Abstract
‘Internet addiction disorder’ (IAD) is rapidly becoming a prevalent mental health concern in many countries around the world. The neurobiological underpinning of internet addiction should be studied to unravel the potential heterogeneity. The present study examines the neural correlates of response inhibition in males with and without IAD using an event-related functional magnetic resonance imaging (fMRI) Stroop task. The IAD group demonstrated greater ‘Stroop effect’-related activity in the anterior and posterior cingulate cortices (pFDR<0.05) compared to their healthy peers. These results may suggest diminished efficiency of response inhibition processes in the IAD group relative to healthy controls.
Keywords: Internet addiction disorder, inhibitory control, Stroop task, impulsivity, fMRI
1. Introduction
Maladaptive internet use, henceforth referred to as ‘internet addiction disorder’ (IAD), is marked by an inability to control one’ s internet use, which eventually causes psychological, social and/or work difficulties (Young, 1998). Despite significant prevalence estimates for IAD and associations with adverse consequences (Dong et al., 2011a; Dong et al., 2011b; Kim et al., 2010; Niemz et al., 2005; Young, 1998), the neurobiological underpinnings of IAD have received relatively little study (Block, 2006; Liu and Potenza, 2007). For instance, high IAD incidence rates have been reported in China (10.6% college students (Wu and Zhu, 2004)), Taiwan (5.9% college students (Chou and Hsiao, 2000)), while 9.8% of German adults report at least one negative consequence of Internet use (Beutel et al., 2011) and South Korea has identified IA as a serious public health issue (Block, 2008).
The nosology and optimal diagnostic criteria for IAD remain controversial. IAD has been proposed to merit inclusion in DSM -V (Block, 2008), yet whether it is most accurately described as a behavioral addiction, impulse control disorder (ICD) or manifestation of other underlying psychiatric disorders continues to be a topic of debate (Young and Rogers, 1998; Beard and Wolf, 2001; Holden, 2001; Beard, 2005). Young’ s (1998) original proposed diagnostic criteria for IAD were modeled on the criteria for pathological gambling (PG) in DSM-IV (Diagnostic and Statistical Manual of Mental Disorders), wherein PG is classified as an Impulse Control Disorder (ICD), although it is currently under consideration for reclassification as a ‘behavioral addiction’ in DSM-V. IAD shares characteristics with impulse control disorders (e.g., intense preoccupation Internet use, lack of control over the amount of time spent online, compulsive Internet use) as well as behavioral addictions (e.g., development of euphoria, craving, tolerance) (Grant et al., 2010).
Regardless of whether IAD is best conceptualized as a behavioral addiction or an impulse control disorder, IAD has been speculated to be related to impaired inhibitory control. While impulsivity has always been recognized as central to ICDs, it is increasingly acknowledged to impact upon risk for and maintenance of addictive disorders. Pre-existing impulsivity may increase one’ s vulnerability to develop addictive disorders and engagement in addictive behaviors may exacerbate aspects of impulsivity (for review (de Wit, 2009; Dick et al., 2010). IAD individuals demonstrate high impulsivity as measured by a behavioral task of response inhibition (GoStop) and a self-report questionnaire (BIS-11), and both behavioral and self-report impulsivity are positively correlated with severity of IAD (Cao et al., 2007). An event-related potential (ERP) study of response inhibition (Go/No-Go task) from our group found higher amplitude and longer peak latency in IAD participants’ nogo-P3 compared with those of healthy controls, suggesting less efficient inhibitory processes in the IAD group (Dong et al., 2010; Dong et al., 2011c).
The present study used a functional magnetic resonance imaging (fMRI) color-word Stroop paradigm to identify the neural correlates of response inhibition in individuals with and without IAD (Leung et al., 2000; Peterson et al., 2002). The Stroop color-word test is a useful tool for assessing inhibitory control, one aspect of cognitive-control which is recruited to respond to attentional or response interference (Carter and van Veen, 2007; Kerns et al., 2004; MacDonald et al., 2000; MacLeod and MacDonald, 2000). The Stroop task has been widely used in behavioral and neuroimaging studies of psychiatric populations, including substance use disorders and proposed behavioral addictions such as pathological gambling (Keilp et al., 2005; Wright et al., 2003; Alvarez-Moya et al., 2009; Bolla et al., 2004; Brewer et al., 2008; MacLeod, 1991; McCusker and Gettings, 1997; Potenza et al., 2003). The incongruent stimuli in the Stroop paradigm require response inhibition to overcome increased cognitive interference, and IAD is thought to involve impaired inhibitory control. As such, we hypothesized that the IAD group would display fMRI activity consistent with diminished efficiency of inhibitory processes, namely greater ‘Stroop effect’-related activity relative to healthy controls in the anterior cingulate cortex, a structure integral to cognitive control and response to conflict.
2. Methods
2.1. Participant Selection
This research was approved by the Human Investigations Committee of Zhejiang Normal University. All participants provided written informed consent. Participants were right handed, non-smoking males (12 IAD, 12 healthy controls (HC)). IAD and HC groups did not significantly differ in age (IAD mean=23.6, SD=3.5 years; HC mean=24.2, SD=3.1 years; t(22) = 0.92, p >0.05). Only males were included due to higher IAD prevalence in men than women. Participants were recruited through advertisements. All participants underwent structured psychiatric interviews (Lecrubier et al., 1997) performed by an experienced psychiatrist. All participants were free of active substance abuse and Axis I psychiatric disorders. Depression was further assessed with the Beck Depression Inventory (Beck et al., 1961) no participants scoring higher than 13 were included. IAD and HC did not fulfill DSM-IV criteria for abuse or dependence of substances, including alcohol. All participants reported having tried at least a “sip” of alcohol in their lifetime with both groups reporting similar numbers of drinking day in the past 30 days (i.e. at least one unit of alcohol) (IAD mean=3.11, SD=0.52; HC mean=3.37, SD=0.61; t=0.83, p>0.05). No participants reported binge-drinking in the past 30 days (i.e. 5 or more units of alcohol within a couple of hours) and none reported having an alcohol problem. All participants were medication free and were instructed not to use any substances of abuse, including coffee, on the day of scanning. No participants reported previous experience with illicit drugs (e.g., cocaine, marijuana), history of Attention Deficit Hyperactivity Disorder (ADHD) or history of mood disorders (e.g., depression, anxiety). Substance use information was based on questionnaires and interviews; no urine toxicology screens were performed.
IAD was determined based on Young’ s online internet addiction test (IAT) (Young, 2009) scores of 80 or higher. Young’ s IAT consists of 20 items associated with online internet use including psychological dependence, compulsive use, withdrawal, related problems in school or work, sleep, family or time management. For each item, a graded response is selected from 1 = “Rarely” to 5 = “Always”, or “Does not Apply”. Scores over 50 indicate occasional or frequent internet-related problems and scores over 80 indicate significant IAD-related life problems (Young, 2009). Additionally, all participants were assessed with a Chinese “Internet addiction test” (IAT) developed by the Beijing Military Region Central Hospital (Tao et al., 2008; Wang et al., 2009), with its validity in distinguishing IAD demonstrated in more than 1,300 clinical trials (Wang et al., 2009). IAD participants met both Chinese IAT requirements; 1) spend more than six hours online everyday aside from work, and 2) show symptoms such as psychological dependence, abstinent reaction, compulsive use, social withdrawal, or negative effect on body and mental health for more than three months. All IAD participants spent most of their time online playing internet games. HCs all scored lower than 20 on Young’ s IAT (mean=15.4, SD=4.2) and did not satisfy either of the Chinese IAT requirements.
2.2. Task and Procedure
An event-related color-word fMRI Stroop task was administered. Three target color words (e.g. red, green, yellow) were presented randomly in congruent (e.g., the word “RED” in red ink) or incongruent (e.g., the word “RED” in green ink) trials. The task was comprised of 2 sessions of 120 trials each. Each trial was presented for 2000 ms, and participants were asked to press a button to indicate to the ink color of the word as soon as possible using three buttons (i.e., green=thumb, red=index finger, yellow=middle finger) of a five-button response box (Invivo Corp.; http://www.invivocorp.com/). A black screen was presented for a random interval of 600-1400 ms (average1000 ms) between trials. Stimuli were presented and behavioral data were collected using E-prime software (Psychology Software Tools, Inc.).
Participants were told that they would be paid a guaranteed 50 dollars for participation and, to encourage quick and accurate task performance, were told they would be rewarded with an additional 0-50 dollars based on their task performance [1/(reaction time * error rate)]. Unbeknownst to them, all participants were paid the full additional amount regardless of task performance, resulting in a total payment of 100 dollars. Participants completed an out-of-scanner practice session which continued until they reached an accuracy rate of 90% or higher. Participants performing below 90% accuracy during the in-scanner Stroop task were excluded from further analyses.
Behavioral data were analyzed with separate analysis of variance (ANOVAs) for reaction time (RT) for correct incongruent trials and mean error rate in two-by-two mixed ANOVAs, with group as a between-subject factor (IAD, HC) and trial type (incongruent, congruent) as a within-subject variable.
2.3. Image Acquisition and Pre-processing
Functional MRI was performed on a 3T system (Siemens Trio) with a gradient-echo EPI T2 sensitive pulse sequence in 33 slices (interleaved sequence, 3 mm thickness, TR=2000 ms, TE=30, flip angle 90°, field of view 220 × 220 mm2, matrix 64 × 64). Stimuli were presented using Invivo synchronous system (Invivo Company, http://www.invivocorp.com/) through a monitor in the head coil, enabling participants to view the stimuli presented on the screen. Structural images were collected using a T1-weighted three-dimensional spoiled gradient-recalled sequence was acquired covering the whole brain (176 slices, repetition time=1700 ms, echo time TE=3.93 ms, slice thickness=1.0 mm, skip=0 mm, flip angle=15, inversion time 1100ms, field of view=240*240mm, in-plane resolution=256* 256). Imaging analysis was conducted using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were slice-timed, reoriented, and realigned to the first volume. T1-co-registered volumes were then normalized to an SPM T1 template resulting in an isometric voxel size of 3 × 3 × 3 mm3 and spatially smoothed using an 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel.
2.4. First-level fMRI Analysis
A general linear model (GLM) was applied to identify blood oxygen level dependence (BOLD) signal in relation to two event types of interest: congruent and incongruent trials. Error trials and six head-movement regressors derived from the realignment stage were included in the GLM design matrix as covariates of no interest. The GLM was independently applied to each voxel to identify voxels that were significantly activated for the events types of interest. A high pass filter (cut-off period=128 sec) was applied to improve the signal-to-noise ratio by filtering out low frequency noise.
2.5. Second-level Group fMRI Analysis
Second level analyses, performed at the group level, treated inter-subject variability as a random effect. Firstly, we determined which voxels showed a main effect of incongruent versus congruent trials within each group (IAD, HC). Secondly, we tested which voxels significantly differed in BOLD signal during Stroop effect between IAD and HC groups ((IADincongruent-IADcongruent)-(HCincongruent-HCcongruent)), after correcting for multiple testing at the whole brain level with false discovery rate (FDR) to a threshold of p<0.05. Finally, we extracted the BOLD signal from the peak voxel within each cluster that demonstrated between-group differences and entered these data for all participants into correlation analyses with correct incongruent trial RT, accuracy rate and total Young IAT score.
3. Results
3.1. Behavioral Performance
The IAD and control HC groups did not significantly differ on accuracy rate [F(1,22)=0.271, p=0.608] although the IAD group showed a non-significant trend towards slower reaction times [F(1,22)=2.735, p=0.112] (See Table 1).
Table 1.
Stroop Task Behavioural results
| Proportion Correct Congruent Incongruent |
Reaction Time (msec) Congruent Incongruent |
||||
|---|---|---|---|---|---|
| IAD | M | 0.97 | 0.94 | 669.77 | 772.81 |
| SD | 0.02 | 0.07 | 145.06 | 163.52 | |
| HC | M | 0.97 | 0.95 | 585.17 | 677.20 |
| SD | 0.02 | 0.05 | 83.51 | 139.59 | |
Notes: M, arithmetic mean; SD, standard deviation; msec, milliseconds; IAD, Internet addiction disorder group; HC, healthy control group.
3.2. fMRI Results
One sample t-tests revealed similar activity patterns in IAD and HC groups during Stroop effect involving the visual regions, posterior cingulate cortex, bilateral super middle frontal gyri, bilateral inferior middle frontal gyri, and right middle temporal region (See Figure1 a, b).
Figure 1.
Activation differences in internet addicts and control groups following incongruent stimuli (p<0.05 corrected and with extent threshold: k>10 voxels.). The figures are shown in different coordinates to give the most complete view of the results.
IAD group demonstrated greater BOLD signal in the anterior cingulate and posterior cingulate cortices relative to the HC group during Stroop process, which was largely attributable to increased activity in the IAD group (See Figure 1c, Table 2).
Table 2.
Regional brain activity changes in Internet addicts minus control subjects following Stroop effect a.
| x,y,zb | Peak intensity |
Number of voxels |
Regionc | Brodmann’s Area |
|---|---|---|---|---|
| Cingulate gyrus; Posterior | ||||
| −6 −27 24 | 4.4097 | 104 | cingulate | 23, 31 |
| 54 −15 −18 | 5.4184 | 49 | Middle temporal gyrus | 21 |
| −3 −24 3 | 4.7841 | 35 | Thalamus; Middle dorsal nucleus | 31 |
| 6 −9 30 | 4.7067 | 24 | Cingulate gyrus | 24, 33 |
| −6 −3 30 | 5.4148 | 14 | Cingulate gyrus | 24 |
| Anterior cingulate; Middle frontal | ||||
| 27 27 33 | 4.3793 | 64 | gyrus | 9,32 |
| Middle frontal gyrus; Inferior | ||||
| 39 6 36 | 4.2585 | 40 | frontal gyrus | 8, 9 |
| −9 9 51 | 4.8828 | 28 | Medial frontal gyrus | 6 |
| −30 18 6 | 4.2098 | 21 | Insula | 13 |
| 21 60 18 | 4.5258 | 13 | Superior frontal gyrus | 10 |
| 30 12 39 | 4.0847 | 11 | Middle frontal gyrus | 8 |
| −18 −21 51 | 4.1644 | 10 | Middle frontal gyrus | 6 |
p<0.05 corrected and at least ten contiguous voxels. Voxel size=3*3*3.
Peak Talairach Coordinates.
The brain region were referenced to the software Xjview (http://www.alivelearn.net/xjview8) and double checked with atlas.
BOLD signal extracted from the peak voxel in the ACC cluster (incongruent minus congruent trials, T value) which differentiated IAD and HC groups was correlated positively with correct incongruent trial RT (r=0.389, p<0.01) and Young’ s IAT scores (r=0.386, p<0.01) but not accuracy rate (r=−0.063, p>0.05) across all participants (See Figure 2).
Figure 2.
Left: Correlation between peak ‘Stroop effect’-related ACC activation and reaction time form incongruent trials. Right: Correlation between peak ‘Stroop effect’-related ACC activation and proportion correct for incongruent trials.
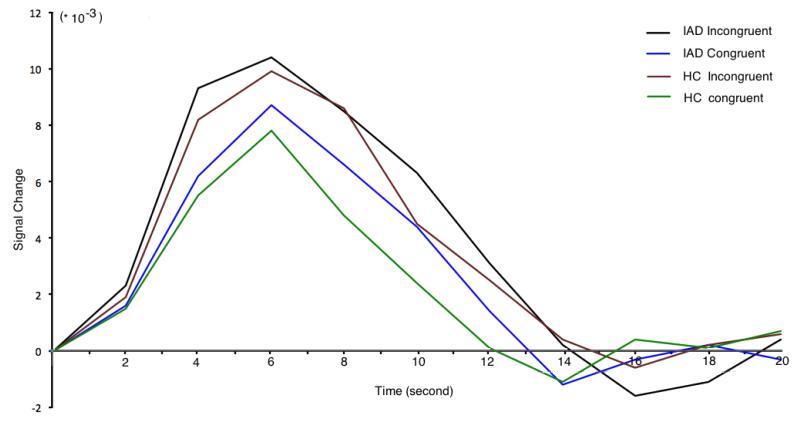
Hemodynamic response functions in congruent/incongruent trials in different groups are shown in figure 3.
Figure 3.
Group average hemodynamic response curves generated from 12 IAD subjects and 12 healthy controls. The hemodynamic response function plot described the temporal changes in the activated brain region in anterior cingulated cortex (circled with green in figure 1) after the stimuli onset. The analyses supposed the activation is 0 in the onset of the stimuli and sampled every 2s (TR=2s) in 20 seconds. The plot was automatically created by NeuroElf (www.neuroelf.net).
4. Discussion
The present study investigated cognitive control in male university students with and without IAD using a fMRI color-word Stroop task. The group with internet addiction disorder demonstrated greater activation during Stroop effect in the anterior and posterior cingulate cortices relative to the healthy control group. Greater BOLD signal in the anterior cingulate cortex was associated with slower incongruent reaction time and more severe scores on Young’ s Internet Addiction Test across all participants.
Both IAD and HC groups demonstrated task-related ACC activation, a brain region commonly activated by the Stroop task (Laird et al., 2005) and previously shown to be involved in conflict monitoring and cognitive control (Floden et al., 2010; Leung et al., 2000; Peterson et al., 2002; Aarts and Roelofs, 2010; Azizian et al., 2009; Botvinick et al., 2004; Floden et al., 2010). The literature is mixed in terms of whether superior Stroop task performance is associated with enhanced (Bush et al., 1999; Kerns et al., 2005; Potenza et al., 2003; Strakowski et al., 2005) or diminished regional BOLD signal (Azizian et al., 2009; Kaufmann et al., 2008; Mohanty et al., 2005), perhaps arising from methodological differences (e.g. color-word Stroop vs. number Stroop). However, the greater ACC recruitment by the IAD relative to the HC group during incongruent trials may reflect diminished ‘cognitive efficiency’ in the IAD group, particularly given the association between higher ACC recruitment and slower incongruent trial reaction time across both groups. Cognitive efficiency refers to notion that performance may be optimized and may require fewer resources when a task is performed quickly (Rypma et al., 2006). The cognitive inefficiency in the IAD could arise from impaired “top-down” cognitive-control processes, which has been associated with increased neural activity in the ACC (Botvinick et al., 1999), and is observed in nicotine-dependent participants performing the Stroop and working memory tasks (Azizian et al., 2009; Xu et al., 2005; Xu et al., 2006).
Robust between-group differences were also observed in the posterior cingulate cortex (PCC, Brodmann areas 23 and 31). As a central component of the proposed Default Mode Network (DMN), the PCC is implicated in attentional processes. The dorsal PCC is functionally connected with the DMN and cognitive control networks (CCN) while at rest, and becomes more anti-correlated with the CCN with as task difficulty increases (Leech et al., 2011). Therefore, greater activation in the dorsal PCC in the IAD group could indicate incomplete disengagement of the DMN resulting in failure to optimize task-related attentional resources. Furthermore, previous studies have demonstrated that PCC neurons respond to reward receipt and magnitude (McCoy et al., 2003; Pearson et al., 2009). In this study, quick and accurate task performance was incentivized by informing participants that they would be rewarded with additional payments based on their performance, which may have led to recruitment of reward-related circuitry during task performance.
Although these findings offer an important contribution to the relatively modest literature on the neurobiological underpinnings of Internet addiction disorder, several limitations should be considered. Firstly, the lack of significant group differences in in-scanner behavior could be said to indicate intact cognitive control processes in the IAD group.
However, a strength of fMRI is the ability to detect meaningful differences in cognitive processes which are yet too subtle to produce robust behavioral effects. Many fMRI researchers believe that in-scanner behavior must be relatively well-matched between groups to allow interpretation of group differences in task-related brain activation. Secondly, the IAD group was only compared with healthy controls. Inclusion of an ‘impulse control disorder’ or ‘behavioral addiction’ control group, such as pathological gamblers, would have contributed valuable information regarding the neurobiological similarities and differences between these phenomenalogically similar disorders. Finally, ongoing debates regarding optimal diagnostic criteria for IAD pose challenges in directly comparing findings across different IAD samples. The IAD participants in this study met strict criteria for IAD based upon two screening instruments and may represent more severe cases of IAD compared with other studies using less stringent cut-offs.
Conclusion
In summary, the group with internet addiction disorder demonstrated greater BOLD signal in the anterior and posterior cingulate cortices during incongruent Stroop trials, relative to healthy controls. These results are consistent with impaired inhibitory control and diminished cognitive efficiency in young men with internet addiction.
Acknowledgements
This research was supported by National Science Foundation of China (30900405). EED was supported by BIRCWH (K12DA031050), which was funded by the National Institute on Drug Abuse (NIDA), Office of Research on Women’ s Health (ORWH) and National Institutes of Health (OD) of the United States. The funders had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Guangheng Dong, Department of Psychology, Zhejiang Normal University, Jinhua City, Zhejiang Province, P.R.China.
Elise E. DeVito, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA
Xiaoxia Du, Department of Physics, Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai. P.R.China.
Zhuoya Cui, Department of Psychology, East China Normal University, Shanghai. P.R.China.
References
- Aarts E, Roelofs A. Attentional Control in Anterior Cingulate Cortex Based on Probabilistic Cueing. Journal of Cognitive Neuroscience. 2011;23:716–727. doi: 10.1162/jocn.2010.21435. [DOI] [PubMed] [Google Scholar]
- Alvarez-Moya EM, Jimenez-Murcia S, Moragas L, Gomez-Pena M, Aymami MN, Ochoa C, Sanchez-Diaz I, Menchon JM, Fernandez-Aranda F. Executive functioning among female pathological gambling and bulimia nervosa patients: preliminary findings. Journal of the International Neuropsycholological Society. 2009;15:302–306. doi: 10.1017/S1355617709090377. [DOI] [PubMed] [Google Scholar]
- Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED. Smoking Reduces Conflict-Related Anterior Cingulate Activity in Abstinent Cigarette Smokers Performing a Stroop Task. Neuropsychopharmacology. 2009;35:775–782. doi: 10.1038/npp.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard KW. Internet addiction: a review of current assessment techniques and potential assessment questions. Cyberpsycholology and Behavior. 2005;8:7–14. doi: 10.1089/cpb.2005.8.7. [DOI] [PubMed] [Google Scholar]
- Beard KW, Wolf EM. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychology and Behavior. 2001;4:377–383. doi: 10.1089/109493101300210286. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Archive General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beutel ME, Bruhler E, Glaesmer H, Kuss DJ, Wulfling K, Muller KW. Regular and Problematic Leisure-Time Internet Use in the Community: Results from a German Population-Based Survey. Cyberpsychology, Behavior, and Social Networking. 2011;14:291–296. doi: 10.1089/cyber.2010.0199. [DOI] [PubMed] [Google Scholar]
- Block JJ. Prevalence Underestimated in Problematic Internet Use Study. CNS Spectrum. 2006;12:14–15. doi: 10.1017/s1092852900020459. [DOI] [PubMed] [Google Scholar]
- Block JJ. Issues for DSM-V: Internet Addiction. American Journal of Psychiatry. 2008;165:306–307. doi: 10.1176/appi.ajp.2007.07101556. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. Journal of Neuropsychiatry and Clinical Neuroscience. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Cao F, Su L, Liu T, Gao X. The relationship between impulsivity and Internet addiction in a sample of Chinese adolescents. European Psychiatry. 2007;22:466–471. doi: 10.1016/j.eurpsy.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive Affective and Behavior Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chou C, Hsiao M-C. Internet addiction, usage, gratification, and pleasure experience: the Taiwan college students’ case. Computers and Education. 2000;35:65–80. [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Huang J, Du X. Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: An fMRI study during a guessing task. Journal of Psychiatric Research. 2011a;45:1525–1529. doi: 10.1016/j.jpsychires.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Dong G, Lu Q, Zhou H, Zhao X. Impulse inhibition in people with Internet addiction disorder: Electrophysiological evidence from a Go/NoGo study. Neuroscience Letters. 2010;485:138–142. doi: 10.1016/j.neulet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Dong G, Lu Q, Zhou H, Zhao X. Precursor or sequela: pathological disorders in people with Internet addiction disorder. PLoS One. 2011b;6:e14703. doi: 10.1371/journal.pone.0014703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Zhou H, Zhao X. Male Internet addicts show impaired executive control ability: Evidence from a color-word Stroop task. Neuroscience Letters. 2011c;499:114–118. doi: 10.1016/j.neulet.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Floden D, Vallesi A, Stuss DT. Task Context and Frontal Lobe Activation in the Stroop Task. Journal of Cognitive Neuroscience. 2011;23:867–879. doi: 10.1162/jocn.2010.21492. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to Behavioral Addictions. The American Journal of Drug and Alcohol Abuse. 2010;36:233–241. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden C. ‘Behavioral’ Addictions: Do They Exist? Science. 2001;294:980. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- Kaufmann L, Ischebeck A, Weiss E, Koppelstaetter F, Siedentopf C, Vogel SE, Gotwald T, Marksteiner J, Wood G. An fMRI study of the numerical Stroop task in individuals with and without minimal cognitive impairment. Cortex. 2008;44:1248–1255. doi: 10.1016/j.cortex.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased Conflict- and Error-Related Activity in the Anterior Cingulate Cortex in Subjects With Schizophrenia. American Journal of Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lau CH, Cheuk K-K, Kan P, Hui HLC, Griffiths SM. Predictors of heavy Internet use and associations with health-promoting and health risk behaviors among Hong Kong university students. Journal of Adolescence. 2010;33:215–220. doi: 10.1016/j.adolescence.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, Janavs J, Dunbar GC. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. [Google Scholar]
- Leung H-C, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An Event-related Functional MRI Study of the Stroop Color Word Interference Task. Cerebral Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Liu T, Potenza MN. Problematic Internet Use: Clinical Implications. CNS Spectr. 2007;12:453–466. doi: 10.1017/s1092852900015339. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade Reward Signals in Posterior Cingulate Cortex. Neuron. 2003;40:1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends in Cognitive Science. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- McCusker CG, Gettings B. Automaticity of cognitive biases in addictive behaviours: further evidence with gamblers. British Journal of Clinical Psychology. 1997;36(Pt 4):543–554. doi: 10.1111/j.2044-8260.1997.tb01259.x. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Herrington J, Koven N, Fisher J, Wenzel E, Webb A, Heller W, Banich M, Miller G. Neural mechanisms of affective interference in schizotypy. Journal of Abnormal Psychology. 2005;114:16–27. doi: 10.1037/0021-843X.114.1.16. [DOI] [PubMed] [Google Scholar]
- Niemz K, Griffiths M, Banyard P. Prevalence of pathological Internet use among university students and correlations with self-esteem, the General Health Questionnaire (GHQ), and disinhibition. CyberPsychology and Behavior. 2005;8:562–570. doi: 10.1089/cpb.2005.8.562. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in Posterior Cingulate Cortex Signal Exploratory Decisions in a Dynamic Multioption Choice Task. Current Biology. 2009;19:1532–1537. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung H-C, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung H-C, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An fMRI Stroop Task Study of Ventromedial Prefrontal Cortical Function in Pathological Gamblers. American Journal of Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Martin Bly B, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. NeuroImage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal fMRI Brain Activation in Euthymic Bipolar Disorder Patients During a Counting Stroop Interference Task. American Journal of Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Tao R, Huang X, Wang J, Liu C, Zhang H, Xiao L, Yao S. A proposed criterion for clinical diagnosis of internet addiction. Medical Journal of Chinese People’s Liberation Army. 2008;33:1188–1191. [Google Scholar]
- Wang W, Tao R, Niu Y, Chen Q, Jia J, Wang X, Kong Q, Tian C. Preliminarily Proposed Diagnostic Criteria of Pathological Internet Use. Chinese Mental Health Journal. 2009;23:890–894. [Google Scholar]
- Wright I, Waterman M, Prescott H, Murdoch-Eaton D. A new Stroop-like measure of inhibitory function development: Typical developmental trends. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:561–575. doi: 10.1111/1469-7610.00145. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhu K. Path analysis on related factors causing internet addiction disorder in college students. Chinese Journal of Public health. 2004;20:1363–1366. [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain Activity in Cigarette Smokers Performing a Working Memory Task: Effect of Smoking Abstinence. Biological Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Jarvik M, Olmstead R, Brody AL, Ernst M, London ED. Effect of Cigarette Smoking on Prefrontal Cortical Function in Nondeprived Smokers Performing the Stroop Task. Neuropsychopharmacology. 2006;32:1421–1428. doi: 10.1038/sj.npp.1301272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS. Internet addiction: The emergence of a new clinical disorder. CyberPsychology and Behavior. 1998;1:237–244. [Google Scholar]
- Young KS. Internet Addiction Test (IAT) 2009 From http://netaddiction.com/index.php?option=combfquiz&view=onepage&catid=46&Itemid=106.
- Young KS, Rogers RC. The relationship between depression and internet addiction. Cyberpsychology and Behavior. 1998;1:25–28. [Google Scholar]