Abstract
Regular consumption of fruits, vegetables, whole grains, and other plant foods has been negatively correlated with the risk of the development of chronic diseases. There is a huge gap between the average consumption of fruits and vegetables in Americans and the amount recommended by the 2010 Dietary Guidelines for Americans. The key is to encourage consumers to increase the total amount to 9 to 13 servings of fruits and vegetables in all forms available. Fresh, processed fruits and vegetables including frozen and canned, cooked, 100% fruit juices and 100% vegetable juices, as well as dry fruits are all considered as servings of fruits and vegetables per day. A wide variety of fruits, vegetables, whole grains, and other plant foods provide a range of nutrients and different bioactive compounds including phytochemicals, vitamins, minerals, and fibers. Potatoes serve as one of the low-fat foods with unique nutrients and phytochemical profiles, particularly rich in vitamin C, vitamin B-6, potassium, manganese, and dietary fibers. Potatoes provide 25% of vegetable phenolics in the American diet, the largest contributors among the 27 vegetables commonly consumed in the United States, including flavonoids (quercetin and kaempferol), phenolic acids (chlorogenic acid and caffeic acid), and carotenoids (lutein and zeaxanthin). More and more evidence suggests that the health benefits of fruits, vegetables, whole grains, and other plant foods are attributed to the synergy or interactions of bioactive compounds and other nutrients in whole foods. Therefore, consumers should obtain their nutrients, antioxidants, bioactive compounds, and phytochemicals from a balanced diet with a wide variety of fruits, vegetables, whole grains, and other plant foods for optimal nutrition, health, and well-being, not from dietary supplements.
Introduction
Increasing evidence suggests that a healthy eating strategy with increased consumption of plant-based foods plays important roles in the prevention of chronic diseases, such as heart disease, cancer, stroke, diabetes, Alzheimer’s disease, cataracts, and age-related function decline (1, 2). It is estimated that one third of all cancer deaths in the United States could be prevented through dietary modification (1, 3, 4). This suggests that changes in dietary patterns and lifestyle, such as increasing the consumption of fruits and vegetables and more balanced intakes of meat and plant foods, are a practical and effective strategy for reducing the incidence of chronic diseases.
The 2010 Dietary Guidelines for Americans recommend that most people, based on a 2000-kcal diet, should eat at least 9 servings of fruits and vegetables per day, 4 servings of fruits and 5 servings of vegetables (5). Actually, a 2010 study found that the average consumption of fruits and vegetables in the United States is only 3.6 servings of fruits and vegetables (1.4 servings of fruits and 2.2 servings of vegetables) per person per day (6). The gap between the recommendation and consumption is huge. To reach the goal of at least 9 servings of fruits and vegetables per day, we should continue educating Americans about the health benefits of fruits and vegetables in a balanced diet and recommend that consumers to eat a wide variety of fruits and vegetables from different sources and including all forms, fresh, frozen, canned, dried, and 100% juices, and the manufacturing of convenient packaging to make fruits and vegetables easy to serve and to store for consumers. A wide variety of fruits and vegetables provides a range of nutrients and different bioactive compounds including phytochemicals (phenolics, flavonoids, and carotenoids), vitamins (vitamin C, folate, and pro-vitamin A), minerals (potassium, calcium, and magnesium), and fibers. One of the hypotheses about the health benefits of fruits and vegetables is attributed to the synergy or interactions of bioactive compounds and other nutrients in whole foods (2).
This review focuses on the bioactive components of fruits and vegetables including potatoes, especially on phytochemicals related to the health benefits.
Phytochemicals
Fruits, vegetables, and other plant-based foods are rich in bioactive phytochemicals that may provide desirable health benefits beyond basic nutrition to reduce the risk of the development of chronic diseases (2).
Phytochemicals are bioactive non-nutrient plant compounds in fruits, vegetables, whole grains, and other plant foods that have been hypothesized to reduce the risk of major chronic diseases (2). More than 5000 individual dietary phytochemicals have been identified in fruits, vegetables, whole grains, legumes, and nuts, but a large percentage of them still remain unknown. These phytochemicals need to be isolated and identified before we can fully understand the health benefits of bioactive compounds in whole foods (7). In addition, recent research suggests that the benefits of bioactive compounds in fruits, vegetables, and other plant foods may be even greater than is currently understood because in vitro and animal studies suggest that they have multiple mechanisms of action beyond antioxidant activity (8). Because bioactive compounds differ widely in composition and ratio from fruits to vegetable to grains and often have mechanisms complementary to one another, it is suggested that, to receive the greatest health benefits, one should consume a wide variety of plant-based foods daily (2, 7).
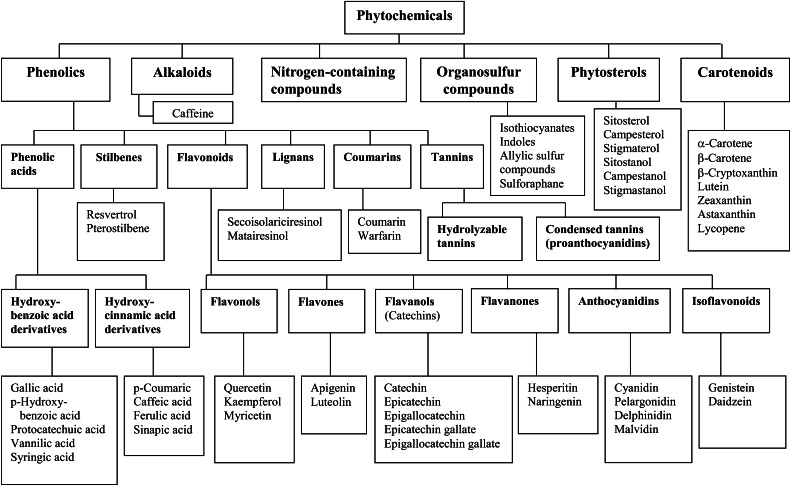
The most important groups of dietary phytochemicals can be divided into general categories as phenolics, alkaloids, nitrogen-containing compounds, organosulfur compounds, phytosterols, and carotenoids (Fig. 1) (2). The most studied groups of dietary phytochemicals related to human health and well-being are phenolics and carotenoids.
Figure 1.
Classification of dietary phytochemicals. Adapted from Reference 2 with permission.
Phenolics
Phenolics are a group of compounds with ≥1 aromatic rings possessing ≥1 hydroxyl groups. Phenolics are generally are classified as subgroups of phenolic acids, flavonoids, stilbenes, coumarins, and tannins (Fig. 1) (2). Phenolics are the products of secondary metabolism in plants; play vital roles in the reproduction, growth, and metabolism of the plants; act as defense mechanisms against pathological virus and fungus infections, parasites, and predators; and contribute to the color of plants. In addition to their functions in plants, phenolic compounds in our diet may reduce the risk of chronic diseases such as cancer, heart disease, and diabetes. Fruits and vegetables are good sources of dietary phenolics. In a study involving the 25 most common fruits consumed in the United States, wild blueberry and blackberry had the highest total phenolic contents, followed by pomegranate, cranberry, blueberry, plum, raspberry, strawberry, red grape, and apple, in order of total phenolic content (9). The remaining fruits in order of phenolic content were pear, pineapple, peach, grapefruit, nectarine, mango, kiwifruit, orange, banana, lemon, avocado, cantaloupe, honeydew, and watermelon. Apples provided 33%, of all fruit phenolics, the largest contributors, to the American diet. Among 27 common vegetables consumed in the United States, spinach had the highest phenolic content, followed by red pepper, beets, broccoli, Brussels sprouts, eggplant, asparagus, and green pepper, in order of phenolic content (10, 11). The rest of the vegetables in order of phenolic content were yellow onion, cauliflower, cabbage, radish, chili pepper, mushroom, sweet potato, carrot, sweet corn, potato, squash, white onion, green pea, tomato, green bean, celery, lettuce, romaine lettuce, and cucumber.
Clearly, phenolic compounds are not found only in fruits and vegetables with bright colors. For example, potatoes are also good sources of dietary phenolics and contain 36 mg of gallic acid equivalents per 150 g of fresh potatoes (10, 11). The total antioxidant activity of 150 g fresh weight potato was estimated to be equivalent to that of 124.5 mg of vitamin C; this is much higher than the total antioxidant activity of the 14.4 of mg of vitamin C in 150 g fresh weight potato, suggesting that the additive and/or synergistic mechanism of phytochemicals in potatoes may contribute to their antioxidant activities (11). Potatoes account for 25% of vegetable phenolics in American diet, the largest contributors among 27 vegetables commonly consumed in the United States (10).
Phenolic acids
Phenolic acids can be divided into 2 major subgroups: hydroxybenzoic acid and hydroxycinnamic acid derivatives. Hydroxybenzoic acid derivatives in plant foods include p-hydroxybenzoic, gallic acids, syringic, protocatechuic, and vanillic acids (2). They are usually present in the bound form in foods as components of complex structures such as lignins and hydrolyzable tannins or attached to cell walls and proteins. They can also be found as derivatives of sugar and organic acids in fruits, vegetables, and whole grains.
Hydroxycinnamic acid derivatives in plant foods include p-coumaric, ferulic, caffeic, and sinapic acids. They are primarily present in the bound form, connected to cell wall structural components such as cellulose, lignin, and proteins through ester bonds (2). Ferulic acids are present mainly in the seeds and leaves of plants, primarily covalently conjugated to mono- and disaccharides, glycoproteins, plant cell wall polysaccharides, polyamines, insoluble carbohydrate polymers, and lignin. Wheat bran is an excellent source of ferulic acids, which are esterified to hemicellulose of the cell walls. It was reported that ferulic acids in whole grains were present in 3 forms: free, soluble conjugated, and bound, in the ratio of 0.1:1:100 (12). Food processing including thermal processing, pasteurization, freezing, and fermentation results in the release of these bound phenolic acids (13).
Caffeic, p-coumaric, ferulic, protocatechuic, and vanillic acids are found in virtually all plant-based foods. Curcumin and chlorogenic acids are major derivatives of hydroxycinnamic acids in plants. Curcumin is made of 2 ferulic acids connected by a methylene in a diketone structure and is the major yellow pigment of the spices turmeric and mustard. Chlorogenic acids are the esters of caffeic acids and are the major substrates for enzymatic oxidation leading to browning in plants, particularly in apples and potatoes.
In potatoes, the most abundant phenolic acids are chlorogenic acid (1.0–2.2 mg/g, dry weight) and caffeic acid (19–62 μg/g, dry weight), followed by p-coumaric, ferulic acid, and gallic acid (14, 15).
Flavonoids
Flavonoids are a major group of phenolic compounds that commonly have a generic structure consisting of 2 aromatic rings (A and B rings) connected by 3 carbons that are usually in an oxygenated heterocycle ring, or C ring (2). Fruits, vegetables, and other plant foods are rich sources of flavonoids, which have been linked to reducing the risk of major chronic diseases, such as heart disease, cancer, stroke, diabetes, Alzheimer’s disease, cataracts, and age-related function decline (2). More than 5000 individual flavonoids have been isolated and identified. Structural differences in the heterocycle C ring categorize them as flavonols (quercetin, kaempferol, and myricetin), flavones (luteolin and apigenin), flavonols (catechin, epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate), flavonones (naringenin), anthocyanidins (cyanidin and malvidin), and isoflavonoids (genistein and daidzein). These are common flavonoids in the diet. Dietary flavonoids are most commonly found in nature as conjugates in glycosylated or esterified forms, but can be present as aglycones, especially in cooked or processed plant foods. Many different glycosylated forms can be found in nature because >80 different sugars have been reported bound to flavonoids in plant foods (16). Anthocyanidins provide unique colors in some fruits, vegetables, and whole grains. Apples are good sources for quercetin, epicatechin, and cyanidin. The major flavonoids in oranges and orange juices are hesperetin and naringenin.
In potatoes, the most abundant flavonoids are quercetin glycoside, quercetin, kaempferol glycoside, and kaempferol, followed by catechin and rutin (14, 15). Color-fleshed potatoes are rich in anthocyanins, mainly derivatives of cyanidin, patanin, delphinidin, and peonidin (15), and total anthocyanin contents in color-fleshed potatoes range from 14 to 16,330 μg/g dry weight (15).
The estimate of human intake of all flavonoids was a few hundred milligrams to 650 mg/d (17, 18). Total average intake of flavonols (quercetin, myricetin, and kaempferol) and flavones (luteolin and apigenin), major flavonoids in human diet, was estimated as 23 mg/d, of which quercetin contributed ∼70%, kaempferol 17%, myricetin 6%, luteolin 4%, and apigenin 3% (19).
Carotenoids
Carotenoids are classified into hydrocarbons (carotenes) and their oxygenated derivatives (xanthophylls), with a 40-carbon skeleton of isoprene units (2). It is estimated that >600 distinct carotenoids have been isolated and identified with yellow, orange, and red colors and are present widely in fruits, vegetables, whole grains, and other plants. In terms of health benefits, carotenoids have received considerable attention because of their unique physiological functions as provitamins and antioxidant effects, especially in scavenging singlet oxygen.
The structures of carotenoids are cyclized at 1 or both ends, have various hydrogenation levels or possess oxygen-containing functional groups. β-Carotene and lycopene are examples of cyclized and acyclized carotenoids, respectively. Carotenoids are primarily in the all-trans form in nature. The central part of the molecule is formed by a long series of conjugated double bonds that provide carotenoids with their shape, chemical reactivity, and light-absorbing properties. β-Carotene, α-carotene, and β-cryptoxanthin have pro-vitamin A activity and can be converted to retinol (vitamin A) after being metabolized in humans. Zeaxanthin and lutein are the essential carotenoids in the macular region (yellow spot) of the retina of eyes in humans. A diet rich in zeaxanthin and lutein has been associated with a reduced risk of the development of cataract and macular degeneration.
Orange and yellow vegetables and fruits, including carrots, spinach, pumpkins, papayas, sweet potatoes, winter squash, mangoes, cantaloupes, and red peppers, are rich sources of β-carotene. Dark green leafy vegetables, including spinach, kale, turnip greens, broccoli, Brussels sprouts, and collards, are rich sources of lutein and zeaxanthin. Tomatoes, watermelons, pink grapefruits, apricots, and pink guavas are the most common sources of lycopene. It has been estimated that 85% of lycopene intake in the United States is from processed tomato products such as ketchup, tomato paste, and tomato soup. The most abundant carotenoids in potatoes are lutein and zeaxanthin, followed by β-carotene and β-cryptoxanthin (15).
Carotenoids play essential functions in photosynthesis and photoprotection in plants. The photoprotection role of carotenoids in plants is due to their ability to quench reactive oxygen species, especially singlet oxygen, which is formed from exposure of light and radiation. Carotenoids can react with free radicals and become radicals themselves. Their reactivity is mainly influenced by the length of the chain of conjugated double bonds and the characteristics of the end functional groups. Carotenoid radicals are stabilized by delocalization of unpaired electrons over the conjugated polyene chain of the molecules. This delocalization also allows additional reactions to occur at many sites on the radical molecules (20). Carotenoids are especially powerful in scavenging singlet oxygen generated from light induced lipid oxidation or radiation. Astaxanthin, zeaxanthin, and lutein are excellent in scavenging free radicals because of the unique end functional groups.
Vitamins
Potatoes are good sources of vitamin C (ascorbic acid). Andre et al. (21) reported that the vitamin C content in potatoes ranged from 22 to 69 mg per 100 g dry weight depending on cultivars. One medium-size baked potato (173 g, fresh weight) provides 16.6 mg of vitamin C (22), which could meet 27.7% of daily value. This is very important for people to obtain sufficient amount of vitamin C in many areas of the world, where potatoes are a dominant vegetable. Vitamin C is an essential nutrient and plays an important function in collagen synthesis to prevent scurvy, a vitamin C deficiency disease. Vitamin C is also an excellent antioxidant to scavenge free radicals and to prevent oxidative stress. Potatoes are also good sources of vitamin B-6, which is essential for regulating nervous system function and metabolism. One medium-size baked potato (173 g, fresh weight) provides 0.54 mg of vitamin B-6, which could meet 27% of daily value (22).
Glycoalkaloids
Glycoalkaloids are natural toxins produced in potatoes during germination. The 2 major glycoalkaloids in potatoes are α-chaconine and α-solanine, and the ratio of these compounds varies (23). Glycoalkaloids are synthesized as natural defense mechanisms against pathogens, insects, parasites, and predators and are mainly localized in the skin with the highest levels around the eyes of outer layer of potatoes. These compounds are toxic to humans and can cause death at concentrations >330-mg/kg sample. The LD50 values of α-chaconine and α-solanine are 23 and 34 mg/kg (23). Removal of sprouts and peels of potatoes before cooking eliminates almost all glycoalkaloids.
Dietary phytochemicals in the prevention of cardiovascular disease
Many epidemiological studies have examined the role of phytochemicals and increased dietary intake of fruits and vegetables in the prevention of cardiovascular disease (CVD). Consumption of flavonoids in humans was significantly inversely correlated with mortality from coronary heart disease (CHD) and with the incidence of myocardial infarction (19). Dietary flavonoid intake was also inversely associated with CHD mortality (24). The total intake of flavonoids (quercetin, myricetin, kaempferol, luteolin, and ficetin) was inversely associated with the LDL cholesterol and plasma total cholesterol concentrations (25). As a single phytochemical, intake of quercetin was inversely correlated with LDL cholesterol and total cholesterol plasma levels. In a study involving subjects from the NHANES Epidemiological Follow-up Study, there was a 27% lower CVD mortality rate with the consumption of fruits and vegetables at least 3 times per day compared with only once per day. Intake of fruits and vegetables was inversely correlated with the incidence of stroke, stroke mortality, CVD mortality, CHD mortality, and all-cause mortality (26). Joshipura et al. (27) reported that total consumption of fruits and vegetables was correlated with a decreased risk of CHD, and an inverse association between total consumption of fruits and vegetables and CHD was observed with an intake of >4 servings per day. The Women’s Health Study subjects had an RR of 0.68 for CVD when comparing the highest versus the lowest quintiles of fruit and vegetable intake, and the RR for myocardial infarction was only 0.47. It was reported that there was a 20–30% reduction in risk of CVD associated with high intake of fruits and vegetables (28).
In a study of 22,043 adults in Greece, a higher degree of adherence to the Mediterranean diet with increased consumption of fruits and vegetables was found to be correlated with a 25% of reduction in total mortality and a 33% of reduction in death due to CHD (29). In a community-dwelling population in Washington County, Maryland, Genkinger et al. (30) found that subjects with the highest quintile of fruit and vegetable intake had a lower risk of all-cause mortality (RR: 0.63) and CVD mortality (RR: 0.76) compared with those in the lowest quintile. A combined analysis of >100,000 participants in the Health Professionals’ Follow-up Study and the Nurses’ Health Study showed that intake of fruits and vegetables was inversely correlated with the risk of CVD, with an RR for an increment of 5 servings daily of 0.88, with green leafy vegetables showing the strongest inverse correlation (31). A 2008 study conducted by Heidemann et al. (32) of 72,113 women without any history of CVD or cancer, it was showed that a more prudent diet with a high intake of fruits and vegetables was associated with a 17% lower risk of all-cause mortality and a 28% lower risk of cardiovascular mortality when comparing individuals the highest to the lowest quintile of diet prudency (32). Furthermore, a population-based cohort study in the Netherlands, in addition to finding that the relative risk of CHD incidence was 0.66 for subjects with a high intake of fruits and vegetables when compared to those with low consumption, also found that this inverse relationship was present regardless of whether the fruits and vegetables were raw or processed (33).
The LDL oxidation hypothesis has been suggested as the atherogenic factor contributing to CVD (34, 35). Circulating LDL infiltrates the artery wall and increase intimal LDL. Intimal LDL can be oxidized by free radicals. The oxidized LDL in the intima is more atherogenic than native LDL and serves as a chemotactic factor recruiting circulating monocytes and macrophages into the intima. Oxidized LDL is taken up by macrophages in the intima, further inducing the formation of inflammatory cytokines and promoting cell proliferation of smooth muscle cells, cholesterol ester accumulation, and foam cell formation. Foam cell accumulation in the blood vessel would form a fatty streak, resulting in further endothelial injury and leading to atherosclerotic disease. LDL oxidation is critical in the initiation and progression of atherosclerosis. Therefore, it has been hypothesized that dietary antioxidants or phytochemicals scavenge free radicals and prevent LDL oxidation and might prevent or delay the progression of atherosclerotic lesions (36). In addition, dietary antioxidants or phytochemicals have been shown to have roles in the reduction of platelet aggregation, modulation of cholesterol synthesis and absorption and lipid profiles, reduction of blood pressure, and anti-inflammation.
Some human clinical trials investigating the effects of fruit and vegetable intake on CVD have been conducted, including known CVD contributing factors and mechanisms. In a randomized, controlled trial conducted by Watzl et al. (37), a group of nonsmoking men consumed a diet of <2 servings per day of fruits and vegetables for 4 wk. After this time period, the men were then randomly assigned to consume either 2 servings per day, 5 servings per day, or 8 servings per day of carotenoid-rich fruits and vegetables for an additional 4-wk period. Compared with the low-intake group (2 servings per day), the high-intake group was found to have significantly increased total carotenoid concentrations in plasma as well as significantly reduced C-reactive protein levels at week 8 (37).
Dietary phytochemicals in the prevention of cancer
It is estimated that one third of all cancer deaths in the United States could be prevented through the dietary modification (1, 3, 38). Increasing bioactive compounds and antioxidant defenses through dietary phytochemicals, present in fruits, vegetables, whole grains, and other plant foods, may prevent, reduce, or delay the oxidation of DNA and affect cellular signal transduction pathways controlling cell proliferation and apoptosis (2). The evidence supporting a high consumption of fruits and vegetables to reduce the risk of the development of cancer is reviewed in the following.
Consumption of ≥28 servings of vegetables per week showed a 35% reduction in the risk of prostate cancer compared with an intake of <14 servings per week (39). In another study, fruit and vegetable consumption correlated with the reduced risk of pancreatic cancer (40). Compared with those in the lowest quartile of total intake of fruits and vegetables, those in the highest quartile had an RR of 0.47. For total fruits and fruit juice, the RR was 0.72, and for total vegetables it was 0.45. There was a significant inverse association found among dark leafy vegetables, cruciferous vegetables, yellow vegetables, carrots, beans, onions, and garlic.
Voorrips et al. (41) reported in a large Dutch study that the intake of fruits and vegetables was inversely associated with reduced risk of colon cancer in women. No relationship was found between fruit and vegetable consumption and colon cancer risk in men. In the Nurses’ Health Study, consumption of fruits was inversely correlated with polyp formation (42). Subjects who ate ≥5 servings of fruits per day had a reduced risk of the development of colorectal adenomas compared with those who ate ≤1 serving (OR: 0.6; 95% CI: 0.44, 0.81). However, the risk reduction for vegetables was not significant (OR: 0.82; 95% CI: 0.65, 1.05).
In a 2003 study of 22,043 adults, a higher degree of adherence to the Mediterranean diet with an increased consumption of fruits and vegetables was found to be associated with a 0.76 RR of death due to cancer (95% CI: 0.47, 0.94) (28). In a community-dwelling population in Washington County, Maryland, in addition to finding that participants in the highest quintile of fruit and vegetable intake had a lower risk of all-cause mortality (RR: 0.63; 95% CI: 0.51, 0.78) and CVD mortality, Genkinger et al. (30) also found that they exhibited a lower risk of cancer-caused mortality (RR: 0.65; 95% CI: 0.45, 0.93) compared with those in the lowest quintile. In a European prospective cohort study, the overall cancer risk was found to be slightly reduced (RR: 0.97; 95% CI: 0.96, 0.99) when the intake of total fruits and vegetables combined was increased by 200 g/d (43).
In 2009, van Duijnhoven et al. (44) reported that increased consumption of fruits and vegetables was correlated with a decreased risk of colon cancer (RR: 0.76; 95% CI: 0.63, 0.91) when comparing those in the highest and lowest quintiles. In a US study, subjects who ate ∼5.8 servings of fruits and vegetables daily had a significantly reduced risk of cancers of the oral cavity, pharynx, and larynx (RR: 0.71; 95% CI: 0.55, 0.92) compared with those who only consumed ∼1.5 servings per day (45).
In our laboratory, rats were fed diets supplemented with whole apple extracts equivalent to human consumption of 1, 3, and 6 apples per day over the 24-wk study (46). Mammary cancer in rats was induced at day 50 with 7,12-dimethylbenz[a]anthracene. Apple supplementation decreased mammary cancer incidence, tumor yield, and tumor burden in a dose-dependent manner, indicating that apple consumption may be an accessible method of cancer prevention.
Carcinogenesis is a multistage process with initiation, promotion, and progression, and oxidative damage and chronic inflammation play important roles in the formation of cancer via different mechanisms (47, 48). Free radicals induce DNA oxidative damage in the stage of initiation. If left unrepaired, the damaged DNA can lead to base mutation, single- and double-strand breaks, DNA cross-linking, and chromosomal breakage and rearrangement (47). This potentially cancer-inducing oxidative damage in the initiation stage might be prevented or retarded by dietary antioxidants or phytochemicals from fruits, vegetables, whole grains, and other plant foods. Dietary phytochemicals also play important roles in the stages of promotion and progression of carcinogenesis by regulating different signal transduction pathways with multiple molecular targets. Dietary phytochemicals from fruits, vegetables, whole grains, and other plant foods have been demonstrated to have complementary and overlapping mechanisms of action for cancer prevention (Table 1), including scavenging free radicals, deactivation of carcinogens, modulation of detoxification phase II enzymes, DNA damage repair, inhibition of cell proliferation, regulation of cell cycle and gene expression through signal transduction pathways, induction of apoptosis, inhibition of nuclear factor κB activation and anti-inflammation, antiangiogenesis, stimulation of the immune system, regulation of hormone metabolism and receptors, and antibacterial and antiviral effects (8).
Table 1.
Potential mechanisms of actions of dietary phytochemicals for cancer prevention
| Antioxidant activity |
| Scavenge free radicals and oxidants |
| Reduce oxidative stress |
| Inhibit nitrosation and nitration |
| Prevent DNA binding and damage |
| Regulation of DNA damage repair |
| Induction of cell differentiation |
| Inhibition of cell proliferation |
| Induction of cell-cycle arrest |
| Inhibition of oncogene expression |
| Induction of tumor suppress gene expression |
| Induction of apoptosis |
| Regulation of signal transduction pathways |
| Enzyme induction and enhancing detoxification |
| Phase II enzyme |
| Glutathione peroxidase |
| Catalase |
| Superoxide dismutase |
| Enzyme inhibition |
| Cyclooxygenase-2 |
| Inducible nitric oxide synthase |
| Xanthine oxide |
| Phase I enzyme (block activation of carcinogens) |
| Anti-inflammation |
| Enhancement of immune function and surveillance |
| Antiangiogenesis |
| Inhibition of cell adhesion and invasion |
| Regulation of steroid hormone metabolism |
| Regulation of estrogen metabolism |
| Antibacterial and antiviral effects |
Adapted from Reference 2 with permission.
Role of whole food in the prevention of chronic diseases
The hypothesis that dietary antioxidants or phytochemicals lower the risk of the development of chronic diseases has been primarily formulated from epidemiological observation studies, which have shown that regular consumption of fruits, vegetables, and whole grains is inversely associated with a reduced risk of the development of chronic diseases. However, this hypothesis has been questioned recently because, taken alone, the individual antioxidants or phytochemicals studied in clinical trials do not appear to have consistent beneficial effects, and the actions of the dietary supplements alone do not explain the observed health benefits of diets rich in fruits, vegetables, and whole grains (49–51).
The isolated compounds as dietary supplements in pure form may not work in the same way as the compounds in whole foods and, in addition to having fewer of the beneficial effects, may also be potentially detrimental. β-Carotene is an excellent dietary antioxidant in fruits and vegetables and was thought to prevent lung cancer based on several epidemiological studies. However, in human clinical trials, smokers did not have positive effect of supplemental β-carotene with respect to lung cancer incidence, and there might even have been a significant increase in the lung cancer rate and total mortality rate (49, 52). Several studies showed that vitamin C supplements had no effect on lowering the incidence of cancer or CHD in human clinical trials (53, 54). Vitamin E supplements failed to show any beneficial effect on the endpoints of myocardial infarction, stroke, or death for the patients who had had a myocardial infarction (55). In the Heart Outcomes Prevention Evaluation (HOPE) study, patients at high risk of CVD were given 400 IU/d vitamin E or a placebo for 4.5 y, but no differences were found in deaths from cardiovascular causes or myocardial infarctions or deaths from CHD or stroke between the placebo and vitamin E groups (56). In a clinical trial to study the potential effect of selenium and vitamin E on prostate cancer prevention [Selenium and Vitamin E Cancer Prevention Trial (SELECT)], an increased risk of prostate cancer was observed in the group taking vitamin E, and an increased risk of type 2 diabetes was found in the group taking selenium (57). Although these increased risks were statistically nonsignificant (P = 0.06 and P = 0.16, respectively), the clinical trial had to be terminated because of ethical and safety concerns. In the follow-up studies of SELECT with additional new cases of prostate cancer since the first report in 2009, the prostate cancer incidence in the vitamin E supplementation group was 17% higher (P = 0.008) compared with the placebo group, indicating that vitamin E supplementation significantly increased the risk of prostate cancer among healthy men (58).
Dietary phytochemicals of fruits, vegetables, whole grains, and other plant foods were shown to have potent antioxidant activity, and the mixture or combination of phytochemicals in fruits, vegetables, and whole grains was proposed to be responsible for their strong antioxidant and anticancer activity (9, 10, 59–61). It was reported that whole apple phytochemical extracts exhibited strong antioxidant activity and inhibited tumor cell growth in vitro in a dose-dependent manner (61). Further studies demonstrated that whole apple phytochemical extracts inhibited mammary cancer in a rat model in a dose-dependent manner at doses comparable to those of human consumption of 1, 3, and 6 apples per day (46). This study demonstrated that whole apple phytochemical extracts effectively inhibited mammary cancer growth in the rat model; thus, consumption of apples may be an effective strategy for cancer protection. In a study examining the possible additive, synergistic, or antagonistic interactions among apple phytochemicals, apple phytochemical extracts and quercetin 3-β-d-glucoside in combination exhibited a synergistic effect against MCF-7 human breast cancer cell proliferation (62). The additive and synergistic effects of phytochemicals in fruits, vegetables and whole grains have been proposed to be responsible for their potent antioxidant and anticancer activities. This partially explains why no single antioxidant can replace the combination of natural phytochemicals in fruits and vegetables to achieve the observed health benefits.
Milenkovic et al. (63) reported a human nutrigenomics study by examining the effects of orange juice and hesperidin, a pure citric phytochemical, on the expression of genes in leukocytes in healthy volunteers after consumption of orange juice, hesperidin, or placebo for 4 wk. The profiles of global gene expression were compared using human whole genome cDNA microarrays. Both orange juice and hesperidin intake significantly affected expression of leukocyte genes. Intake of orange juice modulated changes in expression of 3422 genes, intake of hesperidin induced the expression of 1819 genes, and 1582 genes were in common in both groups. Many genes affected by intake of orange juice and hesperidin are genes involved in lipid transport, adhesion, chemotaxis, and cell infiltration, suggesting lower recruitment and infiltration of circulating cells to vascular wall and intima, lower lipid accumulation, and formation of the atherosclerotic plaque. This study also supported the importance of whole food instead of a single phytochemical.
Fruits, vegetables, whole grains, and other plant foods have their distinctive phytochemical profiles and compositions (11, 12, 59, 60, 64–68). These distinctive phytochemicals are different in molecular size, polarity, solubility, bioavailability, metabolic pathways, and excretion. All these will affect the distribution and concentrations of each phytochemical in different organs, tissues, cells, and subcellular organelles. Therefore, the health benefits of fruits, vegetables, whole grains, and other plant foods cannot be achieved or mimicked by dietary supplements in the form of tables or pills without fully upstanding the balanced natural combination and profiles of phytochemicals present in fruits, vegetables, and whole grains. People should obtain their different antioxidants or phytochemicals from a balanced diet with a wide variety of fruits, vegetables, whole grains, and other plant foods for optimal health benefits and well-being, not from dietary supplements. More importantly, obtaining antioxidants or phytochemicals by eating whole foods with a wide variety of fruits, vegetables, whole grains, and other whole foods is generally considered safe and is not likely to result in consumption of toxic quantities compared with the consumption of pure phytochemicals as dietary supplements in the form of tablets or capsules. Fruits and vegetables eaten in the recommended amounts (9–13 servings of fruits and vegetables per day) are safe.
Conclusions
Regular consumption of fruits, vegetables, whole grains, and other plant foods has been negatively correlated with the risk of the development of chronic diseases. There is a huge gap between the average consumption of fruits and vegetables in Americans and the amount recommended by the 2010 Dietary Guidelines for Americans. The key is to encourage consumers to increase the total amount to 9 to 13 servings of fruits and vegetables in all forms available. Fresh, processed fruits and vegetables including frozen and canned, cooked, 100% fruit juices, and 100% vegetable juices, as well as dry fruits are all considered servings of fruits and vegetables per day.
Potatoes are one of the low-fat foods with unique nutrients and phytochemical profiles and are particularly rich in vitamin C, vitamin B-6, potassium, manganese, and dietary fibers. Potatoes account for 25% of vegetable phenolics in the American diet, the largest contributors among the 27 vegetables commonly consumed in the United States, including flavonoids (quercetin and kaempferol), phenolic acids (chlorogenic acid and caffeic acid), and carotenoids (lutein and zeaxanthin). Increasing evidence suggests that the health benefits of fruits, vegetables, whole grains, and other plant foods are attributed to the synergy or interactions of bioactive compounds and other nutrients in whole foods. Therefore, consumers should obtain their nutrients, antioxidants, bioactive compounds, or phytochemicals from their balanced diet with a wide variety of fruits, vegetables, whole grains, and other plant foods for optimal nutrition, health, and well-being, not from dietary supplements. Further research on the health benefits of whole foods is warranted.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Literature Cited
- 1.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296:695–8 [DOI] [PubMed] [Google Scholar]
- 2.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–85S [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Peto R. Avoidable risks of cancer in the United States. J Natl Cancer Inst. 1981;66:1191–308 [PubMed] [Google Scholar]
- 4.Willett WC. Diet, nutrition, and avoidable cancer. Environ Health Perspect. 1995;103:165–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.USDA. Dietary Guidelines for Americans 2010. USDA Human Nutrition Information Service, Hyattsville, MD. 2010. [Google Scholar]
- 6. PBH, Produce for Better Health Foundation. State of the plate: 2010 Study on America#x2019s consumption of fruits and vegetables. 2010.
- 7.Liu RH. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am J Clin Nutr. 2003;78:517S–20S [DOI] [PubMed] [Google Scholar]
- 8.Liu RH, Finley J. Potential cell culture models for antioxidant research. J Agric Food Chem. 2005;53:4311–4 [DOI] [PubMed] [Google Scholar]
- 9.Wolfe KL, Kang XM, He XJ, Dong M, Zhang QY, Liu RH. Cellular antioxidant activity of common fruits. J Agric Food Chem. 2008;56:8418–26 [DOI] [PubMed] [Google Scholar]
- 10.Song W, Derito CM, Liu KM. Liu H X, Dong M, Liu RH. Cellular antioxidant activity of common vegetables. J Agric Food Chem. 2010;58:6621–9 [DOI] [PubMed] [Google Scholar]
- 11.Chu Y-F, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of vegetables. J Agric Food Chem. 2002;50:6910–6 [DOI] [PubMed] [Google Scholar]
- 12.Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem. 2002;50:6182–7 [DOI] [PubMed] [Google Scholar]
- 13.Dewanto V, Wu X, Liu RH. Processed sweet corn has higher antioxidant activity. J Agric Food Chem. 2002;50:4959–64 [DOI] [PubMed] [Google Scholar]
- 14.Shakya R, Navarre DA. Rapid screening of ascorbic acid, glycoalkaloids, and phenolics in potato using high-performance liquid chromatography. J Agric Food Chem. 2006;54:5253–60 [DOI] [PubMed] [Google Scholar]
- 15.Andre CM, Oufir M, Guignard C, Hoffmann L, Hausman JF, Evers D, Larondelle Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of beta-carotene, alpha-tocopherol, chlorogenic acid, and petanin. J Agric Food Chem. 2007;55:10839–49 [DOI] [PubMed] [Google Scholar]
- 16.Hollman PCH, Arts ICW. Flavonols, flavones and flavonols - nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1081–93 [Google Scholar]
- 17.Hollman PCH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–42 [DOI] [PubMed] [Google Scholar]
- 18.Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–91 [PubMed] [Google Scholar]
- 19.Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–11 [DOI] [PubMed] [Google Scholar]
- 20.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–8 [PubMed] [Google Scholar]
- 21.Andre CM, Ghislain M, Bertin P, Oufir M, Herrera Mdel R, Hoffmann L, Hausman JF, Larondelle Y, Evers D. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J Agric Food Chem. 2007;55:366–78 [DOI] [PubMed] [Google Scholar]
- 22. USDA National Nutrient Database for Standard Reference, Release 24, 2011, accessed on June 12, 2012. http://ndb.nal.usda.gov/
- 23.Friedman M. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem. 2006;54:8655–81 [DOI] [PubMed] [Google Scholar]
- 24.Hertog MGL, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S. Flavonoid intake and long-term risk of coronary heart disease and cancer in the Seven Countries Study. Arch Intern Med. 1995;155:381–6 [PubMed] [Google Scholar]
- 25.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–50 [DOI] [PubMed] [Google Scholar]
- 26.Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–9 [DOI] [PubMed] [Google Scholar]
- 27.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–14 [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Manson JE, Lee I-M, Cole SR, Hennekens CH, Willett WC, Buring JE. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. Am J Clin Nutr. 2000;72:922–8 [DOI] [PubMed] [Google Scholar]
- 29.Trichopoulou A, Costacou T. , Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608 [DOI] [PubMed] [Google Scholar]
- 30.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–33 [DOI] [PubMed] [Google Scholar]
- 31.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84 [DOI] [PubMed] [Google Scholar]
- 32.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all-causes in a prospective cohort of women. Circulation. 2008;118:230–7. [DOI] [PMC free article] [PubMed]
- 33.Oude Griep LM, Geleijnse JM, Kromhout D, Ocké MC, Verschuren WMM. Raw and processed fruit and vegetable consumption and 10-year coronary heart disease incidence in a population-based cohort study in the Netherlands. PLoS ONE. 2010;5:e13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Oxidized lipids in atherogenesis: formation, destruction and action. Thromb Haemost. 1997;78:195–9 [PubMed] [Google Scholar]
- 35.Witztum JL, Berliner JA. Oxidized phospholipids and isoprostanes in atherosclerosis. Curr Opin Lipidol. 1998;9:441–8 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Moreno C, Jimenez-Escrig A, Saura-Calixto F. Study of low-density lipoprotein oxidizability ;Indexes to measure the antioxidant activity of dietary polyphenols. Nutr Res. 2000;20:941–53 [Google Scholar]
- 37.Watzl B, Kulling SE, Möseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82:1052–8 [DOI] [PubMed] [Google Scholar]
- 38.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–8 [DOI] [PubMed] [Google Scholar]
- 40.Chan JM, Wang F, Holly EA. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol Biomarkers Prev. 2005;14:2093–7 [DOI] [PubMed] [Google Scholar]
- 41.Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2000;152:1081–92 [DOI] [PubMed] [Google Scholar]
- 42.Michels KB, Giovannucci E, Chan AT, Singhania R, Fuchs CS, Willett WC. Fruit and vegetable consumption and colorectal adenomas in the Nurses’ Health Study. Cancer Res. 2006;66:3942–53 [DOI] [PubMed] [Google Scholar]
- 43. Boffetta P, Couto E, Wichmann J, Ferrari P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, BüCchner FL, Key T, Boeing H, et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2010;21;102:529–37. [DOI] [PubMed]
- 44.van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–52 [DOI] [PubMed] [Google Scholar]
- 45.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122:2330–6 [DOI] [PubMed] [Google Scholar]
- 46.Liu RH, Liu J, Chen B. Apples prevent mammary tumors in rats. J Agric Food Chem. 2005;53:2341–3 [DOI] [PubMed] [Google Scholar]
- 47.Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res. 1991;250:3–16 [DOI] [PubMed] [Google Scholar]
- 48.Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res. 1995;339:73–89 [DOI] [PubMed] [Google Scholar]
- 49.Omenn GS, Goodman GE, Thomquist MD, Barnes J, Cullen MR. Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5 [DOI] [PubMed] [Google Scholar]
- 50.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet. 1996;347:781–6 [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60 [DOI] [PubMed] [Google Scholar]
- 52.The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-tocopherol, Beta-carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1020–35 [DOI] [PubMed] [Google Scholar]
- 53.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–92 [DOI] [PubMed] [Google Scholar]
- 54.Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Lakka TA, Rissanen T, Leskinen L, et al. Antioxidant supplementation in atherosclerosis prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J Intern Med. 2000;248:377–86 [DOI] [PubMed] [Google Scholar]
- 55.GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55 [PubMed] [Google Scholar]
- 56. Yusuf S, Dagenais G, Pogue J, Bosch J. Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. [DOI] [PubMed]
- 57.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effects of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun J, Chu Y-F, Wu X, Liu RH. Antioxidant and antiproliferative activities of fruits. J Agric Food Chem. 2002;50:7449–54 [DOI] [PubMed] [Google Scholar]
- 60.Chu Y-F, Liu RH. Cranberries inhibit LDL oxidation and induce LDL receptor expression in hepatocytes. Life Sci. 2005;77:1892–901 [DOI] [PubMed] [Google Scholar]
- 61.Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature. 2000;405:903–4 [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Liu RH. Synergistic effect of apple extracts and quercetin 3-β-D-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem. 2009;57:8581–6 [DOI] [PubMed] [Google Scholar]
- 63.Milenkovic D, Deval C, Dubray C, Mazur A, Morand C. Hesperidin displays relevant role in the nutrigenomic effect of orange juice on blood leukocytes in human volunteers: a randomized controlled cross-over study. PLoS ONE. 2011;6:e26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of wheat varieties. J Agric Food Chem. 2003;51:7825–34 [DOI] [PubMed] [Google Scholar]
- 65.Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem. 2005;53:2297–306 [DOI] [PubMed] [Google Scholar]
- 66.Adom KK, Liu RH. A rapid peroxylradical scavenging capacity (PSC) assay for assessing both hydrophilic and lipophilic antioxidants. J Agric Food Chem. 2005;53:6572–80 [DOI] [PubMed] [Google Scholar]
- 67.He X, Liu RH. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J Agric Food Chem. 2007;55:4366–70 [DOI] [PubMed] [Google Scholar]
- 68.He X, Liu RH. Phytochemicals of apple peels: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 2008;56:9905–10 [DOI] [PubMed] [Google Scholar]