Abstract
Toxin-antitoxin (TA) systems have been reported in the genomes of most bacterial species, and their role when located on the chromosome is still debated. TA systems are particularly abundant in the massive cassette arrays associated with chromosomal superintegrons (SI). Here, we describe the characterization of two superintegron cassettes encoding putative TA systems. The first is the phd-docSI system identified in Vibrio cholerae N16961. We determined its distribution in 36 V. cholerae strains and among five V. metschnikovii strains. We show that this cassette, which is in position 72 of the V. cholerae N16961 cassette array, is functional, carries its own promoter, and is expressed from this location. Interestingly, the phd-docSI system is unable to control its own expression, most likely due to the absence of any DNA-binding domain on the antitoxin. In addition, this SI system is able to cross talk with the canonical P1 phage system. The second cassette that we characterized is the ccdVfi cassette found in the V. fischeri superintegron. We demonstrate that CcdBVfi targets DNA-gyrase, as the canonical CcBF toxin, and that ccdVfi regulates its expression in a fashion similar to the ccdF operon of the conjugative plasmid F. We also establish that this cassette is functional and expressed in its chromosomal context in V. fischeri CIP 103206T. We tested its functional interactions with the ccdABF system and found that CcdAVfi is specific for its associated CcdBVfi and cannot prevent CcdBF toxicity. Based on these results, we discuss the possible biological functions of these TA systems in superintegrons.
INTRODUCTION
Toxin-antitoxin (TA) systems were originally discovered on low-copy-number plasmids through the stabilizing role that they play in these replicons (for recent reviews on TA systems, see references 1, 2, and 3). They are generally composed of two genes encoding a toxin and an antitoxin that antagonizes the toxin activity or prevents its synthesis. The antitoxin can be either an RNA (type I and III systems [4]) or a protein (type II systems), while the toxin is always a protein. In type II systems, the antitoxin and toxin genes are organized in operons whose expression is generally autoregulated at the transcriptional level by the toxin-antitoxin complex. The antitoxin is unstable and degraded by ATP-dependent proteases. The toxin is stable and inhibits an essential cellular process (e.g., replication, translation, or peptidoglycan synthesis). These type II systems have more recently been identified as genuine components of the chromosome of most bacteria (5–7), with up to more than 80 predicted TA systems in the Mycobacterium tuberculosis genome (8). Although their stabilization capacity is clearly established when they are located on plasmids, their role when located on the chromosome is much less evident and is still debated (2). There are currently as many as six proposed nonexclusive hypotheses regarding the biological roles of these chromosomal elements (2). The first four roles can be described as physiological or developmental regulators. TA systems were proposed to be in charge of a programmed cell death-like response, allowing altruistic suicide under stressful conditions (reviewed in reference 9). However, this hypothesis is controversial, as several groups failed to reproduce the original observations (see reference 10). A second proposed role, substantiated by the work of Gerdes and colleagues, is that TA systems could act as growth modulators involved in cell survival under unfavorable conditions (11). In relation to a role for survival, TA systems have also been proposed to be involved in the production of persister cells within bacterial populations (12). Persisters consist of a small fraction of cells that are in a dormant state and appear to be resistant to stress conditions, such as antibiotic treatments (for a review, see reference 13). TA systems have also been proposed to play a role of development regulators in Myxococcus xanthus (14). The last two hypothetical roles proposed for chromosomal TA are more in line with their original function in plasmids. First, it has been shown that these systems could protect their host genome from colonization by an incoming mobile element or a plasmid carrying a TA from the same functional family by allowing its harmless loss through neutralization of the toxin of the invading element by the chromosomal antitoxin (15, 16). Also, they have been proposed to stabilize chromosomal regions by preventing accidental deletions, especially when located in unstable segments such as mobile genetic elements (MGE) (17–19), as, for example, in integrative and conjugative elements such as SXT (19, 20). In this line, it is striking to notice that TA systems are extremely common in cassettes of chromosomal integrons, especially in superintegrons (SI), (for a review, see reference 21).
Superintegrons gather hundreds of cassettes in Vibrio genomes (17, 22), mostly of unknown functions. Cassettes are in most cases promoterless and are thought to constitute a silent reservoir of adaptive functions (17, 23). Silent cassettes can be called on for expression through recombination to an expression spot, in most cases when brought to the first position(s) in the array (for a review, see reference 21). Cassette recombination is triggered in times of stress, as integron integrase expression is commonly controlled by the SOS response (24, 25), but also when horizontal gene exchanges occur (26, 27). Thus, between two episodes of stress, the cassette arrays stay steady, and one considers that the selective pressure exerted on distal silent cassettes is low if not absent. Several studies have shown that the Vibrio cholerae SI cassette array is a highly variable region that could be used to discriminate different V. cholerae serogroups and even isolates of the same serogroup. Using primers designed to anneal to the highly conserved V. cholerae cassette attC sites, historically named VCR (Vibrio cholerae repeats [28]), several teams have proposed PCR-based typing applications on SI unique variability (29–33). The V. cholerae N16961 SI carries 13 cassettes encoding putative type II TA systems (5, 17). The TA genes carried in 9 of these 13 cassettes are carried in the opposite orientation to that of other genes in the cassettes, preventing TA expression from the promoter located in attI sites that directs cassette gene expression (34). However, these TA genes are among the rare cassettes that can carry their own promoter, as there is sufficient space within the cassette boundaries to carry expression signals in the region located 5′ of the TA genes. The fact that these cassettes are most likely expressed lead us to propose that they could play a stabilization role by preventing cassette loss through homologous recombination between identical attC sites or between copies of repeated identical cassettes (17, 18). Data on the higBA and parDE V. cholerae TA cassettes gave further support to this role (35–37).
In this work, we describe the characterization of two SI cassettes encoding putative SI TA systems. The first is the phd-docSI cassette, which we identified through the analysis of the V. cholerae N16961 genome sequence (17). We first developed a simple SI PCR typing strategy, based on the previously published SI/VCR typing applications mentioned above, to determine both the SI intIA proximal and distal cassettes in the SI cassette array. We applied this to the characterization of SI variation in 36 strains from the Pasteur Institute collection (CIP) of different origins, isolation dates, and serogroups (15 were from non-O1/non-O139 or unknown serogroup, while the others were of serogroup O1 or O139). This strategy allowed the definition of 15 SI types defined by the sequence of the first cassette(s) in the SI, which are supported by whole-genome sequencing data obtained for different V. cholerae strains. We then determined the distribution of the phd-docSI cassette among these different V. cholerae strains, as well as in 5 Vibrio metschnikovii strains, where this cassette is also found (17). We show that this cassette, which is in position 72 in the V. cholerae N16961 SI array, is expressed and encodes a functional TA system that does not regulate its own expression. We further establish that the SI Phd (PhdSI) can prevent DocP1 toxicity and conversely that PhdP1 can prevent the DocSI deleterious effect.
The second cassette that we characterized is the ccd cassette from V. fischeri SI (17). As for the phd-doc cassette, we demonstrate that this cassette encodes a functional TA system with an active promoter and is expressed when in its original SI context. We show that the CcdBVfi toxin targets DNA gyrase, and we found that the CcdA-BVfi complex regulates the cassette expression, in a fashion similar to the ccdF operon of conjugative plasmid F. We also tested its functional interactions with the ccdF system. We also tested both phd-docSI and ccdVfi for their aptitude to mediate postsegregational killing (PSK) when carried on an unstable vector. Altogether, our results clearly support a role of SI stabilization but also suggest that these cassettes can play a protective role against incoming MGE to a certain extent.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Escherichia coli strains used in this work are described in Table 1, Vibrio cholerae strains in Table 2, and Vibrio metschnikovii and Vibrio fischeri strains in Table 3. E. coli, V. cholerae, and V. metschnikovii strains were grown in Luria-Bertani (LB) or on LB agar at 37°C and Ceria 132 synthetic medium supplemented with 0.1% Casamino Acids (CMM [38]). V. fischeri strains were grown in marine broth (MB) or marine agar (MA) at 30°C. When appropriate, media were supplemented with chloramphenicol (Cm; 5 mg/ml for V. cholerae or 25 mg/ml for E. coli), spectinomycin (Sp; 100 μg/ml for V. cholerae or 50 μg/ml for E. coli), kanamycin (Km; 25 μg/ml for E. coli and V. cholerae or 75 μg/ml for V. fischeri), and ampicillin (Ap; 100 μg/ml).
Table 1.
Escherichia coli strains used in this study
| Strain | Genotype or description | Reference or source |
|---|---|---|
| DH5α | supE44 ΔlacU169(ϕ80dlacZΔM15) ΔargF hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Laboratory collection |
| β2163 | MG1655::Δdap::(erm pir)RP4-2Tc::Mu (Km) | 76 |
| MC1061 | F− araD139 Δ(araA-leu)7697 Δ(ccdB-lacI)3 galK16 galE15 hsdR2 relA1 spoT1 mcrB9999 rpsL150 (Strr) | Laboratory collection |
| B462 | DH2 lacIq gyrA462 zei::Tn10 | 15 |
| CSH50λ sfiA::lacZ | ara Δ(lac-pro) strA thi sfiA::lacZ | 15 |
| SG22622 | MC4100 cpsB::lacZ ara malP::lacIq | 77 |
| SG22622 gyrA462 | SG22622 containing the gyrA462 CcdBF resistance mutation | 77 |
| ω3326 | DH5α/pUC18::phdSI | This work |
| ω3379 | ω3326/pSU18T::docSI | This work |
| ω3761 | ω3730/pSU18::docP1 | This work |
| ω3338 | β2163/pUC18::phdSI | This work |
| ω3481 | ω3338/pSU18T::docSI | This work |
| ωB217 | ω3326/pSU18T | This work |
| CSH50-1 | CSH50/pJL-OPphd-docSI | This work |
| CSH50-2 | CSH50/pJL-OPphd-docP1 | This work |
| CSH50-3 | CSH50/pJL-OPccdF | This work |
| CSH50-4 | CSH50/pJL-OPccdVfi | 15 |
Table 2.
Vibrio cholerae strains included in this studya
| SI subtypeb | Strain | Isolation yr | Source | Origin | Serogroup | Biotype |
|---|---|---|---|---|---|---|
| I | N16961 | 1975 | CIP | Bangladesh | O1 Inaba | Eltor |
| II | MJ-1236 | 1994 | Clinical, Bangladesh | O1 Inaba | Matlab variant of Eltor | |
| 63.32 | 1961 | CIP | Hong Kong | O1 | Eltor | |
| 68.10 | 1968 | CIP | Vietnam | O1 | ND | |
| 254 | 1991 | C. A. Salles | Human, Brazil | O1 | ND | |
| 104151 | 1992 | CIP | Human, Bangladesh | O139 | ND | |
| 104152 | 1993 | CIP | Human, Bangladesh | O139 | ND | |
| 1024 | 1961 | J.-M. Fournier | Philippines | ND | ND | |
| III | M66-2 | 1937 | Indonesia | |||
| 66.2 | 1966 | CIP | Indonesia | O1 | Eltor | |
| 182 | 1983 | C. A. Salles | Australia | O1 | ND | |
| 1023 | 1937 | J.-M. Fournier | Indonesia | ND | ND | |
| IV | 12129 | 1985 | Water, Australia | O1 Inaba | Eltor | |
| A256 | 1953 | CIP | O1 Inaba | Eltor | ||
| V | V52 | 1968 | Clinical, Sudan | O37 | ||
| 335 | 1965 | C.A. Salles | Czechoslovakia | Non-O1 | ND | |
| VI | 723 | 1954 | C.A. Salles | Food, Thailand | Non-O1/O139 | ND |
| VII | 63.40 | 1935 | CIP | Non-O1, non-O139 | Nanking | |
| VIII | 63.41 | 1935 | CIP | Non-O1, non-O139 | Eltor | |
| IX | 104153 | 1989 | CIP | Water, Bangladesh | Non-O1, non-O139 | ND |
| X | 104154 | 1987 | CIP | Water, Bangladesh | Non-O1, non-O139 | ND |
| XI | 151 | 1986 | C. A. Salles | Mexico | Non-O1 | ND |
| XII | VL426 | ? | Water, Maidstone, UK | Non-O1, non-O139 | Albensis | |
| 69.41 | 1958 | CIP | Fish, Germany | Non-O1, non-O139 | ND | |
| XIII | O395 | 1965 | India | O1 Ogawa | Classical | |
| 569B | 1940 | J.-M. Fournier | Human, India | O1 | ND | |
| 62.14 | 1959 | CIP | Thailand | O1 | ND | |
| 62.15 | 1959 | CIP | Thailand | O1 | ND | |
| 62.16 | 1959 | CIP | Thailand | O1 | ND | |
| A269 | 1943 | CIP | O1 | ND | ||
| 55.91 | 1953 | CIP | O1 | ND | ||
| 1143 | 1959 | J.-M. Fournier | India | ND | ND | |
| 1144 | 1947 | J.-M. Fournier | Egypt | ND | ND | |
| 1145 | 1953 | J.-M. Fournier | India | ND | ND | |
| XIV | ME11672 | 1993 | M. Gallas | Water, Argentina | Non-O1, non-O139 | ND |
| XV | BA5 | 1993 | M. Gallas | Water, Argentina | Non-O1, non-O139 | ND |
Genome sequences are accessible for strains in boldface type. ND, not determined.
See the text for definition of SI subtypes; sequences are available at the following GenBank accession numbers: I, KC691400; II, KC691401; III, KC683366; IV, KC683367; V, KC691402; VI, KC683368; VII, KC691399; VIII, KC691403; IX, KC691404; X, KC683369; XI, KC683370; XII, KC691405; XIII, KC691406, XIV, KC683371; XV, KC683372.
Table 3.
Vibrio metschnikovii and Vibrio fischeri strains used in this study
| Species | Strain | Isolation yr | Source | Origin | phd-docSI | ccdABVfi |
|---|---|---|---|---|---|---|
| V. metschnikovii | A267 | 1888 | CIP | Poultry | + | − |
| 69.14T | 1922 | CIP | Diseased fowl | + | − | |
| 69.15 | 1954 | CIP | Unknown | + | − | |
| 8050 | 1980 | CIP | Human urine, Nevers, France | + | − | |
| 104262 | 1977 | CIP | Cooked cockles, Maidston, UK | + | − | |
| V. fischeri | 103206T (ATCC 7744) | 1889 | CIP | Unknown | − | + |
| ES114 | 1990 | P. Dunlap | Light organ symbiont of the squid Euprymna scolopes (78) | − | − |
PCR procedures.
Error-free PCRs were performed in 50-μl mixtures by using Pfu DNA polymerase (Promega) following the manufacturer's instructions. Other PCRs were performed in 50-μl mixtures by using PCR Reddy mix (Abgène, United Kingdom) following the manufacturer's instructions. Primers were obtained from Sigma (Evry, France) and are listed in Table 4. The conditions used for amplification were as follows: 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 60s. The sequence of each cloned PCR product used in the different constructions described in this work was verified. Sequencing was performed by MWG-Biotech AG (Ebersberg, Germany) and with ABI Prism Applied Biosystem 3100 Genetic Analyzer.
Table 4.
Oligonucleotides used in this study
| Primer name | Sequence (5′-3′) | Restriction site |
|---|---|---|
| I4 | GCGAACACTTAACAAAAACTGGG | |
| VCR1 | GTCCCTCT-TGAGGCGTTTGTTA | |
| VCR2 | GCCCCTTAGGCGGGCGTTA | |
| 829-2 | TTCCACTGCGCCATTTACTGA | |
| 829-7 | TCTGTCACTACAGGTTTGCC | |
| 32-2 | GCATTCTTTGCGTCACACTATGGC | |
| VPD1 | CGGGATCCCGTTTGTTTAGAGAGCTGAATTTTGTTCT | BamHI |
| VPD2 | GGAATTCCTTATGTTT TTTATCACGTTTTG | EcoRI |
| VP1 | GGATCCTCATTACATATCAGCCAATTTAGTGAATGT | BamHI |
| VP2 | GGAATTCCAGGAGTGATTATGAATCGAAAAGTTGAAGC | EcoRI |
| VPQF | TGTAGCCGTTGTTCTTGACG | |
| VPQR | GAACGAGCATCTTCGTTTGA | |
| VD1 | CGGGATCCCGTTAGAGAGCTGAATTTTGTTCTTTTGC | BamHI |
| VD2 | GGAATTCCGGAGATCCTAATGGATATCATCTGTTTTCCTTTTGA | EcoRI |
| VD3 | GCTCTAGAGCTTAGAGAGCTGAATTTTGTTCTTTTGC | XbaI |
| pVPI | CGCGGATCCTTTCGATTCATATCACACCTACTATCAATAAAGGTTAGGCCG | BamHI |
| pVP2 | GGGAATTCTAGATTAACCCCTTAGGCGGGCGTTATGTTTTTTATCACGTTTTGGGGCTATAATCGGCCTAACCT | EcoRI |
| PP.D1 | CGGGATCCATCTACTCCGCAGAACCATACA | BamHI |
| PP.D2 | GGAATTCCGTTTATGCAATCCATTAACTTCCGT | EcoRI |
| PP1 | CGGGATCCTATCGGTTAACCAGTTCCTTGTTG | BamHI |
| PP2 | GGAATTCATGAGGCATATATCACCGGAAGAA | EcoRI |
| PDP1 | GCTCTAGAGCCTACTCCGCAGAACCATACA | XbaI |
| 5CcdAVfRI | TTGTGAATTCTATGAGAAATCAATATAATACACAAGCGG | EcoRI |
| 3CcdAVfPI | AGTCTCTGCAGTTAAAATACTCGGTATGA | PstI |
| 5CcdBVfXb | TCTAGAAGGAGGTTTAAATGTCTCAATTTACGCTATATAAAAACAAAGAT | XbaI |
| 3CcdBVfPI | AGTCTCTGCAGTTTA AATGCCAGTGAT | PstI |
| 5O/P-ccdVf | CTGCAGATGCATTTGTTAAATG | PstI |
| 3O/P-ccdVf | AAGCTTACCGCTTGTGTATTATATTG | HindIII |
| pSFN1-1 | AACTGCAGAACCAATGCATTGGCGCGCTATCGCTTGTCGGCC | PstI |
| pSFN1-2 | CCCAAGCTTAAGAGCAGGGCTTTATATAGGACG | HindIII |
| ccdVf1 | GGAATTCCGAGGTATTTTTAAATGTCTCAATTTACGCTAT | EcoRI |
| ccdVf2 | CGGGATCCTTAAATGCCAGTGATTAAAAATCCC | BamHI |
| proVch-for | CCCCCTGCAGCCCTTAGGCGGGCGTTATGTTTTTTATCACGTTTTGGGGCTATAATCGGCCTAACCTTTATTGATAGTAGGTGTGATAAGCTTCCCC | PstI- HindIII |
| proVch-rev | GGGGAAGCTTATCACACCTACTATCAATAAAGGTTAGGCCGATTATAGCCCCAAAACGTGATAAAAAACATAACGCCCGCCTAAGGGCTGCAGGGGG | PstI- HindIII |
| proP1-for | GGGGAAGCTTAAACACCTCGTGTACTCGTTATGTGTACACAATTATAAACTTCACAGGCATAAAGCACCAGCACTGCAGGGGG | PstI- HindIII |
| proP1-rev | CCCCCTGCAGTGCTGGTGCTTTATGCCTGTGAAGTTTATAATTGTGTACACATAACGAGTACACGAGGTGTTTAAGCTTCCCC | PstI- HindIII |
| Oli94 | GGAATTCCATGCATTTGTTAAATG | EcoRI |
| Oli95 | GGATCCTTAAATGCCAGTGATTAA | BamHI |
| Oli96 | CTGCAGATGCATTTGTTAAATG | PstI |
| Oli97 | AAGCTTACCGCTTGTGTATTATATT | EcoRI |
| 5′phd-docVch | CCCCGAATTCCCCTTAGGCGGGCGTTAT | EcoRI |
| 3′phd-docVch | CCCCGAATTCCCCTTAGGCGGGCGTTAT | BamHI |
| 5′phd-docP1′ | CCCCGAATTCTGCTGGTGCTTTATGCCTGT | EcoRI |
| 3″phd-docP1′ | CCCCGGATCCATCTACTCCGCAGAACCATA | BamHI |
| MRV | AGCGGATAACAATTTCACACAGGA | |
| MFD | CGCCAGGGTTTTCCCAGTCACGAC | |
| 16SQF | TCAGCTCGTGTTGTGAAATG | |
| 16SQR | GTAAGGGCCATGATGACTTG |
Characterization of the V. cholerae intIA proximal and distal parts.
The intIA proximal SI cassette(s) was amplified with primers I4 and VCR1 (39) and the distal SI cassette region with primers VCR2 (22) and either 829-2 or 32-2, using the V. cholerae strains listed in Table 2 as the templates. The PCR products were digested with HincII and inserted into pUC18 or pNot218, digested by the same enzyme, and sequenced with MRV and MFD primers.
RNA preparation and RT-PCR.
Total RNA was purified from LB cultures harvested in the middle and at the end of the exponential phase as previously described (40). Reverse transcriptase PCR (RT-PCR) was performed on 500 ng V. cholerae N16961 total RNA with VP1 and VP2 primers (Table 4) using the Access RT-PCR system (Promega) with or without AMV reverse transcriptase. Real-time quantitative RT-PCRs were performed and analyzed as previously described (41), except that a 2-min preincubation at 90°C was replaced by 5 min at 80°C. The expression level of tested genes was normalized using the 16S rRNA gene of V. cholerae (primers 16SQF and 16SQR; Table 4). The experiments were repeated twice independently.
Plasmids.
Plasmids used in this study are listed in Table 5.
Table 5.
Plasmids used and constructed
| Plasmid | Properties | Reference or source |
|---|---|---|
| pNOT218 | oriColE1, Apr | 79 |
| pSU18 | oriP15A, Cmr | 80 |
| pSU18T | pSU18 oriTRP4 | 42 |
| pSU38 | oriP15A, Kmr | 80 |
| pSU38T | pSU38 oriTRP4 | This work |
| pUC18 | oriColE1, Apr | 81 |
| pUC19 | oriColE1, Apr | 81 |
| pCR-XL-TOPO | oriColE1, Apr | 82 |
| pBAD33 | oriP15A, Cmr, PBAD::MCS | 83 |
| pBAD43 | oripSC101, Spr, PBAD::MCS | 83 |
| p3326 | pUC18::phdSI | This work |
| p3730 | pUC18::phdP1 | This work |
| p3178 | pSU18::docSI | This work |
| p3974 | pBAD43::docSI | This work |
| p4009 | pBAD43::docP1 | This work |
| p1400 | pTZ19R::ccdABVfi | 17 |
| pJL207 | p15A, Cmr, lacZ | 84 |
| p3481 | pSU18T::docSI | This work |
| pSFN1 | pB1067 | 46 |
| pSU38TgV | pSU38T-oriVpSFn1 | This work |
| p9939 | pSU38TgV::ccdBVfi | This work |
| p3610 | pSU18T::ccdBVfi | This work |
| pBAD-ccdBF | pBAD33::ccdBF | 15 |
| pBAD-ccdBVfi | pBAD33::ccdBVfi | This work |
| pKK223-3 | ColE1, Apr, PTAC promoter | 85 |
| pKK-ccdAVfi | pKK223-3::ccdAVfi | This work |
| pJL-OPccdVfi | pJL207::O/P ccdBVfi::lacZ | This work |
| pJL-OPccdF | pULB2006 | 45 |
| pJL-OPphd-docSI | pJL207::O/P phdSI::lacZ | This work |
| pJL-OPphd-docP1 | pJL207::O/P phdP1::lacZ | This work |
| pMLO59 | pGB2 ts derivative, Spr | 15 |
| pMLO-phd-docSI | pMLO59::phd-docSI | This work |
| pMLO-phd-docP1 | pMLO59::phd-docP1 | This work |
| pMLO-ccdVfi | pMLO59::ccdVfi | This work |
| pMLO-ccdF | pULB2710 | 46 |
Plasmid constructions.
All plasmids and intermediate constructs were sequenced.
(i) Plasmids expressing phdSI or phdP1.
phd genes were amplified from V. cholerae N16961 and P1 phage chromosomal DNA, using primers VP1 and VP2 or PP1 and PP2. PCR products were digested with EcoRI and BamHI and inserted into pUC18, digested by the same enzymes.
(ii) Plasmids expressing docSI or docP1.
doc genes were amplified from V. cholerae N16961 and P1 phage chromosomal DNA, using the primers VD2 and VD3 or PD2 and PD3. PCR products were digested with EcoRI and XbaI and inserted into pBAD43, digested by the same enzymes. These plasmids, pBAD43::docSI and pBAD43::docP1, do not express doc in the presence of 1% glucose but do express doc in the presence of 0.2% arabinose. pSU18::docSI was constructed by cloning of the docSI gene after amplification using primers VD2 and VD1 for cloning of the product into pSU18 using EcoRI and BamHI. The ligation mix was transformed into a DH5α strain containing plasmid p3326, which expresses phdSI to prevent Doc toxicity and is compatible with pSU18.
(iii) Plasmids expressing ccdBF and ccdBVfi.
The expression plasmids are isogenic, i.e., all the open reading frames (ORFs) were cloned using the same restriction sites in the expression vectors and all the regulatory sequences added by PCR. The Shine-Dalgarno box (SD) and the sequence between the SD and the ATG of the ORFs were identical. pBAD-ccdBF plasmid has been described previously (15). pBAD-ccdBVfi plasmid was constructed as follows: the ccdB gene from V. fischeri, ccdBVfi, was amplified by PCR using p1400 (17) as the template and the primers 5CcdBVfXb and 3CcdBVfPI. The PCR fragment XbaI site was blunted with the Klenow enzyme and then restricted by PstI. The resulting fragment was inserted into pUC19 digested by SmaI and PstI. The resulting plasmid was then digested by XbaI and PstI. The fragment containing ccdBVfi was inserted into pBAD33 digested by the same enzymes. The ligation mixture was transformed in B462.
For pKK-ccdAVfi plasmid, the ccdA gene from V. fischeri, ccdAVfi, was amplified by PCR using p1400 (17) as the template and the primers 5CcdAVfRI and 3CcdAVfPI. The PCR product was cloned into the TOPO-XL vector (Invitrogen). The resulting plasmid was then digested by EcoRI and PstI. The fragment containing ccdAVfi was inserted into pKK223-3 using the same enzymes.
(iv) Conjugative plasmids expressing docSI, ccdBVfi, or ccdBF.
An expression vector able to be transferred by conjugation and to replicate in V. fischeri was constructed starting from the p15A derivative pSU38, by cloning the oriTRP4 as described previously for pSU18T (42). As neither ColE1 nor p15A derivatives are stably replicated in V. fischeri, we added in pSU38T, a fragment containing the replication origin of the Vibrio nigripulchritudo plasmid pSFn1 (43), also called pB1067 (44). This plasmid is the paradigm of a plasmid group commonly found in Vibrio species and that has been shown to replicate in all Vibrio species, including V. fischeri, when tested (44). OriVpSFn1 was amplified from pSFn1 using primers pSFN1-1 and pSFN1-2. The PCR product was digested with PstI and HindIII and inserted into pSU38T (p3337) digested by the same enzyme to give pSU38TgV (p9887). The ccdBVfi gene was amplified from V. fischeri CIP103206T, using primers Ccdvf1 and Ccdvf2. PCR products were digested with EcoRI and BamHI and inserted into pSU38TgV, digested by the same enzymes, and transformed in B462, leading to plasmid p9939 (pSU38TgV::ccdBVfi).
(v) Promoter activity reporter plasmids.
To construct the pJL-OPphd-docSI plasmid, 2 complementary oligonucleotides, proVch-for and proVch-rev, encompassing the operator/promoter region of the phd-docSI operon were synthesized. Annealing of these primers (1 μg each) was performed by slow cooling at room temperature after heating. The double-strand product was then restricted by HindIII and PstI and cloned in the pJL207 vector restricted by the same restriction enzymes. Recombinant clones were screened on minimal medium containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside).
To construct the pJL-OPphd-docP1 plasmid, a similar procedure was followed with primers proP1-for and proP1-rev.
To construct the pJL-OPccdVfi plasmid, the operator/promoter region of the ccdVfi operon was amplified by PCR using the p1400 plasmid (17) as a template and the primers Oli96 and Oli97. The PCR product was cloned into the pCR-XL-TOPO vector (Invitrogen). A resulting plasmid with the insert in the proper orientation was then digested by HindIII and PstI (sites from the TOPO-XL vector). The fragment containing the operator/promoter region of the ccdVfi operon was inserted into pJL207 digested with the same enzymes. Recombinant clones were screened on MacConkey lactose medium.
Plasmid pJL-OPccdF has been described in reference 45 under the designation pULB2006.
(vi) Postsegregational killing plasmids.
To construct the pMLO-phd-docSI plasmid, the phd-docSI operon was amplified by PCR using primers 5′phd-docVch and 3′phd-docVch. The PCR product was cloned into the pMLO59 plasmid digested by EcoRI and BamHI.
To construct the pMLO-phd-docP1 plasmid, the phd-docP1 operon was amplified by PCR using a P1vir as a template and primers 5′phd-docP1 and 3′phd-docP1. The PCR product was cloned into the pCR-XL-TOPO vector. The resulting plasmid was then digested by EcoRI and BamHI. The fragment containing the phd-docP1 operon was inserted into pMLO59 digested with the same enzymes.
To construct the pMLO-ccdVfi plasmid, the ccdVfi operon was amplified by PCR using the p1400 plasmid (17) as a template and primers Oli94 and Oli95.
The PCR product was cloned into the TopoXL vector. The resulting plasmid was then digested by EcoRI and BamHI. The fragment containing the ccdVfi operon was inserted into pMLO59 digested with the same enzymes.
Plasmid pMLO-ccdF has been described previously under the designation pULB2710 (46).
Conjugations.
Conjugations were performed as previously described (47) and repeated at least three times. Briefly, overnight cultures of donor and recipient cells were diluted 100-fold in LB and grown to an optical density (OD) of ∼0.5. Donor and recipient cells were then mixed in a 1:1 ratio on 0.45-μm conjugation filters on LB plates preheated at 37°C when the recipient was V. cholerae or at 30°C when it was V. fischeri. After overnight incubation at 37°C (V. cholerae) or 30°C (V. fischeri), the filter was suspended in 5 ml LB, and dilutions were plated on selective plates to determine (i) the number of conjugants and (ii) the total number of recipients. Plasmid presence in transconjugant was checked by PCR with primers pSFN1-1 and pSFN1-2 on 8 randomly chosen clones. The new broad-host-range mobilizable vector pSU38TgV, constructed for this study (see above), was transferred from E. coli strain ω3480 to V. fischeri strain CIP 103206T at 1.6 × 10−4 ± 0.5 × 10−4 and to strain ES114 at 3.1 × 10−4 ± 0.5 × 10−4.
Toxicity and antitoxicity assays.
Strains carrying the toxin-expressing plasmids and/or the antitoxin-expressing plasmid were grown overnight at 37°C in CCM supplemented with glucose (0.4%) and the appropriate antibiotics. Overnight cultures were diluted in the same medium to an OD at 600 nm (OD600) of ∼0.01 and grown at 37°C to an OD600 of ∼0.1 to 0.2. The cultures were centrifuged at 4,000 rpm for 10 min at room temperature. The bacterial pellets were resuspended in CCM, prewarmed at 37°C, and supplemented with glycerol (0.4%) and the appropriate antibiotics. Arabinose was then added (0.25% or 1%), and the cultures were grown at 37°C. Samples were removed at indicated time points, diluted in MgSO4 (10 mM), and plated on CCM plates supplemented with glucose (0.4%) and the appropriate antibiotics. Plates were incubated overnight at 37°C.
Postsegregational killing assay.
CSH50λsfiA::lacZ strains containing the pMLO59 vector and its derivatives were grown overnight at 30°C in LB containing Sp (100 μg/ml). Overnight cultures were centrifuged at 4,000 rpm for 10 min at room temperature and suspended in LB. Cultures were then diluted 400-fold in LB at 42°C. Cultures were diluted every 50 min to maintain an OD600 of 0.1 to 0.2. Samples were removed at indicated times, diluted in MgSO4 (10 mM), and plated on LB plates and on LB plates supplemented with Sp. Plates were incubated overnight at 30°C. Numbers of CFU/ml on LB Sp plates at 42°C were similar for each pMLO59 derivative, indicating that these plasmids were lost at the same frequency (not shown).
Promoter activity assay.
CSH50/pJL-OPccdF, CSH50/pJL-OPccdVfi, CSH50/pJL-OPphd-docP1, and CSH50/pJL-OPphd-docP1 containing the pMLO59 vector or its derivatives were grown at 30°C in LB containing Cm (20 μg/ml) and Sp (100 μg/ml) to an OD600 of 0.3. Samples were removed, and β-galactosidase assays were performed as described in reference 48. Promoter activity was calculated as the ratio of β-galactosidase activity in the absence of the TA systems to that in the presence of the corresponding TA systems.
RESULTS
Determination of the different SI subtypes carried in V. cholerae strains.
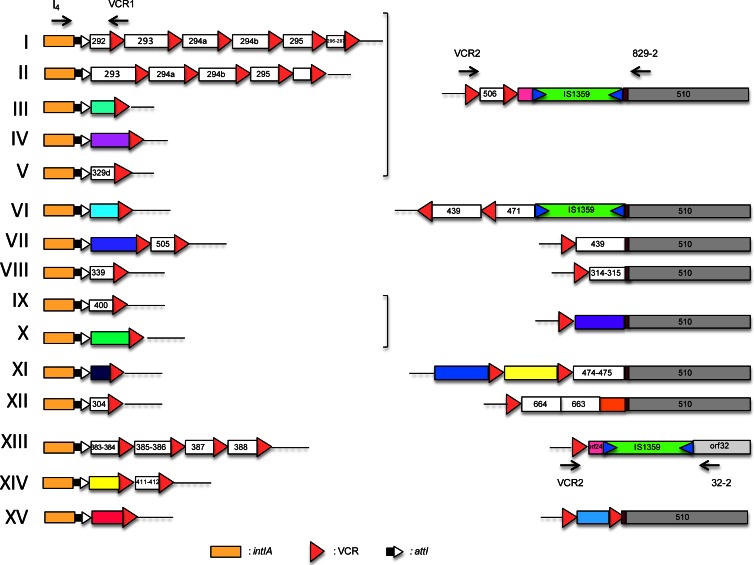
Several studies have shown that the SI cassette array was highly remodeled between different V. cholerae serogroups, and even among isolates of the same serogroup. Several laboratories have proposed PCR-based typing applications on this unique variability (29–33). In order to get a better view of SI variability and, in parallel, of the phd-docSI cassette distribution, we designed a simple PCR strategy (see Fig. S1 in the supplemental material) to characterize the intIA proximal cassette(s), the distal cassettes content of the SI, and the phd-docSI cassette presence and context among 36 V. cholerae strains (Table 2). Using a primer specific for the intIA gene (I4) and the primer VCR1 (39), we were able to amplify the very first cassette(s) present in all the strains tested. Indeed, VCR1 hybridizes with one of the two most conserved parts of the VCR, the V. cholerae-specific attC site, and thus allows the amplification of most V. cholerae cassettes as previously shown (39). The different amplicons were sequenced and allowed the retrieval of 15 different types of identical first-cassette arrangement and sequences (Fig. 1). In parallel, we performed a second PCR assay in order to characterize the distal part of the SI. For each strain, two PCRs were performed, which both used primer VCR2 (22), which hybridizes with the other most conserved sequence of the VCR and is oriented inversely to VCR1, and either primer 829-2 or 32-2, which are specific to the first gene downstream of the SI cassette array VCA0510 in N16961 or of Orf-32 in 569B (49). In all strains, one of these two PCRs gave rise to an amplification product, which after sequencing allowed the definition of 9 different distal parts (Fig. 1). As several V. cholerae strains' genome sequences became available during the course of this study, we also analyzed their intIA proximal SI cassette structure, determined to which of the 15 types they belong, and included this information in Table 2.
Fig 1.
Vibrio cholerae superintegron subtypes. Each of the 15 subtypes is defined by a superintegron having both a specific intIA proximal cassette(s) and a distal end of the cassette array. Subtypes are numbered in Roman numerals; intIA, VCR, and attI symbols are described in the figure. ORFs carried in cassettes that are found in the N16961 reference genome sequence are identified as white boxes, and the corresponding VCA0XXX identification is given by the last 3 digits; when the carried cassettes are not found in the N16961 genome, they are symbolized with colored boxes. The primers used for the subtype determination (see the text), their orientation, and their locations are indicated by black arrows. Insertion sequences (IS) are symbolized by boxes carrying two triangles pointing inside the box.
phd-docSI cassette occurrence in the different V. cholerae and V. metschnikovii strains.
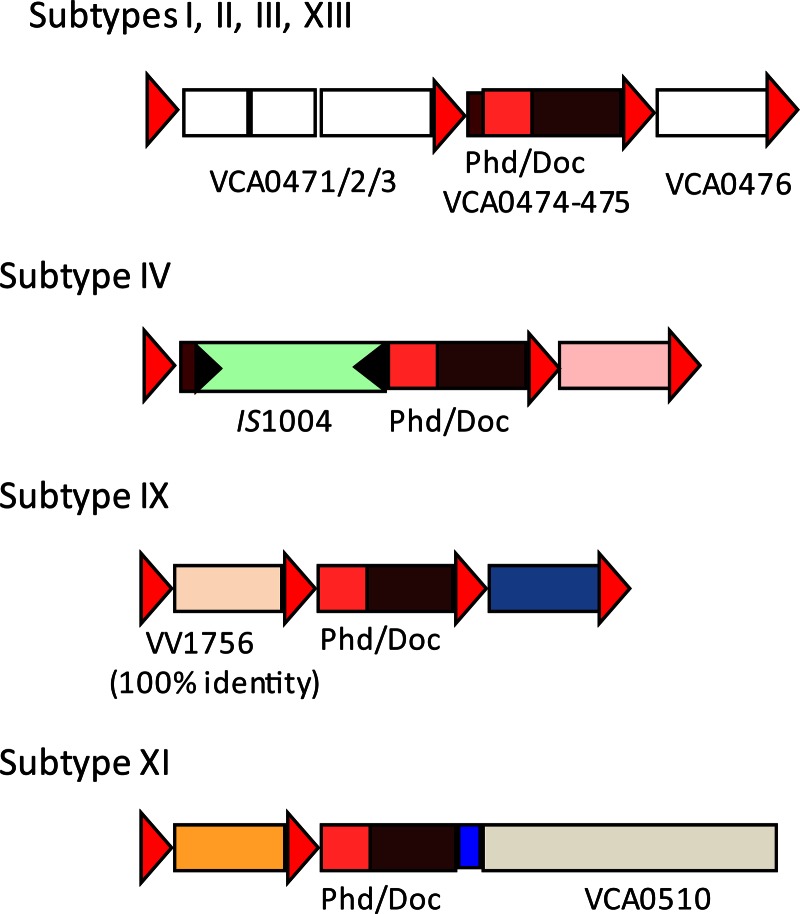
We further established the distribution of the phd-docSI cassette in these different V. cholerae SI subtypes and in the five different V. metschnikovii strains available at the Pasteur Institute collection (CIP) (Table 3). We performed two PCRs for each of the SI subtype strains, the first with primer PD1, a primer located upstream of the phd gene and oriented toward the inside of the cassette, and VCR1, and the second with PD1 and PD2, a primer located inside doc oriented toward the 5′ end of the gene. We found that only strains from subtypes VI, VII, and XII did not carry the phd-docSI cassette (not shown), while strains from the other 12 V. cholerae subtypes carried this cassette. In subtypes I, II, III, V, and XIII, the phd-docSI cassette context was identical to the one of N16961 (Fig. 2), where a cassette that has lost most of its VCR site precedes it (17). We have been able to characterize the phd-docSI cassette context in three other SI subtypes (Fig. 2). In strain 104153 (subtype IX), the cassette is preceded by a cassette with a canonical attC site, also found in V. vulnificus, while in strain A256 (subtype IV) the cassette contains an IS1004 copy inserted 21 bp upstream of the phd gene (Fig. 2). In the latter, analysis of the sequence also revealed that the doc gene carried a mutation in codon 40, and that led us to test its functionality (see below). Finally, we found that in subtype XI, the phd-docSI cassette was the last of the SI cassette array. Moreover, this cassette was now immobilized due to the loss of its associated VCR (Fig. 2). All V. metschnikovii strains were found to carry the cassette (Table 3). We found that strains 69.14T and 69.15 carried the phd-docSI cassette downstream of the same cassette (Orf253 to -255) in strain A267, where we previously described this TA cassette (17), while its context was different, but unknown, in the 2 other strains (data not shown).
Fig 2.
Genetic context of the phd-doc cassette in different V. cholerae superintegron subtypes. Subtypes are identified by their Roman numeral (Fig. 1). ORFs carried in cassettes that are found in the N16961 reference genome sequence are identified as white boxes, and the corresponding VCA0XXX identification is given by the last 3 digits; when the carried cassettes are not found in the N16961 genome, they are symbolized with colored boxes. Red triangles correspond to the VCRs of the different cassettes, and black triangles symbolize the inverted repeats (IRs) of IS1004.
Functional characterization of the phd-docSI system in E. coli.
In order to establish if the phd-docSI-encoded proteins were functional, we tested it in E. coli, which does not naturally carry a phd-doc TA system. After PCR amplification from V. cholerae N16961 genomic DNA with appropriate primers, we cloned the phdSI gene, which encodes the putative antidote to the DocSI toxin in pUC18 (Apr), under the control of the Plac promoter, leading to plasmid pUC18::phd (p3326). In parallel, we amplified the docSI gene and cloned it in a compatible plasmid, pSU18T, which carries a chloramphenicol resistance marker (Cmr), and transformed strain ω3326 (DH5α pUC18::phd). Transformation of DH5α with the same ligation mix did not produce any Cmr clones (not shown). One ω3326 transformant, carrying both compatible plasmids carrying docSI and phdSI, was named ω3379. In parallel, ω3326 was also transformed with the empty pSU18T, giving rise to ωB217. The plasmids carried in both strains, pUC18::phd and pSU18T::doc in ω3379 and pUC18::phd and pSU18T in ωB217, were extracted. These plasmid mixes were then digested by endonuclease NdeI, which specifically cleaves pUC18::phd but neither pSU18T nor pSU18T::doc. These NdeI-treated samples, which contain only propagative pSU18T derivatives, were then used to transform either DH5α or ω3326 (DH5 α/pUC18::phd). Both samples gave approximately the same number of Cmr clones in ω3326, while the Cmr clone frequency in DH5α dropped to 1% after transformation with the ω3379 sample (pSU18T::doc plus linearized pUC18::phd) compared to what is obtained with the ωB217 sample (pSU18T plus linearized pUC18::phd) (Table 6). Replica plating of those DH5α Cmr clones obtained after transformation with ω3379 NdeI-treated sample on LB Ap plates, showed that all Cmr clones were also Apr. The lack of ApS Cmr clones demonstrates the functionality of both genes in E. coli: docSI, encoding the toxin, and phdSI, encoding its antidote.
Table 6.
Demonstration of the functionality of DocSI/PhdSI functionality as a toxin-antitoxin couple
| DNA | Frequencya for CMR transformants coupled with: |
|
|---|---|---|
| DH5α | DH5α(pUC18::phd) | |
| ωB217 DNA (pSU18T + linearized pUC18::phd) | (1.12 ± 0.3) × 104 | (3.8 ± 0.3) × 104 |
| ω3379 DNA (pSU18T::doc + linearized pUC18::phd) | (2.7 ± 0.2) × 102 | (1.9 ± 0.3) × 104 |
Normalized for 1 μg of NdeI-treated plasmid mix.
The phd-docSI cassette is functional and expressed from the V. cholerae superintegron.
In order to establish the functionality of this TA module in V. cholerae, we used a strategy similar to the one described above, using conjugation to deliver the docSI gene in a strain carrying (N16961, subtype I) or lacking (CIP 63-40, subtype VII) the phd-docSI cassette. We used two different donor strains, both carrying a nonmobilizable pUC18::phdSI and either the compatible and mobilizable Cmr plasmid pSU18T::docSI or an empty pSU18T. We then made a conjugation between these two donors and either N16961 or CIP 63-40 as the recipient and selected for Cmr transconjugants. Under these conditions, with N16961, we obtained Cmr transconjugants for both the empty pSU18-oriT and its docSI derivative, at the same frequency (10−2). This shows that the phdSI gene is expressed from its cassette context in N16961 and could prevent DocSI toxicity. With CIP 63-40, Cmr transconjugants were obtained at the same frequency as in N16961 with the empty pSU18T, while with pSU18T::docSI, Cmr clones were only obtained at a frequency of 10−6. This difference suggested that DocSI was toxic in strain CIP 63-40. To further understand the origin of the few Cmr clones in strain CIP 63-40 after transfer of the docSI plasmid, we replicated these clones on Ap plates and found that a fraction of them were Apr (data not shown). We then analyzed the plasmid content of a sample of the Aps and Apr transconjugants and found that Apr clones all carried cointegrates of pSU18T::docSI and pUC18::phdSI, while the Aps clones carried a pSU18T::docSI containing a deletion covering most of the docSI gene, likely due to a replication slippage between two 11-bp-long repeats (not shown), inactivating its product. On the other hand, when we extracted the plasmids carried by the Cmr clones obtained in N16961, the pSU18T::docSI did not show any modification and were found to still be toxic when transformed in E. coli.
In order to formally demonstrate the expression of phd-doc cassette, we performed a semiquantitative RT-PCR, with or without AMV reverse transcriptase, and found a single band only after reverse transcription (see Fig. S2 in the supplemental material), demonstrating its efficient transcription. Furthermore, expression of the phd-doc operon was analyzed by real-time quantitative RT-PCR on total RNA extracted from cells in mid-log or early stationary phases, and a 3-fold-higher expression of the cassette was observed in exponential growth than in stationary phase. These results demonstrate that the phd-docSI cassette is expressed and functional in V. cholerae, DocSI being toxic and PhdSI able to prevent its deleterious activity.
In strain A256, in which the phd-docSI cassette is flanked by an IS1004, inserted 21 bp upstream of phd, analysis of the cassette sequence also revealed a mutation in codon 40 of the doc gene (CGA→TGA), leading to a premature stop. We checked that this mutation impaired the toxicity of the encoded DocSIA256, by transformation of E. coli with a plasmid expressing docSIA256 in the absence of phdSI in the recipient. We obtained transformants at similar rates with either the pDocSIA256 or the empty vector, showing that this mutation (R40*) inactivates Doc. We also tested if the IS1004 insertion modifies phdSIA256 in the A256 SI context. In order to do that, we cotransformed strain A256 with pSU18::docSI (Cmr) and pUC18::phdSI (Apr), selected Cmr transformants, and tested if they were also Apr. We found that all Cmr clones were also Apr, showing that the phdSIA256 expression in the A256 SI context was extinct.
The phd-doc systems of phage P1 and V. cholerae are able to cross talk.
The hypothesis that chromosomal TA could also play a role in the protection against incoming mobile elements, such as plasmids, interleukin-converting enzymes (ICEs), or lysogenic phages, implies that chromosomal systems should be able to counteract the TA systems brought by the invading element. As the Doc superfamily appears to be widespread in bacteria both on chromosomes and on plasmids (7), we tested the cross-interactions between the phd-docSI of the vibrio superintegron and the canonical phd-docP1 of the P1 phage in E. coli. The two Doc proteins show 32% identity, while the two Phd proteins are less related (17% identity) and do not hit each other by BLASTP (see Fig. S3 in the supplemental material). The two phd genes were cloned in pUC18 under the PLAC promoter, while the two doc genes were cloned in pBAD43, a low-copy-number SpR plasmid, under the PBAD promoter. The two pBAD43::doc plasmids could be maintained in E. coli only in the presence of 1% glucose, while transfer to LB plus 0.2% arabinose killed the cells. We then transformed each of these two strains independently with either the pUC18::phdP1 or the pUC18::phdSI and selected transformants in parallel on LB Sp Ap plus arabinose or plus glucose. We obtained the same number of transformants on all media, demonstrating that the two systems were able to interact and prevent the toxicity of the noncognate TA toxin.
The V. fischeri SI ccdVfi system is a toxin-antitoxin gene pair and is expressed from the V. fischeri superintegron.
We had previously obtained indirect evidence of the CcdBVfi toxic activity (17). To demonstrate the functionality of the two components, we used a strategy similar to the one used for phd-docSI and for another ccd homologous system (15). The ccdBvfi gene was cloned in the pBAD33 vector (PBAD promoter) and the ccdAvfi gene in the compatible pKK223-3 vector (PTAC promoter). E. coli was first transformed by the pKK223-3 vector or its derivative carrying the ccdAVfi gene. These strains were subsequently transformed with the pBAD33 vector or the pBAD33 plasmid carrying the ccdBVfi gene. Transformation mixtures were plated on LB plates containing the appropriate antibiotics in the absence of inducer. The transformation efficiency for each plasmid was comparable to that of the pBAD33 control vector in each strain (data not shown).
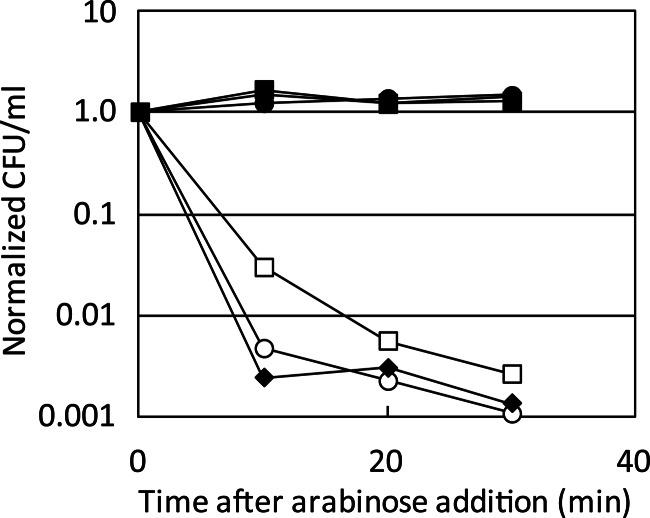
The toxic activity of the toxin and the ability of its antitoxin to counteract its toxic activity were assayed in liquid cultures (Fig. 3). Production of the CcdBVfi protein resulted in a dramatic loss of viability after 10 min of induction. Viability was not affected in the presence of its antitoxin, CcdAVfi. These results show that the V. fischeri SI ccdVfi system is a toxin-antitoxin gene pair.
Fig 3.
Interactions between the ccdVfi and ccdF systems. SG22622/pKK223-3/pBAD-ccdBF (open square), SG22622/pKK223-3/pBAD-ccdBVfi (open circle), SG22622/pKK-ccdAF/pBAD-ccdBF (filled square), SG22622/pKK-ccdAVfi/pBAD-ccdBVfi (filled circle), SG22622/pKK-ccdAF/pBAD-ccdBVfi (filled triangle), and SG22622/pKK-ccdAVfi/pBAD-ccdBF (filled diamond) were grown as described in Materials and Methods. After addition of 1% arabinose, serial dilutions of the cultures were plated without arabinose and incubated overnight at 37°C. The number of CFU per ml at each time point was normalized to that of time zero (just before arabinose addition) for each strain. This experiment was performed at least in duplicate.
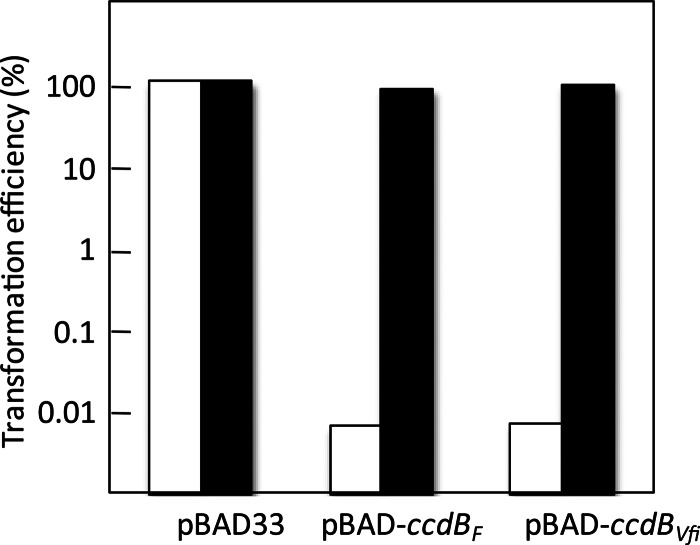
To determine whether the CcdBVfi toxin targets the DNA gyrase as shown for the CcdBF toxin (50), we transformed the pBAD-ccdBVfi and pBAD-ccdBF plasmids in SG22622 and in the isogenic strain carrying the CcdBF-resistant mutation gyrA462 (strain SG22622 gyrA462). Figure 4 shows that the relative transformation efficiency for the pBAD-ccdBF and pBAD-ccdBVfi plasmids in the wild-type strain was very low in the presence of 1% arabinose (<10−4), while it was comparable to that of the vector in SG22622 gyrA462 (about 100) (Fig. 4). Thus, the gyrA462 mutation enables transformation of pBAD-ccdBF, a pBAD33 derivative expressing ccdBF (15), as well as of the pBAD-ccdBVfi plasmid upon induction, showing that the CcdBVfi toxins also target the DNA gyrase.
Fig 4.
The CcdBVfi toxin targets the DNA-gyrase. SG22622 (white bars) and SG22622gyrA462 (black bars) were transformed with the control vector pBAD33 or the pBAD-ccdBF and pBAD-ccdBVfi plasmids as indicated. Transformation mixtures were plated onto LB plates without arabinose or with 1% arabinose. The efficiency of transformation for each plasmid is the ratio of the number of transformants obtained on 1% arabinose plates to the number of transformants on plates without arabinose. This experiment was performed at least in triplicate.
We originally identified the ccdVfi cassette through the partial characterization of the integron cassette array found in the V. fischeri type strain (CIP 103206T) (17). Analysis of the genome of V. fischeri ES114, the Euprymna scolopes squid light organ symbiont, which was determined in 2005 (51), revealed the absence of the ccdVfi cassette in this strain. In order to determine if this TA module was expressed in its original genetic context, we introduced by conjugation a plasmid able to replicate in V. fischeri and expressing ccdBVfi, pSU38TgV::ccdBVfi (p9939), in both V. fischeri strains CIP 103206T and ES114, and selected transconjugants on Km, the resistance brought by the plasmid marker. Kmr clones carrying plasmid p9939 were obtained in V. fischeri CIP 103206T only at a frequency (1.7 × 10−4 ± 0.54 × 10−4) similar to the one obtained for the empty pSU38TgV vector (1.6 × 10−4 ± 0.5 × 10−4). Strain ES114 Kmr clones were obtained only at low frequency (10−6) and likely corresponded to spontaneous Kmr mutants, as these clones were all found to lack p9939 by PCR. In order to check that CIP 103206T Kmr transconjugants were carrying p9939 with a functional ccdBVfi, we purified the plasmid from several clones and transformed E. coli strains B462, a CcdB-resistant strain, and DH5α. We obtained transformants in B462 only, demonstrating that ccdBVfi was still functional and expressed in these plasmids. Altogether, these results strongly support that ccdVfi is expressed from the SI context.
Ability of the CcdAF and CcdAVfi antitoxins to counteract their noncognate toxins.
It is not clear whether the ccdVfi system coexists with the ccdF system, although plasmid conjugation between E. coli and Vibrio fischeri has already been demonstrated in a laboratory context (52). However, it is unlikely that F can replicate in V. fischeri, as it cannot be maintained in V. cholerae (53). We tested the functional interactions between the antitoxins and the toxins of the ccdF and the ccdVfi systems. As for Phd-Doc, here too the two toxins are more related to each other than the two antitoxins, with 41% identity between the CcdBs and 22% identity between the CcdAs (see Fig. S4 in the supplemental material). The ability of the different antitoxins to counteract the toxic activity of the different toxins was assayed in liquid cultures. Expression of CcdBF and CcdBVfi toxins resulted in a dramatic loss of viability, while viability was restored by coexpression of CcdAF (Fig. 3). This shows that the CcdAF antitoxin is able to counteract the toxic activity of the SI cassette toxin as efficiently as its cognate toxin. However, expression of the CcdAVfi antitoxin was not able to counteract CcdBF efficiently (Fig. 3).
Autoregulation of the ccdVfi and phd-docVch systems.
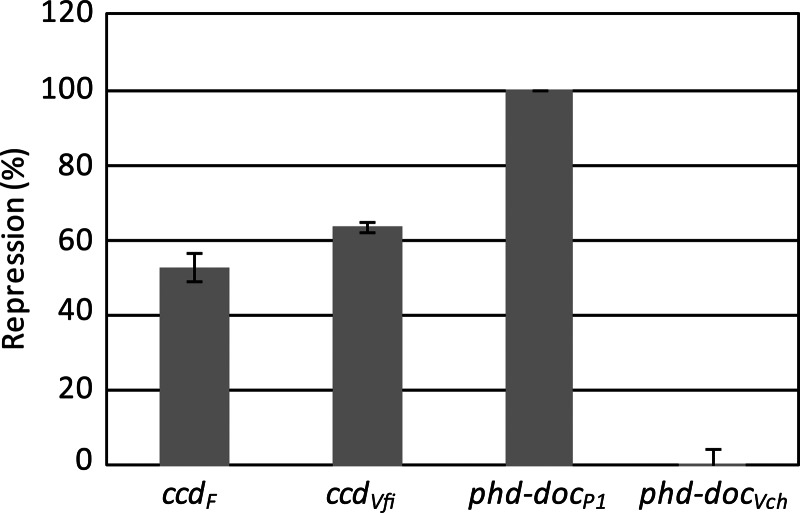
The putative promoter regions of the ccdVfi and phd-docSI systems were cloned in the pJL207 plasmid carrying a promoter-free lacZ gene in a fashion similar to what has been previously published for the ccdF system (45) (see Materials and Methods). Interestingly, while the promoter activity of phd-docSI was comparable to that of the P1 system, the activity of the ccdVfi was 3.4-fold higher than that of its F plasmid counterpart (data not shown). Figure 5 shows that the ccdVfi system is autoregulated, as expected, by the CcdAVfi and CcdBVfi proteins expressed in trans. The repression appears to be similar to that of the ccdVfi system (40 to 60% repression). For phd-docSI, we were unable to detect any repression, while repression of phd-docP1 was very efficient, being nearly complete.
Fig 5.
Autoregulation of the ccdVfi and phd-docSI systems. Strain CSH50 containing the different promoter-lacZ fusions and the pMLO59 vector or its derivatives carrying the corresponding TA systems were grown at 30°C, and promoter activity was measured at mid-log phase. The graph represents the percentage of repression of each promoter in the presence of the corresponding TA system. The experiments were performed in triplicate.
Ability of the ccdVfi and phd-docVch systems to mediate postsegregational killing.
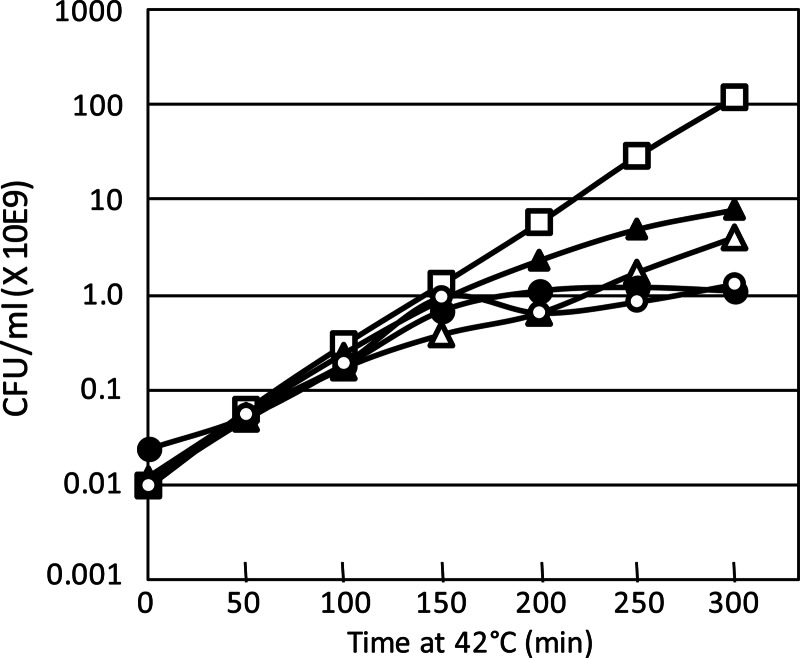
The ccdVfi and phd-docSI systems were cloned in a conditionally replicating (thermosensitive) plasmid, pGB2 (see Materials and Methods). Figure 6 shows that after 150 min of culture at 42°C, the ability of the strains carrying the four systems to form colonies decreased in comparison with that of the control strain. Efficiency levels of the phd-docSI and phd-docP1 systems to mediate postsegregational killing (PSK) were comparable, and after 300 min at 42°C, more than 99% of the population was unable to form colonies. The ccdVfi system was slightly more efficient than ccdF between 150 and 250 min at 42°C, but overall, at 300 min, the two were comparable.
Fig 6.
Ability of the ccdVfi and phd-docSI systems to mediate postsegregational killing. CSH50 λsfiA::lacZ strains containing the pMLO59 vector (open square) and its derivative carrying the ccdF (filled triangle), ccdVfi (open triangle), phd-docP1 (closed circle), or phd-docSI (open circle) systems were grown at 30°C in LB as described in Materials and Methods. Cultures were sampled at the times indicated in the figure, and viability was measured (in CFU/ml). These experiments were performed in duplicate.
Postsegregational killing relies on the Lon-dependent degradation of the CcdAF antitoxin (46). We therefore compared the postsegregational SOS induction mediated by the loss of the pMLO59-ccdVfi plasmid at 42°C in CSH50λsfiA::lacZ and in CSH50 lon::Tn10 λsfiA::lacZ strains. Postsegregational SOS induction was completely abolished in the CSH50 lon::Tn10 λsfiA::lacZ strain (data not shown). This shows, though indirectly, that like the CcdAF antitoxin, CcdAVfi is also a substrate of the Lon ATP-dependent protease.
DISCUSSION
In order to be able to couple the detection of the presence of phd-docSI cassette with the variability of the SI cassette array, we devised a simple PCR-typing strategy, based on three sets of primers that allowed the determination of the variability in the intIA proximal and distal parts of the SI cassette array of V. cholerae strains and isolates. After sequencing the different PCR products corresponding to the intIA proximal SI cassette(s), we were able to define 15 SI subtypes from the 36 strains from the Pasteur Institute collection (CIP) of different origins, isolation dates, and serogroups (15 were from non-O1/non-O139 or unknown serogroup, while the others were of serogroup O1 or O139) (Table 2 and Fig. 1). Our results confirm the highly discriminative power of such SI-based typing (32), as O1 strains were distributed among 5 SI subtypes, though it cannot be substituted for serogroup typing, as SI subtype II gathers O1 and O139 strains. However, inside a given serogroup it can help to discriminate among different clones and be useful for epidemiological studies. It has been observed in the class 1 integron that cassette integration preferentially occurs at the attI site and leads to more variability in the first position than in remote positions (54). The fact that less variability is observed in the distal part of the SI than in the attI proximal part suggests that this is also likely true in the V. cholerae SI. Analysis of the phd-docSI cassette presence in the 36 strains showed a perfect match between the subtyping and the presence or absence of this cassette, showing that loss of the cassette was coupled with reordering mediated by extensive cassette recombination. This cassette was absent in only 3 of the 15 SI subtypes, which are defined by only one strain (SI subtypes VI and VII; Table 2) or two strains (SI subtypes XII; Table 2). We also demonstrated the presence of this cassette in the six V. metschnikovii strains that we tested.
We functionally characterized this phd-docSI cassette, as well as the ccdVfi cassette so far found only in V. fischeri. These two type II TA systems belong to the families that are the least represented among the SI TA cassettes, compared to relBE or parDE, for example (5). We demonstrated that the two cassettes encode functional proteins and carry their own active promoters. We further showed that the two cassettes were efficiently expressed from their SI context, in V. cholerae N16961 for phd-docSI, and in V. fischeri CIP 103206T for ccdVfi. The phd-docSI cassette is likely expressed in all V. cholerae and V. metschnikovii strains when present, with one notable exception in V. cholerae strain A256. Indeed, in this strain, we found that an IS1004 insertion a few base pairs upstream of phd reduces its expression to a level that does not allow inhibition of DocSI toxicity when expressed in trans from a p15A vector, while in the absence of IS1004, the PhdSI level of expression from the SI is sufficient to prevent the DocSI toxicity when expressed from the plasmid. In addition, in this strain the doc toxin gene is inactivated by a premature stop codon, while the phd sequence is native.
There are only 2 other TA systems from the phd-doc family that have been characterized to a certain extent, one in the Yersinia pestis chromosome (55) and the original one, discovered in the phage P1 genome (56), which has been extensively studied. DocP1 has been shown to interact with the 30S ribosomal subunit, and its toxicity is the result of inhibition of translation elongation, possibly at the translocation step (57). The DocSI toxin shares 30% identity to DocP1, while the PhdSI is only distantly related to PhdP1 (17% identity), and they are even further less related to the Y. pestis proteins, 21% and <10%, respectively. However, BLASTP screening of databases with PhdSI as query detects 3 related proteins, which are parts of other putative phd-doc TAs, with an encoded Doc much more related to the SI TA than to the P1 one. Two are found in marine bacteria. The first is carried in the genome of Desulfotalea psychrophila LSv54 (accession number for Phd, YP065779-1, and for Doc, YP65772-1) (58), and its products share 66% and 85% identity to PhdSI and DocSI, respectively; however, both encoded proteins are truncated in their C-terminal part and are likely inactive. The second TA is carried in the large plasmid pSba102 found in Shewanella baltica OS155 (accession number for Phd, YP00104727-1, and for Doc, EHY 78006-1) (73% and 62% identity to PhdSI and DocSI, at the protein level). The third one is carried in the genome of the plant pathogen Pseudomonas syringae pv. mori str. 301020 (accession number for Phd, EGH24474-1, and for Doc, EGH24475-1) and shows 73 and 77% identities with PhdSI and DocSI, respectively. Analysis of the genetic contexts of these different phd-doc stystems does not reveal any cassette structural features. Interestingly, Docs are generally much more constrained, and BLASTP screening of databases with DocSI is able to hit the DocP1 protein, while as mentioned above, PhdSI detects only three related proteins and not PhdP1. The PhdSI and the Phd proteins encoded by both S. baltica and P. syringae are 56 amino acids (aa) long (the D. psychrophila LSv54 Phd protein is truncated at the C terminus and lacks 5 aa) and thus much shorter that the 73-aa-long PhdP1. Interestingly, the alignment of these proteins suggests that the difference in size is due to an N-terminal truncation in PhdSI and its two related Phd (see Fig. S1 in the supplemental material). The crystal structure shows that PhdP1 forms a heterotetrameric 2:2 complex with DocP1 (59). It has been shown for PhdP1 that the C-terminal domain (residues 52 to 73) harbors the site of interaction with DocP1 and that this domain is sufficient to prevent Doc-mediated growth arrest (60). On the other hand, the N-terminal region (residues 1 to 51) of PhdP1 corresponds to the dimerization/DNA-binding domain that has been shown to bind to the operator site of the phd-docP1 operon (60, 61) and to regulate the TA operon expression (62). Operator binding and repression of the phd-docP1 operon by PhdP1 are enhanced by the presence of DocP1 in a cooperative manner (63, 64). Thus, the N-terminal truncation found in PhdSI may explain why there is no transcriptional autoregulation. The C-terminal part of the PhdSI is only poorly related to the one of PhdP1, but several of the amino acids identified as important for the PhdP1-DocP1 interaction through genetic or structural studies are conserved (59–61, 65, 66) (see Fig. S1 in the supplemental material). The demonstration we made that PhdSI can prevent the DocP1 toxicity and, vice versa, that PhdP1 can prevent the DocSI toxicity shows that the overall structure and biochemical properties of the interaction regions are conserved.
The characterization of the ccdVfi system further confirmed that this operon encodes a functional TA system, which has the same characteristics as the ccdF system. We demonstrated here that even if only remotely related to the CcdBF (37% identity), CcdBVfi targets DNA gyrase in a similar fashion, as inferred from the insensitivity of the E. coli gyrA462 mutant toward the toxin activity, even if a recent structural study suggests that the CcdBvfi binding to GyrA involves slightly different interactions than those made by CcdBF (67). The three-dimensional (3D) structure of crystals of CcdBVfi in complex with a GyrA fragment (GyrA14, amino acids 362 to 493) has been recently published (68), showing that CcdBVfi and CcdBF recognize the same surface of DNA gyrase fragment (67). Furthermore, the key amino acids of the toxic active site of CcdBF that had been previously identified as glycine100 and isoleucine101 (69) are conserved in CcdBVfi (see Fig. S2 in the supplemental material).
We obtained evidence that CcdAVfi is degraded by the Lon protease pathway, similarly to what has been found for CcdAF and its homologue CcdAO157 (15, 46). We found that the ccdVfi system is at least as efficient to trigger postsegregational SOS induction as is the ccdF system (15) and as efficient to mediate plasmid stabilization. Finally, we found that the ccdVfi operon expression is tightly autoregulated by the CcdAVfi and CcdBVfi proteins, similarly to what has been observed in the ccdF system.
Chromosomal TA systems have been proposed to stabilize chromosomal regions by preventing accidental deletions (17), especially when located in unstable segments such as mobile genetic elements (MGE) (18, 19). The study made by Rowe-Magnus and collaborators on the RelBE1 and ParDE1 cassettes carried in the Vibrio vulnificus SI has substantiated this role. Indeed, they showed that both systems were expressed in vivo and that the activity of the proteins encoded in the two TAs counterbalanced the extent of deletions catalyzed by the class 1 integron integrase IntI1 (18). Furthermore, the presence of TA gene cassettes in SIs did not preclude integrase-mediated microevolution from occurring; en masse gene cassette loss was suppressed, but the exchange of individual cassettes continued. The net effect was stabilization of SI gene cassette arrays in the absence of selection, and this activity may in part explain why gene cassettes coding for TA systems are common in large SIs but are absent from smaller SIs, like the resistance integrons (21). The studies of the V. cholerae cassettes encoding TA systems of the HigBA and ParDE families also support this role (35–37, 70). Here, we demonstrated that two other TA cassettes, carried in the SI of different Vibrio species, are also expressed in their original context, suggesting that all the SI TA cassettes share this stabilization role. It would be interesting to determine if, when multiple TA cassettes from the same functional family are found in the same SI, all are expressed and if they all evolved in such a way that they do not cross-interact, as has been already shown for the three ParDE and the two HigBA cassettes of the V. cholerae N16961 SI (35, 37).
These chromosomal TA systems can also potentially protect their host genome from colonization by an incoming mobile element or a plasmid carrying a TA from the same functional family, by allowing its harmless loss through the neutralization of the invading toxin by the chromosomal antitoxin (15, 17, 71). In order to substantiate this hypothesis, we tested the functional interactions between these two SI TA systems and those carried in MGE. These two systems are among the least represented, and unfortunately we did not find related systems carried in the MGE characterized in these species, or in close relatives. However, we tested the interaction of the phd-docSI with the phage P1 system, and the ccdABVfi with the plasmid F homologous system. In the Phd-Doc cross talk experiment, we found that both Doc toxins could be antagonized by both antitoxins, showing that if a P1 phage was able to somehow infect V. cholerae its lysogenic state would be destabilized by the products expressed from the SI phd-doc cassette. As mentioned above, we identified in silico a closely related and likely functional phd-doc operon in the unpublished sequence of a large plasmid, pSba102, from a marine bacterium, Shewanella baltica OS155 (accession number, NC_009036). As V. metschnikovii and V. cholerae are also marine bacteria, it is possible that such a plasmid or a related one can be exchanged among these species, and it would have been interesting to establish the cross talks between these two phd-doc systems. In the case of the ccd systems, we obtained different results in similar cross talk experiments between the V. fischeri and plasmid F systems. Indeed, we have shown that the CcdAVfi antitoxin is unable to counteract the toxic activity of CcdBF while the CcdAF antitoxin is able to counteract the CcdBVfi toxin (Fig. 3). We had previously observed similar one-way interaction between the ccdF system and the chromosomal system found in E. coli O157:H7 (15), while Matsuba and colleagues had made a similar observation for the PemK toxin of the pem (parD) TA system located on the R100 (R1) plasmid of E. coli and the two antitoxins of its chromosomal homologues, which are unable to counteract the toxicity of the plasmid-encoded toxin (72), except when mutated (71). YefM-YoeB homologues carried either on plasmids or on chromosomes have also been found to poorly cross talk, at most (73, 74). Thus, it appears that the plasmidic systems are “dominant” over their chromosomal homologues in many cases. One may imagine that evolution has selected that type of hierarchy between homologous TA systems. Chromosomal TA systems like those found in the SI cassettes might thus also serve as exclusion systems to protect bacteria from being loaded with an excess of exogenous DNA-carrying related TA systems (plasmids, transposons, and phages), a function related to the one of restriction-modification systems (see reference 75). Our results and previous observations described above suggest that selective pressure on plasmidic TA systems to evade chromosomal TA cross-interaction is extremely strong. However, some chromosomal systems are able to mediate antiaddiction and protect the cell against PSK (16). It is very unlikely that F can replicate in V. fischeri, as we found that if F can be conjugated in vibrios, such as V. cholerae, it cannot replicate in this species (53). It is also very unlikely that phage P1 can infect Vibrio species. However, as these two TAs are embedded in integron cassettes, they may also exist in different hosts, where F or P1 can be more likely encountered.
From the structural point of view, the fact that the PhdSI proteins counteract the DocP1 proteins and vice versa is surprising, especially as the CcdAVfi proteins do not counteract the CcdBF proteins. Indeed PhdSI and PhdP1 are only remotely related, both in size and in sequence, and the two Doc proteins share only 30% identity, while the two CcdB proteins share a higher identity (37%), as do the two CcdA proteins, which share 20% identity over the whole length of the proteins. These cross talk pattern differences may reflect intrinsic properties of the two different TA systems or a selective constraint linked to the fact that they naturally compete in common hosts. In this line, it would be very interesting to determine the interactions between the PhD-Doc of pSab102 and those encoded in the Vibrio SI, to see if, while more closely related among them than with the P1 proteins, they evolved to prevent cross-interactions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jason Bland for helpful reading of the manuscript.
Work in the Mazel laboratory is funded by the Institut Pasteur, the Centre National de la Recherche Scientifique (CNRS-UMR 3525), the French National Research Agency (ANR-08-MIE-016), the French Government's Investissement d'Avenir program, Laboratoire d'Excellence “Integrative Biology of Emerging Infectious Diseases” (grant no. ANR-10-LABX-62-IBEID) and the European Union Seventh Framework Programme (FP7-HEALTH-2011-single-stage) “Evolution and Transfer of Antibiotic Resistance” (EvoTAR), and the Fondation pour la Recherche Médicale (équipe FRM 2007). Work in the laboratory of L.V.M. is supported by the Fonds de la Recherche (FRSM-3.4530.04), Fondation Van Buuren, and Fonds Jean Brachet. N.I. was supported by a Franco-Pakistani doctoral fellowship.
Footnotes
Published ahead of print 8 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01389-12.
REFERENCES
- 1. Gerdes K, Christensen SK, Lobner-Olesen A. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 3:371–382 [DOI] [PubMed] [Google Scholar]
- 2. Van Melderen L, Saavedra De Bast M. 2009. Bacterial toxin-antitoxin systems: more than selfish entities? PLoS Genet. 5:e1000437 doi:10.1371/journal.pgen.1000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamaguchi Y, Park JH, Inouye M. 2011. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45:61–79 [DOI] [PubMed] [Google Scholar]
- 4. Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, Salmond GP. 2009. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc. Natl. Acad. Sci. U. S. A. 106:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33:966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guglielmini J, Szpirer C, Milinkovitch MC. 2008. Automated discovery and phylogenetic analysis of new toxin-antitoxin systems. BMC Microbiol. 8:104 doi:10.1186/1471-2180-8-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leplae R. 2011. Diversity of bacterial type II toxin-antitoxin systems: a comprehensive search and functional analysis of novel families. Nucleic Acids Res. 39:5513–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramage HR, Connolly LE, Cox JS. 2009. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution. PLoS Genet. 5:e1000767 doi:10.1371/journal.pgen.1000767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. 2006. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2:e135 doi:10.1371/journal.pgen.0020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Melderen L. 2010. Toxin-antitoxin systems: why so many, what for? Curr. Opin. Microbiol. 13:781–785 [DOI] [PubMed] [Google Scholar]
- 11. Christensen SK, Pedersen K, Hansen FG, Gerdes K. 2003. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J. Mol. Biol. 332:809–819 [DOI] [PubMed] [Google Scholar]
- 12. Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186:8172–8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis K. 2010. Persister cells. Annu. Rev. Microbiol. 64:357–372 [DOI] [PubMed] [Google Scholar]
- 14. Nariya H, Inouye M. 2008. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 132:55–66 [DOI] [PubMed] [Google Scholar]
- 15. Wilbaux M, Mine N, Guerout AM, Mazel D, Van Melderen L. 2007. Functional interactions between coexisting toxin-antitoxin systems of the ccd family in Escherichia coli O157:H7. J. Bacteriol. 189:2712–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saavedra De Bast M, Mine N, Van Melderen L. 2008. Chromosomal toxin-antitoxin systems may act as antiaddiction modules. J. Bacteriol. 190:4603–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowe-Magnus DA, Guerout AM, Biskri L, Bouige P, Mazel D. 2003. Comparative analysis of superintegrons: engineering extensive genetic diversity in the Vibrionaceae. Genome Res. 13:428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szekeres S, Dauti M, Wilde C, Mazel D, Rowe-Magnus DA. 2007. Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection. Mol. Microbiol. 63:1588–1605 [DOI] [PubMed] [Google Scholar]
- 19. Wozniak RA, Waldor MK. 2009. A toxin-antitoxin system promotes the maintenance of an integrative conjugative element. PLoS Genet. 5:e1000439 doi:10.1371/journal.pgen.1000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dziewit L, Jazurek M, Drewniak L, Baj J, Bartosik D. 2007. The SXT conjugative element and linear prophage N15 encode toxin-antitoxin-stabilizing systems homologous to the tad-ata module of the Paracoccus aminophilus plasmid pAMI2. J. Bacteriol. 189:1983–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cambray G, Guerout AM, Mazel D. 2010. Integrons. Annu. Rev. Genet. 44:141–166 [DOI] [PubMed] [Google Scholar]
- 22. Mazel D, Dychinco B, Webb VA, Davies J. 1998. A distinctive class of integron in the Vibrio cholerae genome. Science 280:605–608 [DOI] [PubMed] [Google Scholar]
- 23. Boucher Y, Labbate M, Koenig JE, Stokes HW. 2007. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 15:301–309 [DOI] [PubMed] [Google Scholar]
- 24. Guerin E, Cambray G, Sanchez-Alberola N, Campoy S, Erill I, Da Re S, Gonzalez-Zorn B, Barbe J, Ploy MC, Mazel D. 2009. The SOS response controls integron recombination. Science 324:1034. [DOI] [PubMed] [Google Scholar]
- 25. Cambray G, Sanchez-Alberola N, Campoy S, Guerin E, Da Re S, Gonzalez-Zorn B, Ploy MC, Barbe J, Mazel D, Erill I. 2011. Prevalence of SOS-mediated control of integron integrase expression as an adaptive trait of chromosomal and mobile integrons. Mobile DNA 2:6 doi:10.1186/1759-8753-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baharoglu Z, Mazel D. 2011. Vibrio cholerae triggers SOS and mutagenesis in response to a wide range of antibiotics: a route towards multiresistance. Antimicrob. Agents Chemother. 55:2438–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baharoglu Z, Krin E, Mazel D. 2012. Connecting environment and genome plasticity in the characterization of transformation-induced SOS regulation and carbon catabolite control of the Vibrio cholerae integron integrase. J. Bacteriol. 194:1659–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barker A, Clark CA, Manning PA. 1994. Identification of VCR, a repeated sequence associated with a locus encoding a hemagglutinin in Vibrio cholerae O1. J. Bacteriol. 176:5450–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Labbate M, Boucher Y, Joss MJ, Michael CA, Gillings MR, Stokes HW. 2007. Use of chromosomal integron arrays as a phylogenetic typing system for Vibrio cholerae pandemic strains. Microbiology 153:1488–1498 [DOI] [PubMed] [Google Scholar]
- 30. Feng L, Reeves PR, Lan R, Ren Y, Gao C, Zhou Z, Cheng J, Wang W, Wang J, Qian W, Li D, Wang L. 2008. A recalibrated molecular clock and independent origins for the cholera pandemic clones. PLoS One 3:e4053 doi:10.1371/journal.pone.0004053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon YS, Kim DW, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR. 2009. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 106:15442–15447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chowdhury N, Asakura M, Neogi SB, Hinenoya A, Haldar S, Ramamurthy T, Sarkar BL, Faruque SM, Yamasaki S. 2010. Development of simple and rapid PCR-fingerprinting methods for Vibrio cholerae on the basis of genetic diversity of the superintegron. J. Appl. Microbiol. 109:304–312 [DOI] [PubMed] [Google Scholar]
- 33. Gao Y, Pang B, Wang HY, Zhou HJ, Cui ZG, Kan B. 2011. Structural variation of the superintegron in the toxigenic Vibrio cholerae O1 El Tor. Biomed. Environ. Sci. 24:579–592 [DOI] [PubMed] [Google Scholar]
- 34. Jove T, Da Re S, Denis F, Mazel D, Ploy MC. 2010. Inverse correlation between promoter strength and excision activity in class 1 integrons. PLoS Genet. 6:e1000793 doi:10.1371/journal.pgen.1000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christensen-Dalsgaard M, Gerdes K. 2006. Two higBA loci in the Vibrio cholerae superintegron encode mRNA cleaving enzymes and can stabilize plasmids. Mol. Microbiol. 62:397–411 [DOI] [PubMed] [Google Scholar]
- 36. Budde PP, Davis BM, Yuan J, Waldor MK. 2007. Characterization of a higBA toxin-antitoxin locus in Vibrio cholerae. J. Bacteriol. 189:491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuan J, Yamaichi Y, Waldor MK. 2011. The three Vibrio cholerae chromosome II-encoded ParE toxins degrade chromosome I following loss of chromosome II. J. Bacteriol. 193:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glansdorff N. 1965. Topography of cotransducible arginine mutations in Escherichia coli K-12. Genetics 51:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rowe-Magnus DA, Guerout AM, Mazel D. 2002. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol. Microbiol. 43:1657–1669 [DOI] [PubMed] [Google Scholar]
- 40. Krin E, Danchin A, Soutourina O. 2010. RcsB plays a central role in H-NS-dependent regulation of motility and acid stress resistance in Escherichia coli. Res. Microbiol. 161:363–371 [DOI] [PubMed] [Google Scholar]
- 41. Krin E, Derzelle S, Bedard K, Adib-Conquy M, Turlin E, Lenormand P, Hullo MF, Bonne I, Chakroun N, Lacroix C, Danchin A. 2008. Regulatory role of UvrY in adaptation of Photorhabdus luminescens growth inside the insect. Environ. Microbiol. 10:1118–1134 [DOI] [PubMed] [Google Scholar]
- 42. Le Roux F, Binesse J, Saulnier D, Mazel D. 2007. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl. Environ. Microbiol. 73:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reynaud Y, Saulnier D, Mazel D, Goarant C, Le Roux F. 2008. Correlation between detection of a plasmid and high-level virulence of Vibrio nigripulchritudo, a pathogen of the shrimp Litopenaeus stylirostris. Appl. Environ. Microbiol. 74:3038–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le Roux F, Davis BM, Waldor MK. 2011. Conserved small RNAs govern replication and incompatibility of a diverse new plasmid family from marine bacteria. Nucleic Acids Res. 39:1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salmon MA, Van Melderen L, Bernard P, Couturier M. 1994. The antidote and autoregulatory functions of the F plasmid CcdA protein: a genetic and biochemical survey. Mol. Gen. Genet. 244:530–538 [DOI] [PubMed] [Google Scholar]
- 46. Van Melderen L, Bernard P, Couturier M. 1994. Lon-dependent proteolysis of CcdA is the key control for activation of CcdB in plasmid-free segregant bacteria. Mol. Microbiol. 11:1151–1157 [DOI] [PubMed] [Google Scholar]
- 47. Bouvier M, Demarre G, Mazel D. 2005. Integron cassette insertion: a recombination process involving a folded single strand substrate. EMBO J. 24:4356–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou Y, Gottesman S. 1998. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J. Bacteriol. 180:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clark CA, Purins L, Kaewrakon P, Focareta T, Manning PA. 2000. The Vibrio cholerae O1 chromosomal integron. Microbiology 146:2605–2612 [DOI] [PubMed] [Google Scholar]
- 50. Bernard P, Couturier M. 1992. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J. Mol. Biol. 226:735–745 [DOI] [PubMed] [Google Scholar]
- 51. Ruby EG, Urbanowski M, Campbell J, Dunn A, Faini M, Gunsalus R, Lostroh P, Lupp C, McCann J, Millikan D, Schaefer A, Stabb E, Stevens A, Visick K, Whistler C, Greenberg EP. 2005. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. U. S. A. 102:3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dunlap PV, Kuo A. 1992. Cell density-dependent modulation of the Vibrio fischeri luminescence system in the absence of autoinducer and LuxR protein. J. Bacteriol. 174:2440–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baharoglu Z, Bikard D, Mazel D. 2010. Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation. PLoS Genet. 6:e1001165 doi:10.1371/journal.pgen.1001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Collis CM, Recchia GD, Kim MJ, Stokes HW, Hall RM. 2001. Efficiency of recombination reactions catalyzed by class 1 integron integrase IntI1. J. Bacteriol. 183:2535–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goulard C, Langrand S, Carniel E, Chauvaux S. 2010. The Yersinia pestis chromosome encodes active addiction toxins. J. Bacteriol. 192:3669–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lehnherr H, Maguin E, Jafri S, Yarmolinsky MB. 1993. Plasmid addiction genes of bacteriophage P1: doc, which causes cell death on curing of prophage, and phd, which prevents host death when prophage is retained. J. Mol. Biol. 233:414–428 [DOI] [PubMed] [Google Scholar]
- 57. Liu M, Zhang Y, Inouye M, Woychik NA. 2008. Bacterial addiction module toxin Doc inhibits translation elongation through its association with the 30S ribosomal subunit. Proc. Natl. Acad. Sci. U. S. A. 105:5885–5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rabus R, Ruepp A, Frickey T, Rattei T, Fartmann B, Stark M, Bauer M, Zibat A, Lombardot T, Becker I, Amann J, Gellner K, Teeling H, Leuschner WD, Glockner FO, Lupas AN, Amann R, Klenk HP. 2004. The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environ. Microbiol. 6:887–902 [DOI] [PubMed] [Google Scholar]
- 59. Arbing MA, Handelman SK, Kuzin AP, Verdon G, Wang C, Su M, Rothenbacher FP, Abashidze M, Liu M, Hurley JM, Xiao R, Acton T, Inouye M, Montelione GT, Woychik NA, Hunt JF. 2010. Crystal structures of Phd-Doc, HigA, and YeeU establish multiple evolutionary links between microbial growth-regulating toxin-antitoxin systems. Structure 18:996–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith JA, Magnuson RD. 2004. Modular organization of the Phd repressor/antitoxin protein. J. Bacteriol. 186:2692–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McKinley JE, Magnuson RD. 2005. Characterization of the Phd repressor-antitoxin boundary. J. Bacteriol. 187:765–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gazit E, Sauer RT. 1999. Stability and DNA binding of the Phd protein of the phage P1 plasmid addiction system. J. Biol. Chem. 274:2652–2657 [DOI] [PubMed] [Google Scholar]
- 63. Magnuson R, Lehnherr H, Mukhopadhyay G, Yarmolinsky MB. 1996. Autoregulation of the plasmid addiction operon of bacteriophage P1. J. Biol. Chem. 271:18705–18710 [DOI] [PubMed] [Google Scholar]
- 64. Magnuson R, Yarmolinsky MB. 1998. Corepression of the P1 addiction operon by Phd and Doc. J. Bacteriol. 180:6342–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garcia-Pino A, Christensen-Dalsgaard M, Wyns L, Yarmolinsky M, Magnuson RD, Gerdes K, Loris R. 2008. Doc of prophage P1 is inhibited by its antitoxin partner Phd through fold complementation. J. Biol. Chem. 283:30821–30827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garcia-Pino A, Dao-Thi MH, Gazit E, Magnuson RD, Wyns L, Loris R. 2008. Crystallization of Doc and the Phd-Doc toxin-antitoxin complex. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 64:1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Jonge N, Simic M, Buts L, Haesaerts S, Roelants K, Garcia-Pino A, Sterckx Y, De Greve H, Lah J, Loris R. 2012. Alternative interactions define gyrase specificity in the CcdB family. Mol. Microbiol. 84:965–978 [DOI] [PubMed] [Google Scholar]
- 68. De Jonge N, Hohlweg W, Garcia-Pino A, Respondek M, Buts L, Haesaerts S, Lah J, Zangger K, Loris R. 2010. Structural and thermodynamic characterization of Vibrio fischeri CcdB. J. Biol. Chem. 285:5606–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bahassi EM, Salmon MA, Van Melderen L, Bernard P, Couturier M. 1995. F plasmid CcdB killer protein: ccdB gene mutants coding for non-cytotoxic proteins which retain their regulatory functions. Mol. Microbiol. 15:1031–1037 [DOI] [PubMed] [Google Scholar]
- 70. Yuan J, Sterckx Y, Mitchenall LA, Maxwell A, Loris R, Waldor MK. 2010. Vibrio cholerae ParE2 poisons DNA gyrase via a mechanism distinct from other gyrase inhibitors. J. Biol. Chem. 285:40397–40408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Santos Sierra S, Giraldo R, Diaz Orejas R. 1998. Functional interactions between chpB and parD, two homologous conditional killer systems found in the Escherichia coli chromosome and in plasmid R1. FEMS Microbiol. Lett. 168:51–58 [DOI] [PubMed] [Google Scholar]
- 72. Masuda Y, Miyakawa K, Nishimura Y, Ohtsubo E. 1993. chpA and chpB, Escherichia coli chromosomal homologs of the pem locus responsible for stable maintenance of plasmid R100. J. Bacteriol. 175:6850–6856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grady R, Hayes F. 2003. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system specified by a multidrug-resistant, clinical isolate of Enterococcus faecium. Mol. Microbiol. 47:1419–1432 [DOI] [PubMed] [Google Scholar]
- 74. Nieto C, Cherny I, Khoo SK, de Lacoba MG, Chan WT, Yeo CC, Gazit E, Espinosa M. 2007. The yefM-yoeB toxin-antitoxin systems of Escherichia coli and Streptococcus pneumoniae: functional and structural correlation. J. Bacteriol. 189:1266–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kobayashi I. 2001. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res. 29:3742–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Demarre G, Guerout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marliere P, Mazel D. 2005. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPalpha) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 156:245–255 [DOI] [PubMed] [Google Scholar]
- 77. Allali N, Afif H, Couturier M, Van Melderen L. 2002. The highly conserved TldD and TldE proteins of Escherichia coli are involved in microcin B17 processing and in CcdA degradation. J. Bacteriol. 184:3224–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boettcher KJ, Ruby EG. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rowe-Magnus DA, Guerout A-M, Ploncard P, Dychinco B, Davies J, Mazel D. 2001. The evolutionary history of chromosomal super-integrons provides an ancestry for multi-resistant integrons. Proc. Natl. Acad. Sci. U. S. A. 98:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bartolome B, Jubete Y, Martinez E, de la Cruz F. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75–78 [DOI] [PubMed] [Google Scholar]
- 81. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 82. Bernard P, Gabant P, Bahassi EM, Couturier M. 1994. Positive-selection vectors using the F plasmid ccdB killer gene. Gene 148:71–74 [DOI] [PubMed] [Google Scholar]
- 83. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Light J, Molin S. 1982. The sites of action of the two copy number control functions of plasmid R1. Mol. Gen. Genet. 187:486–493 [DOI] [PubMed] [Google Scholar]
- 85. Brosius J, Holy A. 1984. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc. Natl. Acad. Sci. U. S. A. 81:6929–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.