Abstract
With the phase-out of polybrominated diphenyl ether (PBDE) flame retardants, the use of new and alternate flame retardants has been increasing. 2,2-bis(chloromethyl)propane-1,3-diyltetrakis(2-chloroethyl) bisphosphate, known as V6, is a flame retardant applied to polyurethane foam commonly found in furniture and automobile foam. However, to the authors’ knowledge, no research has been conducted on V6 levels in the environment. The intention of this study was to measure the concentration of V6 in foam collected from baby products where it was recently detected, and measure levels in dust samples collected from homes and automobiles in the Boston, MA area. To accomplish this a pure V6 commercial standard was purchased from a Chinese manufacturer and purified (> 98%). An analytical method to measure V6 in dust samples using liquid chromatography tandem mass spectrometry (LC/MS-MS) was developed. Extraction was conducted using Accelerated Solvent Extraction (ASE) and extracts were purified using an ENVI-Florisil SPE column (500 mg, 3mL). V6 was measured in foam samples collected from baby products with a concentration ranging from 24,500,000 to 59,500,000 ng/g of foam (n = 12, average ± sd: 46,500,000 ± 12,000,000 ng/g; i.e., on average, 4.6 % of the foam mass was V6). V6 was also detected in 19 of 20 car dust samples and 14 of 20 house dust samples analyzed. The concentration of V6 in the house dust ranged from < 5 ng/g to 1,110 ng/g with a median of 12.5 ng/g, and < 5 ng/g to 6,160 ng/g in the car dust with a median of 103.0 ng/g. Concentrations in car dust were significantly higher than the house dust, potentially indicating higher use of V6 in automobiles compared to products found in the home. Furthermore, tris (2-chloroethyl) phosphate (TCEP), a known carcinogen, was found in the V6 commercial mixture (14% by weight) as an impurity and was consistently detected with V6 in the foam samples analyzed. A significant correlation was also observed between V6 and TCEP in the dust samples, suggesting that the use of V6 is a significant source of TCEP in the indoor environment.
INTRODUCTION
Over the past decade there have been increasing concerns about exposure to brominated flame retardants (BFRs), which are largely used in plastics, furniture, electronic products, etc. Many studies have reported the ubiquitous presence and persistence of these compounds in the environment and their bioaccumulation in human tissues1,2. More recent toxicology studies have suggested that exposure to these chemicals could be linked to disruption of thyroid hormone regulation and neurodevelopment 3,4. Long-term epidemiological studies have also observed negative associations between polybrominated diphenyl ether (PBDE) exposures at birth and neurodevelopment in children5,6. Two commercial PBDE mixtures, PentaBDE and OctaBDE, were voluntarily phased out and the last commercial mixture, DecaBDE, will undergo a voluntary phase out by manufacturers in the United States by 20137.
Since the phase-out of PBDE commercial mixtures, additional types of flame retardants (FRs) have been increasingly used as replacements. Potential replacements include decabromodiphenyl ethane (DBDPE), tetrabromobisphenol-A (TBBPA), bis(2,4,6,-tribromphenoxy)ethane (BTBPE), and several phosphate based compounds8. In 2005, the Environmental Protection Agency (EPA) evaluated flame retardant alternatives for low-density polyurethane foam (PUF), in which PentaBDEs was previously widely used9. The alternatives included Firemaster® 550/552 (which includes bis(2-ethylhexyl)tetrabromophthalate (TBPH) and ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB)), and some additional organophosphate flame retardants (PFRs) such as triphenyl phosphate (TPP), chloroalkyl phosphates (such as tris(1,3-dichloro-2-propyl) phosphate (TDCPP)), or alkylated triaryl phosphates (e.g. non chlorinated PFRs). However, it is likely that this report does not represent all the FRs currently in use today.
In our recent study investigating the use of FRs in polyurethane foam (PUF) used in baby products, a new chlorinated organophosphate 2,2-bis(chloromethyl)propane-1,3-diyltetrakis(2-chloroethyl) bisphosphate (V6) was identified in 12 of 101 samples10. Though it has been in use since 1990s11, there is little information known about the environmental levels of V6. According to an EU risk assessment report, V6 is primarily used in flexible PUF and is particularly suited to expensive and durable articles, e.g. automotive and furniture applications due to its high price and low mobility in the foam11. The report also suggests that 50 – 75% of the total V6 demand is used in PUF for automotive applications and 25 – 50% is used in furniture. The total production of V6 was less than 5,000 tonnes in 2000 in the EU, but its global production was increasing by approximately 10% per annum11. The production of V6 in the USA was about 454 – 4500 tonnes in 199812. V6 is also widely available in Chinese flame retardant commercial markets, which was confirmed by a product search on several Chinese online trade platforms such as Alibaba, though no specific production information is available. One limited report describes the possible detection of V6 in air, effluent, sediment, and biological samples analyzed by a gas chromatograph using a nitrogen phosphorus detector13. However, in terms of its toxicity, very limited information is available. In the EU risk assessment report on V6, no acute toxicity to fish, algae and other invertebrates has been observed11. However, V6 was reported to significantly decrease serum cholinesterase activity in rats of both sexes following oral administration; the largest decrease of cholinesterase activity observed at 500 mg/kg body weight was 59% for males and 91% for females after 8 hours, suggesting possible neurotoxicity similar to some organophosphate pesticides11. Interestingly, a case study reported on the death of pet dogs locked in cars overnight, and suggested that one cause of death may be due to organophosphate flame retardants present in the foam of the car that the dogs were chewing14. In that study, they detected tris (2-chloroethyl) phosphate (TCEP) as well as an unknown bis-phosphate chemical in the dog’s stomach contents. The structure of V6 is similar to a “dimer” of TCEP (Figure 1), which has been reported to be a carcinogenic compound15. TCEP is a known impurity (a byproduct of the synthesis) in the commercial V6 mixture with a percentage of 4.5 – 7.5% according to the EU risk assessment report11. Given this impurity, the use of V6 commercial mixtures in consumer products may be an important source of TCEP, especially after the phase-out of TCEP in the 1980s15. Therefore, further investigation of V6 and TCEP levels in products and environment compartments is warranted.
FIGURE 1.
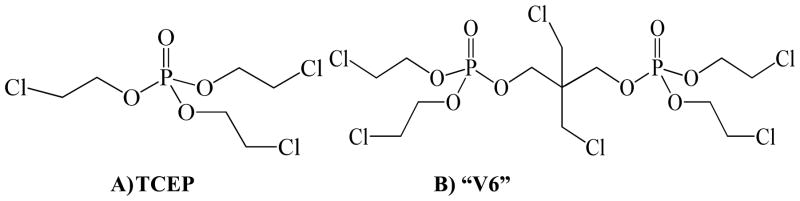
Structures of (A) TECP and (B) “V6”
House dust has been widely used as an important environment matrix to monitor the contamination level of various FRs, and inadvertent dust ingestion is an important exposure pathway into humans1,16,17,18. Considering that the primary use of V6 is believed to be in automobile foams, it may likely be present in car dust. In this study, we therefore investigated levels of V6 in both car dust and house dust samples for comparison. To the authors’ best knowledge, there is no commercially available pure V6 standard and no analytical methods for V6 have been developed for dust samples. Therefore, the goals of this study were: 1) to prepare and purify a V6 commercial standard; 2) develop a method to analyze V6 in dust samples; 3) measure V6 levels in foam from baby products previously identified as having V6; 4) measure V6 in house and car dust samples to provide more information on its detection and abundance in indoor environments; and 5) examine the relationship between TCEP and V6 in foam products and indoor dust to determine the importance of V6 as a source for TCEP.
MATERIALS AND METHODS
Materials
The V6 commercial mixture was purchased from a flame retardant manufacturer in China (Hongming Auxiliaries CO., LTD, Jiande, Zhejiang Province, China). Deuterium labeled d15-TDCPP was synthesized by Dr. Vladimir Belov (Max Planck Institute for Biophysical Chemistry, Goettingen, Germany) and was used as an internal quantification standard. TCEP was purchased from Sigma Aldrich (St. Louis, MI). All solvents used throughout this study were HPLC grade.
Sample Collection
Information of the dust collection is described in previously published studies19,20. Briefly, dust samples were collected using a Eureka Mighty-Mite vacuum cleaner (model 3670) and crevice tool attachment. House dust samples were obtained in the bedroom from 20 Boston (MA) area houses during 2009. For car dust, 20 samples were collected from the surfaces of the front and back seats of participants’ cars. The information on the car manufacturer, production year, and sampling date were recorded. The detailed sampling information of car dust samples are shown in Table S1 in supplementary information (SI). Field blanks for the house dust samples were prepared using sodium sulfate powder as a surrogate for dust and collected using the Mighty-Mite vacuum. The foam samples collected from baby products and analyzed here were from our previous study10.
Sample Extraction
The dust samples (~ 0.1 g) were extracted using either hexane or dichloromethane (DCM) (described in results section) by accelerated solvent extraction (ASE, Dionex) at a temperature of 100 °C and pressure of 1500 psi. The final extract was evaporated to approximately 1 mL using an automated nitrogen evaporation system (Turbo Vap II, Zymark Inc.). After the cleanup (described in results section), extracts was again reduced to 0.5 mL and spiked with d15-TDCPP as the internal standard due to their similar retention times and physicochemical properties. Unfortunately, a labeled standard (or another similarly structured compound) was not available for use in this study, which may contribute to uncertainties in the measurements of V6 reported here. Measurement of TCEP in dust samples was conducted in 0.1 grams of dust using a method reported in Van den Eede et al. 201221 and using d15-TDCPP as an internal standard. The extraction of foam samples was conducted using the same extraction method reported in our previous studies10,22,23. No further cleanup steps were performed for the foam sample extracts except filtration through 0.2 μm PTFE filters before analysis.
Sample Analysis
V6 and its internal standard d15-TDCPP were analyzed by liquid chromatography tandem mass spectrometry (LC/MS-MS) (Agilent 6410 Triple Quad LCMS) in positive atmospheric pressure chemical ionization (APCI) ionization mode using multiple reaction monitoring (MRM). Ion transition m/z 582.9 to 360.8 was used for quantification of V6 and m/z 582.9 to 296.8 and m/z 582.9 to 98.9 were qualifier ions. Ion transition m/z 446.0 to 102.0 was monitored to qualify and quantify d15-TDCPP. In the ion source, gas (N2) temperature was set to 350 °C, vaporizer temperature was set to 250 °C, the gas flow rate was 10 mL/min, the nebulizer pressure was 345 kPa (50 psi), the capillary voltage was +2,500 V and the corona charge was 4 μA. A ZORBAX Eclipse XDB C18 column (4.6 × 50 mm, 1.8 μm) was used for separation with a flow rate of 0.4 mL/min. Gradient elution was applied as follows: 0–6 min: start with 60% methanol and increase to 100%; maintain at 100% methanol until 12min; 12–15min: decrease from 100% to 60% methanol and 5 mins was used as a post run equilibrium time.
Quality Assurance
Sodium sulfate powder and clean PUF were used as laboratory blanks for dust samples, and foam samples, respectively. As part of our quality assurance criteria, levels of V6 were analyzed in field (for house dust) and laboratory blanks (n = 3), duplicate dust samples (n=3), and house dust Standard Reference Material (SRM) 2585 (National Institute of Standards and Technology (NIST), Gaithersburg, MD). Matrix spikes were prepared by spiking different amounts of V6 into SRM 2585 (n = 12) and into sodium sulfate powder (n = 3). V6 was not detected in either field or laboratory blanks. Therefore, the method detection limit (MDL) was first estimated by measuring the instrumental detection limit (IDL), which was calculated by using a signal to noise (S/N) ratio of 3, and an average dust mass of 0.1 g. Then the MDL in dust was confirmed by spiking 0.5 ng V6 into three dust samples (0.1g) that did not originally contain V6 and measuring recovery, which was 70 ± 10% (Table 1). The calculated MDL for V6 in this study was therefore 5.0 ng/g of dust. In a similar way, the calculated MDL for V6 in foam samples was 5,000 ng/g of foam, and is much higher due to the significant dilution (100 fold) of the extract to allow for measurements within our calibration curve.
Table 1.
Measured Recovery of V6 in Dust Matrix Spikes
| Percent recovered | |
|---|---|
| Very Lowa (close to MDL) | 70 ± 10 (n = 3) |
| Lowb | 89 ± 7 (n = 3) |
| Mediumc | 82 ± 3 (n = 6) |
| Highd | 72 ± 12 (n = 3) |
Low (close to MDL): 0.5 ng spikes into three house dust samples that did not contain V6;
Low: 100 ng spikes into SRM 2585;
Medium: 200 ng and 600 ng spikes into SRM 2585;
High: 1000 ng spikes into SRM 2585.
Statistical Analysis
SPSS 12.0.1 was used for the statistical analyses. V6 and TCEP levels in the dust and foam samples were tested for normality and log-normality using the Shapiro–Wilk test. As neither condition held for TCEP and V6 in the dust, we used non-parametric statistics in our analyses (the Mann-Whitney test for comparison of means between samples and a Spearman correlation analysis). Dust concentrations of V6 and TCEP below the MDLs were replaced by MDL/2 for correlation analyses.
RESULTS AND DISCUSSION
Purchase and purification of a “V6” commercial product
After purchasing the V6 commercial mixture, a solution was prepared and analyzed by full scan gas chromatography-mass spectrometry operated in electron ionization mode (GC/EI-MS) and using full scan LC/MS operating in electron spray ionization (ESI), to check the purity. Analysis revealed several significant impurities in the mixture. TCEP and other unknown impurities were observed in the product similar to what was reported in the EU risk assessment11. TCEP was quantified in the V6 commercial mixture by GC/EI-MS with a percentage of 13.5 ± 0.4 % (average ± sd, n = 5) by weight in the original mixture, which was higher than the reported 4.5 – 7.5% in the EU risk assessment report11.
Purification of the V6 mixture was performed using silica gel flash column chromatography and confirmed with thin layer chromatography (TLC). Gradient elution starting from 100% DCM to 50% ethyl acetate in DCM (v/v) gave V6 as a translucent, viscous oil (~ 1.1 g). Analysis of the purified material by TLC using DCM:ethylacetate (1:1, v/v) as a mobile phase indicated the desired product (Rf = 0.23, visualized with KMnO4 stain) had been successfully separated from the major impurity TCEP (Rf = 0.38). The purity of V6 was further checked by full scan in LC/MS-MS operating in both APCI and ESI. As shown in Figure S1, all the significant peaks other than V6 in the original mixtures were removed and there was no difference between the purified standard and the methanol blank except the single peak of pure V6. The purity of V6 was also reconfirmed by full scan GC/EI-MS. In the end, ~1.1 g V6 was purified to greater than 98% and quantification of V6 in foam and dust was completed using the purified standard.
Method Development for V6 in dust by LC/MS-MS
Several different types of solvents were tested for extraction efficiency of SRM 2585. DCM showed the highest recovery of V6 (> 95%). Hexane, and mixtures of hexane:DCM (1:1, v/v) could not completely extract V6 from the dust samples (< 70%, n = 3). Therefore, 100% DCM was used as the extraction solvent throughout this study.
Since an ENVI-Florisil SPE column (500 mg, 3 mL) is commonly used by our laboratory and others21 during the analysis of flame retardants in dust (e.g. PBDEs, HBCDs, and several PFRs, including TCEP, and TDCPP), we optimized a clean-up method using this SPE as shown in Figure S2. In brief, the SPE column was first conditioned with 5 mL methanol and rinsed with 3 mL hexane. Then the dust extract (in hexane) was loaded on the SPE using 0.5 mL hexane and 4 mL hexane was used to elute hydrophobic FRs (e.g. PBDEs) in fraction one (F1). Subsequently, most PFRs were eluted in fraction two (F2) using 10mL ethyl acetate. In our preliminary experiments, we found that <10% of “V6” (n = 3) could be collected in F1 and F2. Therefore, more polar solvents were tested in an F3 fraction, including acetone and methanol, which were found to elute V6 from the column. However, we found that hexane was not efficient in completely transferring all the V6 in the dust extract to the SPE column (i.e. some V6 remained in the test tube). Therefore, the test tube that originally contained the dust extract was rinsed with 0.5 mL methanol and reloaded onto the SPE (after F1 and F2 were collected) and then a final F3 elution with methanol was collected. The solvent volume for fraction three was optimized using 3 mL, 6 mL and 10 mL methanol. It was found that 6 mL methanol could elute most of the V6, but 10 mL was used in this study to guarantee complete elution of V6.
V6 and its internal standard, d15-TDCPP, were then analyzed by LC/MS-MS. V6 could be ionized using either APCI or ESI but the latter provided better ionization efficiency. However, significant ion suppression of V6 and its internal standard d15-TDCPP were observed in ESI mode, as shown in Figure S3. In contrast, no significant matrix effect was observed in the APCI mode. Therefore, APCI was used for the quantification of V6. Furthermore, the data using APCI were consistent with those obtained by the standard addition method (Table S2).
Matrix Spike Tests
The recovery of V6 in dust samples was assessed by spiking different levels of the pure V6 standard onto a homogenous dust sample (SRM 2585). In this experiment, 0.5ng (very low, near detection limits), 100 ng (low), 200 ng (medium), 600 ng (medium), and 1000 ng (high) of V6 were spiked onto 0.1 g of SRM 2585 (in triplicate). The measured recovery is shown in Table 1. A non-spiked SRM 2585 were run alongside to correct for any background level of V6 in the SRM. The average recovery in spiked dust samples was 70, 89, 82 and 72% for the very low, low, medium and high spiking samples, respectively. The lower recovery in the high spike may be due to breakthrough in the ENVI-Florisil SPE column during collection of the F1 and F2 fractions. Step-wise recovery tests were also investigated in the evaporation/concentration steps but losses were negligible.
SRM measurements of V6 (intra and inter day variability)
As part of our quality control, the native level of V6 in SRM 2585 was measured. The concentration of V6 in SRM 2585 was determined to be 117.0 ± 5.5 ng/g (mean ± s.d.) and small intra and inter day variability was observed with relative standard deviations of 6.8 and 3.7%, respectively. It was interesting to note that V6 was detected in SRM 2585, which was collected in the mid- to late 1990s, indicating that V6 has probably been used since the 1990s.
Measurement of V6 in Polyurethane Foam from Baby products
In our previous study of FRs in baby products, V6 was identified (but not quantified due to lack of a standard) in 12 of 101 baby products. Most detects were in nursing pillows but it was also detected in one sleep positioner, one portable mattress and one baby carrier10. Furthermore, it was also observed in one foam sample collected from residential furniture22. Samples of baby foam previously suspected of containing V6 were extracted and analyzed in this study and its presence was confirmed. As shown in Table 2, the concentration of V6 in baby products ranged from 24,500,000 to 59,500,000 ng/g of foam, with an average concentration of 46,500,000 ng/g (i.e., 4.6 %). A similar level of V6 was detected in the one sample of couch foam from our previous study (out of 102 samples) with a concentration of 36,300,000 ng/g22. These values were comparable with previous reports indicating that V6 was applied at rates of 5.3% in automotive foams11.
Table 2.
Summary Statistics for V6 measurements in Baby products (n = 101), House Dust (n = 20) and Car Dust Samples (n = 20)
| Baby Products (106 ng/g) | House dust (ng/g) | Car dust (ng/g) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| V6 | TCEP | V6 | TCEP | V6 | TCEP | |
|
| ||||||
| % detect | NA | NA | 70% | 48% | 95% | 95% |
| Concentration | 46.5 ±12.0a | 3.6 ± 1.6a | 12.5c | 50.2c | 103.0c | 1080.0c |
| Range | 24.5 – 59.4b | 1.1 – 5.9b | < 5 – 1,110 | < 20 – 1350 | < 5 – 6,160 | < 20 – 50,120 |
Average was calculated based on 12 baby products in which V6 was detected (Average ± SD).
Range is only from the 12 baby products in which V6 was measured.
Median.
NA indicates not applicable.
Measurement of V6 and TCEP in car and house dust samples
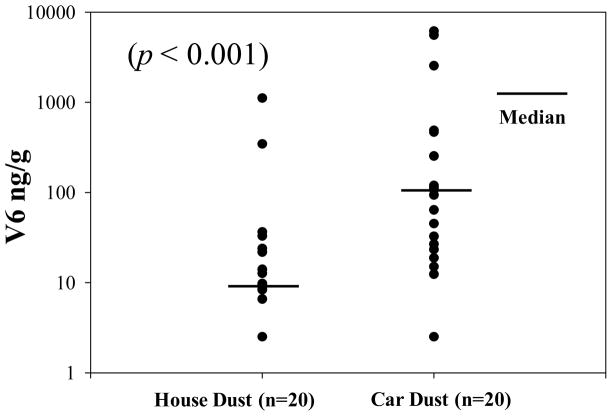
Since the primary use of V6 was suggested to be in automobiles, we expected to find higher level of V6 in car dust than in house dust. As shown in Table 2, V6 was detected in 70% and 95% of the house dust and car dust, respectively; indicating V6 is common in dust. As shown in Figure 2, the concentration of V6 in the house dust samples ranged from < 5 to 1,110 ng/g with a median of 12.5 ng/g. The concentration of V6 in the car dust ranged from < 5 to 6,160 ng/g with a median of 103.0 ng/g. Since TCEP was detected in the V6 commercial mixture examined here, and in the foam samples, we measured TCEP in the dust samples investigated in this study. The median concentrations of TCEP were 50.2 and 1,080 ng/g for house dusts and car dusts, respectively (Table 2). Levels of V6 and TCEP both in house dust and car dust samples were not normally distributed. After logarithmic transformation, V6 showed a normal distribution in the car dust samples, but not in the house dust samples. The levels of V6 and TCEP (evaluated individually) in the car dust samples were significantly higher than levels measured in the house dust samples (p < 0.001, Mann-Whitney test), suggesting a greater use of V6 in automobiles. Concentrations in cars may also be higher in part due to differences in how dust samples were collected, directly from the seats in the cars vs floors in homes. In term of car production year, V6 was detected in five dust samples from cars that were made in 1990s, further suggesting long historical use of V6 in car manufacturing.
FIGURE 2.
Scatter plot representing the distribution of V6 in house dust (n = 20) and car dust samples (n = 20).
Correlation between V6 and TCEP in baby products and dust samples
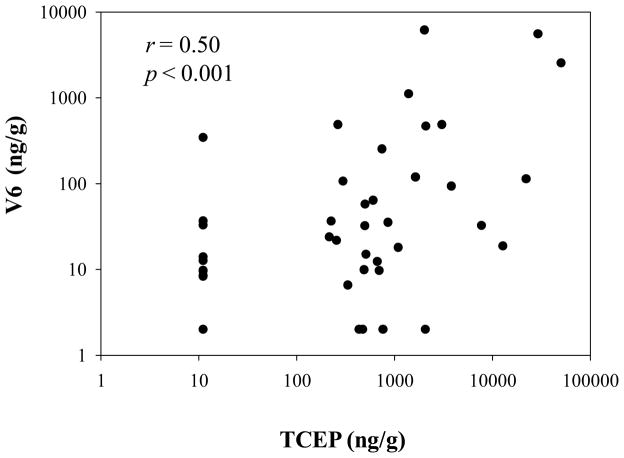
As we found in our earlier study, V6 was always detected in foam samples in combination with TCEP. The relative contribution of TCEP in the foam varied from about 2–18% of the sum flame retardant burden (i.e. TCEP + V6), suggesting that TCEP is likely a common impurity in the V6 formulation, but the impurity likely varies among different commercial formulations. To determine if V6 may be a source of TCEP to indoor environments, we conducted a correlation analysis between V6 and TCEP in the dust samples analyzed here. As shown in Figure 3, the concentrations of V6 and TCEP in dust samples (n = 40) were significantly correlated (r = 0.50, p < 0.001). A spearman test excluding the samples (n = 13) in which the levels of TCEP and V6 were below the MDL was additionally conducted and the result was also significant (r = 0.50, p = 0.015). This result suggests that V6 is likely an important source of TCEP in the environment. TCEP has a higher vapor pressure than V6, which may result in greater migration from treated products over time and higher concentrations in indoor dust relative to V611. This may be a concern due to the reported carcinogenic properties of TCEP.
FIGURE 3.
Correlation between the V6 and TCEP concentrations in dust samples (n = 40).
Comparison of V6 levels in house dust and car dust with other alternate FRs
To evaluate the abundance of V6 in the environment relative to other FRs commonly used in polyurethane foam, we compared our measurements here in dust with other reported FRs measurements in US dust samples. As shown in Table 3, the level of V6 in house dust was much lower than most BFRs and PFRs previously measured23–27. Unfortunately, there are very few measurements of FRs in car dust samples in the US for comparison. Here, the level of V6 in car dust was several orders of magnitude lower than measured levels of PentaBDE reported in other studies. There are several possible reasons for the low level of V6 in car dust comparing with other FRs. For one thing, V6 may be used in combination with other flame retardants in automobile foam. V6 was known to be primarily used in car seats made of low-density PUF; while other FRs, e.g. TDCPP, may be used both as a flame retardant and plasticizer. In addition, the mobility of V6 in the foam may be much less than that of the other FRs given its chemical structure and high molecular weight11. Further studies investigating the FR composition in car foam samples should be conducted.
Table 3.
Comparison of V6 Levels in House Dust and Car Dust with Other FRs (commonly used in polyurethane foam) Detected in Other Studies in the US
| House dust (ng/g) | Car dust (ng/g) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Range | GM | Sampling Year | Range | GM | Sampling Year | |
| TPP | < 150 – 1,798,00023 | 7360 | 2006 | NAa | NA | NA |
| TCEP | 330 – 110,00025 | 2700b | 2011 | < 20 – 50,120 (This study) | 1,466 | 2009 |
| TCPP | < 140 – 5,49023 | 572 | 2006 | NA | NA | NA |
| TDCPP | < 90 – 56,09023 | 1890 | 2006 | < 30 – 326,00026 | 12,500 | 2009 |
|
| ||||||
| PentaBDEc | 141 – 61,30027 | 2170 | 2009 | 151– 137,35024 | 1,779 | NA |
| 110 – 44,30027 | 2,610 | 2009 | ||||
| TBB | < 450 – 75,00023 | 840 | 2006 | NA | NA | NA |
| TBPH | < 300 – 47,11023 | 650 | 2006 | NA | NA | NA |
| “V6” | < 5 – 1,117 (This study) | 12b | 2009 | < 5 – 6,160 (This study) | 103 | 2009 |
NA indicates not available.
Median was used.
PentaBDE is Sum of BDE 28/33, 47, 99, 100, and 153.
< LOQ indicates below limit of quantification.
In conclusion, V6 was detected and measured in foam collected from baby products, house dust, and car dust samples. The ubiquitous distribution of V6 in dust samples indicates V6 may also be used as a replacement for PentaBDE in PUF, especially in automobiles. Furthermore, the presence of TCEP, a known carcinogen, in the V6 commercial mixture may be contributing to exposure in indoor environments as these flame retardants migrate out of foam and contaminate indoor dust. There may also be additional concern regarding the presence of TCEP in baby products treated with V6. Because TCEP has a higher vapor pressure than most PFRs, PBDEs and particularly V6, additional studies may wish to assess infant’s exposure to TCEP during use of products treated with V6. For example, this may be of special concern for infants sleeping on sleep positioners that may be treated with V6. Infants spend long periods of time in close proximity to the surfaces of the foam that may be off-gassing TCEP, and air concentrations of TCEP may be higher at the surface of the foam than in bulk air within the room if off gassing is occurring. Considering the very limited data on V6, and potential increased use due to the phase out of PBDEs, further studies on this compound should be performed.
Supplementary Material
Acknowledgments
The authors thank the study participants and the following BUSPH research assistants and students: Jennifer Ames, Kim Burke, Erin Collins, Ashley Miller, and Steve Nicholson. We also acknowledge Research funding provided by National Institute of Environmental Health Sciences (Grant numbers R01 ES016099, R01 ES015829 and T32 ES014562). Partial support was also provided by a private donation from Fred and Alice Stanback.
Footnotes
Supporting Information Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 2.Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T, Takasuga T, Senthilkumar K, Yamashita F, Koizumi A. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. Environ Health Perspect. 2006;114(8):1179–85. doi: 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noyes PD, Hinton DE, Stapleton HM. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol Sci. 2011;122(2):265–74. doi: 10.1093/toxsci/kfr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci. 2011;124(2):339–47. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber T, Gassmann K, Gotz C, Hubenthal U, Moors M, Krause G, Merk HF, Nguyen NH, Scanlan TS, Abel J, Rose CR, Fritsche E. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118(4):572–8. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EPA. DecaBDE phaseout initiative. 2009 http://www.epa.gov/opptintr/existingchemicals/pubs/actionplans/deccadbe.html.
- 8.Covaci A, Harrad S, Abdallah MA, Ali N, Law RJ, Herzke D, de Wit CA. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int. 2011;37(2):532–56. doi: 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.EPA. Furniture Flame Retardancy Partnership: Environmental Profiles of Chemical Flame Retardant Alternatives; EPA 742-R-05-002A. U.S. EPA; Washington, DC: 2005. [Google Scholar]
- 10.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Union. Risk Assessment Report of 2,2-bis(chloromethyl)trimethylene bis[bis(2-chloroethyl)phosphate] (V6) 2007. CAS No: 38051-10-4. [Google Scholar]
- 12.Van der Veen I, De Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 13.Green N, Schlabach M, Bakke T, Brevik EM, Dye C, Herzke D, Huber S, Plosz B, Remberger M, Schøyen M, Uggerud HT, Vogelsang C. NIVA Report 5569-2008, SPFO-Report 1014/2008 TA-2367/2008. 2008. Screening of Selected Metals and New Organic Contaminants 2007. [Google Scholar]
- 14.Lehner AF, Samsing F, Rumbeiha WK. Organophosphate ester flame retardant-induced acute intoxications in dogs. J Med Toxicol. 2010;6(4):448–58. doi: 10.1007/s13181-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Union. Risk Assessment Report of Tri (2-Chloroethyl) Phosphate. 2008. CAS No: 115-96-8 EINECS No: 204-118-5. [Google Scholar]
- 16.Harrad S, Hazrati S, Ibarra C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environ Sci Technol. 2006;40(15):4633–8. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ Sci Technol. 2010;44(14):5627–32. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120(7):1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ Int. 2008;34(8):1085–91. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 2008;42(18):6910–6. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 21.Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta. 2012;89:292–300. doi: 10.1016/j.talanta.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol Environ Sci Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagalante AF, Shedden CS, Greenbacker PW. Levels of polybrominated diphenyl ethers (PBDEs) in dust from personal automobiles in conjunction with studies on the photochemical degradation of decabromodiphenyl ether (BDE-209) Environ Int. 2011;37(5):899–906. doi: 10.1016/j.envint.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46(24):13056–66. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carignan CCM, MD, Cooper EM, Watkins DJ, Fraser AJ, Bernays WH, Stapleton HM, Webster TF. Predictors of Tris(1,3-dichloro-2-propyl) phosphate Metabolite in the Urine of Office Workers. Environ Int. 2012 doi: 10.1016/j.envint.2013.02.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjödin A, Webster TF. Impact of dust from multiple microenvironments and diet on PentaBDE body burden. Environ Sci Technol. 2012;46(2):1192–200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.