Abstract
Vitamin D status has been implicated in insulin resistance, type 2 diabetes mellitus, and hypertension, but the range of vitamin D status values over which the association can be found is unknown. Our objective was to define this range in a cohort of nondiabetic adult Canadians. We used a regression modeling strategy, first adjusting insulin-response variables and systolic and diastolic blood pressure for BMI, waist circumference, weight, age, and sex. The resulting residuals were regressed against serum 25-hydroxyvitamin D [25(OH)D] concentration using successive 40% data blocks ranging from the 0th to the 60th percentile of 25(OH)D values. All of the predictor variables were significantly associated with each of the dependent variables, with BMI and waist circumference accounting for >98% of the explained variance. The vitamin D association was localized to the serum 25(OH)D range extending from ∼40 to ∼90 nmol/L (16–36 μg/L). We conclude that vitamin D status is inversely associated with insulin responsiveness and blood pressure. Consistent with the threshold response characteristic typical of nutrients, the association was strongest in a circumscribed region of the range of 25(OH)D values. There was no association at 25(OH)D values >80–90 nmol/L (32–36 μg/L), indicating that the vitamin D association applied principally to values below that level. The differences observed, if they can be further confirmed in prospective studies, are of a magnitude that would be clinically important.
Introduction
A large and rapidly growing literature suggests that vitamin D status is a factor in various chronic conditions such as autoimmune disorders and cardiovascular disease (1). Of the several suggested extraskeletal activities of vitamin D, perhaps the best attested is insulin resistance. Increased insulin resistance is found associated with low vitamin D status in a variety of observational study designs (2–22), including population-based studies in countries as geographically diverse as Norway (20), Australia (17), and the United States (5, 19). Low vitamin D status is predictive of incident metabolic syndrome (5, 10, 17), the features of which include insulin resistance and hypertension. That this association is most likely causal has been shown by several recently published randomized, controlled trials evaluating vitamin D effects on insulin resistance (23–25) as well as blood pressure (BP)7 (26–28). The BP association has a clear basis in biology because evidence from vitamin D knockout mice indicates that hypertension is a consequence of vitamin D deprivation (29).
Although more sophisticated mathematical models are potentially available, all of the observational studies of which we are aware have used a simple linear (or sometimes logarithmic) relationship between vitamin D status and the various indices of insulin responsiveness or BP. Such models explicitly assume that the association is operative across the full range of vitamin D intakes. This is arguably not the best analytic strategy because nutrient responses tend to be sigmoidal, with response reaching a plateau at some point within the plausible intake range (30, 31). To our knowledge, no study to date has sought specifically to find the “sensitive” segment of the vitamin D status range or to focus its analysis on that range.
As with insulin response, large population studies looking for associations between vitamin D and BP have yielded inconsistent results (32–38). This inconsistency could be because the actual effect is small (39), resulting in a correspondingly low power to detect a real association. Or, as we hypothesize here for both insulin and BP, results of large observational studies may have been influenced by a failure to use an analytic strategy that corresponds to the unique features of nutrient response (30, 31).
Thus, although the association is reasonably well established, existing data do not provide information on the range of vitamin D status values over which it is operative, nor how much vitamin D is sufficient to produce the reported difference in insulin responsiveness or BP. In this article, we report the results of application of an analytic strategy specifically designed to fit sigmoid curves. The predictor variable was 25-hydroxyvitamin D [25(OH)D]8, and the outcome variables were fasting plasma insulin, homeostatic model assessment–insulin resistance (HOMA-IR) (40), insulin responsiveness (QUICKI) (41), and both systolic and diastolic BP.
Methods
Subjects
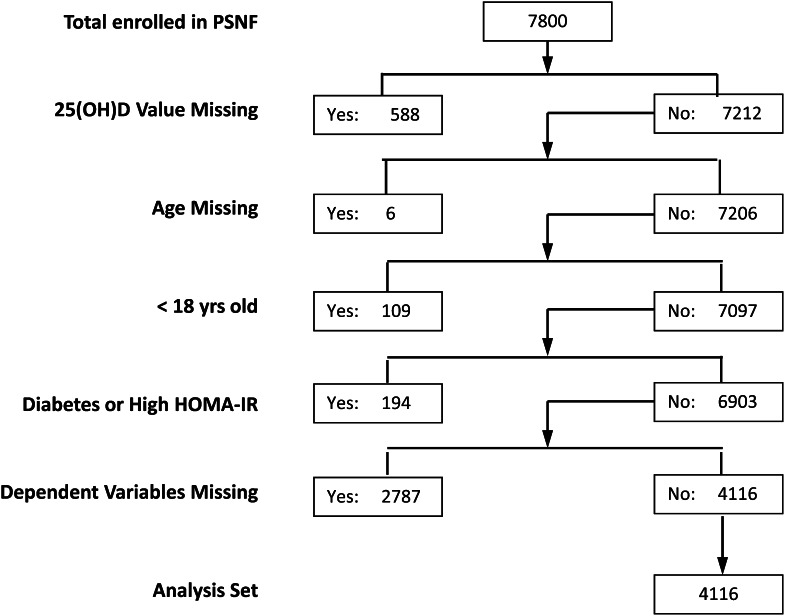
We had the opportunity to examine this matter in a hitherto untapped databank developed by a not-for-profit Canadian health foundation, Pure North S’Energy Foundation (PNSF), located in Calgary, Alberta. This foundation was created to address health issues among workers in the Canadian oil and gas industry (and their families). Its program includes promoting such outcomes as healthy weight and improved nutrition among its members. Membership is voluntary and without cost. Participants are recruited into the foundation’s program typically through their employers. Foundation enrollment consists of gathering health information from participants through a questionnaire and interview, making anthropometric measurements, and providing both blood and urine samples for analysis. For this study, data were obtained for members enrolled from October 2007 through December 2011, using measurements made on entry (or as close to the entry visit as possible to include the desired biochemical data). Planned analysis of the anonymized data were reviewed by the Western Institution Review Board and determined to be “exempt.” Nevertheless, the study was registered at clinicaltrials.gov and given the clinical trial number NCT01692028. The only exclusions were age younger than 18 y, a clinical diagnosis of diabetes, and a HOMA-IR value of ≥16. Of a total of 7800 enrollees, 588 were missing values for 25(OH)D, the principal predictor variable; 115 were younger than 18 y of age or age could not be determined, and 194 had a clinical diagnosis of diabetes, leaving 6903 potentially analyzable records. Of these, 2787 were missing values for the outcome variables, leaving 4116 participants with sufficient data for analysis. (This information is shown in CONSORT form as Figure 1.) The large gap between foundation enrollment and study enrollment was due to the fact that participants entered the foundation for personal reasons and not as study participants. Data needed for this study were not obtained for many of them. Where available data permitted, we compared individuals included in the analysis set with those excluded for the foregoing reasons. Except for the fact that there were more men in the excluded group (68% vs. 58%) and their serum 25(OH)D values were lower (72.4 nmol/L vs. 88.7 nmol/L), the 2 groups did not differ appreciably.
Figure 1.
Generation of the analysis sample from the total adult cohort enrolled in the Pure North S'Energy Foundation (PNSF) program during the period October 2007 through December 2011. The list on the left gives the sorting criteria for each level of sample generation, and the designations “Yes” and “No” in the boxes to the right refer to the number of individuals meeting or not meeting that criterion, respectively. HOMA-IR, homeostasis model assessment–insulin resistance; 25(OH)D, 25-hydroxyvitamin D.
PNSF had publically promoted vitamin D consciousness, and nearly 90% of enrollees were taking vitamin D supplements at the time of enrollment. Nonusers exhibited the same distribution of men and women as the group as a whole, but had lower 25(OH)D values, as would be expected (data not shown).
Procedures
Participants were instructed to fast at least 8 h before a scheduled appointment. Blood samples for 25(OH)D, fasting insulin, and fasting glucose were collected by a PNSF staff phlebotomist and sent to Calgary Laboratory Services for analysis. The analytical methods for determining glucose, insulin, and 25(OH)D were the Roche Hexokinase UV assay, performed on the Roche Modular P800 analyzer, the Abbott Architect Insulin Chemiluminescent assay, and the DiaSorin Liaison chemiluminescent assay, respectively. The fasting serum insulin and glucose values were combined to yield the commonly used measure of insulin resistance ([HOMA-IR (39)] and a measure of insulin responsiveness [QUICKI (41)], using a conversion factor for insulin of 6.945 pmol/mU. Cuff BP was measured using a standardized protocol with the participant sitting, relaxed and silent, with cuff size matched to arm circumference.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 20. Variables that were appreciably skewed to the right [HOMA-IR, insulin, glucose, and 25(OH)D] were loge transformed before incorporation into the various models. This transformation effectively normalized all 4 variables. Sex was coded as a 0/1 variable. All variables of interest were analyzed separately for men and women.
Multiple linear models were generated for the dataset as a whole as well as for men and women separately. Values of the 5 dependent variables (or their loge transforms) were adjusted for BMI, waist circumference, weight, age, and sex using a linear regression model. Modeling was confined to cases for which there were valid values for all of the involved variables. Values for the dependent variables, adjusted for BMI, waist, sex, age, and weight, were plotted against the loge transform of 25(OH)D using first the locally weighted scatterplot smoothing (LOESS) method, and then a method similar to LOESS, but reporting actual slopes for sequential data blocks. To do this, we performed a series of linear regressions using data blocks comprising 40% of the fully adjusted residual values for the dependent variables from the entire dataset (∼1646 records per block), stepping them up from the lowest 25(OH)D value by 5 percentile increments. The output of this exercise was a series of values for the coefficient for the natural logarithm (ln)25(OH)D term in the bivariate linear regression equations for each of the dependent variables. This approach, as noted, is similar to that used by LOESS, with the exception of the fact that we assumed a quasisigmoid pattern for the underlying relationship between the outcome variable and vitamin D status, whereas LOESS is model free. Coefficients with P < 0.05 were considered statistically significant.
Results
Pertinent features of the analyzable members of the cohort are set forth in Table 1. There were 2406 males and 1710 females, with ages spanning a range from 18 to 95 y, BMI ranging from 14.7 to 55.5 kg/m2, and serum 25(OH)D values ranging from 10.0 to 361.0 nmol/L (4 to 144 μg/L). Thus, the individuals in this cohort exhibited values that were approximately coextensive with the range of values for these variables in the general population. As would be expected, the men had larger body size than the women. For all variables except age, the values for men and women differed significantly, which is not surprising given the large sample sizes. Overall, however, the sex-related differences were small. Nevertheless, the differences justified treating sex as a cofactor in the subsequent analyses.
Table 1.
Values for demographic and biochemical variables in the analyzed sample.1
| Variable | Men (n = 2406) | Women (n = 1710) | P value |
| Age, y | 41.4±11.6 (18–85) | 41.3±12.6 (18–95) | >0.05 |
| Weight, kg | 90.0±15.5 (40–140) | 71.8±16.3 (37.9–136.5) | <0.001 |
| Height, m | 1.78±0.070 (1.52–1.98) | 1.64±0.069 (1.42–1.93) | <0.001 |
| BMI, kg/m2 | 28.5±4.52 (15.7–50.2) | 26.5±5.84 (14.7–55.5) | <0.001 |
| Waist circumference, cm | 98.3±13.1 (70–185.4) | 88.4±15.4 (55–195) | <0.001 |
| Plasma glucose, mmol/L | 4.89±0.72 (3–16) | 4.60±0.64 (3–13) | <0.001 |
| Plasma insulin, pmol/L | 49.3±34.5 (2.6–343.6) | 46.1±28.5 (7–301) | 0.001 |
| HOMA-IR | 1.58±1.25 (0.1–12.1) | 1.39±1.00 (0.1–11.4) | <0.001 |
| QUICKI | 0.37±0.040 (0.27–0.63) | 0.380±0.040 (0.27–0.57) | <0.001 |
| Systolic BP, mm Hg | 126.1±13.5 (86–198) | 119.3±15.3 (75–223) | <0.001 |
| Diastolic BP, mm Hg | 81.2±10.0 (48–150) | 76.0±10.2 (49–123) | <0.001 |
| 25(OH)D, nmol/L | 85.2±43.5 (10–361) | 93.6±40.2 (10.1–353) | <0.001 |
All values are expressed as mean ± SD (range). QUICKI, quantitative insulin sensitivity check index.
Table 2 presents the numbers of individuals in the cohort who had values above the upper reference limit for fasting insulin and systolic BP (120 pmol/L and 140 mm Hg, respectively), arrayed by 25(OH)D quartile. It shows a significant excess of individuals with elevated values at the lowest vitamin D status quartile. Contrasting the lowest and highest quartiles, the RR (95% CI) for an elevated fasting insulin value was 2.08 (1.31–3.29; P < 0.01) and for elevated BP, 1.36 (1.08–1.71; P < 0.01). However, because of the strong interrelationship of obesity and waist circumference, on the one hand, with both BP and insulin responsiveness on the other, the relative excess of values at the lower vitamin D status quartiles would likely have been influenced by factors such as obesity in those same quartiles. It was necessary, therefore, to dissect out an independent effect (if any) for 25(OH)D after adjusting for the recognized and strongly influential body size and fat distribution variables.
Table 2.
Distribution (N) of elevated Insulin and blood pressure values by 25(OH)D quartile.1
| Value | 25(OH)D quartile23 |
|||
| 1st | 2nd | 3rd | 4th | |
| Insulin >120 pmol/L4 | 54 | 40 | 34 | 26 |
| Systolic BP ≥140 mm Hg4 | 148 | 128 | 121 | 109 |
BP, blood pressure; 25(OH)D, 25-hydroxyvitamin D.
Quartile boundaries: 59.9, 82.1, 109.0 (nmol/L).
Quartile totals: 1: 1032; 2: 1027; 3: 1035; 4: 1022.
Upper limit of reference range.
Table 3 sets forth the resulting multiple linear regression models for the 5 dependent variables of interest, using the entire dataset, with sex entered as a cofactor and including ln25(OH)D. Stepwise methods were used, but for each dependent variable except ln(insulin), the same set of predictor variables was found to be statistically significant (Table 3), although not always in the same order. These were BMI, waist circumference, weight, age, sex, and ln25(OH)D. These 5 variables accounted for almost one fourth of the total variance for the 3 insulin-related outcome variables (insulin, HOMA-IR, and QUICKI), and for approximately one fifth of the variance of the BP variables. For the insulin-related dependent variables, BMI and waist circumference alone accounted for almost 98% of the explained variance, more or less as expected. The coefficients for ln25(OH)D in these models, together with their 95% CIs, are also shown in Table 3. Except for QUICKI, the sign of the coefficient for all models was negative, indicating an inverse correlation. For QUICKI, which measures insulin sensitivity rather than resistance, the sign was positive. The coefficients of ln25(OH)D in these models were for the most part statistically highly significant (P < 0.001) for all outcome variables except systolic BP, for which P = 0.09.
Table 3.
Multiple regression models for insulin response and blood pressure.1
| Dependent variable | Significant predictor variables | R2 for model | Coefficient for ln25(OH)D2 | P value3 |
| ln[insulin] | BMI, waist, sex, ln25(OH)D | 0.262 | −0.084 (−0.117 to −0.051) | <0.001 |
| ln[HOMA-IR] | BMI, waist, weight, age, sex, ln25(OH)D | 0.274 | −0.075 (−0.112 to −0.038) | <0.001 |
| QUICKI | BMI, waist, weight, age, sex, ln25(OH)D | 0.238 | +0.0029 (0.00143– 00437) | <0.001 |
| Systolic BP | BMI, waist, weight, age, sex, ln25(OH)D | 0.227 | −0.755 (−1.63 to +0.123) | 0.092 |
| Diastolic BP | BMI, waist, weight, age, sex, ln25(OH)D | 0.184 | −1.480 (−2.12 to −0.84) | <0.001 |
BP, blood pressure; HOMR-IR, homeostasis model assessment–insulin resistance; ln, natural logarithm; QUICKI, quantitative insulin sensitivity check index; 25(OH)D, 25-hydroxyvitamin D.
Mean (95% CI).
Probability that the confidence interval for the coefficient for ln[25(OH)D] includes 0.
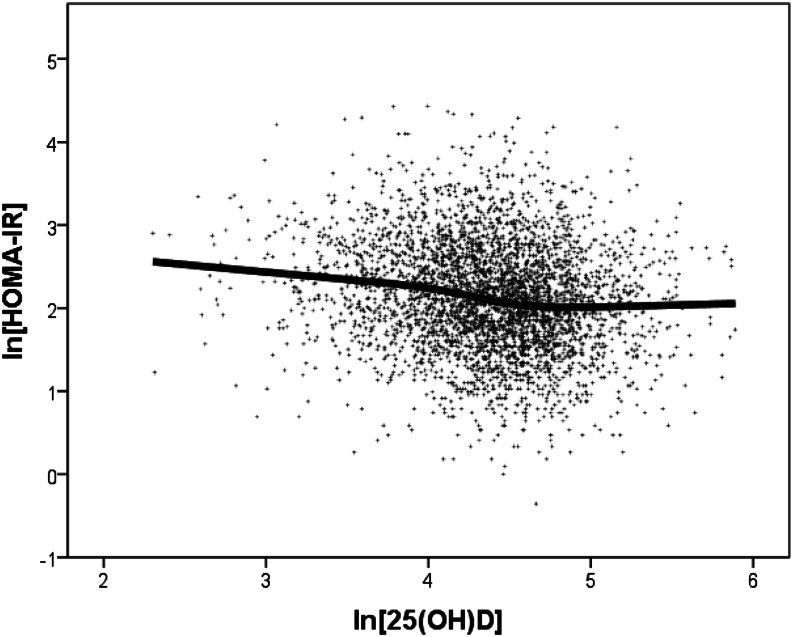
These analyses establish an independent association of vitamin D status with the dependent variables in this cohort, accounting for ∼2% of their variance. But strictly linear models, as their name suggests, generally presume that the relationship, if any, prevails across the full range of the data analyzed. Significant departures from this assumption could appreciably alter, and even obscure, an underlying relationship. Because the underlying hypothesis for nutrients is that the response is approximately sigmoid shaped and because sigmoid curves have 2 flat regions at low- and high-exposure values (with the transition being sandwiched between them), we first developed LOESS plots such as Figure 2 [lnHOMA-IR on ln25(OH)D]. This plot shows, in the middle of the ln25(OH)D range, a clear offset between low and high ln25(OH)D values. It is this offset that we explored in the subsequent analyses. The evident smallness of the offset in Figure 2 (relative to the range of the HOMA-IR values) is a graphic reflection of the relative smallness of the contribution of ln25(OH)D to the total variance (as shown in Table 3).
Figure 2.
Locally weighted scatterplot smoothing plot of the relationship between ln(HOMA-IR) and ln25(OH)D, showing an offset in the middle of the range of ln25(OH)D values. HOMA-IR, homeostasis model assessment–insulin resistance; ln, natural logarithm; 25(OH)D, 25-hydroxyvitamin D.
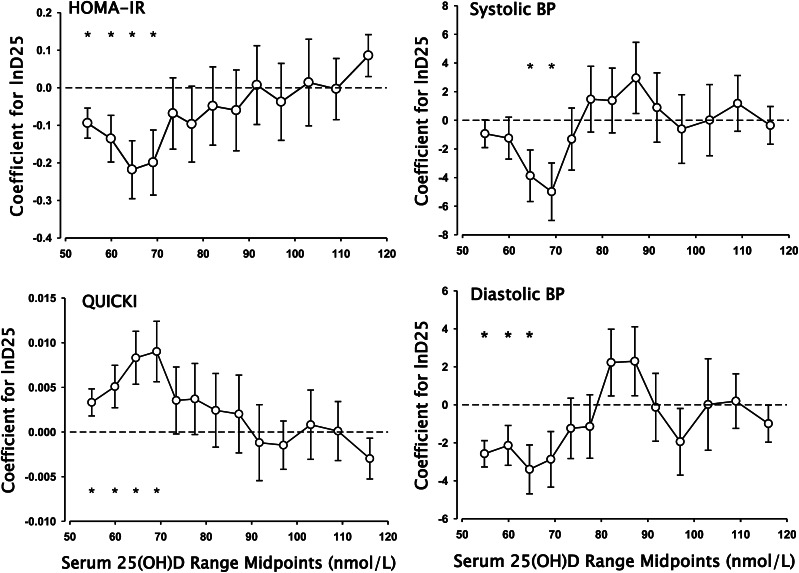
In this analysis, we prepared a set of residuals, adjusting the raw values of the outcome variables for BMI, waist, age, sex, and weight. We then repeated the modeling exercise by regressing these residuals against ln25(OH)D, using a series of 40% contiguous data blocks, stepping up from the 0th percentile for serum 25(OH)D through the 60th percentile. For each iteration, we determined the value of the fitted coefficient of the 25(OH)D variable and its 95% confidence limits. Figure 3 plots the values for the resulting ln25(OH)D coefficients for ln(HOMA-IR), QUICKI, systolic BP, and diastolic BP as the dependent variables. In each case, these coefficients are plotted at the midpoints of the 25(OH)D data ranges evaluated. [A curve very similar to that of HOMA-IR was produced for ln(insulin) (not shown).] Coefficients significantly different from 0 are marked with an asterisk.
Figure 3.
Plots of the coefficients of the ln25(OH)D term in bivariate regression equations using as dependent variables the residuals for ln(HOMA-IR), QUICKI, systolic BP, and diastolic BP derived after fitting to a multivariate model using BMI, waist circumference, age, sex, and weight. For each plot, the points represent the computed coefficients for successive 40% data blocks stepping upward from the lowest 25(OH)D value by increments of 5 percentiles. Error bars are 1 SEM. Asterisks designate coefficient values that are significantly different from 0. Note that the x-axis values are plotted at the midpoint of the ranges over which the relationships were calculated. The boundaries of those ranges extend upward and downward by ~20 nmol/L. BP, blood pressure; D25, 25-hydroxyvitamin D; HOMA-IR, homeostasis model assessment–insulin resistance; ln, natural logarithm; QUICKI, quantitative insulin sensitivity check index; 25(OH)D, 25-hydroxyvitamin D.
The patterns evident in Figure 3 show that there was indeed a statistically significant correlation between vitamin D status and each of the outcome variables, but that it was confined to only a portion of the serum 25(OH)D continuum, consistent with the sigmoid model. In regions either lower or higher, the coefficients were smaller (and for HOMA-IR and systolic BP were not statistically different from 0). However, in the response region, the absolute magnitude of the coefficients increased, reaching values that were 2 to 7 times greater than those produced using the full range of serum 25(OH)D values (i.e., those shown in Table 3). This pattern is what would be predicted for a relationship that was embedded in a continuum but applicable in only a portion of its range.
For all 5 outcome variables, the third and fourth data blocks [i.e., 25(OH)D values between 42 and 82 nmol/L] produced the numerically largest value for the coefficient of ln25(OH)D. For systolic BP, the earlier and later blocks produced 0 or near 0 values, indicating the absence of any association of ln25(OH)D with outcome at those 25(OH)D concentrations. For the others, particularly diastolic BP, there was a strong suggestion of a vitamin D effect beginning in the lowest quantiles evaluated.
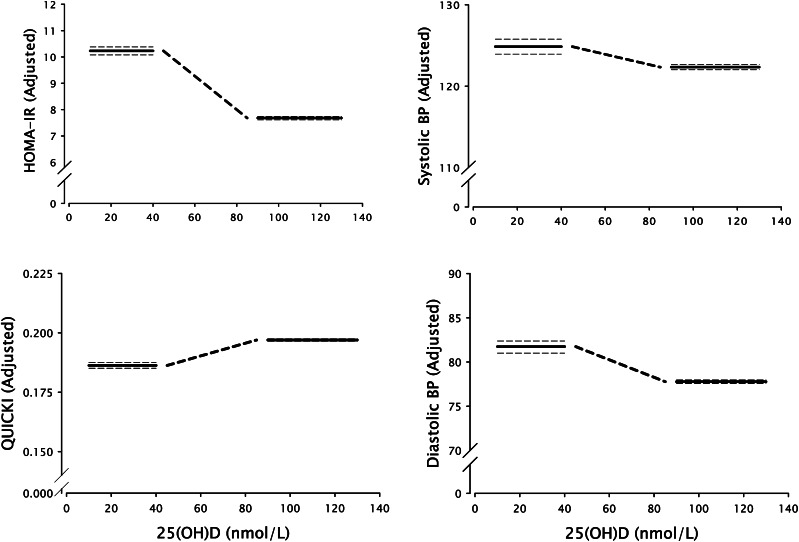
Figure 4 shows the quantitative offset between the values of the 4 dependent variables of Figure 3, below and above the transition zone. This mode of presenting the data displays the underlying inverse sigmoid character of the relationship and corresponds directly to the LOESS plot (Fig. 1). In each case, the heavy horizontal lines represent the mean values for the residuals for the 4 dependent variables (after adjusting for BMI, weight, waist circumference, sex, and age), plotted over the ranges over which there was no significant association with ln25(OH)D (i.e., <40 and >90 nmol/L), and the heavy dashed diagonal line represents a schematic approximation of the transition.
Figure 4.
Plots of quasi-integrals of the data in Figure 2 showing the underlying inverse sigmoid character of the relationship between vitamin D status and HOMA-IR, insulin, and BP. In each plot, the heavy horizontal lines represent the flat portions of the sigmoid curve above and below the transition zone (together with their 95% CI, the horizontal dashed lines). The heavy diagonal dashed line represents the transition and is an approximation, as the data indicate only that the transition occurs somewhere in the range of 40–90 nmol/L (16–36 μg/L). BP, blood pressure; HOMA-IR, homeostasis model assessment–insulin resistance; QUICKI, quantitative insulin sensitivity check index; 25(OH)D, 25-hydroxyvitamin D.
Table 4 presents the set of maximum coefficients for the vitamin D term derived from the various models for all 5 dependent variables, together with the data ranges over which the magnitude of the coefficient was found to be maximal. Because some of the variables had been log-transformed, actual effect size over the ranges where the association is detectable is nonlinear. Nevertheless, the aggregate effects across the cognate transition zones can be estimated from the difference between the 2 steady-state values in the plots of Figure 4. For insulin, the total magnitude of the vitamin D–related difference was −12.0 pmol/L, −0.37 for HOMA-IR, +0.023 for QUICKI, –2.56 mm Hg for systolic BP, and –3.96 mm Hg for diastolic BP (P < 0.001 for all).
Table 4.
Magnitude of the coefficients of the ln25(OH)D term over the indicated 25(OH)D range.1
| Outcome Variable | Maximum Coefficient2 | 25(OH)D, range |
| nmol/L | ||
| ln[Insulin] | −0.246 (−0.403 to −0.0892) | 49.4–87.2 |
| ln[HOMA-IR] | −0.218 (−0.369 to −0.067) | 42.4–82.1 |
| QUICKI | +0.00901 (0.0234–0.0157) | 49.4–87.2 |
| Systolic BP | −4.99 (−8.93 to −1.050) | 49.4–87.2 |
| Diastolic BP | −3.40 (−5.928 to −0.8716) | 42.4–82.1 |
BP, blood pressure; ln, natural logarithm; 25(OH)D, QUICKI, quantitative insulin sensitivity check index; 25-hydroxyvitamin D.
Point estimate (95% CI).
Discussion
These analyses add further support to the hypothesis that vitamin D status contributes significantly to the maintenance of glucose homeostasis and normal BP in free-living North American adults. Its effect is much smaller than the effects of fatness and fat distribution, both of which could easily obscure the vitamin D effect in cross-sectional studies. The data summarized in Table 3 (which is how this issue is usually approached) effectively established the role of vitamin D status as an independent predictor, at least for this cohort. Although we could have stopped there, the subsequent segmented analysis added new information to the description of the vitamin D effect. It resulted in substantially improved sensitivity, permitting both localization of the association to the operative exposure range and better estimation of its true magnitude than would have been possible using ordinary linear modeling methods (with their typical assumption of monotonic relationships between variables across the span of analyzed values). This latter point is illustrated clearly for systolic BP, which exhibited only a marginal significance (P = 0.09) when the entire range of 25(OH)D values was used to generate a model (Table 3). By contrast, when the focus was confined to the middle of the 25(OH)D range, the absolute value of the coefficient of ln25(OH)D increased substantially and became clearly significant (P = 0.015).
The recently published study of Gagnon et al. (17) contained a LOESS plot of the risk of incident metabolic syndrome against vitamin D status, showing a clear offset at 25(OH)D values ranging from ∼50 nmol/L to ∼100 nmol/L, very similar to where we found the maximum association for our 5 dependent variables. As with our data, 25(OH)D concentration explained ∼1% of the variance in risk of the outcome variable (incident metabolic syndrome) (17).
Although our study represents what is, to our knowledge, the first explicit modeling of observational data using the sigmoid character of nutrient response, it must be recognized that such a model is implicit in the process involved in setting intake requirements and is explicitly diagrammed, for example, in Chapter 1 of Willett’s Nutritional Epidemiology (42). [A decision that X mg/d of a particular nutrient is sufficient to meet the average needs is equivalent to saying that the benefit produced by the nutrient is maximized at this intake and that further increases produce no additional health benefit, i.e., the curve has reached the upper flat region of its sigmoid shape. For example, the Institute of Medicine decision that a vitamin D status of 50 nmol/L (20 μg/L) was sufficient (43) was tantamount to saying that the transition from inadequate to adequate had occurred at <50 nmol/L (20 μg/L).]
Other recent reports using variants of a segmental analysis analogous to ours have found results congruent with those described here. Hyppönen and Power (14) using a “broken-stick” method, instead of assuming linearity of response, reported a statistically significant negative association between vitamin D status and hemoglobin A1c, with a break point at ∼65 nmol/L. Larsen et al. (28), in a randomized, controlled trial, found an inverse effect on BP at 25(OH)D values mainly <80 nmol/L. Both inflection points are within the range of vitamin D status values where we found an association between 25(OH)D and the insulin and BP variables. Further, these findings, together with those presented here, are consistent with the recommendation from the systematic review by Cavalier et al. (44) that serum 25(OH)D be maintained at >75 nmol/L.
The very recent study of Belenchia et al. (25) provides strong corroboration of the findings that we report here. Using a randomized, controlled trial design in 35 obese adolescents and administering a vitamin D dose of 4000 iu/d, these investigators increased serum 25(OH)D from 47 to 95 nmol/L and found a significant decrease in HOMA-IR (−1.63) and a significant increase in QUICKI (+0.016). These changes are very similar to those we observed in adults and span precisely the region of the 25(OH)D continuum in which our association occurs.
A principal strength of our study lies not so much in the relationship that we found (which is to some extent confirmatory), but in the additional inferential power conferred by the segmented analysis, permitting in observational studies better localization of the effect transition than has heretofore been possible. In this dataset, that transition lies somewhere in the 25(OH)D range of 40–90 nmol/L. To localize it more precisely would have required smaller percentile blocks, which in turn would have required a much larger sample size if we were to have adequate power within each block. In any case, these data provide information concerning the serum 25(OH)D concentration that must be achieved to ensure the cognate effects. In this cohort and with this analytic approach, our results suggest that that value must be at least as high as 80 nmol/L (32 μg/L).
A second strength is seen by way of contrast with usual studies of such associations, which report estimated changes in some endpoint variable per some arbitrary amount of change in the predictor variable, e.g., a decrease of 1 mm Hg in systolic BP for each 10 nmol/L increase in serum 25(OH)D. That approach clearly assumes not only linearity, but independence of the response and the basal exposure, i.e., that any 10-nmol/L increase will produce the specified response, irrespective of starting level. That is clearly not valid for a sigmoid type of response. By contrast, the approach we have taken here yields the total response produced, not by a certain unit quantity of the independent variable, but by the full transition from inadequate to adequate.
A weakness of the study lies in its observational character and the corresponding inability to attribute a causal role to vitamin D status, at least from these data alone. However, given that there are randomized trial data showing an effect of vitamin D on both HOMA-IR (23–25) and BP (26–28), the concordant evidence from this study strongly suggests that elevating vitamin D status to a 25(OH)D level of at least 80 nmol/L would be expected to result in small but useful improvements in both glucose homeostasis and BP control.
It must also be noted that we did not attempt to evaluate the linearity of the relationship between the confounders (e.g., BMI, waist) and the endpoint variables as we did for vitamin D. For them, there is no a priori reason to doubt that linearity, which is usually assumed in the literature on this topic. Nevertheless, that assumption is not evidence of possible underlying nonlinearity nor that some nonlinear method would not have removed more of the variance in the endpoint variables. Finally, the database on which these analyses are based did not include values for serum leptin or other cytokines associated with obesity and insulin resistance. Hence, we were unable to explore these possibly mechanistic associations.
Acknowledgments
The authors thank Pure North S'Energy Foundation for providing access to their data. Persons in PNSF providing specific assistance and support throughout the project include Allan Markin, Founder, Wendy Paramchuk, Executive Director, Stuart Wilkinson, Medical Director, Eric Arrata, Naturopathic Director, Peter Faris, Biostatistician (Alberta Health Services). All authors have read and approved the final manuscript.
Footnotes
Abbreviations used: BP: blood pressure; ln: natural logarithm; LOESS, locally weighted scatterplot smoothing; PNSF, Pure North S’Energy Foundation; QUICKI, quantitative insulin sensitivity check index; 25(OH)D, 25-hydroxyvitamin D.
25(OHD concentration is expressed as nmol/L; to convert to ng/mL, multiply given values by 0.4.
Literature Cited
- 1.Borges MC, Martini LA, Rogero MM. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition. 2011;27:399–404 [DOI] [PubMed] [Google Scholar]
- 2.Kaviani M, Abdollahian M, Almasi V, Amini M, Yamini AA. Effects of vitamin D on insulin resistance in nursing home residents: an interventional study. Endocrynol Pol. 2012;63:191–5 [PubMed] [Google Scholar]
- 3.Miñambres I, Sánchez-Hernández J, Sánchez-Quesada JL, Rodríguez J, de Leiva A, Pérez A. The association of hypovitaminosis D with the metabolic syndrome is independent of the degree of obesity. ISRN Endocrinology 2012; 2012:691803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan H, Knuutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97 [DOI] [PubMed] [Google Scholar]
- 5.Jialal I. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metab Syndr Relat Disord. 2012;10:1–2 [DOI] [PubMed] [Google Scholar]
- 6.Simha V, Mahmood M, Ansari M, Spellman CW, Shah P. Effect of vitamin D replacement on insulin sensitivity in subjects with vitamin D deficiency. J Investig Med. 2012;60:1214–8 [DOI] [PubMed] [Google Scholar]
- 7.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29:e142–50 [DOI] [PubMed] [Google Scholar]
- 8.Brouwer-Brolsma EM, Feskens EJM, Steegenga WT, de Groot LCPGM. Associations of 25-hydroxyvitamin D with fasting glucose, fasting insulin, dementia and depression in European elderly: the SENECA study. Eur J Nutr. 2012. Epub 2012 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick MF. D-iabetes and D-eath D-efying vitamin D. Nat Rev Endocrinol. 2012;8:388–90 [DOI] [PubMed] [Google Scholar]
- 10.Fung GJ, Steffen LM, Zhou X, Harnack L, Tang W, Lutsey PL, Loria CM, Reis JP, Van Horn LV. Vitamin D intake is inversely related to risk of developing metabolic syndrome in African American and white men and women over 20 y: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. 2012;96:24–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deleskog A, Hilding A, Brismar K, Hamsten A, Efendic S, Östenson CG. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55:1668–78 [DOI] [PubMed] [Google Scholar]
- 12.Patra SK, Nasrat H, Goswami B, Jain A. Vitamin D as a predictor of insulin resistance in polycystic ovarian syndrome. Diabetes Metab Syndr. 2012;6:146–9 [DOI] [PubMed] [Google Scholar]
- 13.Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilz S, Prioletta A, Scragg R, Volpe SL, Witham MD, Giaccari A. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr Metab Cardiovasc Dis. 2012;22:81–7 [DOI] [PubMed] [Google Scholar]
- 14.Hyppönen E, Power C. Vitamin D status and glucose homeostasis in the 1058 British Birth Cohort. Diabetes Care. 2006;29:2244–6 [DOI] [PubMed] [Google Scholar]
- 15.Chiu KC, Chu A, Go LW, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79:820–5 [DOI] [PubMed] [Google Scholar]
- 16.Tzotzas T, Papadopoulou FG, Tziomalos K, Karras S, Gastaris K, Perros P, Krassas GE. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab. 2010;95:4251–7 [DOI] [PubMed] [Google Scholar]
- 17.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab. 2012;97:1953–61. [DOI] [PubMed] [Google Scholar]
- 18.Forouhi NG, Ye Z, Rickard AP, Shaw KT, Luben R, Langenberg C, Wareham NJ. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55:2173–82 [DOI] [PubMed] [Google Scholar]
- 19.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001–2006. Diabetes Care. 2012;35:2048–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson MS, Figenschau Y, Almås B, Njølstad I, Jorde R. Serum 25-hydroxyvitamin D levels in subjects with reduced glucose tolerance and type 2 diabetes – the Tromsø OGTT-study. Int J Vitam Nutr Res. 2011;81:317–27 [DOI] [PubMed] [Google Scholar]
- 21.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham NM, Akter S, Kurotani K, Nanri A, Sato M, Hayabuchi H, Yasuda K, Mizoue T. Serum 25-hydroxyvitamin D and markers of insulin resistance in a Japanese working population. Eur J Clin Nutr. 2012;66:1323–8 [DOI] [PubMed] [Google Scholar]
- 23.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6 [DOI] [PubMed] [Google Scholar]
- 24.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomized placebo-controlled trial. Br J Nutr. 2010;103:549–55 [DOI] [PubMed] [Google Scholar]
- 25.Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. Epub 2013 Feb 13. [DOI] [PubMed] [Google Scholar]
- 26.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7 [DOI] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Orav EJ, Stähelin HB, Wolfram S, Jetter A, Schwager J, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27:160–9 [DOI] [PubMed] [Google Scholar]
- 28.Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens. 2012;25:1215–22 [DOI] [PubMed] [Google Scholar]
- 29.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1-alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–9 [DOI] [PubMed] [Google Scholar]
- 30.Heaney RP, Vitamin D. Basal status and effective dose. N Engl J Med. 2012;367:77–8 [DOI] [PubMed] [Google Scholar]
- 31.Biesalski HK, Aggett PJ, Anton R, Bernstein PS, Blumberg J, Heaney RP, Henry J, Nolan JM, Richardson DP, van Ommen B, et al. 26th Hohenheim Consensus Conference, September 11, 2010: Scientific substantiation of health claims: evidence-based nutrition. Nutrition. 2011;27:S1–20 [DOI] [PubMed] [Google Scholar]
- 32.Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225–36 [DOI] [PubMed] [Google Scholar]
- 33.Geleijnse JM. Vitamin D and the prevention of hypertension and cardiovascular diseases: a review of the current evidence. Am J Hypertens. 2011;24:253–62 [DOI] [PubMed] [Google Scholar]
- 34.Pilz S, Tomaschitz A. Role of vitamin D in arterial hypertension. Expert Rev Cardiovasc Ther. 2010;8:1599–608 [DOI] [PubMed] [Google Scholar]
- 35.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29:636–45 [DOI] [PubMed] [Google Scholar]
- 36.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9 [DOI] [PubMed] [Google Scholar]
- 37.Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;32:195–201 [DOI] [PubMed] [Google Scholar]
- 38.Lamendola CA, Ariel D, Feldman D, Reaven GM. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am J Clin Nutr. 2012;95:1055–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Gobbo LC, Song Y, Dannenbaum DA, Dewailly E, Egeland GM. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta cell function in Canadian Cree. J Nutr. 2011;141:290–5 [DOI] [PubMed] [Google Scholar]
- 40.Bonora E, Saggiani F, Targher G, Zenere MB, Alberiche M, Monauni T, Bonadonna RC, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity – studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63 [DOI] [PubMed] [Google Scholar]
- 41.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10 [DOI] [PubMed] [Google Scholar]
- 42.Willett WC. Nutritional epidemiology. 2nd ed. New York, NY: Oxford University Press; 1998. p. 13. [Google Scholar]
- 43.IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 44.Cavalier E, Delanaye P, Souberbielle J-C, Radermecker R-P. Vitamin D and type 2 diabetes mellitus: where do we stand? Diabetes Metab. 2011;37:265–72 [DOI] [PubMed] [Google Scholar]