Abstract
The second messenger cyclic di-GMP (c-di-GMP) is a nearly ubiquitous intracellular signal molecule known to regulate various cellular processes, including biofilm formation, motility, and virulence. The intracellular concentration of c-di-GMP is inversely governed by diguanylate cyclase (DGC) enzymes and phosphodiesterase (PDE) enzymes, which synthesize and degrade c-di-GMP, respectively. The role of c-di-GMP in the plant pathogen and causal agent of fire blight disease Erwinia amylovora has not been studied previously. Here we demonstrate that three of the five predicted DGC genes in E. amylovora (edc genes, for Erwinia diguanylate cyclase), edcA, edcC, and edcE, are active diguanylate cyclases. We show that c-di-GMP positively regulates the secretion of the main exopolysaccharide in E. amylovora, amylovoran, leading to increased biofilm formation, and negatively regulates flagellar swimming motility. Although amylovoran secretion and biofilm formation are important for the colonization of plant xylem tissues and the development of systemic infections, deletion of the two biofilm-promoting DGCs increased tissue necrosis in an immature-pear infection assay and an apple shoot infection model, suggesting that c-di-GMP negatively regulates virulence. In addition, c-di-GMP inhibited the expression of hrpA, a gene encoding the major structural component of the type III secretion pilus. Our results are the first to describe a role for c-di-GMP in E. amylovora and suggest that downregulation of motility and type III secretion by c-di-GMP during infection plays a key role in the coordination of pathogenesis.
INTRODUCTION
Erwinia amylovora is the causal agent of fire blight and a devastating phytopathogen that infects plant species of the family Rosaceae, most notably apple and pear trees (1). E. amylovora can infect flowers, fruits, actively growing shoots, and rootstock crowns (2). During the primary infection via the flower (1), E. amylovora cells multiply rapidly on the stigma. Motility and free moisture are important factors in the subsequent dissemination of cells down the outside of the stigma to nectarthodes, which provide entry into the plant (3–5).
Following flower infection, E. amylovora cells spread systemically through host vascular tissues and cortical parenchyma. The wilting symptoms of fire blight are the result of bacterial invasion, the secretion of extracellular polysaccharide (EPS), and the formation of biofilms within host xylem that plug these tubes, restricting water flow (6, 7). E. amylovora secretes two distinct EPSs, amylovoran and levan, both of which contribute to plant infection (6). Amylovoran is an acidic polysaccharide composed of repeating units of galactose and glucuronic acid (8–10), while levan is a homopolymer of fructose residues synthesized from sucrose by the secreted enzyme levansucrase (11). Biofilm formation by E. amylovora is required for effective colonization of host xylem tissues, the exit of pathogen cells from infected leaves into host stems, and systemic spread within trees (6, 7). The impact of biofilm formation on xylem colonization has also been noted for several other plant pathogens, including Clavibacter michiganensis, Pantoea stewartii, and Xylella fastidiosa (12–14), and the ability to form biofilms appears to be a common strategy for the survival or transmission of phytopathogens (6, 15).
The second messenger cyclic di-GMP (c-di-GMP) regulates biofilm formation in the majority of bacteria. In general, a high level of intracellular c-di-GMP positively regulates biofilm formation and negatively regulates swimming motility (16–19). c-di-GMP is synthesized by diguanylate cyclase (DGC) enzymes encoding GGDEF domains and is degraded by phosphodiesterase (PDE) enzymes encoding either an EAL or a HD-GYP domain. c-di-GMP exhibits diverse functions in plant pathogens: it negatively regulates the pathogenesis of Xanthomonas campestris and Dickeya dadantii in plants (20) but positively influences plant colonization by Xylella fastidiosa (15).
Because c-di-GMP is an essential signaling molecule that is necessary for EPS secretion and biofilm formation in many bacteria, and because amylovoran secretion and biofilm formation are critical for E. amylovora virulence (6), we hypothesized that c-di-GMP signaling would positively influence E. amylovora virulence. We systematically determined that two of the five DGCs in E. amylovora, edcC and edcE, encode proteins that synthesize c-di-GMP and positively regulate amylovoran production and biofilm formation while negatively regulating flagellum-based motility. Importantly, although biofilm formation and amylovoran secretion levels were reduced in E. amylovora edcC and edcE mutants, these mutants exhibited increased tissue necrosis in two plant infection models. This result could be explained partly by repression of the type III secretion system (T3SS) gene hrpA by c-di-GMP. Our results suggest that c-di-GMP signaling plays a key role in the establishment and development of plant infections by limiting the virulence of E. amylovora.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Unless otherwise mentioned, E. amylovora strain Ea1189 and Escherichia coli strains were grown in Luria-Bertani (LB) broth and plates at 28°C and 37°C, respectively. Amylovoran secretion assays for wild-type (WT) and mutant strains were conducted in MBMA medium [per liter, 3 g KH2PO4, 7 g K2HPO4, 1 g (NH4)2SO4, 2 ml glycerol, 0.5 g citric acid, and 0.03 g MgSO4] amended with 1% sorbitol, while those for the overexpression strains were conducted in MBMA-LB (3:1) medium. For biofilm formation assays, WT, mutant, and overexpression strains were grown in 0.5× LB medium. Media were amended with kanamycin (Km; 100 μg/ml), ampicillin (Ap; 100 μg/ml), chloramphenicol (Cm; 10 μg/ml), tetracycline (Tet; 10 μg/ml), or gentamicin (Gm; 10 μg/ml) as necessary.
Table 1.
Bacterial strains and plasmids used in this study and their relevant characteristics
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| E. coli S17-λpir | 21 | |
| E. amylovora | ||
| Ea1189 | Wild type | 22 |
| Ea1189Δams | Deletion of the ams operon | 23 |
| Ea1189ΔedcC | Deletion of EAM_1504; edcC::FRT | This study |
| Ea1189ΔedcE | Deletion of EAM_2435; edcE::FRT | This study |
| Ea1189ΔedcCE | edcC edcE deletion mutant; Cmr | This study |
| Ea1189ΔedcACE | edcA edcC edcE deletion mutant; Cmr Kmr | This study |
| Ea1189ΔflhC | flhC deletion mutant; Kmr | 24 |
| Plasmids | ||
| pKD3 | Contains Cmr cassette and flanking FRT sites; Cmr | 25 |
| pKD4 | Contains Kmr cassette and flanking FRT sites | 25 |
| pKD46 | l-Arabinose-inducible lambda Red recombinase; Apr | 25 |
| pTL17 | IPTG-inducible FLPase; Kmr | 26 |
| pTL18 | IPTG-inducible FLPase; Tetr | 26 |
| pEVS141 | pEVS143 with the pTac promoter removed; vector control; Kmr | 27 |
| pEVS143 | Broad-host-range cloning vector; inducible Cmr and GFP; Kmr | 27 |
| pCMW75 | V. harveyi DGC gene qrgB; overexpression vector; Kmr | 28 |
| pCMW98 | Active-site mutant of qrgB in pCMW75 | 28 |
| pACE-edcA | pEVS143 cmR::edcA; overexpression vector; Kmr | This study |
| pACE-edcB | pEVS143 cmR::edcB; overexpression vector; Kmr | This study |
| pACE-edcC | pEVS143 cmR::edcC; overexpression vector; Kmr | This study |
| pACE-edcD | pEVS143 cmR::edcD; overexpression vector; Kmr | This study |
| pACE-edcE | pEVS143 cmR::edcE; overexpression vector; Kmr | This study |
| pBBR1MCS-1 | Broad-host-range cloning vector; R6K ori; Cmr | 29 |
| pBBR1MCS-5 | Broad-host-range cloning vector; R6K ori; Gmr | 29 |
| pLFC19 | edcC gene and native promoter in pBBR1MCS-5; Gmr | This study |
| pLFC13 | edcE gene and native promoter in pBBR1MCS-1; Cmr | This study |
| pACYCDuet-1 | Expression vector containing two MCS; P15A ori; Cmr | Novagen |
| pLFC11 | edcC and edcE genes with native promoter in pACYCDuet-1; Cmr | This study |
MCS, multiple cloning sites.
DNA manipulations.
DNA manipulations were performed using standard techniques (30). The E. amylovora genome sequence was obtained from GenBank (accession no. FN666575) (31). Native DGCs were amplified from E. amylovora Ea1189 genomic DNA with the primers listed in Table 2, digested with restriction enzymes, and cloned into plasmid pEVS143 (27) to generate isopropyl-β-d-1-thiogalactopyranoside (IPTG)-inducible overexpression plasmids.
Table 2.
Oligonucleotide primers used in this study
| Primer designation | Sequence |
|---|---|
| 70-mer primers used to make a 1.1-kb DNA segment for deletion by recombination | |
| edcA.ko.us | 5′-GTCATTTTTCGTATTACGGGAAGTCACTGTCAATGCCAGGCGAATGAATCGTGTAGGCTGGAGCTGCTTC |
| edcA.ko.ds | 5′-CGCAGGCTGACCCGCGATATCTTCAGGTCAGCCCGCCGCATTGCACTGGACATATGAATATCCTCCTTA |
| edcB.ko.us | 5′-CTTAACGGTGCTGGCACGTTGATATTTCAGGGCAGTCACGAGTAAATATAGTGTAGGCTGGAGCTGCTTC |
| edcB.ko.ds | 5′-ATAATAAGTTAAATCAATGTGGCTGGCCCGCAGAGCAGTTCGGTTGGCTACATATGAATATCCTCCTTA |
| edcC.ko.us | 5′-AACCCGGCCGGCTGGTGAGCGATTCATTTTTACCGTCCTGAGCCTGAAAGGTGTAGGCTGGAGCTGCTTC |
| edcC.ko.ds | 5′-GGACAGTTGTTGCTCTGCAATGCCAACCCGCGGTGAGCGGGTTGGCATCACATATGAATATCCTCCTTA |
| edcD.ko.us | 5′-ATGGTGCAAGATTTTAAGATTATGCCTAAAAGGCACCATGCCGGAGAGTAGTGTAGGCTGGAGCTGCTTC |
| edcD.ko.ds | 5′-CCGTTGCGCCCGGCGACTGACCAGCCGCGGCCTTGAGGTTGATGGCGGTACATATGAATATCCTCCTTA |
| edcE.ko.us | 5′-CCAGCACCCGCCAGGAAAGAAATGGCTAAAACATCTATGCTTTAAGCCGAGTGTAGGCTGGAGCTGCTTC |
| edcE.ko.ds | 5′-CAACGGCCTGCGGATCGCCGTATTGACCTGGAAAACATATAAAGCACAGCCATATGAATATCCTCCTTA |
| Primers flanking target genes by 500 bp (used for confirming deletions) | |
| edcA .5 us | 5′-GATAACCACGCTGCTGAAAAAC |
| edcA .5 ds | 5′-TTCGTCAGACGGGATTAGCCGC |
| edcB .5 us | 5′-TCTTCACCGCCCATTAACCG |
| edcB .5 ds | 5′-AAACGTTTATCGCAGCCATT |
| edcC .5 us | 5′-CGCATTGTTTCGTCAACGAATG |
| edcC .5 ds | 5′-CGCCGACATCCGCCATTACG |
| edcD .5 us | 5′-CGGCTGAGCATTGGCTGGCG |
| edcD .5 ds | 5′-CATGTACTGTGCCATCGCCG |
| edcE .5 us | 5′-AGCGCCGAGTGCAGAACTAC |
| edcE .5 ds | 5′-CGACTTCCAGGGACAGCGCG |
| Primers used to clone expression vectors for edcA to edcE | |
| edcA us | 5′-TAGGAATTCAGGAGCTAAGGAAGCTAAAATGAATGAAGACTCAGATGT |
| edcA ds | 5′-ATAAGATCTCTAAAATTCTTTGAGGCGGC |
| edcB us | 5′-TAGGAATTCAGGAGCTAAGGAAGCTAAAATGAATTTGCAAAGCTACGA |
| edcB ds | 5′-ATAGGATCCTTATACGATATCTGCAGGTT |
| edcC us | 5′-TAGGAATTCAGGAGCTAAGGAAGCTAAAATGCCAAACAATACTTATCT |
| edcC ds | 5′-ATAGGATCCTTACAACATAAATTCGGGTC |
| edcD us | 5′-TAGGAATTCAGGAGCTAAGGAAGCTAAAATGTCGACAGATAATTCAAG |
| edcD ds | 5′-ATAGGATCCTCAGTACACGGAAAGCCGAC |
| edcE us | 5′-TAGGAATTCAGGAGCTAAGGAAGCTAAAATGCTGCCAACGGTAGCCTG |
| edcE ds | 5′-ATAGGATCCCTACTGGCGCGAAGGTTCAT |
| Primers used to clone edcC, edcE, and edcCE complementation vectors | |
| EdcE pBBR1 Fw | 5′-GAATCTCGAGGAATGCTGCCAACGGTAG |
| EdcE pBBR1 Rv | 5′-CATAGGATCCATCGCCGTATTGACCTGG |
| EdcC pBBR1 Fw | 5′-GAATGAATTCTCGATAAATTGCCTGCAAC |
| EdcC pBBR1 Rv | 5′-CATAGGATCCGATCGACCGCGTTATCTTC |
| EdcE pACYC Fw | 5′-GATCGAATTCGAATGCTGCCAACGGTAG |
| EdcE pACYC Rv | 5′-CATAGTCGACATCGCCGTATTGACCTGG |
| EdcC pACYC Fw | 5′-GATCCATATGGTCGATAAATTGCCTGCAAC |
| EdcC pACYC Rv | 5′-CTCAGGTACCGATCGACCGCGTTATCTTC |
Insertional mutagenesis and complementation.
Chromosomal mutation of each gene predicted to be involved in c-di-GMP synthesis was carried out as described previously (7, 25). Briefly, the 1.1-kb chloramphenicol resistance (Cmr) cassette with flanking identical flippase recognition target (FRT) sites was amplified from plasmid pKD3 (25) using primers encoding 20 bp of homology to the Cmr cassette and 50 bp of homology to the regions immediately upstream and downstream of the target gene. All primer sequences are listed in Table 2. PCR products were purified and electroporated into E. amylovora expressing the Red, β, λ, and Exo recombinase genes from the pKD46 plasmid (25). After recovery, colonies were selected on LB agar plates amended with the appropriate antibiotics. Cells with the mutation were identified by colony PCR using primers located 500 bp upstream and downstream of the mutation. Mutant colonies containing the Cm or Km resistance cassette were transformed with plasmid pTL17 or pTL18 (26), each of which encodes an IPTG-inducible site-specific recombinase that triggers recombination between the FRT sites, leading to excision of the antibiotic resistance gene. Isolated colonies were tested for Cm or Km sensitivity, and the loss of antibiotic cassettes was confirmed by colony PCR using the same flanking primers originally used to confirm the insertion. For complementation assays, ΔedcC and ΔedcE strains were transformed with plasmids pLFC19 and pLFC13, containing the edcC and edcE genes along with their native promoters, ligated into pBBR1MCS-1 and pBBR1MCS-5 (29), respectively. The ΔedcCE double mutant was complemented with plasmid pLFC11, which contains the edcC and edcE genes and native promoters, ligated into pACYCDuet-1 (Novagen).
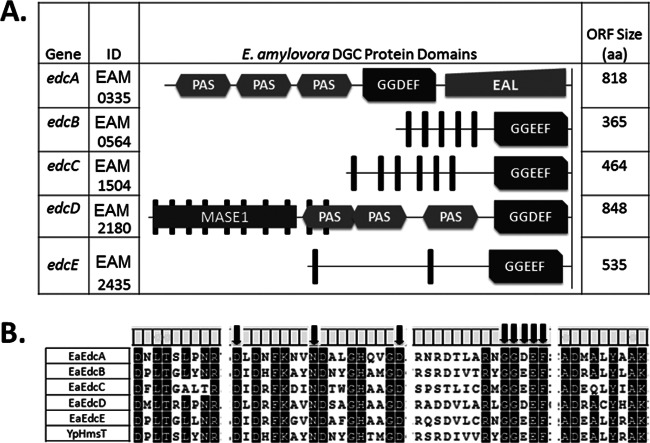
Bioinformatics.
The search for open reading frames (ORFs) in E. amylovora that contain GGDEF, EAL, and/or HD-GYP domains was carried out using the Motif Alignment and Search Tool (MAST), version 4.6.1 (32). The presence and organization of conserved protein domains were predicted using Pfam, version 25.0 (33), and transmembrane (TM) domains were identified using TMHMM, version 2.0 (34) (see Fig. 1A). Amino acid alignment using ClustalW in MEGA5.0 (35) was utilized to examine the conserved sequences of the GGDEF domains (see Fig. 1B).
Fig 1.
The five putative DGC enzymes present in the genome of Erwinia amylovora Ea1189. (A) The EAL and GGDEF domains of these proteins are shown with the protein lengths (in amino acids) and gene locus tags. Protein domains were predicted using Pfam, version 25.0, and are drawn to scale. Membrane-spanning domains were predicted by TMHMM, version 2.0, and are shown as vertical filled bars. (B) The GGDEF domain proteins from E. amylovora were aligned with HmsT, an active DGC from Yersinia pestis. Protein sequences were aligned using ClustalW on the MEGA 5.0 platform. Conserved amino acids (>80%) are highlighted in black. Residues required for enzymatic activity from this domain are indicated by black arrows above the amino acid alignment. It should be noted that the third amino acid of the GGDEF sequence of active DGCs can be either an aspartate or a glutamate residue.
Determination of intracellular c-di-GMP concentration.
The procedure for the determination of intracellular c-di-GMP concentrations by use of ultra performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS-MS) has been described in detail elsewhere (36). Specifically, for these experiments, overnight cultures were grown in LB medium and were then used to inoculate 7 ml fresh medium in a 25-ml Erlenmeyer flask with a starting optical density at 600 nm (OD600) of 0.05. At an OD600 of about 0.8, corresponding to mid- to late-exponential growth, the CFU counts per milliliter were calculated by serial dilution and colony counts on LB agar plates, and cells were harvested by centrifugation of 5 ml of cells in 35-ml polystyrene centrifuge tubes at 4°C for 10 min at 8,000 × g. The supernatant was removed, and the pellet was resuspended with 1 ml phosphate-buffered saline (PBS) and was transferred to a fresh 1.5-ml polystyrene Eppendorf tube. The cell suspension was centrifuged at 10,000 × g for 1 min, and the PBS was removed by aspiration. The cells were then lysed with 0.1 ml extraction buffer (40% acetonitrile–40% methanol in 0.1 N formic acid), left at −20°C for 20 min, and then centrifuged at 4°C for 1 min at 15,000 × g. The debris-free liquid was then analyzed by UPLC-MS-MS. By use of a standard curve of chemically synthesized c-di-GMP (Axxora), the total amount of c-di-GMP extracted was determined. An estimate of the intracellular c-di-GMP concentration was obtained by dividing the total amount of c-di-GMP extracted by the estimated volume of cytoplasm extracted. The length and width of one cell were quantified using ImageJ software (37), and cell volume was estimated by using the formula for calculating the volume of a cylinder (volume = π · height · radius squared). The average intracellular volume of an E. amylovora cell in LB medium during exponential growth was estimated to be 1.88 × 10−12 ml. The total cellular volume was obtained by multiplying the intracellular volume of one cell by the total number of cells harvested, and the intracellular c-di-GMP concentration was estimated by dividing the total quantity of c-di-GMP by the total intracellular volume.
Motility assays.
Swimming motility was examined by immersing a 10-μl Pipetman plastic tip in overnight bacterial cultures, followed by stab-inoculation onto a 0.3% agar LB plate. The inoculated plates were incubated at 28°C for 20 h. For the overexpression strains, the low-density agar plates were amended with 1.0 mM IPTG and Km. Motility plates were photographed under white light using a Red imaging system (Alpha Innotech), and the files were analyzed with ImageJ software (37). From the “Threshold” window, each motility plate image was converted to dark background, and the threshold was adjusted until the area of the colony was roughly translated into pixels. This same technique was used for a reference sticker, and by normalizing the motility pixel area by the 1-cm2 reference sticker, we determined the motility area (in square centimeters). This assay was repeated at least three times.
CPC binding assay for turbidometric quantification of amylovoran production.
The concentration of amylovoran in supernatants of bacterial cultures was determined quantitatively as described previously (24, 38). Briefly, E. amylovora strains were grown overnight, pelleted, and washed with 0.5× PBS. Cultures were inoculated into 3 ml of MBMA medium with 1% sorbitol or into a 3:1 mixture of LB-MBMA medium to a starting OD600 of 0.1 and were grown for either 20 or 48 h at 28°C with agitation. The OD600 of the bacterial suspensions was measured, and 1 ml of bacterial suspension was pelleted. A 0.8-ml portion of the supernatant was transferred to a new tube, mixed with 40 μl of 50-mg/ml cetylpyridinium chloride (CPC; Sigma), and incubated at room temperature for 10 min. The amylovoran concentration was determined by measuring the OD600 of the suspensions and was normalized to the OD600 of the corresponding culture. The Δams strain, containing a deletion of the entire ams operon, is unable to produce amylovoran (23) and was thus used as a negative control. This assay was repeated at least three times.
Biofilm formation.
Biofilm formation by the overexpression, mutant, and complemented strains was determined by using a previously described method (6). Briefly, 100 μl of equilibrated overnight cultures was added to 2 ml of 0.5× LB medium in individual wells of 24-well plates, each well containing a glass coverslip at a ≈30° angle. After incubation at 28°C for 48 h, planktonic cells and medium were removed, and coverslips were stained with 10% crystal violet (CV) for 1 h. Stained coverslips were then washed three times with water and were air dried for 1 h. The CV stain was then dissolved in 200 μl 40% methanol–10% acetic acid, and the OD600 of the solution was recorded. This experiment was repeated at least three times.
Virulence assays.
Virulence assays using immature pears were conducted as described previously (39). Briefly, immature pears were surface sterilized with 10% bleach, rinsed with sterile distilled water, and dried. Overnight bacterial cultures were adjusted to ≈1 × 104 CFU/ml in 0.5× PBS. Pears were stab-inoculated with 3 μl of the bacterial suspensions and were incubated at 28°C in a humidified chamber. Calipers were used to quantify the lesion diameter at 4 days postinoculation (dpi). Each experiment included 10 replicates, and this experiment was repeated at least three times. Apple shoot infection assays were conducted as described previously (7). Briefly, overnight bacterial cultures were adjusted to ≈2 × 108 CFU/ml with 0.5× PBS. Two-year-old apple trees (Malus X domestica cv. Gala) on M9 rootstock were inoculated by cutting the youngest leaves of central shoots with scissors previously dipped in the bacterial suspensions. Symptoms were monitored at 4 dpi. This experiment was repeated at least twice, with four replicates for each experiment.
Impact of c-di-GMP on the transcription of type III secretion and ams promoters.
Promoter regions and ribosomal binding sites (RBS) of hrpA, hrpS, and amsG (∼500-bp fragments upstream of the start codon) were amplified by PCR with primers incorporating BamHI and SalI restriction sites. The hrpA and hrpS PCR products were purified, digested with restriction enzymes, and cloned into the pPROBE-AT plasmid (40), which contains the coding region of the gfp reporter gene without the promoter sequence. The amsG PCR product was inserted into pBBRlux-1 (41). Recombinant fusion products were confirmed by PCR and sequencing. Reporter plasmids were electroporated into E. amylovora wild-type and overexpression strains. To evaluate promoter activity, cultures were grown overnight in LB medium at 28°C, pelleted, and washed twice with 0.5× PBS. For analysis of hrp gene expression, 5 μl of bacterial culture was transferred to 150 μl of an hrp-inducing minimal medium (hrpMM) (42) supplemented with ampicillin, kanamycin, and 1.0 mM IPTG in a 96-well plate. After 9 h of induction, promoter activity was determined by measuring the relative fluorescence of green fluorescent protein (GFP) and was normalized to the OD600 of the corresponding culture by using a Safire plate reader (Tecan). This assay was repeated at least three times with four technical replicates. Analysis of amsG-lux was performed similarly; cultures were grown overnight in 150 μl LB medium supplemented with chloramphenicol and kanamycin in a 96-well plate with agitation at 28°C and were then transferred to 150 μl LB medium supplemented with chloramphenicol, kanamycin, and 0.1 mM IPTG in a new 96-well plate with a 96-pin replicator tool (V&P Scientific). Cultures on plates were grown at 28°C with agitation, and maximum luminescence was recorded using a SpectraMax M2 multimode microplate reader at 8 h and was normalized to the OD600 of the culture.
RESULTS
Erwinia amylovora encodes five putative diguanylate cyclase enzymes.
Bioinformatic analysis of the E. amylovora wild-type (WT) strain Ea1189 genome revealed four genes encoding GGDEF domains, one gene encoding both an EAL and a GGDEF domain, and two genes with only EAL domains. No genes with HD-GYP domains were identified. We named the five GGDEF-encoding genes edcA to edcE (for Erwinia diguanylate cyclases), and their domain structures are shown in Fig. 1A. Prediction of conserved domains using the Pfam database revealed that both EdcA and EdcD contain three PAS domains, which have been widely characterized as receptors of different stimuli/signals in Archaea and Bacteria (43). In addition, EdcD also harbors a MASE1 (membrane-associated sensor) domain, usually found in bacterial signaling proteins and associated with GGDEF and EAL domains (44). EdcA also contains an EAL domain, suggesting that this protein could function as either a DGC or a PDE. All of the edc genes, except edcA, are predicted to encode inner membrane proteins based on predicted membrane-spanning domains. Bacteria often encode degenerate DGC enzymes that may function as receptors for c-di-GMP (45). However, based on sequence analysis, all five GGDEF-encoding proteins in E. amylovora are predicted to be enzymatically active, since they contain the residues critical for DGC activity (Fig. 1B).
Three of the E. amylovora DGCs synthesize c-di-GMP.
To determine if the putative DGCs mentioned above can synthesize c-di-GMP, we overexpressed the five edc genes in E. amylovora from a plasmid under the control of the Ptac promoter following induction with isopropyl-β-d-1-thiogalactopyranoside (IPTG) (28). As a positive control, we analyzed the expression of QrgB, a DGC from Vibrio harveyi that has been shown to synthesize c-di-GMP in several bacterial species (28). An active-site mutant of QrgB (QrgB*) in which the GGEEF active site was mutated to AAEEF and the WT strain containing the plasmid vector served as negative controls. Metabolites were extracted, and the concentration of c-di-GMP was determined using LC-MS-MS. c-di-GMP was not observed in either the WT strain containing the vector control or the strain expressing QrgB*, suggesting that the basal intracellular levels of this molecule were below the detection limit of our method (Table 3). Failure to detect basal levels of c-di-GMP in bacteria is not uncommon, since the intracellular concentration of this molecule is typically in the submicromolar range (46; unpublished results). Overexpression of qrgB resulted in detectable levels of c-di-GMP at an intracellular concentration of approximately 1 μM (Table 3), showing that c-di-GMP can be synthesized and detected in E. amylovora. Overexpression of edcA, edcC, and edcE resulted in 76, 176, and 4.6 nM c-di-GMP, respectively (Table 3). No c-di-GMP was detectable in strains overexpressing either edcB or edcD.
Table 3.
Intracellular c-di-GMP levels in E. amylovora Ea1189 containing various DGC overexpression plasmids
| Plasmid | c-di-GMP level (nM) |
|---|---|
| Vector control | NDb |
| pCMW75 (qrgB) | 1,050 ± 248 |
| pCMW98 (qrgB*) | ND |
| pACE-edcA | 76.6 ± 3.39 |
| pACE-edcB | ND |
| pACE-edcC | 176 ± 71.8 |
| pACE-edcD | ND |
| pACE-edcEa | 4.6 ± 0.420 |
A 15-ml culture volume was used for this strain, while 5 ml was used for the other strains.
ND, none detected.
c-di-GMP negatively regulates swimming motility in E. amylovora.
Swimming motility in most plant-pathogenic bacteria, including E. amylovora, is facilitated by the helical rotation of peritrichous flagella (47). Since c-di-GMP represses flagellar motility in several bacterial species (16, 48–51), we asked whether c-di-GMP inhibits the flagellum-mediated swimming motility of E. amylovora through low-density agar plates. Overexpression of QrgB strongly repressed swimming motility, while the WT strain containing the vector control and a strain expressing QrgB* were highly motile (Fig. 2A). Like the positive control, strains overexpressing EdcC or EdcE were essentially nonmotile, suggesting that production of c-di-GMP from these enzymes inhibited swimming motility. Surprisingly, EdcA overexpression did not repress motility (Fig. 2A), even though overexpression of this enzyme in liquid medium generated a higher concentration of c-di-GMP than overexpression of EdcE (Table 3).
Fig 2.

c-di-GMP inhibits flagellar motility in E. amylovora. Motility was examined in strain Ea1189 overexpressing DGC genes (A) or in DGC mutant strains (B). Values are normalized to the value for pEVS141, the vector control (Vector) (similarly labeled in all subsequent figures), or to the value for wild-type Ea1189. The complemented gene was expressed from a plasmid (indicated by a lowercase “p”). Data represent three biological replicates, and error bars indicate the standard errors of the means. Different letters above bars indicate statistically significant differences (P < 0.05 by Student's t test).
Heterologous expression of DGCs is a powerful approach to determining which enzymes have the potential to contribute to c-di-GMP signaling, because it overrides any native transcriptional control of the corresponding genes. However, overexpression can lead to unnaturally high concentrations of c-di-GMP, which may disrupt signaling specificity mechanisms. To further address the role of the edc genes in E. amylovora motility, we examined the swimming motility of mutants carrying whole-gene deletions of edcA, edcC, and edcE, since these three DGCs synthesized measurable c-di-GMP upon overexpression (Table 3). We hypothesized that mutation of these DGCs would decrease the intracellular c-di-GMP concentration, leading to increased motility. In support of this hypothesis, mutation of edcC and edcE significantly increased motility over that of the WT strain, whereas a double mutation of both edcC and edcE increased motility even further (Fig. 2B). Deletion of edcA in the edcCE mutant did not alter motility, showing that edcA does not impact flagellar motility under the conditions we examined. The changes in motility in the edcC, edcE, and edcCE mutants were complemented by heterologous expression of the corresponding genes (Fig. 2B). Complementation resulted in lower motility than that of the WT strain due to the expression of the complementing genes on multicopy plasmids, results similar to those in Fig. 2A. These experiments revealed that c-di-GMP synthesized by both EdcC and EdcE represses motility in E. amylovora.
c-di-GMP positively regulates amylovoran production.
Biosynthesis of amylovoran, the major EPS produced by E. amylovora, was quantified in culture supernatants by using the turbidometric cetylpyridinium chloride (CPC) binding assay as described previously (38). For this experiment, bacteria are typically grown in the glycerol-based MBMA defined medium. However, we observed that WT E. amylovora overexpressing DGCs exhibited slower growth in MBMA medium (data not shown), presumably due to increased levels of c-di-GMP. Growth inhibition caused by c-di-GMP overproduction has been observed in both E. coli and Vibrio cholerae (36, 52). It should be noted that c-di-GMP impacted the growth of E. amylovora only in MBMA medium, and thus, this finding does not impact the interpretation of the other experiments described here. We determined that a medium containing 3 parts MBMA and 1 part LB medium did not exhibit c-di-GMP-dependent growth inhibition upon overexpression of DGCs, and this medium was used for the studies for which results are presented in Fig. 3A. As expected, the negative-control Δams strain was deficient in amylovoran production (Fig. 3A). Overexpression of QrgB, EdcC, or EdcE led to amylovoran production levels higher than those with the WT empty-vector and QrgB* negative controls (Fig. 3A), whereas overexpression of EdcA, EdcB, or EdcD did not impact amylovoran secretion compared to that with the vector control.
Fig 3.
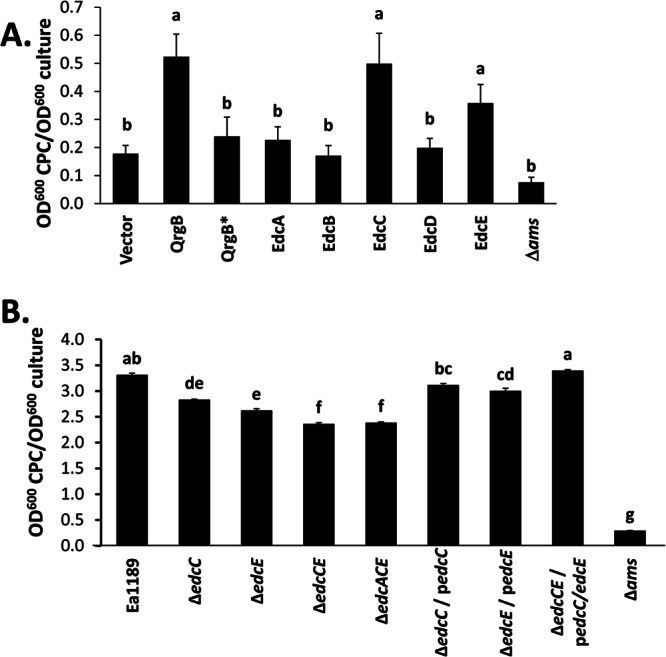
c-di-GMP increases the production of amylovoran in E. amylovora. (A) Overexpression strains were grown in 3 parts MBMA medium with 1% sorbitol and 1 part LB medium supplemented with Km and 0.1 mM IPTG. (B) The WT, deletion mutants, and corresponding complemented strains were grown in MBMA medium amended with 1% sorbitol for 48 h at 28°C. The complemented gene was expressed from a plasmid (indicated by a lowercase “p”). Amylovoran production was quantified using the turbidometric CPC-binding assay, and the values were normalized to the cell density. The Ea1189Δams mutant is deficient in amylovoran production and was used as a negative control. Data represent three biological replicates, and error bars indicate the standard errors of the means. Different letters above bars indicate statistically significant differences of the means (P < 0.05 by Student's t test).
We similarly examined amylovoran production in the edcA, edcC, and edcE deletion mutant strains and observed correlations with our findings from the overexpression studies. For these experiments, the bacteria were grown in complete MBMA medium, which resulted in higher levels of amylovoran production by the WT Ea1189 control. Mutation of edcC or edcE significantly reduced amylovoran production levels, while an edcCE double mutant exhibited a further decrease (Fig. 3B). Introduction of the edcA mutation into the edcCE double mutant did not significantly alter amylovoran production (Fig. 3B), suggesting that edcA is not involved in this process. The amylovoran production level was significantly increased in each of the mutants upon complementation with the corresponding edc gene(s) in trans. In these studies, no growth inhibition was observed during complementation, presumably due to removal of the DGCs expressed from the chromosome. Of note, the production of amylovoran from the ΔedcCE mutant strain remained significantly higher than that from the Δams control, showing that although amylovoran secretion is reduced at low c-di-GMP levels, a significant amount of this EPS is still synthesized (Fig. 3B). These results show that c-di-GMP positively regulates amylovoran production in E. amylovora and that, as with motility, EdcC and EdcE are the dominant DGCs regulating this process.
c-di-GMP positively regulates biofilm formation.
To further examine the role of c-di-GMP in E. amylovora, we measured the impact of overexpression and mutation of DGCs on biofilm formation. In agreement with our studies of amylovoran production, overexpression of qrgB, edcC, or edcE resulted in significant increases in biofilm formation over levels for the WT empty-vector control and the QrgB* mutant (Fig. 4A). Mutation of edcE alone, but not edcC, significantly reduced biofilm formation, although the ΔedcCE double mutant formed significantly less biofilm than the ΔedcE mutant (Fig. 4B). Like flagellar motility and amylovoran production, biofilm formation by a ΔedcACE mutant was indistinguishable from that by a ΔedcCE mutant, suggesting that edcA does not contribute to biofilm formation in E. amylovora. Each of the mutants was complemented with the corresponding gene expressed in trans, and as expected, the Δams mutant deficient in amylovoran production exhibited significantly less biofilm formation than all the other strains examined (Fig. 4B). Therefore, the regulation of amylovoran production through c-di-GMP synthesis by EdcC and EdcE positively impacts biofilm formation by E. amylovora.
Fig 4.

c-di-GMP induces biofilm formation of E. amylovora grown under static conditions. Biofilm formation by DGC overexpression strains (A) and DGC mutants (B) was determined by quantifying crystal violet (CV) binding as described in Materials and Methods. For the quantification of biofilm formation, E. amylovora Ea1189, deletion mutants, and complemented strains were grown in 0.5× LB medium for 48 h at 28°C. The complemented gene was expressed from a plasmid (indicated by a lowercase “p”). Biofilm formation was quantified as the absorbance after CV staining. Values are means for 12 replicates from one representative experiment. This assay was repeated three times with similar results. Error bars indicate the standard errors of the means, and different letters above the bars indicate statistically significant differences of the means (P < 0.05 by Student's t test).
c-di-GMP inhibits the virulence of E. amylovora in two plant infection models.
Motility, amylovoran secretion, and biofilm formation are all behaviors of E. amylovora that have been associated with plant infection (6, 53, 54). To determine the impact of c-di-GMP on E. amylovora virulence, we inoculated immature pears with the WT strain Ea1189, edc deletion mutants, or complemented strains and examined the development of tissue necrosis. Inoculation of the WT strain resulted in a necrotic lesion surrounding the inoculation point at 4 days postinfection (dpi) (Fig. 5B). Based on our previous studies indicating that amylovoran production positively influences E. amylovora virulence for the pear (6), we hypothesized that mutations of active DGCs that reduce amylovoran secretion would lead to decreased disease. However, while single mutations of edcC and edcE did not significantly impact the lesion size, the ΔedcCE double mutant produced a significantly larger area of necrosis (Fig. 5A and B). In accordance with our results presented above, mutation of edcA had no significant effect: the area of tissue necrosis induced by the ΔedcACE mutant was not significantly different from that with the ΔedcCE mutant. Expression of edcC and edcE in the ΔedcCE mutant exhibited only partial complementation (Fig. 5). We determined that the complementation plasmids are maintained in E. amylovora during the course of the experiment; however, since overexpression of the edcC and edcE genes from the complementation vector was dependent on IPTG induction, we speculate that the lack of complete complementation is likely due to decreases in the concentrations of IPTG (added only at the inoculation time) in the pears during the 4-day experiment.
Fig 5.
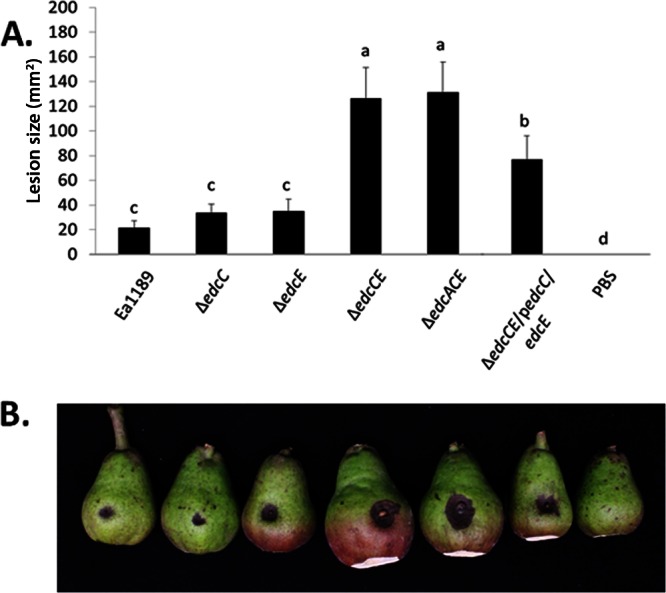
Virulence of E. amylovora DGC mutant strains in an immature-pear infection model. (A) Sizes of lesions on immature pears inoculated with 2 ml of the indicated strains at ≈1 × 104 CFU ml−1. Lesion size was measured using calipers at 4 dpi. The experiment was repeated three times with similar results. The ΔedcCE mutant was complemented with edcC and edcE expressed from a plasmid (indicated by a lowercase “p”). Values are means for 10 replicates from one representative experiment, and error bars represent the standard errors. Different letters above the bars indicate statistically significant differences (P < 0.05 by Student's t test). (B) Representative pears illustrate symptom development at 4 dpi for Ea1189 and the mutant strains.
To determine if the negative influence of c-di-GMP on virulence and tissue necrosis was specific to the immature-pear assay or relevant to other plant infection models as well, the impact of c-di-GMP on bacterial virulence was also evaluated in apple shoots inoculated with the WT, mutant, and complemented strains. Inoculation with the ΔedcC or ΔedcE mutant resulted in increased tissue necrosis and migration through the central vein of the leaf, in comparison with the lesion elicited by the WT strain (Fig. 6). This disease phenotype was more pronounced in the ΔedcCE and ΔedcACE double and triple mutants. Complementation of these mutants partially restored WT levels of tissue necrosis and bacterial migration. The results from both the immature-pear assay and the apple shoot infection assay indicate that EdcC and EdcE possess DGC activity in both pears and apple shoots and that c-di-GMP negatively modulates the acute virulence of E. amylovora.
Fig 6.

c-di-GMP inhibits acute virulence and migration of E. amylovora in an apple shoot infection model. Symptom development on apple shoots at 4 dpi is shown. The youngest leaves of central shoots were clip-inoculated with scissors previously dipped in a bacterial suspension of the indicated strains at a concentration of ≈5 × 108 CFU ml−1. The mutations were complemented with genes expressed from a plasmid (indicated by a lowercase “p”). This assay was repeated three times with similar results.
c-di-GMP regulates transcription of amylovoran synthesis and type III secretion genes.
c-di-GMP controls bacterial behaviors at multiple levels, including the induction of transcription, posttranscriptional gene regulation, and direct modulation of protein activity (45). To begin to explore the mechanism by which c-di-GMP inversely controls biofilm formation and virulence, we constructed a transcriptional fusion of the amsG promoter to a luciferase (lux) reporter. amsG is the first gene of the 12-gene ams operon, which encodes the enzymatic machinery necessary for synthesizing amylovoran (55). We observed that induction of qrgB, edcC, or edcE significantly increased transcription from the amsG promoter over that with a vector control (Fig. 7A).
Fig 7.

c-di-GMP controls the transcription of genes involved in amylovoran production and type III secretion. The expression of an amsG-lux transcriptional fusion (A) and of hrpA and hrpS transcriptional fusions to gfp (B) was examined upon expression of the indicated DGCs. Luciferase expression is shown as relative light units (RLU), determined by dividing luminescence by the OD600 of the culture. Similarly, gfp is expressed in normalized units (fluorescence units/OD600). Error bars indicate the standard errors. Symbols above the bars indicate statistically significant differences from the vector controls (WT) by Student's t test (*, P < 0.05).
The negative effect of c-di-GMP on bacterial virulence in immature pears and apple shoots led us to hypothesize that this second messenger repressed the expression of type III secretion system (T3SS)-related genes. To test this hypothesis, the expression of the hrpA gene, which encodes the major structural protein of the T3SS pilus in E. amylovora (56), was evaluated by using a promoter-gfp transcriptional fusion in the WT and in strains overexpressing qrgB, qrgB*, edcC, or edcE. Overexpression of the active DGC qrgB, edcC, or edcE led to levels of promoter activity significantly reduced from those of the WT containing the vector control and the strain expressing qrgB* (Fig. 7B).
We also evaluated the expression of hrpS, a σ54-dependent enhancer-binding protein (EBP) that regulates the expression of HrpL, the alternative sigma factor required for the transcription of T3SS-related genes such as hrpA (57). In contrast to the reduced levels of hrpA promoter activity, qrgB and edcE did not significantly affect hrpS promoter activity (Fig. 7B). Although overexpression of edcC resulted in a statistically significant increase in the level of hrpS promoter activity over that for the WT strain, this result may be not biologically relevant, since this strain synthesizes c-di-GMP at lower levels than the qrgB-overexpressing control strain. Together, these results indicate that c-di-GMP synthesized by EdcC and EdcE negatively regulates the expression of the T3SS hrpA gene and that this regulation does not involves changes in the expression levels of the HrpL regulator, HrpS.
DISCUSSION
Our results indicate that c-di-GMP is a positive regulator of amylovoran production and biofilm formation in E. amylovora and a negative regulator of swimming motility, T3SS gene expression, and virulence. Using overexpression studies and mutagenesis, we systematically determined that the dominant DGCs in E. amylovora are EdcC and EdcE, while EdcB and EdcD are not capable of producing c-di-GMP under the conditions examined. Although overexpression of edcA generated measurable levels of c-di-GMP, we conclude that this DGC does not impact the phenotypes we examined. c-di-GMP signaling has been shown to be a highly specific process whereby different DGC enzymes specifically influence c-di-GMP-mediated behaviors (36, 58, 59). It is possible that the c-di-GMP generated by EdcA does not control biofilm formation, motility, or virulence as examined here but rather modulates other bacterial behaviors not tested in this study. The intracellular level of c-di-GMP was below the level of detection in the WT strain Ea1189, suggesting that under the conditions we examined, the levels of this molecule are relatively low in E. amylovora compared with those in other bacteria. For reference, we were able to detect and quantify c-di-GMP levels in a number of other bacterial species, including but not limited to V. cholerae, Yersinia pestis, Salmonella enterica serovar Typhimurium, and Clostridium difficile (36, 60–63).
Analysis of the regulatory roles of elevated c-di-GMP levels revealed insight into the interplay between biofilm formation, motility, and T3SS gene expression in E. amylovora pathogenesis. A previous study demonstrated that biofilm-deficient mutants in which amylovoran production was unaffected, but genes encoding specific attachment factors, such as type I fimbriae, were mutated, exhibited reductions in virulence when inoculated into apple leaves, revealing a distinct role for biofilm formation independent of EPS secretion in plant disease (7). Moreover, it has been reported previously that the amount of amylovoran produced correlated with virulence in the host (64). Our results contrasted with these conclusions, since we observed that the ΔedcCE and ΔedcACE mutants, which exhibited decreased biofilm formation and amylovoran EPS production, showed an increased virulence phenotype. We conclude that the decreased levels of amylovoran production and biofilm formation at reduced c-di-GMP levels are sufficient to facilitate E. amylovora infection and systemic invasion of leaves, while altered regulation of other behaviors, such as motility and T3SS gene expression, leads to increased virulence.
The increased motility of the ΔedcCE and ΔedcACE mutants is likely one behavior responsible for the enhanced virulence of these mutants. Motility is an important virulence factor contributing to the infection of flowers by E. amylovora (47, 53). However, E. amylovora cells are thought to be nonmotile in the apoplast, although these data come from one study in which cells were examined in vitro immediately after recovery from infected stems (65). More recently, E. amylovora strains demonstrating increased swarming motility in vitro were shown to be more virulent than less-motile strains in apple genotypes exhibiting high levels of fire blight resistance (66). The ΔedcCE and ΔedcACE mutants exhibited increased swimming motility in vitro due to a lack of c-di-GMP production. We similarly determined that c-di-GMP inhibited the swarming of E. amylovora (data not shown). Thus, it is likely that increased motility in planta following leaf inoculation enabled these mutants to gain more rapid access to host cells over a larger area, leading to the enhanced necrosis phenotype observed.
We determined that, in addition to inhibiting motility, c-di-GMP represses T3SS-related genes, as has been reported for the plant pathogen Dickeya dadantii and the animal pathogen Salmonella enterica serovar Typhimurium (20, 67, 68). Our results suggest that this regulation is not associated with a negative effect of c-di-GMP on the expression of HrpS, but it could be associated with downregulation of other key players in the hrp signaling cascade, such as the two-component transduction system HrpX/HrpY, RpoN (σ54), or with direct negative regulation of HrpL, the alternative sigma factor required for the transcription of hrp genes (57, 69).
Although the regulation of the transition between infection stages in E. amylovora is poorly understood, recent studies have demonstrated that coinfection of a strain lacking the entire ams operon with a T3SS deletion mutant leads to full virulence (23). This finding suggests that E. amylovora specifically controls the expression of pathogenicity determinants, among which T3SS is required in host colonization and the early stages of infection while the biosynthesis of amylovoran is required for establishment in the host vascular tissues during late stages of the disease. Moreover, regulatory elements of T3SS expression negatively control the production of amylovoran, since a T3SS deletion mutant produced 3- to 4-fold-larger amounts of this EPS than the WT strain (23). In addition, HrpL regulates the expression of non-T3SS-related virulence factors (70). Our results suggest that c-di-GMP is involved in the orchestration of bacterial pathogenesis, since T3SS-related genes and amylovoran production are inversely modulated by this second messenger. However, further studies are needed to elucidate the specific role of c-di-GMP in the coordination of expression of pathogenesis-related traits.
c-di-GMP has now been implicated in the ability of a number of plant pathogens to cause infections. Our results suggest that E. amylovora appears to most closely resemble Dickeya dadantii, in which mutation of two PDE enzymes, which presumably increased c-di-GMP levels, enhanced biofilm formation while reducing plant virulence (20). The decreased virulence of D. dadantii was attributed to downregulation of T3SS-related genes and decreased secretion of pectate lyase by c-di-GMP (20). Similarly, a recent study demonstrated that high levels of c-di-GMP promote the activation of a biofilm-forming phenotype in Pectobacterium atrosepticum and positively regulate the expression of poly-β-1,6-N-acetyl-d-glucosamine (PGA), an EPS essential for biofilm formation (71). In Xanthomonas campestris pv. campestris, increased levels of c-di-GMP similarly inhibit plant virulence and increase biofilm formation (72). However, c-di-GMP negatively influences biosynthesis of the EPS xanthan, which is necessary for plant disease, in X. campestris pv. campestris, whereas we determined that c-di-GMP positively regulated amylovoran biosynthesis in E. amylovora. From the perspective of c-di-GMP signaling, Xylella fastidiosa appears to be the most distinct from other bacterial plant pathogens; in this organism, c-di-GMP positively influences the expression of secreted virulence factors and type IV pili while negatively impacting biofilm formation (15). Therefore, each of these phytopathogens has integrated c-di-GMP as a central regulator in the control of virulence, EPS secretion, and biofilm formation in unique ways presumably optimally adapted to their different disease progressions.
In summary, we have identified five genes that potentially encode DGC enzymes in the plant pathogen E. amylovora. Analysis of these DGCs through overexpression or deletion indicated that two of them, EdcC and EdcE, regulate motility, amylovoran production, biofilm formation, expression of the T3SS, and plant virulence. E. amylovora is a powerful model system for the study of c-di-GMP signaling in a bacterial plant pathogen, since its pathway is relatively simple, encoding only seven DGCs and PDEs. Moreover, E. amylovora allows the determination of the role of c-di-GMP in the pathogenesis of a bacterial pathogen during the infection of its native host. The coordination of pathogenesis by E. amylovora and the transition between infection stages that require motility, T3SS, amylovoran production, or biofilm formation suggest that continued investigation into the regulatory networks controlled by c-di-GMP will be essential to full understanding of the pathogenesis of E. amylovora.
ACKNOWLEDGMENTS
This work was supported by funding from the USDA-CSREES (to G.W.S.), NIH grants K22-AI080937 and U54-AI057153 (to C.M.W.), and the Michigan State University Center for Microbial Pathogenesis.
We thank Quan Zeng for providing the hrpA and hrpS transcription fusions.
Footnotes
Published ahead of print 8 March 2013
REFERENCES
- 1. Eden-Green SJ, Billing E. 1974. Fireblight. Rev. Plant Pathol. 53:353–365 [Google Scholar]
- 2. Norelli JL, Jones AL, Aldwinckle HS. 2003. Fire blight management in the twenty-first century: using new technologies that enhance host resistance in apple. Plant Dis. 87:756–765 [DOI] [PubMed] [Google Scholar]
- 3. Wilson M, Sigee DC, Epton HAS. 1990. Erwinia amylovora infection of hawthorn blossom. III. The nectary. J. Phytopathol. 128:62–74 [Google Scholar]
- 4. Wilson M, Epton HAS, Sigee DC. 1989. Erwinia amylovora infection of hawthorn blossom. II. The stigma. J. Phytopathol. 127:15–28 [Google Scholar]
- 5. Vanneste JL. 1995. Erwinia amylovora, p 21–46 In Singh US, Singh RP, Kohmoto K. (ed), Pathogenesis and host specificity in plant diseases: histopathological, biochemical, genetic, and molecular bases. Pergamon Press, Oxford, United Kingdom [Google Scholar]
- 6. Koczan JM, McGrath MJ, Zhao Y, Sundin GW. 2009. Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: implications in pathogenicity. Phytopathology 99:1237–1244 [DOI] [PubMed] [Google Scholar]
- 7. Koczan JM, Lenneman BR, McGrath MJ, Sundin GW. 2011. Cell surface attachment structures contribute to biofilm formation and xylem colonization by Erwinia amylovora. Appl. Environ. Microbiol. 77:7031–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jumel K, Geider K, Harding SE. 1997. The solution molecular weight and shape of the bacterial exopolysaccharides amylovoran and stewartan. Int. J. Biol. Macromol. 20:251–258 [DOI] [PubMed] [Google Scholar]
- 9. Nimtz M, Mort A, Domke T, Wray V, Zhang Y, Qiu F, Coplin D, Geider K. 1996. Structure of amylovoran, the capsular exopolysaccharide from the fire blight pathogen Erwinia amylovora. Carbohydr. Res. 287:59–76 [DOI] [PubMed] [Google Scholar]
- 10. Politis DJ, Goodman RN. 1980. Fine structure of extracellular polysaccharide of Erwinia amylovora. Appl. Environ. Microbiol. 40:596–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gross M, Geier G, Rudolph K, Geider K. 1992. Levan and levansucrase synthesized by the fireblight pathogen Erwinia amylovora. Physiol. Mol. Plant Pathol. 40:371–381 [Google Scholar]
- 12. Chalupowicz L, Zellermann EM, Fluegel M, Dror O, Eichenlaub R, Gartemann KH, Savidor A, Sessa G, Iraki N, Barash I, Manulis-Sasson S. 2012. Colonization and movement of GFP-labeled Clavibacter michiganensis subsp. michiganensis during tomato infection. Phytopathology 102:23–31 [DOI] [PubMed] [Google Scholar]
- 13. Koutsoudis MD, Tsaltas D, Minogue TD, von Bodman SB. 2006. Quorum-sensing regulation governs bacterial adhesion, biofilm development, and host colonization in Pantoea stewartii subspecies stewartii. Proc. Natl. Acad. Sci. U. S. A. 103:5983–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tyson GE, Stojanovic B, Kuklinski RF, Divittoria T, Sullivan ML. 1985. Scanning electron microscopy of Pierce's disease bacterium in petiolar xylem of grape leaves. Phytopathology 75:264–269 [Google Scholar]
- 15. Chatterjee S, Killiny N, Almeida RP, Lindow SE. 2010. Role of cyclic di-GMP in Xylella fastidiosa biofilm formation, plant virulence, and insect transmission. Mol. Plant Microbe Interact. 23:1356–1363 [DOI] [PubMed] [Google Scholar]
- 16. Jenal U, Malone J. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40:385–407 [DOI] [PubMed] [Google Scholar]
- 17. Römling U, Gomelsky M, Galperin MY. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57:629–639 [DOI] [PubMed] [Google Scholar]
- 18. Cotter P, Stibitz S. 2007. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 10:17–23 [DOI] [PubMed] [Google Scholar]
- 19. Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263–273 [DOI] [PubMed] [Google Scholar]
- 20. Yi X, Yamazaki A, Biddle E, Zeng Q, Yang CH. 2010. Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii. Mol. Microbiol. 77:787–800 [DOI] [PubMed] [Google Scholar]
- 21. de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386–405 [DOI] [PubMed] [Google Scholar]
- 22. Burse A, Weingart H, Ullrich MS. 2004. NorM, an Erwinia amylovora multidrug efflux pump involved in in vitro competition with other epiphytic bacteria. Appl. Environ. Microbiol. 70:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Y, Sundin GW, Wang D. 2009. Construction and analysis of pathogenicity island deletion mutants of Erwinia amylovora. Can. J. Microbiol. 55:457–464 [DOI] [PubMed] [Google Scholar]
- 24. Zhao Y, Wang D, Nakka S, Sundin G, Korban S. 2009. Systems level analysis of two-component signal transduction systems in Erwinia amylovora: role in virulence, regulation of amylovoran biosynthesis and swarming motility. BMC Genomics 10:245 doi:10.1186/1471-2164-10-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long T, Tu KC, Wang YF, Mehta P, Ong NP, Bassler BL, Wingreen NS. 2009. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 7:e68 doi:10.1371/journal.pbio.1000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV. 2006. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 72:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Waters CM, Lu W, Rabinowitz JD, Bassler BL. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 190:2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 30. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 31. Sebaihia M, Bocsanczy AM, Biehl BS, Quail MA, Perna NT, Glasner JD, DeClerck GA, Cartinhour S, Schneider DJ, Bentley SD, Parkhill J, Beer SV. 2010. Complete genome sequence of the plant pathogen Erwinia amylovora strain ATCC 49946. J. Bacteriol. 192:2020–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bailey TL, Gribskov M. 1998. Combining evidence using p-values: application to sequence homology searches. Bioinformatics 14:48–54 [DOI] [PubMed] [Google Scholar]
- 33. Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A. 2010. The Pfam protein families database. Nucleic Acids Res. 38(Database issue):D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580 [DOI] [PubMed] [Google Scholar]
- 35. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM. 2012. Quantification of high-specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. U. S. A. 109:12746–12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellemann P, Bereswill S, Berger S, Geider K. 1994. Visualization of capsule formation by Erwinia amylovora and assays to determine amylovoran synthesis. Int. J. Biol. Macromol. 16:290–296 [DOI] [PubMed] [Google Scholar]
- 39. Zhao Y, Blumer SE, Sundin GW. 2005. Identification of Erwinia amylovora genes induced during infection of immature pear tissue. J. Bacteriol. 187:8088–8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller WG, Leveau JH, Lindow SE. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant Microbe Interact. 13:1243–1250 [DOI] [PubMed] [Google Scholar]
- 41. Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69–82 [DOI] [PubMed] [Google Scholar]
- 42. Huynh T, Dahlbeck D, Staskawicz B. 1989. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245:1374–1377 [DOI] [PubMed] [Google Scholar]
- 43. Taylor BL, Zhulin IB. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nikolskaya AN, Mulkidjanian AY, Beech IB, Galperin MY. 2003. MASE1 and MASE2: two novel integral membrane sensory domains. J. Mol. Microbiol. Biotechnol. 5:11–16 [DOI] [PubMed] [Google Scholar]
- 45. Ryan RP, Tolker-Nielsen T, Dow JM. 2012. When the PilZ don't work: effectors for cyclic di-GMP action in bacteria. Trends Microbiol. 20:235–242 [DOI] [PubMed] [Google Scholar]
- 46. Simm R, Morr M, Remminghorst U, Andersson M, Römling U. 2009. Quantitative determination of cyclic diguanosine monophosphate concentrations in nucleotide extracts of bacteria by matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry. Anal. Biochem. 386:53–58 [DOI] [PubMed] [Google Scholar]
- 47. Cesbron S, Paulin J-P, Tharaud M, Barny M-A, Brisset M-N. 2006. The alternative σ factor HrpL negatively modulates the flagellar system in the phytopathogenic bacterium Erwinia amylovora under hrp-inducing conditions. FEMS Microbiol. Lett. 25:221–227 [DOI] [PubMed] [Google Scholar]
- 48. Simm R, Morr M, Kader A, Nimtz M, Römling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134 [DOI] [PubMed] [Google Scholar]
- 49. Römling U, Amikam D. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9:218–228 [DOI] [PubMed] [Google Scholar]
- 50. Wolfe AJ, Visick KL. 2008. Get the message out: cyclic di-GMP regulates multiple levels of flagellum-based motility. J. Bacteriol. 190:463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Merritt JH, Brothers KM, Kuchma SL, O'Toole GA. 2007. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J. Bacteriol. 189:8154–8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bayot R, Ries S. 1986. Role of motility in apple blossom infection by Erwinia amylovora and studies of fire blight control with attractant and repellent compounds. Phytopathology 76:441–445 [Google Scholar]
- 54. Bellemann P, Geider K. 1992. Localization of transposon insertions in pathogenicity mutants of Erwinia amylovora and their biochemical characterization. J. Gen. Microbiol. 138:931–940 [DOI] [PubMed] [Google Scholar]
- 55. Bugert P, Geider K. 1995. Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol. Microbiol. 15:917–933 [DOI] [PubMed] [Google Scholar]
- 56. Jin Q, Hu W, Brown I, McGhee G, Hart P, Jones AL, He SY. 2001. Visualization of secreted Hrp and Avr proteins along the Hrp pilus during type III secretion in Erwinia amylovora and Pseudomonas syringae. Mol. Microbiol. 40:1129–1139 [DOI] [PubMed] [Google Scholar]
- 57. Wei Z, Kim JF, Beer SV. 2000. Regulation of hrp genes and type III protein secretion in Erwinia amylovora by HrpX/HrpY, a novel two-component system, and HrpS. Mol. Plant Microbe Interact. 13:1251–1262 [DOI] [PubMed] [Google Scholar]
- 58. Newell PD, Yoshioka S, Hvorecny KL, Monds RD, O'Toole GA. 2011. Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1. J. Bacteriol. 193:4685–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kader A, Simm R, Gerstel U, Morr M, Römling U. 2006. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 60:602–616 [DOI] [PubMed] [Google Scholar]
- 60. Bobrov AG, Kirillina O, Ryjenkov DA, Waters CM, Price PA, Fetherston JD, Mack D, Goldman WE, Gomelsky M, Perry RD. 2011. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol. Microbiol. 79:533–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Amarasinghe JJ, D'Hondt RE, Waters CM, Mantis NJ. 2013. Exposure of Salmonella enterica serovar Typhimurium to a protective monoclonal IgA triggers exopolysaccharide production via a diguanylate cyclase-dependent pathway. Infect. Immun. 81:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 194:3307–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bellows LE, Koestler BJ, Karaba SM, Waters CM, Lathem WW. 2012. Hfq-dependent, co-ordinate control of cyclic diguanylate synthesis and catabolism in the plague pathogen Yersinia pestis. Mol. Microbiol. 86:661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ayers AR, Ayers SB, Goodman RN. 1979. Extracellular polysaccharide of Erwinia amylovora: a correlation with virulence. Appl. Environ. Microbiol. 38:659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Raymundo AK, Ries SM. 1981. Motility of Erwinia amylovora. Phytopathology 71:45–49 [Google Scholar]
- 66. Wang D, Korban SS, Zhao Y. 2010. Molecular signature of differential virulence in natural isolates of Erwinia amylovora. Phytopathology 100:192–198 [DOI] [PubMed] [Google Scholar]
- 67. Ahmad I, Lamprokostopoulou A, Le Guyon S, Streck E, Barthel M, Peters V, Hardt WD, Römling U. 2011. Complex c-di-GMP signaling networks mediate transition between virulence properties and biofilm formation in Salmonella enterica serovar Typhimurium. PLoS One 6:e28351 doi:10.1371/journal.pone.0028351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lamprokostopoulou A, Monteiro C, Rhen M, Römling U. 2010. Cyclic di-GMP signalling controls virulence properties of Salmonella enterica serovar Typhimurium at the mucosal lining. Environ. Microbiol. 12:40–53 [DOI] [PubMed] [Google Scholar]
- 69. Wei ZM, Beer SV. 1995. hrpL activates Erwinia amylovora hrp gene transcription and is a member of the ECF subfamily of sigma factors. J. Bacteriol. 177:6201–6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McNally RR, Toth IK, Cock PJA, Pritchard L, Hedley PE, Morris JA, Zhao Y, Sundin GW. 2012. Genetic characterization of the HrpL regulon of the fire blight pathogen Erwinia amylovora reveals novel virulence factors. Mol. Plant Pathol. 13:160–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pérez-Mendoza D, Coulthurst SJ, Sanjuán J, Salmond GPC. 2011. N-Acetylglucosamine-dependent biofilm formation in Pectobacterium atrosepticum is cryptic and activated by elevated c-di-GMP levels. Microbiology 157:3340–3348 [DOI] [PubMed] [Google Scholar]
- 72. Ryan RP, Fouhy Y, Lucey JF, Jiang BL, He YQ, Feng JX, Tang JL, Dow JM. 2007. Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris. Mol. Microbiol. 63:429–442 [DOI] [PubMed] [Google Scholar]