 2011; Mao et al., 2011; Yang et al., 2011). However, it is unclear whether such factories exist in keratinocytes and how they contribute to terminal differentiation-associated gene silencing in the epidermis remain to be clarified.
Cross-talk between the epigenetic and transcription factor-mediated regulatory mechanisms in the control of keratinocyte differentiation
The program of epidermal differentiation and barrier formation is tightly controlled by a number of transcription regulators including the p63, c-Myc, AP-2, Klf4, Arnt, PPAR-alpha, m-Ovo, etc. (Blanpain and Fuchs, 2009; Geng et al., 2006; Koster and Roop, 2007; Truong and Khavari, 2007). The p63 transcription factor serves as a master regulator of epidermal development, and p63 knockout (-/-) mice fail to form stratified epithelium and to express a number of epidermis-specific genes [reviewed in (Koster and Roop, 2007; Vanbokhoven et al., 2011)]. In keratinocytes, p63 regulates a large number of genes that encode distinct adhesion/signalling molecules, transcription factors, cell cycle-associated proteins, as well as tissue-specific proteins, such as keratins, involucrin, and loricrin [reviewed in (Vigano and Mantovani, 2007)].
Recent data revealed that in the epidermis of p63-null mice the expression of a large number of genes involved in the control of nuclear/chromatin assembly and remodeling is significantly changed compared to wild-type controls (Fessing et al., 2011). These genes include several key regulators of the chromatin structure and remodeling: histone methytransferases Ehmt1/2, components of the Polycomb complexes Cbx2/4/6/7 and Jarid2, ATP-dependent chromatin remodellers Mi-2α/β, Brg1, Chd1 and Brm, as well as genome organizer Satb1 (Fessing et al., 2011). Whereas involvement of p63 in regulation of the majority of these genes remain to be clarified, it is shown that p63 directly controls the expression of Satb1 and promotes establishing of specific higher-order chromatin structure in central domain of the EDC required for maintenance of the proper balance of gene expression during terminal keratinocyte differentiation (Fessing et al., 2011). These data demonstrate that Satb1 is an important, hitherto unrecognized component of the global regulatory network of p63, and suggest a novel fundamental mechanism on how master regulators of tissue morphogenesis establish tissue-specific patterns of higher-order chromatin structure and remodeling to control the fate of multi-potent progenitor cells.
p63 expression in keratinocytes is regulated by histone methyltransferase Setd8, which, in turn, is a target of c-Myc transcription factor and mediates its effects on epidermal differentiation (Driskell et al., 2011). c-Myc is an important regulator of epidermal homeostasis and promotes keratinocyte proliferation and differentiation into epidermal and sebaceous gland lineages (Lo Celso et al., 2008; Nascimento et al., 2011; Waikel et al., 2001). In addition to direct involvement in the control of expression of epidermis-specific genes, c-Myc also exhibits a potent activity as a modulator of local chromatin structure: it regulates expression of histone acetyltransferase Gcn5 and promotes histone acetylation (Knoepfler et al., 2006). Furthermore, c-Myc regulates expression of RNA methyltransferase Misu, which modulate c-Myc effects on cell proliferation and differentiation (Driskell et al., 2011; Frye and Watt, 2006).
Transcription factors also directly interact with chromatin remodeling factors and target them to gene promoters: c-Myc targets histone acetyltransferases Gcn5 to chromatin and promotes global euchromatisation (Knoepfler et al., 2006), whereas NFATc1 transcription factor forms complex with ATP-dependent chromatin remodeler Brg1 and stimulates formation of DNAse1 hypersensitive sites and recruitment of other transcription factors to target genes (Pham et al., 2010; Wurster and Pazin, 2008). However, transcription factors might also operate as the repressors of transcription by interacting with HDACs and targeting repressive complexes to gene promoters: for instance, transcriptional repressor NFX1 interacts with mSin3A/HDAC and recruits the repression complex to the hTERT promoter in keratinocytes, while p53 inhibits expression of its target genes during DNA damage response via interaction and targeting Sin3B/HDAC complex to the promoters (Bansal et al., 2011; Xu et al., 2008).
Thus, chromatin remodeling factors operate as integral part of the genetic programs governed by transcription factors and modulate their effects on local or global chromatin structure (Fig. 3). Moreover, transcription factors might directly interact with chromatin remodelers to target them to the specific genomic sites and regulate gene expression. However, chromatin remodeling factors might, in turn, regulate expression of the distinct transcription factors via formation of the active or repressive local chromatin structure at their promoter regions.
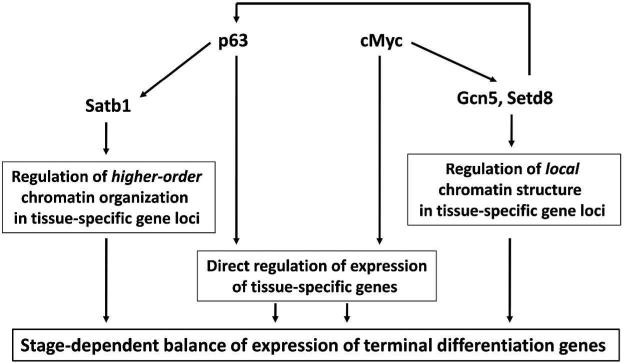
Figure 3. Model illustrating cross-talk between transcription factors and epigenetic regulators in the control of expression of tissue-specific genes in keratinocytes.
In addition to direct regulation of tissue-specific genes, p63 and c-Myc transcription factors promote formation of the permissive chromatin structure in tissue-specific gene loci. p63 via regulation of genome organizer Satb1 support formation of specific higher-order organization of the tissue-specific EDC locus, whereas c-Myc via regulation of histone acetyltrasferase Gcn5 and histone methyltransferase Setd8 promotes formation of the transcription-friendly local chromatin structure. In turn, Setd8 is capable of positively influence p63 expression (based on data published (Driskell et al., 2011; Fessing et al., 2011; Knoepfler et al., 2006; Nascimento et al., 2011).
Conclusions and future directions
In summary, epigenetic regulators modulate both local and higher-order chromatin structure in epidermal keratinocytes and exhibit their effects on cell proliferation and differentiation via at least three mechanisms: 1) Controlling the expression of cell cycle-associated genes; 2) Regulating expression of the terminal differentiation-associated genes; 3) Modulating gene expression programs governed by distinct transcription factors, such as p63, c-Myc, NF-kappaB, etc.
Group of factors that predominantly operate as repressive chromatin regulators (DNA methyltransferase DNMT1, histone deacetylases HDAC1/2, Polycomp components Cbx4, Bmi1, Ezh1/2) stimulate proliferation of progenitor cells via repression of the genes encoding cell-cycle inhibitors p16INK4A, p15INK4B, p19ARF and p21. Some of the repressive chromatin regulators (DNMT1, Ezh1/2, Jarid2) also inhibit premature activation of terminal differentiation-associated genes, whereas HDAC2 inhibits expression of deltaNp63 and interfere with deltaNp63-regulated gene expression program in keratinocytes.
Vice versa, a group of chromatin remodelers that generally support active transcription (histone demethylase Jmjd3, ATP-dependent chromatin remodeler Brg1, genome organizer Satb1) promote terminal keratinocyte differentiation and also exhibit effects on cell proliferation. It is likely that expression of terminal differentiation-associated genes including those that constitute EDC in epidermal keratinocytes requires tightly controlled coordination of action of several epigenetic regulators operating at the levels of both local (DNMT1, Ezh1/2, Bmi1, Jarid2, Jmjd3, Brg1) and higher-order (Satb1) chromatin structures (Fig. 2 C; Fig. 3).
Epigenetic modifications play an important role in the development of many disorders, such as cancer, immunodeficiency, and autoimmune diseases (Bjornsson et al., 2007; Feinberg, 2010). Epigenetic status of skin epithelial and non-epithelial cells is altered in various types of skin cancers and psoriasis (Brown et al., 2004; Chiles et al., 2003; Li et al., 2009; Murao et al., 2006). Comprehensive knowledge of epigenetic mechanisms and their alterations in health and disease, has become a growing priority in biomedical research (Portela and Esteller, 2010). However, additional efforts are required to fully understand how epigenetic mechanisms contribute to interpret different environmental insults applied to the skin, as well as how these mechanisms are altered in skin disorders.
In particular, at least the following important issues need to be resolved:
Cross-talk between distinct signaling pathways that control keratinocyte proliferation/differentiation/migration and epigenetic regulatory mechanisms need to be investigated;
Mechanisms that control specificity of distinct classes of epigenetic regulators in targeting different genes/gene loci in the genome of keratinocytes remain unclear;
Roles of the non-coding and microRNAs and their interaction with epigenetic regulators in modulating gene expression programs need to be clarified;
Topological organization of the keratinocyte genome, links between the higher-order and local chromatin organization and the role of each nuclear sub-organelle in keratinocyte differentiation process, as well as in the response to various environmental stimuli need to be carefully studied;
Roles of distinct classes of epigenetic regulators in the pathological skin conditions associated with alterations of keratinocyte differentiation, their expansion or loss, as well as during DNA damage response remain to be further defined.
Recent advances of pharmacogenomics resulted in the development of a number of molecules that are capable of modulating distinct epigenetic regulatory mechanisms, whereas some of them are already under clinical trials [reviewed in (Heightman, 2011)]. Future research in this direction will help to bridge the gap between our current knowledge of basic epigenetic mechanisms and potential applications of epigenetic modulators, which hopefully will serve as new generation of drugs for treatment of skin disorders, protection of skin against environmental stressors and aging.
Acknowledgements
Work on this review was supported from the grants from Medical Research Council (UK) and NIH to V.A.B. and via K01 Mentored Scientist Award to A.A.S. Critical comments on the manuscript from Dr. Barbara A. Gilchrest and Dr. Chris Lewis are gratefully acknowledged.
Abbreviations
- DNMT
DNA methyltransferase
- EDC
Epidermal Differentiation Complex
- HDAC
Histone deacetylase
- Pol II
RNA Polymerase II
Footnotes
The authors state no conflict of interest.
References
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–6. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Amano T, Sagai T, Tanabe H, Mizushina Y, Nakazawa H, Shiroishi T. Chromosomal dynamics at the Shh locus: limb bud-specific differential regulation of competence and active transcription. Dev Cell. 2009;16:47–57. doi: 10.1016/j.devcel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Andrews AJ, Luger K. Nucleosome structure(s) and stability: variations on a theme. Annu Rev Biophys. 2011;40:99–117. doi: 10.1146/annurev-biophys-042910-155329. [DOI] [PubMed] [Google Scholar]
- Araki Y, Wang Z, Zang C, Wood WH, 3rd, Schones D, Cui K, et al. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30:912–25. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal N, Kadamb R, Mittal S, Vig L, Sharma R, Dwarakanath BS, et al. Tumor suppressor protein p53 recruits human Sin3B/HDAC1 complex for down-regulation of its target promoters in response to genotoxic stress. PLoS One. 2011;6:e26156. doi: 10.1371/journal.pone.0026156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantignies F, Cavalli G. Polycomb group proteins: repression in 3D. Trends Genet. 2011;27:454–64. doi: 10.1016/j.tig.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Bantignies F, Roure V, Comet I, Leblanc B, Schuettengruber B, Bonnet J, et al. Polycomb- dependent regulatory contacts between distant Hox loci in Drosophila. Cell. 2011;144:214–26. doi: 10.1016/j.cell.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Fantauzzo KA, Richardson GD, Jahoda CA, Christiano AM. Transcriptional profiling of developing mouse epidermis reveals novel patterns of coordinated gene expression. Dev Dyn. 2007;236:961–70. doi: 10.1002/dvdy.21099. [DOI] [PubMed] [Google Scholar]
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–3. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore WA, Mahy NL, Chambeyron S. Do higher-order chromatin structure and nuclear reorganization play a role in regulating Hox gene expression during development? Cold Spring Harb Symp Quant Biol. 2004;69:251–7. doi: 10.1101/sqb.2004.69.251. [DOI] [PubMed] [Google Scholar]
- Bjornsson HT, Brown LJ, Fallin MD, Rongione MA, Bibikova M, Wickham E, et al. Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J Natl Cancer Inst. 2007;99:1270–3. doi: 10.1093/jnci/djm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JC, Whetstine JR. Chromatin landscape: methylation beyond transcription. Epigenetics. 2011;6:9–15. doi: 10.4161/epi.6.1.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkareva NV. MicroRNA/mRNA regulatory networks in the control of skin development and regeneration. Cell Cycle. 2012;11:468–74. doi: 10.4161/cc.11.3.19058. [DOI] [PubMed] [Google Scholar]
- Bowman GD. Mechanisms of ATP-dependent nucleosome sliding. Curr Opin Struct Biol. 2010;20:73–81. doi: 10.1016/j.sbi.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco MR, Pombo A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 2006;4:e138. doi: 10.1371/journal.pbio.0040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brianna Caddle L, Grant JL, Szatkiewicz J, van Hase J, Shirley BJ, Bewersdorf J, et al. Chromosome neighborhood composition determines translocation outcomes after exposure to high-dose radiation in primary cells. Chromosome Res. 2007;15:1061–73. doi: 10.1007/s10577-007-1181-7. [DOI] [PubMed] [Google Scholar]
- Brown JM, Green J, das Neves RP, Wallace HA, Smith AJ, Hughes J, et al. Association between active genes occurs at nuclear speckles and is modulated by chromatin environment. J Cell Biol. 2008;182:1083–97. doi: 10.1083/jcb.200803174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VL, Harwood CA, Crook T, Cronin JG, Kelsell DP, Proby CM. p16INK4a and p14ARF tumor suppressor genes are commonly inactivated in cutaneous squamous cell carcinoma. The Journal of investigative dermatology. 2004;122:1284–92. doi: 10.1111/j.0022-202X.2004.22501.x. [DOI] [PubMed] [Google Scholar]
- Brunmeir R, Lagger S, Seiser C. Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol. 2009;53:275–89. doi: 10.1387/ijdb.082649rb. [DOI] [PubMed] [Google Scholar]
- Cai S, Han HJ, Kohwi-Shigematsu T. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat Genet. 2003;34:42–51. doi: 10.1038/ng1146. [DOI] [PubMed] [Google Scholar]
- Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–88. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–99. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- Chakalova L, Fraser P. Organization of transcription. Cold Spring Harb Perspect Biol. 2010;2:a000729. doi: 10.1101/cshperspect.a000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavanas S, Adoue V, Mechin MC, Ying S, Dong S, Duplan H, et al. Long-range enhancer associated with chromatin looping allows AP-1 regulation of the peptidylarginine deiminase 3 gene in differentiated keratinocyte. PLoS One. 2008;3:e3408. doi: 10.1371/journal.pone.0003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiles MC, Ai L, Zuo C, Fan CY, Smoller BR. E-cadherin promoter hypermethylation in preneoplastic and neoplastic skin lesions. Mod Pathol. 2003;16:1014–8. doi: 10.1097/01.MP.0000089779.35435.9D. [DOI] [PubMed] [Google Scholar]
- Chuang CH, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–31. doi: 10.1016/j.cub.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- Cook PR. The organization of replication and transcription. Science. 1999;284:1790–5. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- Cordisco S, Maurelli R, Bondanza S, Stefanini M, Zambruno G, Guerra L, et al. Bmi-1 reduction plays a key role in physiological and premature aging of primary human keratinocytes. J Invest Dermatol. 2010;130:1048–62. doi: 10.1038/jid.2009.355. [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- Cremer T, Cremer M. Chromosome territories. In: Misteli T, Spector DL, editors. The Nucleus. Cold Spring Harbor Laboratory Press; Cold-Spring Harbor, N.Y.: 2011. pp. 93–114. [Google Scholar]
- Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Serna IL, Ohkawa Y, Higashi C, Dutta C, Osias J, Kommajosyula N, et al. The microphthalmia-associated transcription factor requires SWI/SNF enzymes to activate melanocyte-specific genes. J Biol Chem. 2006;281:20233–41. doi: 10.1074/jbc.M512052200. [DOI] [PubMed] [Google Scholar]
- Denslow SA, Wade PA. The human Mi-2/NuRD complex and gene regulation. Oncogene. 2007;26:5433–8. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- Dimitri P, Caizzi R, Giordano E, Carmela Accardo M, Lattanzi G, Biamonti G. Constitutive heterochromatin: a surprising variety of expressed sequences. Chromosoma. 2009;118:419–35. doi: 10.1007/s00412-009-0211-y. [DOI] [PubMed] [Google Scholar]
- Driskell I, Oda H, Blanco S, Nascimento E, Humphreys P, Frye M. The histone methyltransferase Setd8 acts in concert with c-Myc and is required to maintain skin. EMBO J. 2011 doi: 10.1038/emboj.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M, Misteli T. Biogenesis of nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000711. doi: 10.1101/cshperspect.a000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Adhikary G, Rorke EA, Chew YC, Balasubramanian S. Polycomb group proteins are key regulators of keratinocyte function. J Invest Dermatol. 2011;131:295–301. doi: 10.1038/jid.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu D, Brickner JH. Gene positioning and expression. Curr Opin Cell Biol. 2011;23:338–45. doi: 10.1016/j.ceb.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JT, Zhao X. Evidence for local control of gene expression in the epidermal differentiation complex. Exp Dermatol. 2002;11:406–12. doi: 10.1034/j.1600-0625.2002.110503.x. [DOI] [PubMed] [Google Scholar]
- Eskiw CH, Cope NF, Clay I, Schoenfelder S, Nagano T, Fraser P. Transcription factories and nuclear organization of the genome. Cold Spring Harb Symp Quant Biol. 2010;75:501–6. doi: 10.1101/sqb.2010.75.046. [DOI] [PubMed] [Google Scholar]
- Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–98. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–35. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachinetti D, Bermejo R, Cocito A, Minardi S, Katou Y, Kanoh Y, et al. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol Cell. 2010;39:595–605. doi: 10.1016/j.molcel.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–40. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Genome-scale approaches to the epigenetics of common human disease. Virchows Arch. 2010;456:13–21. doi: 10.1007/s00428-009-0847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–53. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–7. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrai C, de Castro IJ, Lavitas L, Chotalia M, Pombo A. Gene positioning. Cold Spring Harb Perspect Biol. 2010;2:a000588. doi: 10.1101/cshperspect.a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessing MY, Mardaryev AN, Gdula MR, Sharov AA, Sharova TY, Rapisarda V, et al. p63 regulates Satb1 to control tissue-specific chromatin remodeling during development of the epidermis. J Cell Biol. 2011;194:825–39. doi: 10.1083/jcb.201101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Scherz J, Szabo S, Mildner M, Benarafa C, Torriglia A, et al. DNase 2 is the main DNA-degrading enzyme of the stratum corneum. PLoS One. 2011;6:e17581. doi: 10.1371/journal.pone.0017581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CL, Fisher AG. Chromatin states in pluripotent, differentiated, and reprogrammed cells. Curr Opin Genet Dev. 2011;21:140–6. doi: 10.1016/j.gde.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–7. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- Frye M, Fisher AG, Watt FM. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS ONE. 2007;2:e763. doi: 10.1371/journal.pone.0000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Watt FM. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr Biol. 2006;16:971–81. doi: 10.1016/j.cub.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–42. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K. Structural analyses of chromosomes and their constituent proteins. Cytogenet Genome Res. 2009;124:215–27. doi: 10.1159/000218127. [DOI] [PubMed] [Google Scholar]
- Galande S, Purbey PK, Notani D, Kumar PP. The third dimension of gene regulation: organization of dynamic chromatin loopscape by SATB1. Curr Opin Genet Dev. 2007;17:408–14. doi: 10.1016/j.gde.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Geng S, Mezentsev AV, Kalachikov S, Raith K, Roop D, Panteleyev AA. Targeted ablation of Arnt in mouse epidermis results in profound defects in desquamation and epidermal barrier function. J Cell Sci. 2006;119:4901–12. doi: 10.1242/jcs.03282. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–3. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heermann DW. Physical nuclear organization: loops and entropy. Curr Opin Cell Biol. 2011;23:332–7. doi: 10.1016/j.ceb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Heightman TD. Therapeutic prospects for epigenetic modulation. Expert Opin Ther Targets. 2011;15:729–40. doi: 10.1517/14728222.2011.561786. [DOI] [PubMed] [Google Scholar]
- Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington's canal. Nat Rev Mol Cell Biol. 2009;10:526–37. doi: 10.1038/nrm2727. [DOI] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–84. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JW, Brock HW. Are polycomb group bodies gene silencing factories? Cell. 2011;144:170–1. doi: 10.1016/j.cell.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Hu Q, Kwon YS, Nunez E, Cardamone MD, Hutt KR, Ohgi KA, et al. Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatin granules. Proc Natl Acad Sci U S A. 2008;105:19199–204. doi: 10.1073/pnas.0810634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner MR, Spector DL. Chromatin dynamics. Annu Rev Biophys. 2010;39:471–89. doi: 10.1146/annurev.biophys.093008.131348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Dupe V, Bornert JM, Messaddeq N, Yaniv M, Mark M, et al. Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation. Development. 2005;132:4533–44. doi: 10.1242/dev.02019. [DOI] [PubMed] [Google Scholar]
- Joffe B, Leonhardt H, Solovei I. Differentiation and large scale spatial organization of the genome. Curr Opin Genet Dev. 2010;20:562–9. doi: 10.1016/j.gde.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Morgan BA, Georgopoulos K. The chromatin remodeler Mi-2beta is required for establishment of the basal epidermis and normal differentiation of its progeny. Development. 2007;134:1571–82. doi: 10.1242/dev.001750. [DOI] [PubMed] [Google Scholar]
- Khalil A, Grant JL, Caddle LB, Atzema E, Mills KD, Arneodo A. Chromosome territories have a highly nonspherical morphology and nonrandom positioning. Chromosome Res. 2007;15:899–916. doi: 10.1007/s10577-007-1172-8. [DOI] [PubMed] [Google Scholar]
- Khavari DA, Sen GL, Rinn JL. DNA methylation and epigenetic control of cellular differentiation. Cell Cycle. 2010;9:3880–3. doi: 10.4161/cc.9.19.13385. [DOI] [PubMed] [Google Scholar]
- Kim RH, Lieberman MB, Lee R, Shin KH, Mehrazarin S, Oh JE, et al. Bmi-1 extends the life span of normal human oral keratinocytes by inhibiting the TGF-beta signaling. Exp Cell Res. 2010;316:2600–8. doi: 10.1016/j.yexcr.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006;25:2723–34. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;9:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Kumaran RI, Thakar R, Spector DL. Chromatin dynamics and gene positioning. Cell. 2008;132:929–34. doi: 10.1016/j.cell.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper K, Kolbl A, Biener D, Dittrich S, von Hase J, Thormeyer T, et al. Radial chromatin positioning is shaped by local gene density, not by gene expression. Chromosoma. 2007;116:285–306. doi: 10.1007/s00412-007-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Workman JL. The changing faces of HP1: From heterochromatin formation and gene silencing to euchromatic gene expression: HP1 acts as a positive regulator of transcription. BioEssays. 2011;33:280–9. doi: 10.1002/bies.201000138. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet. 2007;8:104–15. doi: 10.1038/nrg2041. [DOI] [PubMed] [Google Scholar]
- Landeira D, Fisher AG. Inactive yet indispensable: the tale of Jarid2. Trends Cell Biol. 2011;21:74–80. doi: 10.1016/j.tcb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzuolo C, Roure V, Dekker J, Bantignies F, Orlando V. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat Cell Biol. 2007;9:1167–74. doi: 10.1038/ncb1637. [DOI] [PubMed] [Google Scholar]
- LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, et al. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell. 2010;19:807–18. doi: 10.1016/j.devcel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Adhikary G, Balasubramanian S, Gopalakrishnan R, McCormick T, Dimri GP, et al. Expression of Bmi-1 in epidermis enhances cell survival by altering cell cycle regulatory protein expression and inhibiting apoptosis. J Invest Dermatol. 2008;128:9–17. doi: 10.1038/sj.jid.5700949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, et al. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007;318:447–50. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- Li Y, Sawalha AH, Lu Q. Aberrant DNA methylation in skin diseases. J Dermatol Sci. 2009;54:143–9. doi: 10.1016/j.jdermsci.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–93. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Guo X, Polak L, Lawton LN, Young RA, Zheng D, et al. Genome-wide Maps of Histone Modifications Unwind In Vivo Chromatin States of the Hair Follicle Lineage. Cell Stem Cell. 2011;9:219–32. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Berta MA, Braun KM, Frye M, Lyle S, Zouboulis CC, et al. Characterization of bipotential epidermal progenitors derived from human sebaceous gland: contrasting roles of c-Myc and beta-catenin. Stem Cells. 2008;26:1241–52. doi: 10.1634/stemcells.2007-0651. [DOI] [PubMed] [Google Scholar]
- Luis NM, Morey L, Mejetta S, Pascual G, Janich P, Kuebler B, et al. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of Cbx4. Cell Stem Cell. 2011;9:233–46. doi: 10.1016/j.stem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Mahy NL, Perry PE, Bickmore WA. Gene density and transcription influence the localization of chromatin outside of chromosome territories detectable by FISH. J Cell Biol. 2002;159:753–63. doi: 10.1083/jcb.200207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C, Quivy JP, Probst AV, Almouzni G. Heterochromatin at mouse pericentromeres: a model for de novo heterochromatin formation and duplication during replication. Cold Spring Harb Symp Quant Biol. 2010;75:155–65. doi: 10.1101/sqb.2010.75.013. [DOI] [PubMed] [Google Scholar]
- Mao YS, Zhang B, Spector DL. Biogenesis and function of nuclear bodies. Trends Genet. 2011;27:295–306. doi: 10.1016/j.tig.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella NV, Bhattacharya S, Mukherjee L, Xu J, Berezney R. Cell type specific chromosome territory organization in the interphase nucleus of normal and cancer cells. J Cell Physiol. 2009;221:130–8. doi: 10.1002/jcp.21836. [DOI] [PubMed] [Google Scholar]
- Marfella CG, Imbalzano AN. The Chd family of chromatin remodelers. Mutat Res. 2007;618:30–40. doi: 10.1016/j.mrfmmm.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markaki Y, Gunkel M, Schermelleh L, Beichmanis S, Neumann J, Heidemann M, et al. Functional Nuclear Organization of Transcription and DNA Replication: A Topographical Marriage between Chromatin Domains and the Interchromatin Compartment. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2010.75.042. [DOI] [PubMed] [Google Scholar]
- Markova NG, Karaman-Jurukovska N, Pinkas-Sarafova A, Marekov LN, Simon M. Inhibition of histone deacetylation promotes abnormal epidermal differentiation and specifically suppresses the expression of the late differentiation marker profilaggrin. J Invest Dermatol. 2007;127:1126–39. doi: 10.1038/sj.jid.5700684. [DOI] [PubMed] [Google Scholar]
- McKeown PC, Shaw PJ. Chromatin: linking structure and function in the nucleolus. Chromosoma. 2009;118:11–23. doi: 10.1007/s00412-008-0184-2. [DOI] [PubMed] [Google Scholar]
- Mehta IS, Amira M, Harvey AJ, Bridger JM. Rapid chromosome territory relocation by nuclear motor activity in response to serum removal in primary human fibroblasts. Genome Biol. 2010;11:R5. doi: 10.1186/gb-2010-11-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister P, Mango SE, Gasser SM. Locking the genome: nuclear organization and cell fate. Curr Opin Genet Dev. 2011;21:167–74. doi: 10.1016/j.gde.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejetta S, Morey L, Pascual G, Kuebler B, Mysliwiec MR, Lee Y, et al. Jarid2 regulates mouse epidermal stem cell activation and differentiation. EMBO J. 2011;30:3635–46. doi: 10.1038/emboj.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M. Cohesin: a global player in chromosome biology with local ties to gene regulation. Curr Opin Genet Dev. 2010;20:555–61. doi: 10.1016/j.gde.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Muchardt C, Bourachot B, Reyes JC, Yaniv M. ras transformation is associated with decreased expression of the brm/SNF2alpha ATPase from the mammalian SWI-SNF complex. EMBO J. 1998;17:223–31. doi: 10.1093/emboj/17.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K, Kubo Y, Ohtani N, Hara E, Arase S. Epigenetic abnormalities in cutaneous squamous cell carcinomas: frequent inactivation of the RB1/p16 and p53 pathways. Br J Dermatol. 2006;155:999–1005. doi: 10.1111/j.1365-2133.2006.07487.x. [DOI] [PubMed] [Google Scholar]
- Nandakumar V, Vaid M, Tollefsbol TO, Katiyar SK. Aberrant DNA hypermethylation patterns lead to transcriptional silencing of tumor suppressor genes in UVB-exposed skin and UVB-induced skin tumors of mice. Carcinogenesis. 2011;32:597–604. doi: 10.1093/carcin/bgq282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nascimento EM, Cox CL, Macarthur S, Hussain S, Trotter M, Blanco S, et al. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat Cell Biol. 2011;13:1395–405. doi: 10.1038/ncb2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova N, Dekker J. Integrating one-dimensional and three-dimensional maps of genomes. J Cell Sci. 2010;123:1979–88. doi: 10.1242/jcs.051631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson R, Bartkuhn M, Renkawitz R. CTCF shapes chromatin by multiple mechanisms: the impact of 20 years of CTCF research on understanding the workings of chromatin. Chromosoma. 2010;119:351–60. doi: 10.1007/s00412-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada LA, McQueen PG, Misteli T. Tissue-specific spatial organization of genomes. Genome Biol. 2004;5:R44. doi: 10.1186/gb-2004-5-7-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Tamayo AT, Li C, Bueso-Ramos C, Ford RJ. An epigenetic chromatin remodeling role for NFATc1 in transcriptional regulation of growth and survival genes in diffuse large B-cell lymphomas. Blood. 2010;116:3899–906. doi: 10.1182/blood-2009-12-257378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–68. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Probst AV, Almouzni G. Heterochromatin establishment in the context of genome-wide epigenetic reprogramming. Trends Genet. 2011;27:177–85. doi: 10.1016/j.tig.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- Razin SV, Gavrilov AA, Pichugin A, Lipinski M, Iarovaia OV, Vassetzky YS. Transcription factories in the context of the nuclear and genome organization. Nucleic Acids Res. 2011;39:9085–92. doi: 10.1093/nar/gkr683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–32. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Rishi V, Bhattacharya P, Chatterjee R, Rozenberg J, Zhao J, Glass K, et al. CpG methylation of half-CRE sequences creates C/EBPalpha binding sites that activate some tissue-specific genes. Proc Natl Acad Sci U S A. 2010;107:20311–6. doi: 10.1073/pnas.1008688107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Weir L, Romanowska M, Leigh IM, Panteleyev AA. ARNT controls the expression of epidermal differentiation genes through HDAC- and EGFR-dependent pathways. J Cell Sci. 2012 Apr 14; doi: 10.1242/jcs.095125. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Roix JJ, McQueen PG, Munson PJ, Parada LA, Misteli T. Spatial proximity of translocation-prone gene loci in human lymphomas. Nat Genet. 2003;34:287–91. doi: 10.1038/ng1177. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Bau D, Marti-Renom MA, Dekker J. Chromatin globules: a common motif of higher order chromosome structure? Curr Opin Cell Biol. 2011;23:325–31. doi: 10.1016/j.ceb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N, Dicker A, Popa C, Jones S, Dahler A. Histone deacetylase inhibitors as potential anti-skin cancer agents. Cancer Res. 1999;59:399–404. [PubMed] [Google Scholar]
- Schoenfelder S, Clay I, Fraser P. The transcriptional interactome: gene expression in 3D. Curr Opin Genet Dev. 2010a;20:127–33. doi: 10.1016/j.gde.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Schoenfelder S, Sexton T, Chakalova L, Cope NF, Horton A, Andrews S, et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet. 2010b;42:53–61. doi: 10.1038/ng.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–8. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitan VC, Hao B, Tachibana-Konwalski K, Lavagnolli T, Mira-Bontenbal H, Brown KE, et al. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature. 2011;476:467–71. doi: 10.1038/nature10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–7. doi: 10.1038/nature08683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–70. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Christiano AM. Biology and genetics of hair. Annu Rev Genomics Hum Genet. 2010;11:109–32. doi: 10.1146/annurev-genom-021610-131501. [DOI] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem. 2011;80:473–99. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- Szczerbal I, Bridger JM. Association of adipogenic genes with SC-35 domains during porcine adipogenesis. Chromosome Res. 2010;18:887–95. doi: 10.1007/s10577-010-9176-1. [DOI] [PubMed] [Google Scholar]
- Talbert PB, Henikoff S. Histone variants--ancient wrap artists of the epigenome. Nat Rev Mol Cell Biol. 2010;11:264–75. doi: 10.1038/nrm2861. [DOI] [PubMed] [Google Scholar]
- Tamai K, Kaneda Y, Uitto J. Molecular therapies for heritable blistering diseases. Trends Mol Med. 2009;15:285–92. doi: 10.1016/j.molmed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Truong AB, Khavari PA. Control of keratinocyte proliferation and differentiation by p63. Cell Cycle. 2007;6:295–9. doi: 10.4161/cc.6.3.3753. [DOI] [PubMed] [Google Scholar]
- Vanbokhoven H, Melino G, Candi E, Declercq W. p63, a story of mice and men. J Invest Dermatol. 2011;131:1196–207. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- Vigano MA, Mantovani R. Hitting the numbers: the emerging network of p63 targets. Cell Cycle. 2007;6:233–9. doi: 10.4161/cc.6.3.3802. Epub 2007 Feb 3. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–8. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schones DE, Zhao K. Characterization of human epigenomes. Curr Opin Genet Dev. 2009;19:127–34. doi: 10.1016/j.gde.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Christensen J, Helin K. DNA methylation: TET proteins-guardians of CpG islands? EMBO Rep. 2011;13:28–35. doi: 10.1038/embor.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RR, Broad S, Sheer D, Ragoussis J. Subchromosomal positioning of the epidermal differentiation complex (EDC) in keratinocyte and lymphoblast interphase nuclei. Exp Cell Res. 2002;272:163–75. doi: 10.1006/excr.2001.5400. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhang Y. Tet1 and 5-hydroxymethylation: A genome-wide view in mouse embryonic stem cells. Cell Cycle. 2011;10:2428–36. doi: 10.4161/cc.10.15.16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Pazin MJ. BRG1-mediated chromatin remodeling regulates differentiation and gene expression of T helper cells. Mol Cell Biol. 2008;28:7274–85. doi: 10.1128/MCB.00835-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Luo W, Elzi DJ, Grandori C, Galloway DA. NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes. Mol Cell Biol. 2008;28:4819–28. doi: 10.1128/MCB.01969-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Lin C, Liu W, Zhang J, Ohgi KA, Grinstein JD, et al. ncRNA- and Pc2 Methylation-Dependent Gene Relocation between Nuclear Structures Mediates Gene Activation Programs. Cell. 2011;147:773–88. doi: 10.1016/j.cell.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Fuchs E. MicroRNAs and their roles in mammalian stem cells. J Cell Sci. 2011;124:1775–83. doi: 10.1242/jcs.069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhu P, Wang J, Pascual G, Ohgi KA, Lozach J, et al. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol Cell. 2008;29:69–80. doi: 10.1016/j.molcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J, Caiafa P. CTCF and its protein partners: divide and rule? J Cell Sci. 2009;122:1275–84. doi: 10.1242/jcs.039990. [DOI] [PubMed] [Google Scholar]