Abstract
The plasmid-encoded type three secretion system (TTSS) of Yersinia spp. is responsible for the delivery of effector proteins into cells of the innate immune system, where these effectors disrupt the target cells' activity. Successful translocation of effectors into mammalian cells requires Yersinia to both insert a translocon into the host cell membrane and sense contact with host cells. To probe the events necessary for translocation, we investigated protein-protein interactions among TTSS components of the needle-translocon complex using a chemical cross-linking-based approach. We detected extracellular protein complexes containing YscF, LcrV, and YopD that were dependent upon needle formation. The formation of these complexes was evaluated in a secretion-competent but translocation-defective mutant, the YscFD28AD46A strain (expressing YscF with the mutations D28A and D46A). We found that one of the YscF and most of the LcrV and YopD cross-linked complexes were nearly absent in this mutant. Furthermore, the YscFD28AD46A strain did not support YopB insertion into mammalian membranes, supporting the idea that the LcrV tip complex is required for YopB insertion and translocon formation. However, the YscFD28AD46A strain did secrete Yops in the presence of host cells, indicating that a translocation-competent tip complex is not required to sense contact with host cells to trigger Yop secretion. In conclusion, in the absence of cross-linkable LcrV-YscF interactions, translocon insertion is abolished, but Yersinia still retains the ability to sense cell contact.
INTRODUCTION
Type three secretion systems (TTSS) are employed by a number of Gram-negative pathogens to transfer effector proteins from the bacterial cytosol across the plasma membrane of eukaryotic cells, a process called translocation (1). These systems are essential virulence mechanisms for pathogens because they enable the pathogen to interfere with host defenses and thereby establish a replication niche inside a host (2, 3). In the pathogenic Yersinia spp., the plasmid-encoded TTSS translocates a set of five to six effector proteins, called Yops, which antagonize the functions of innate immune cells during animal infection (4–7). The Yersinia TTSS resembles a syringe-like apparatus with three distinct parts: the base, which spans both the inner and outer membranes; the needle, which protrudes from the base and forms a hollow tube that is a YscF protein polymer; and the tip complex, which rests at the distal end of the needle (8). The tip complex has been visualized by electron microscopy (EM) (9–11) and appears to be a homopentamer of LcrV (11, 12). This observation has been corroborated by the modeling of an LcrV pentamer onto the tip of the homologous Shigella polymer (9) and by oligomerization studies of LcrV and its Pseudomonas aeruginosa homologue, PcrV, which show that when these proteins are purified, they form a pentameric ring structure (13). The base and needle are sufficient for secretion of Yops into the extracellular environment although the regulation of secretion is altered in the absence of LcrV (14–16). Translocation of effectors across host cell membranes requires LcrV (17–19). In addition to its location at the needle tip, LcrV is secreted and is also found in the bacterial cytosol, where it plays a regulatory role in Yop secretion (14–16, 20, 21).
For translocation of Yops to occur, Yersinia must insert two proteins, YopB and YopD, into the membranes of targeted cells (1, 22). YopD, but not YopB, has been found in purified needle preparations from Yersinia enterocolitica; however, its location on or within these needles has not be visualized by EM (10, 11). While it has been speculated that the YopD in these complexes is due to secreted but insoluble YopD which copurifies with needles (11, 12), recent work has also shown that YopD copurifies with LcrV-capped needles (10). YopB and YopD form a hetero-oligomeric pore in mammalian plasma membranes, termed the translocon, and are critical for the formation of a pore through which effector proteins are thought to travel (1, 22). Purified YopB and YopD can insert into lipid bilayers (23, 24), most likely because of their hydrophobic nature. However, by themselves, they do not form a functional translocon that permits entry of effector proteins into cells (23, 25). Extracellular LcrV is essential for the assembly and insertion of the translocon (11, 26) and has been implicated in determining the channel size of the translocation pore (12, 25, 27). In the absence of LcrV, YopD, but not YopB, still inserts into membranes (12). This suggests an active role for LcrV in the insertion of YopB. Combined, these observations lead to a model in which LcrV interacts with YopB and/or YopD and guides them to form a functional translocon, permitting the translocation of effector Yops. It is currently unclear, however, if the role of LcrV in YopB insertion and/or translocon assembly requires LcrV association with the needle tip or if this activity is accomplished by secreted LcrV.
While the TTSS needle is generally hypothesized to function as a conduit through which effectors traverse (28), there is also evidence that the needle aids in the regulation of both Yop secretion and Yop translocation (29, 30). Secretion of Yops can be modulated by altering the levels of calcium in the growth medium (31). A calcium concentration of 1 mM or greater shuts down the secretion of Yops into culture supernatants but does not inhibit Yop translocation into mammalian cells upon host cell contact. In the absence of calcium, Yops are secreted at high levels into the supernatant. In Y. pestis and Y. pseudotuberculosis, several mutations in YscF cause constitutive secretion of Yops in the presence of high calcium concentrations, suggesting that these YscF mutants interfere with calcium regulation (29, 30). In addition, a number of mutations in YscF permit secretion under low-calcium conditions but do not support translocation into host cells. This suggests that the needle also plays a role in translocation distinct from its role in Yop secretion (29, 30). Because Y. pseudotuberculosis appears to sense host cell contact prior to the initiation of Yop translocation, it has been hypothesized that the YscF polymer and/or tip complex senses this contact (9). Translocation-defective yscF mutants might have defects in their association with LcrV and thus fail to form a tip complex, which may be critical for translocation. Alternatively, these needle mutants might lead to structural defects in the needle itself, such as being too short or bent, preventing the TTSS from reaching host cells and initiating secretion in response to cell contact.
To probe how YscF, LcrV, YopB, and YopD collaborate in translocation, we characterized interactions between these proteins using a chemical cross-linking-based approach. In addition, we hypothesized that some YscF mutants that fail to support translocation might have an altered association with LcrV and thus lead to the translocation-negative phenotype. Here, we show that Y. pseudotuberculosis expressing yscFD28AD46A (representing D-to-A changes at positions 28 and 46 encoded by yscF), a translocation-defective mutant (30), failed to form YscF-LcrV cross-linked complexes. Also it failed to insert YopB into host cell plasma membranes yet still sensed host cell contact, as indicated by Yop secretion. These results support a model in which an LcrV tip complex is critical for translocon insertion but is not required for Y. pseudotuberculosis to detect the presence of host cells and initiate secretion of Yops.
MATERIALS AND METHODS
Strains and bacterial culture conditions.
Bacterial strains are listed in Table 1. Y. pseudotuberculosis cells were cultured in Luria broth (L-broth) at 26°C overnight with aeration. Unless otherwise indicated, Y. pseudotuberculosis cultures were diluted 1:40 into secretion medium (2× yeast extract-tryptone [YT] medium supplemented with 20 mM Na-oxalate and 20 mM MgCl2) and incubated at 26°C for 1.5 h with aeration and then at 37°C for 1.5 h with aeration to induce synthesis of the TTSS. Isopropyl β-d-1-thiogalactopyranoside (IPTG; 30 μM) was added at the shift to 37°C to induce expression of yscF when necessary. To monitor the sensitivity of the wild type (WT), the ΔyopN strain, and a strain expressing YscF with the mutations D28A and D46A (YscFD28AD46A) to a range of calcium concentrations, bacteria were grown in 2× YT medium supplemented with 5 mM EGTA and 20 mM MgCl2, and the medium was supplemented with various concentrations of CaCl2 as indicated in the figures. To monitor whether Y. pseudotuberculosis secreted Yops in RPMI medium, Y. pseudotuberculosis cells were grown in 2× YT medium plus 5 mM CaCl2 for 2 h at 26°C and shifted to growth at 37°C, and then the bacteria were gently spun down and resuspended in RPMI medium and grown for 1 h at 37°C. M9 minimal medium supplemented with 0.4% glucose and a 1% solution of alanine, aspartate, cysteine, glutamate, glycine, histidine, isoleucine, leucine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine was used in cross-linking experiments as indicated in the figure legends. When cells were grown in M9 medium, the time for secretion induction was increased from 1.5 h to 3 h to compensate for the reduced growth rate.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | Cloning strain | Invitrogen |
| Y. pseudotuberculosis | ||
| IP2666/pIB1 | Wild type | 32 |
| IP2666 ΔyopB | Deletion of yopB (codons 19–346) | 33 |
| IP2666 ΔyscF | Deletion of yscF (codons 2–86) | 29 |
| IP2666 ΔlcrV | Deletion of lcrV (codons 19–326) | This study |
| IP2666 ΔyopN | Deletion of yopN (codons 2–287) | 29 |
| IP2666 Δ5 | Deletion of yopHEMOJ | 34 |
| IP2666 Δ5V | Deletion of yopHEMOJ and lcrV | This study |
| IP2666 Δ5F | Deletion of yopHEMOJ and yscF | This study |
| IP2666 Δ5N | Deletion of yopHEMOJ and yopN | This study |
| IP2666 Δ5NV | Deletion of yopHEMOJN and lcrV | This study |
| IP2666 Δ5NF | Deletion of yopHEMOJN and yscF | This study |
| Plasmids | ||
| pCVD442 | Suicide vector; Apr | 35 |
| pCVD442-lcrVKO | Suicide vector for deleting lcrV; Apr | This study |
| pCVD442-yscFKO | Suicide vector for deleting yscF; Apr | 29 |
| pCVD442-yopNKO | Suicide vector for deleting yopN; Apr | 29 |
| pTRC99A | IPTG-inducible vector, Apr | 36 |
| pTRC99A-yscF | Expresses yscF | 29 |
| pTRC99A-yscFD28AD46A | Expresses yscFD28AD46A | This study |
The pTRC99A-yscF plasmid (29) was used as a template for site-directed mutagenesis to generate the plasmid pTRC99A-yscFD28AD46A. Primers used are listed in Table 2. The lcrV gene and the yscF genes were deleted in the IP2666 Δ5 (IP2666 ΔyopHEMOJ) and IP2666 Δ5N (IP2666 ΔyopHEMOJN) strain backgrounds by allelic exchange to create IP2666 Δ5F (IP2666 ΔyopHEMOJ ΔyscF), Δ5NF (IP2666 ΔyopHEMOJN ΔyscF), Δ5V (IP2666 ΔyopHEMOJ ΔlcrV), and Δ5NV (IP2666 ΔyopHEMOJN ΔlcrV) strains. pCVD442-lcrVKO or pCVD442-yscFKO (29) was introduced into Y. pseudotuberculosis as described previously (34).
Table 2.
Primers used in this study
| Primer name | Sequence |
|---|---|
| yscF D28AF | 5′-CTCAAGAAGCCAGCAGCCGATGCAAACAAAGCG |
| yscF D28AR | 5′-CGCTTTGTTTGCATCGGCTGCTGGCTTCTTGAG |
| yscF D46AF | 5′-ATTGAAAGATAAGCCTGCCAACCCGGCGCTACTTG |
| yscF D46AR | 5′-CAAGTAGCGCCGGGTTGGCAGGCTTATCTTTCAAT |
Cross-linking of Y. pseudotuberculosis protein.
For cross-linking experiments, Y. pseudotuberculosis cells were washed and resuspended in warm phosphate-buffered saline (PBS) at an optical density at 600 nm (OD600) of 0.5, except where it is indicated in the text that Y. pseudotuberculosis cells were not washed before exposure to cross-linking agents. Fifty microliters of the Y. pseudotuberculosis suspension was subjected to cross-linking with 1 mM bis(sulfosuccinimidyl) suberate (BS3), 1 mM ethylene glycol bis(sulfosuccinimidylsuccinate) (sulfo-EGS), or 1 mM 3,3′-dithiobis(sulfosuccinimidylpropionate) (DTSSP) dissolved in water. Cross-linking reactions were allowed to proceed for 30 min at 37°C. After the cross-linking step, cells were pelleted and resuspended in 2× sample buffer (100 mM Tris, pH 6.8, 4% SDS, 30% glycerol, 1% bromophenol blue). In addition, 4% β-mercaptoethanol (BME) was added to the sample buffer unless DTSSP was used. Proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and visualized by Western blot analysis. Western blotting was carried out using anti-rabbit antibodies to YopB (1:10,000) (gift from J. Bliska, Stony Brook), YopD (1:10,000), LcrV (1:10,000), or YscF (1:15,000). Both Cy3 (1:2500)-conjugated and horseradish peroxidase (HRP; 1:10,000)-conjugated secondary anti-rabbit antibodies (Invitrogen) were used for visualization.
Immunoprecipitation.
Y. pseudotuberculosis cells were grown in 2× YT medium for 1.5 h at 26°C and then shifted to 37°C for an additional 1.5 h with aeration. Cells were treated with cross-linking reagents as described above. Bacteria were then pelleted and resuspended in 100 μl of boiling buffer (50 mM Tris-Cl, pH 7.5, 1 mM EDTA, 1% SDS) and heated for 5 min at 95°C. Lysates were centrifuged to remove insoluble proteins, and supernatants were added to 900 μl of ice cold TNET buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 5 mM EDTA). SigmaFast protease inhibitor cocktail (1×) was added to each reaction mixture. Fifty microliters of a 1:1 solution of protein A beads in TNET buffer was incubated with 2 μl of anti-LcrV serum and then added to the buffered lysate. Mixtures were allowed to incubate at 4°C for ∼18 h, and then the beads were removed, washed in TNET buffer, and eluted by the addition of 2× sample buffer with 4% BME. Samples were heated to 95°C, and proteins were separated by SDS-PAGE and visualized by Western blotting. In some cases, blots were stripped in acid stripping buffer (25 mM glycine, 1% SDS, pH 2.0) for 30 min at room temperature and then reprobed with another antibody.
Secretion of Yops into culture supernatants.
Secretion of Yops into the culture supernatant was performed as described previously (29). The secreted proteins were separated by SDS-PAGE and visualized by Coomassie staining. For determination of YopE secretion into tissue culture supernatants of infected cells, HEp-2 cells were infected as described below in RPMI 1640 medium lacking fetal bovine serum (FBS). Following infection, supernatants were removed, clarified by centrifugation, and precipitated with trichloroacetic acid (TCA) as described previously (29). Secreted proteins were separated by SDS-PAGE, and YopE protein was visualized by Western blot analysis with anti-YopE antibody (1:10,000).
Indirect IF microscopy.
Y. pseudotuberculosis was grown in Yop secretion medium at 37°C for 90 min, fixed, and analyzed by immunofluorescence (IF) as described previously (37). Micrographs were taken with a Nikon inverted TE2000-U microscope with a Photometrics charge-coupled-device (CCD) camera at a magnification of ×60 using MetaVue software (Molecular Devices, Sunnyvale, CA). DAPI (4′,6′-diamidino-2-phenylindole) and Alexa Fluor-594 were visualized using Nikon UV-2E/C and G-2E/C filters, respectively. Images were pseudocolored and merged using NIS-Elements software.
Tissue culture.
HEp-2 cells were maintained in RPMI 1640 medium (Cellgro) with 5% FBS at 37°C and 5% CO2. For plasma membrane isolation experiments, cells were grown to confluence in T-75 flasks (approximately 1 × 107 cells). For all other tissue culture experiments, HEp-2 cells were seeded into either 6- or 96-well tissue culture treated plates at 6 × 105 or 1.5 × 104 cells per well, respectively, 24 h prior to the experiment.
The following procedure for HEp-2 cell infections was used for all tissue culture experiments. Y. pseudotuberculosis cells were gently washed in PBS and resuspended in 37°C RPMI medium with 5% FBS supplemented with 30 μM IPTG. This medium was then used to replace culture medium on HEp-2 cells. Infection was initiated by centrifuging the bacteria onto the cells at 290 × g for 5 min at room temperature (RT). Plates were incubated at 37°C in 5% CO2 for the remainder of the experiment.
Cell-rounding assay.
Cell-rounding experiments were performed as described previously (38). The infection proceeded for 60 min before imaging. Cells were examined on a Nikon Eclipse TE2000-U microscope (Melville, NY).
Plasma membrane isolation and cross-linking.
Y. pseudotuberculosis cells were grown in 2× YT medium at 26°C for 2 h, 30 μM IPTG was then added, and the bacteria were shifted to 37°C for 2 h. A total of 5 × 108 Y. pseudotuberculosis cells were gently washed in warm PBS and resuspended in 5 ml of RPMI medium containing 30 μM IPTG. HEp-2 cells were grown to confluence of approximately 1 × 107 cells in a T-75 flask and washed gently with warm PBS. Five milliliters of RPMI medium containing Y. pseudotuberculosis and IPTG was added to the confluent flask, resulting in a multiplicity of infection (MOI) of ∼50:1. Flasks were incubated without centrifugation for 1 h at 37°C in 5% CO2, after which time flasks were placed on ice for 5 min. The medium was removed from the flasks, the monolayer was gently washed with cold PBS, and the cells were removed by scraping into 5 ml of cold PBS. Cells were centrifuged at 290 × g for 5 min, resuspended in ice-cold swelling buffer (1 mM EGTA, 1 mM MgCl2, and 1 mM dithiothreitol [DTT]), and allowed to sit on ice for 5 min. After swelling, cells were again pelleted at 290 × g for 5 min and resuspended in 1 ml of sucrose buffer (250 mM sucrose, 3 mM imidazole, pH 7.4) and 1× SigmaFast protease inhibitor cocktail. The cells were transferred to a Dounce homogenizer and stroked gently 10 times with the loose pestle. Cell lysates were then transferred to a 15-ml conical tube and centrifuged at 900 × g for 5 min to pellet insoluble material and bacteria. Supernatants were collected, and pellets were washed with 1 ml of sucrose buffer and centrifuged at 900 ×g for 10 min. Supernatants were combined, transferred to a 2-ml Eppendorf tube, and centrifuged at 14,000 × g to remove any remaining insoluble material. For cross-linked samples, a 250 mM stock solution of 1,5-difluoro-2,4-dinitrobenzene (DFDNB) in dimethylformamide (DMF) was prepared and added to the lysate at a final concentration of 500 μM. Samples were incubated on ice for 2 h before being quenched with the addition of 25 mM Tris, pH 8. Both cross-linked and nontreated samples were centrifuged at 125,000 × g to pellet membrane vesicles. Pellets were resuspended in 2× sample buffer with 4% BME and separated by SDS-PAGE. Proteins were visualized by Western blotting.
RESULTS
HMW complexes of LcrV and YopD, but not YopB, can be detected in the presence of Y. pseudotuberculosis needles.
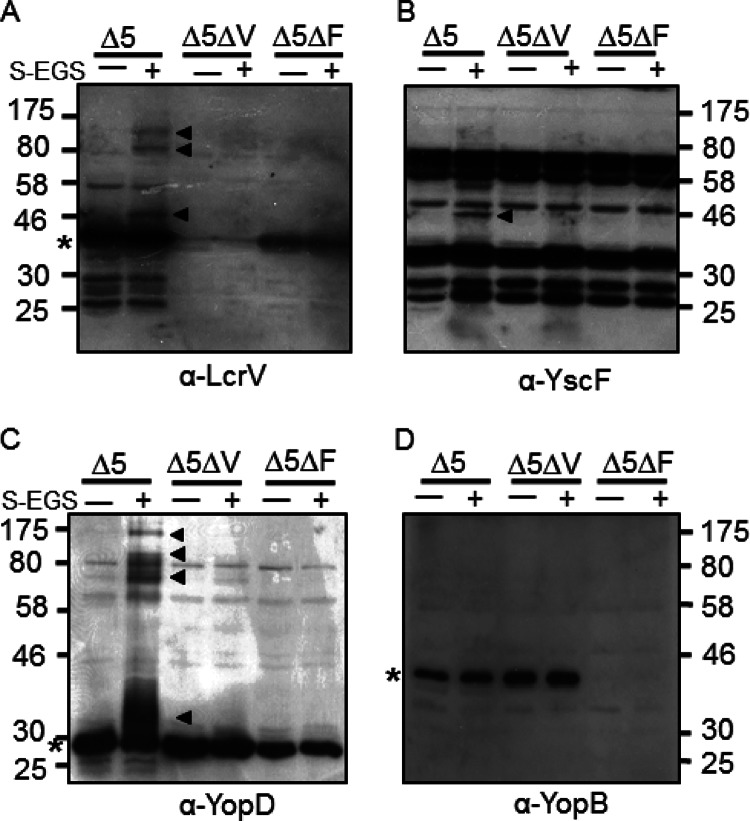
To probe for protein-protein interactions occurring between YscF, LcrV, YopB, and YopD, we established a chemical cross-linking assay using amine-to-amine cross-linkers. In order to eliminate complications from potential cross-links between effector Yops and TTSS components, a Y. pseudotuberculosis strain that lacks five effector Yops, the ΔyopHEMOJ strain (Δ5) was used. Y. pseudotuberculosis cells grown under Yop-secreting conditions were washed gently in PBS and exposed to cross-linkers. Cells were lysed, and YscF, LcrV, YopD, or YopB was analyzed by Western blotting for cross-linked complexes. Sulfo-EGS, a 16.1-Å, non-membrane-permeable NH3-NH3 cross-linker, generated reproducible cross-links containing YscF, LcrV, or YopD (Fig. 1A to C). High-molecular-weight (HMW) species were detected that correspond to multimers of LcrV or LcrV with other proteins (Fig. 1A, arrowheads). One particular LcrV-containing band corresponds to the molecular weight of an LcrV-YscF cross-link (Fig. 1A). A band of similar molecular weight was also recognized by the YscF antibody (Fig. 1B), supporting the idea that this band represents an association between surface-localized LcrV and YscF proteins. The LcrV complexes were detected only when YscF was present (Fig. 1A). This indicates that formation of these complexes requires the presence of needles and suggests that that these complexes are both extracellular and associated with Y. pseudotuberculosis. In addition, HMW cross-linked protein complexes containing YopD were also detected when Y. pseudotuberculosis was grown under secretion-inducing conditions (Fig. 1C). These multimers were dependent on both YscF and LcrV, indicating that external YopD complexes may require both needle formation and tip complex formation in order to form. We were unable to observe HMW structures containing YopB with this or other cross-linkers (Fig. 1D and data not shown), suggesting either that YopB does not associate on the surface of Y. pseudotuberculosis under Yop-secreting conditions or that YopB cannot be cross-linked by these cross-linkers. In summary, the sulfo-EGS cross-linker provides us with a tool to investigate whether the putative YscF-LcrV and/or the HMW complexes of LcrV and YopD are formed when TTSS proteins with various mutations are expressed.
Fig 1.
Cross-linking analysis of secreted but cell-associated LcrV, YscF, YopD, and YopB. The ΔyopHEMOJ (Δ5), ΔyopHEMOJ ΔlcrV (Δ5ΔV), or ΔyopHEMOJ ΔyscF (Δ5ΔF) Y. pseudotuberculosis strain was induced for Yop secretion for 1.5 h at 37°C, washed once in PBS, and then exposed to sulfo-EGS for 30 min at 37°C. Whole cells were solubilized in sample buffer and then subjected to Western blot analysis with antibodies against LcrV (A), YscF (B), YopD (C), or YopB (D). Filled arrowheads indicate cross-linked bands; asterisks indicate the monomeric protein. YscF is not visible on the gel. Molecular mass (kDa) is indicated on the sides of the gels.
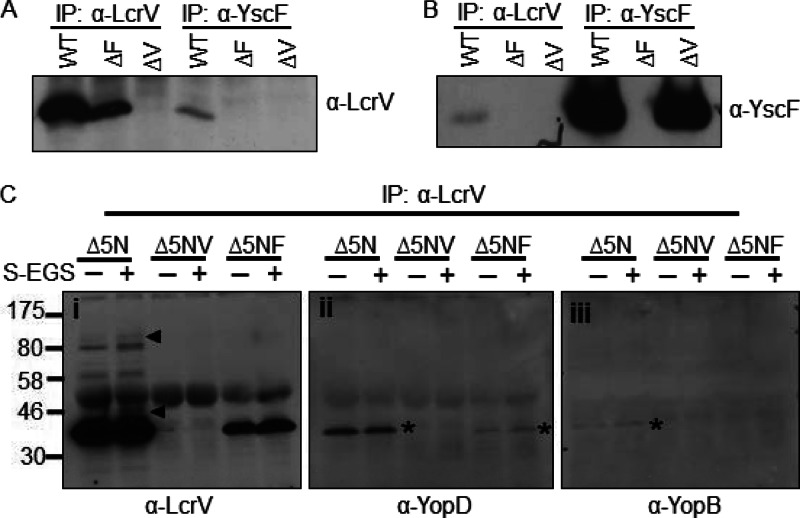
To confirm that the ∼46-kDa band was a cross-linked species consisting of LcrV and YscF (Fig. 1A and B), we exposed Y. pseudotuberculosis to a membrane-impermeable, amine-to-amine, cleavable cross-linker, DTSSP (12 Å). A 46-kDa band was detected by Western blotting with DTSSP (see Fig. 5A; also data not shown). Cross-linked LcrV and YscF were immunoprecipitated, the cross-links were cleaved with BME, and the immunoprecipitates were probed by Western blotting (Fig. 2A and B). We found that YscF was detected in immunoprecipitates of cross-linked LcrV and, likewise, that LcrV was detected in the YscF immunoprecipitates, confirming that LcrV and YscF were directly cross-linked to each other (Fig. 2A and B).
Fig 5.
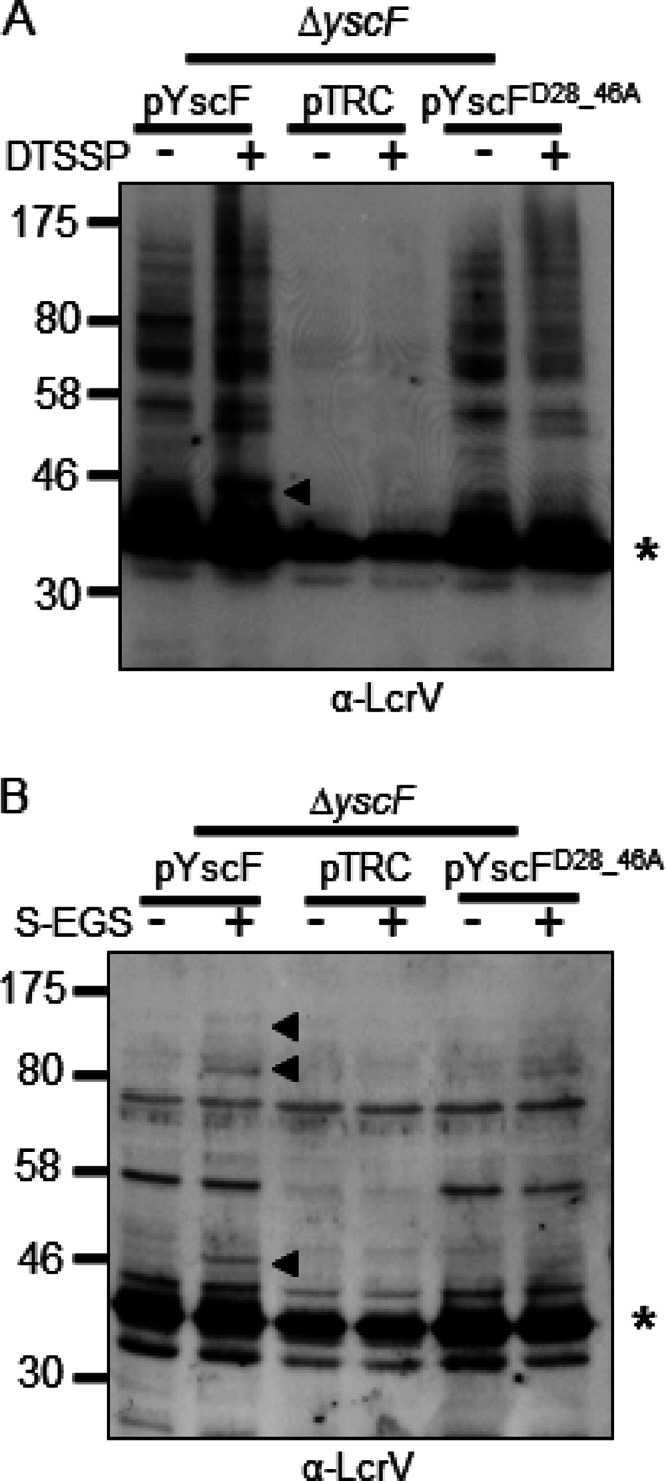
YscF-LcrV and HMW LcrV complexes from a YscFD28AD46A strain are poorly detected with DTSSP or when grown in minimal medium. (A) A ΔyscF strain expressing pTRC99A (pTRC), pTRC99A-yscF (pYscF), or pTRC99A-yscFD28AD46A (pYscFD28_46A) was grown in secretion medium at 37°C. Expression from pTRC99A was induced with the addition of 30 μM IPTG followed by incubation at 37°C for 1.5 h. Cells were incubated with 1 mM DTSSP or water for 30 min at 37°C. Bacteria were solubilized, and proteins were analyzed by Western blotting with anti-LcrV antibody. (B) Strains expressing pTRC99A, pTRC99A-yscF, or pTRC99A-yscFD28AD46A were grown in M9 minimal medium supplemented with 0.4% glucose and 1% defined amino acid mix lacking arginine, glutamine, lysine, and asparagine. Bacteria were incubated for 2 h at 26°C; 30 μM IPTG was added, and bacteria were grown at 37°C for 3 h. Cultures were exposed to 1 mM sulfo-EGS for 30 min at 37°C. Proteins were analyzed by Western blot analysis. Arrows indicate YscF-LcrV and HMW complexes formed by the addition of cross-linking agents. Asterisks indicate monomeric LcrV. Each experiment was repeated twice and a representative blots are shown.
Fig 2.
YscF and LcrV can be immunoprecipitated (IP) after cross-linking. (A and B) A WT, ΔyscF (ΔF), or ΔlcrV (ΔV) Y. pseudotuberculosis strain was exposed to DTSSP and then lysed. Lysates were split and immunoprecipitated with LcrV or YscF antibodies. Cross-links within the immunoprecipitates were cleaved with BME. Proteins were separated by SDS-PAGE and analyzed by Western blotting with anti-LcrV (A) or anti-YscF (B). (C) LcrV was immunoprecipitated from lysates of Y. pseudotuberculosis exposed to sulfo-EGS with anti-LcrV antibody. Proteins were eluted from protein A beads by boiling in sample buffer. Proteins were analyzed by Western blotting with anti-LcrV (i), anti-YopD (ii), and anti-YopB (iii) antibodies. Each experiment was repeated twice, and representative blots are shown. Asterisks indicate incompletely stripped LcrV. Δ5N, ΔyopHEMOJN; Δ5NV, ΔyopHEMOJN ΔlcrV; Δ5NF, ΔyopHEMOJN ΔyscF; α, anti.
We next investigated whether LcrV associates with YopD or YopB under Yop-secreting conditions. After chemical cross-linking with sulfo-EGS, LcrV was immunoprecipitated and analyzed by Western blotting. The LcrV-YscF and one other HMW LcrV-containing complex were immunoprecipitated (Fig. 2C, frame i), consistent with HMW complexes observed in whole bacterial lysates. Our inability to detect the highest HMW complex after immunoprecipitation seen in whole-cell lysates (Fig. 1A) may be because the LcrV antibody is unable to immunoprecipitate the complex, or because its low abundance and HMW make it difficult to visualize by Western blotting. To determine if YopD or YopB was present in the immunoprecipitated complexes, the blot was reprobed with antiserum recognizing YopD or YopB. Neither YopD nor YopB (Fig. 2C, frames ii and iii) was detected in these complexes, suggesting that the HMW complex consists of either multimers of LcrV or LcrV associated with other secreted proteins. Given the observations that LcrV forms HMW complexes only when needles are generated, that LcrV cross-links with YscF, and that a pentamer of LcrV is found on the needle tip (11, 12), it is plausible that these HMW LcrV complexes are cross-linked tip complexes.
The translocation-defective YscFD28AD46A strain secretes Yops in a calcium-regulated manner.
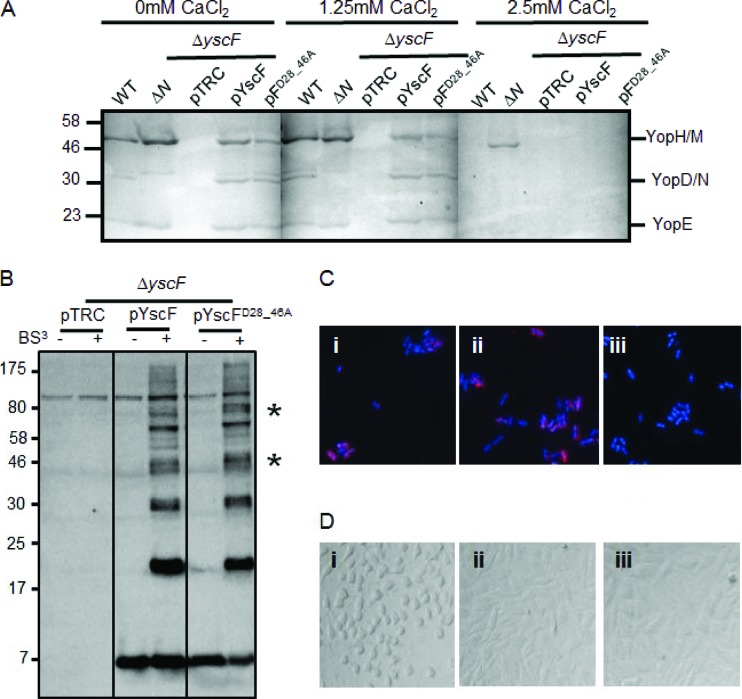
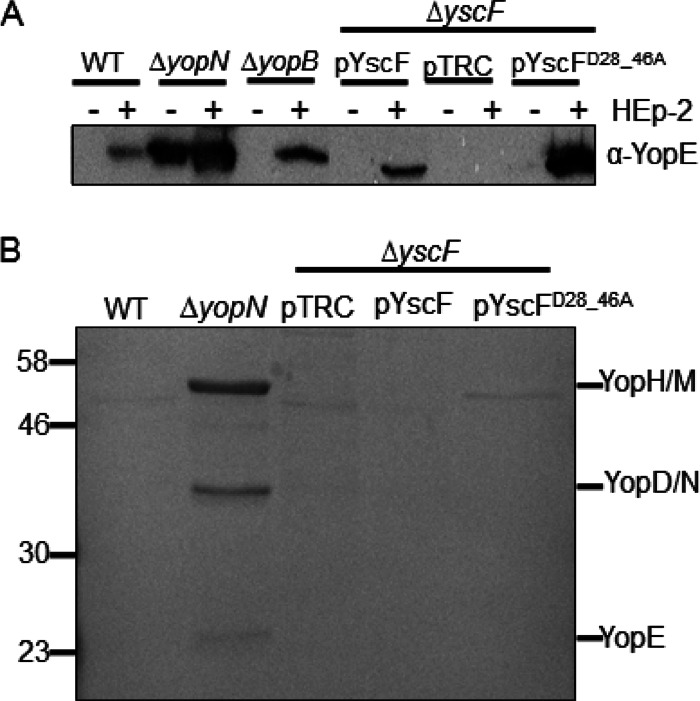
Having established that LcrV cross-linked to YscF and that secreted LcrV and YopD form HMW complexes in association with Y. pseudotuberculosis, we investigated whether a translocation-defective mutant of Yersinia had altered tip complex formation or altered YopD complex formation by studying the cross-linking patterns of these proteins. A yscF mutant, the yscFD28AD46A, strain, which was originally isolated and characterized by Torruellas et al. in Y. pestis (30), was unable to translocate Yops into mammalian cells. This yscF mutant in Y. pestis was also unresponsive to calcium since Yops were secreted into supernatants at calcium concentrations up to 7 mM (30). However, it formed typical needle polymers as measured by BS3 cross-linking. To investigate the phenotypes of this mutation in Y. pseudotuberculosis, we expressed yscFD28AD46A in a ΔyscF strain (29). Surprisingly, when expressed in Y. pseudotuberculosis, the yscFD28AD46A-expressing strain was still sensitive to calcium (Fig. 3A) as the yscFD28AD46A mutant strain secreted WT levels of Yops when it was grown in a calcium-free medium (Fig. 3A). When calcium was added back to the medium, both the WT and the yscFD28AD46A-expressing strains stopped secreting detectable levels of Yops in the presence of 2.5 mM CaCl2, whereas the ΔyopN mutant continued to secrete Yops in the presence of 5.0 and 7.5 mM CaCl2 (Fig. 3A and data not shown). To investigate if Y. pseudotuberculosis expressing yscFD28AD46A formed YscF polymers, Y. pseudotuberculosis was grown in the absence of calcium and exposed to the cross-linker BS3 (Fig. 3B). No differences in the molecular weights of cross-linked complexes were observed between the WT YscF and YscFD28AD46A strains. Interestingly, however, we consistently observed variation in the intensity of specific bands (Fig. 3B, asterisks), suggesting that there may be differences in the polymers formed by this mutant. We also verified that YscF localized to the surface of Y. pseudotuberculosis cells by immunofluorescence (IF) microscopy. In both ΔyscF strains expressing WT YscF and expressing YscFD28AD46A, punctate staining was observed on nonpermeabilized Y. pseudotuberculosis cells, consistent with extracellular localization of YscF polymers (Fig. 3C) (37, 38). We next assessed the ability of this mutant to support translocation of YopE using an HEp-2 cell-rounding assay, which detects the activity of the effector YopE on the actin cytoskeleton of translocated cells (39). Notably, the yscFD28AD46A-expressing strain was incapable of causing HEp-2 cell rounding, suggesting that it is unable to translocate YopE (Fig. 3D). Combined, these observations indicate that the YscFD28AD46A mutant in Y. pseudotuberculosis is calcium regulated and generates needles but cannot support Yop translocation into mammalian cells.
Fig 3.
Characterization of the YscFD28AD46A needle in Y. pseudotuberculosis. (A) The WT IP2666, ΔyopN, or ΔyscF strain carrying pTRC99A (pTRC), pTRC99A-yscF (pYscF), or pTRC99A-yscFD28AD46A (pFD28_46A) was grown in 2× YT medium plus 5 mM EGTA and 20 mM MgCl2 supplemented with the indicated amounts of CaCl2 for 2 h at 26°C. Expression of YscF from pTRC99A was induced with the addition of IPTG when bacteria were shifted to 37°C. Supernatants from cultures grown for 2 h at 37°C were precipitated with TCA. Secreted proteins were separated by SDS-PAGE and visualized by Coomassie blue. (B) Bacteria were grown under secretion-inducing conditions for 90 min and then exposed to 1 mM BS3 or water for 30 min. Whole cells were solubilized in sample buffer and subjected to Western blot analysis with antibody against YscF. Asterisks indicate HMW bands that vary in intensity between the WT YscF- and YscFD28AD46A-expressing strains. pYscFD28_46A, pTRC99A-yscFD28AD46A. (C) Y. pseudotuberculosis cells were grown under secretion-inducing conditions for 1.5 h and then fixed and mounted onto coverslips. Cells were labeled with anti-YscF antibody and visualized with Alexa Fluor-594-conjugated anti-rabbit secondary (red). Bacteria were counterstained with DAPI (blue). Frame i, ΔyscF(pTRC99A-yscF); frame ii, ΔyscF (pTRC99A-yscFD28AD46A); frame iii, ΔyscF(pTRC99A). (D) HEp-2 cells were seeded into 96-well plates and then infected with Y. pseudotuberculosis at an MOI of 50:1. Images were taken after 1 h of incubation at 37°C. Frame i, ΔyscF(pTRC99A-yscF); frame ii, ΔyscF(pTRC99A-yscFD28AD46A); frame iii, ΔyscF(pTRC99A). Each experiment was repeated a minimum of two times. Representative experiments are shown for each.
Translocation-defective YscFD28AD46A needles associate aberrantly with LcrV.
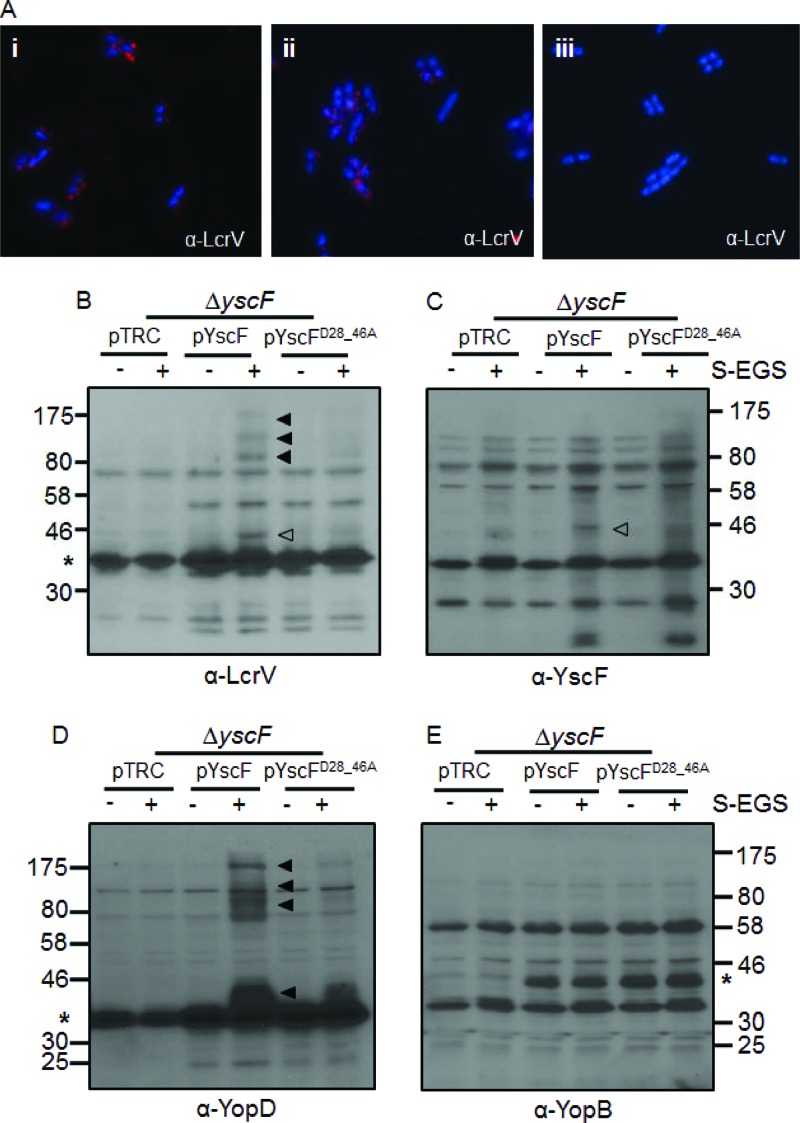
To further probe why needles carrying the YscFD28AD46A mutation fail to translocate Yops, we used IF and chemical cross-linking to investigate whether LcrV was found in association with needles. Both the nonpermeabilized WT yscF-expressing strain and the yscFD28AD46A-expressing strain had punctate staining patterns consistent with an extracellular LcrV location (Fig. 4A). Furthermore, the punctate pattern of staining of both YscF and LcrV on WT and yscFD28AD46A needles suggests that LcrV and YscF have similar arrangements on the surface of both WT and mutant bacteria. Unfortunately, simultaneous staining with YscF and LcrV was not successful, perhaps because the antibodies interfere with each other (unpublished observations). Nonetheless, these results suggest that LcrV is associated in discrete extracellular locations in both the WT and yscFD28AD46A-expressing strains.
Fig 4.
LcrV cross-linked complexes are altered on Y. pseudotuberculosis expressing YscFD28AD46A polymers. (A) Y. pseudotuberculosis cells were grown under secretion-inducing conditions for 1.5 h, fixed, mounted onto coverslips, and labeled with anti-LcrV antibody and then with Alexa Fluor-594-conjugated anti-rabbit secondary antibody (red). Frame i, ΔyscF(pTRC99A-yscF); frame ii, ΔyscF(pTRC99A-yscFD28AD46A); frame iii, ΔyscF(pTRC99A). (B to E) ΔyscF strains expressing pTRC99A, pTRC99A-yscF, or pTRC99A-yscFD28AD46A were grown in secretion medium at 37°C. Expression of YscF was induced with the addition of 30 μM IPTG followed by incubation at 37°C. Cross-linking with sulfo-EGS (S-EGS) was performed as described in the legend for Fig. 1. Proteins were detected with anti-LcrV (B), anti-YscF (C), anti-YopD (D), or anti-YopB (E) antibodies. Open arrowheads indicate the YscF-LcrV complex; filled arrowheads indicate HMW cross-linked bands. Asterisks indicate the monomeric protein; YscF is not visible on the gel. The experiment was repeated twice, and a representative blot is shown.
In contrast to our findings with IF, the cross-linking data revealed several notable differences between WT yscF-expressing and yscFD28AD46A-expressing Y. pseudotuberculosis. When yscFD28AD46A-expressing cells were exposed to sulfo-EGS, there was a stark reduction in the amount of the LcrV-YscF cross-linked product and in the HMW complexes containing LcrV compared to levels in Y. pseudotuberculosis expressing WT YscF (Fig. 4B to E). Furthermore, while YopD was secreted (Fig. 3A), we detected almost no HMW complexes containing YopD associated with yscFD28AD46A-expressing bacteria (Fig. 4D). These observations, combined with the IF results, indicate that although LcrV is secreted and detected on the surface of the mutant, the YscFD28AD46A needles have an altered association with LcrV. Furthermore, this altered association interferes with the formation of the LcrV-YscF cross-link and with HMW forms of LcrV and YopD with associated Y. pseudotuberculosis.
To test if the conformation of the LcrV cross-linked complexes had been only modestly altered such that sulfo-EGS was no longer able to cross-link LcrV to YscFD28AD46A and if another cross-linker might still reveal an association, we examined whether LcrV-YscFD28AD46A cross-links were detectable using DTSSP. The WT yscF-expressing strain had a strong LcrV-YscF cross-link with DTSSP. However, no LcrV-YscF cross-linked product was observed in the strain expressing yscFD28AD46A (Fig. 5A). This indicates that the interaction between LcrV and YscFD28AD46A polymers is altered enough so that it cannot be detected by two different cross-linkers. Curiously, the HMW LcrV complexes that were detected with sulfo-EGS (Fig. 1) were not readily detected with DTSSP (Fig. 5), indicating that these complexes cannot be detected with a shorter cross-link arm. However, a different cross-linked product of approximately 55 kDa was observed (Fig. 5A), which could be an LcrV dimer or LcrV cross-linked with another protein.
We found that the LcrV-YscF cross-linked product was not detected with either sulfo-EGS or DTSSP, but LcrV was detected in a punctate pattern on the surface of Y. pseudotuberculosis cells by IF. Therefore, we investigated whether our manipulations prior to cross-linking dislodged a tip complex that may have a weaker association with the YscFD28AD46A needle and be washed away prior to exposure to a cross-linker. To address this possibility, Y. pseudotuberculosis was grown in a minimal medium in the absence of the amino acids that quench sulfo-EGS, i.e., lysine, glutamine, arginine, and asparagine, and then Y. pseudotuberculosis was exposed to sulfo-EGS without a prior washing step. Again, under these conditions, an LcrV-YscF cross-link was detected in the strain carrying a WT YscF allele but was not detected in the strain carrying the yscFD28AD46A allele (Fig. 5B). In summary, these results suggest that the YscFD28AD46A needles still associate with LcrV but that the conformation of the needle-tip complex is sufficiently different to change the availability of the lysines in YscF and/or LcrV for cross-linking with two different reagents. Furthermore, the YscFD28AD46A needles are unable to support the formation of the HMW complexes containing LcrV and YopD.
YscFD28AD46A needles target YopB and YopD aberrantly to the plasma membrane of mammalian cells, phenocopying a ΔlcrV mutant.
LcrV is critical for YopB and YopD to form productive pores in mammalian cell membranes (12). However, how LcrV facilitates the insertion of the translocon is unknown. The observation that YscFD28AD46A needles have an altered association with LcrV, coupled with the observation that this mutant fails to translocate Yops, suggested that the association of LcrV with YscF may be crucial for insertion of YopB and/or YopD into host cell membranes. To test whether the yscFD28AD46A strain could target YopB and YopD to the plasma membrane of infected cells, plasma membranes were purified from infected cells, and the amount of YopD, YopB, and LcrV associated with the membrane was measured by Western blotting (Fig. 6). For these assays, a strain of Y. pseudotuberculosis that lacks the five effectors and the regulatory protein YopN (Δ5N strain) was used because it was previously reported to increase the detectable amount of membrane-associated pore-forming proteins in sheep red blood cells (SRBCs) (12, 23). This phenomenon was also observed for our Y. pseudotuberculosis Δ5N strain after infection of HEp-2 cells (unpublished observation). YopB, YopD, and LcrV copurified with plasma membranes of HEp-2 cells infected with the Δ5NF strain expressing WT yscF (Fig. 6A). In contrast, plasma membranes from HEp-2 cells infected with the yscFD28AD46A-expressing strain did not contain YopB, and the amount of YopD and LcrV associated with these membranes was reduced (Fig. 6A). The reduction of YopD and the absence of YopB in plasma membranes from cells infected with YscFD28AD46A strain represented the same phenotype as that seen with strains lacking LcrV (Fig. 6B) (12). In summary, these results suggest that the altered association of LcrV with YscFD28AD46A needles leads to a failure to insert YopB into plasma membranes and defective translocon formation, which accounts for the translocation-negative phenotype observed in this strain.
Fig 6.
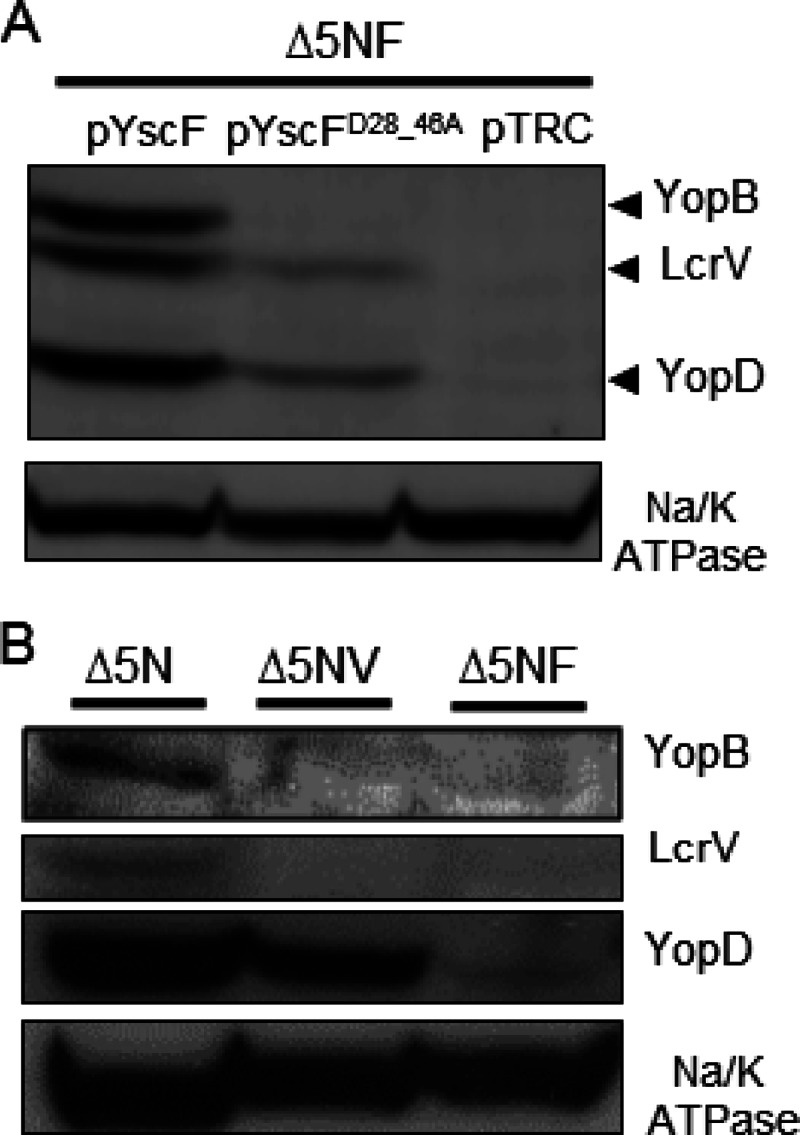
Association of LcrV, YopB, and YopD with HEp-2 plasma membranes after infection with the WT YscF or YscFD28AD46A strain. HEp-2 cells were infected with Y. pseudotuberculosis strains at an MOI of 50:1. After 1 h, the plasma membranes were collected, and the proteins were separated by SDS-PAGE. Proteins were visualized by Western blot analysis with anti-YopB, anti-LcrV, anti-YopD, and anti-Na/K ATPase antibodies. (A) Strains lacking yopHEMOJN yscF (Δ5NF) and expressing pYscF, pYscFD28AD46A, or pTRC99A were induced in 30 μM IPTG at 37°C. (B) Western blot analysis of the ΔyopHEMOJN (Δ5N), ΔyopHEMOJN ΔlcrV (Δ5NV), and ΔyopHEMOJN ΔyscF (Δ5NF) strains. The experiments were performed twice, and a representative blot is shown.
Plasma membrane-associated LcrV oligomerizes despite aberrant interactions with YscFD28AD46A needles.
Since LcrV was associated with plasma membranes from HEp-2 cells infected with either yscF or yscFD28AD46A-expressing Y. pseudotuberculosis (Fig. 6), we asked whether LcrV was oligomerized when it associated with membranes. To address this question, the plasma membranes of HEp-2 cells infected with Y. pseudotuberculosis harboring either the WT yscF allele or the mutant yscFD28AD46A allele were collected following exposure to DFDNB, a 3-Å amine-to-amine cross-linker. After the membranes were concentrated, the presence of HMW complexes of LcrV was assessed by Western blotting (Fig. 5). DFDNB was used because only very weak cross-linked products were detected with sulfo-EGS (data not shown). At least four HMW LcrV-containing complexes were observed in both strains (Fig. 7, asterisks). Interestingly, the LcrV-YscF cross-link was not detected with plasma membrane-associated LcrV. This suggests that this pool of LcrV is no longer associated with the needle or that DFDNB cannot cross-link LcrV with YscF or that the LcrV-YscF cross-link band is obscured by a comigrating band at 46 kDa. In summary, these results indicate that LcrV can form HMW complexes in association with mammalian membranes despite having abnormal association with needles when it is secreted from a YscFD28AD46A strain. These HMW LcrV complexes, however, are not sufficient to support YopB insertion into the membrane, but rather an association of LcrV with the YscF polymer appears necessary for proper insertion of YopB.
Fig 7.
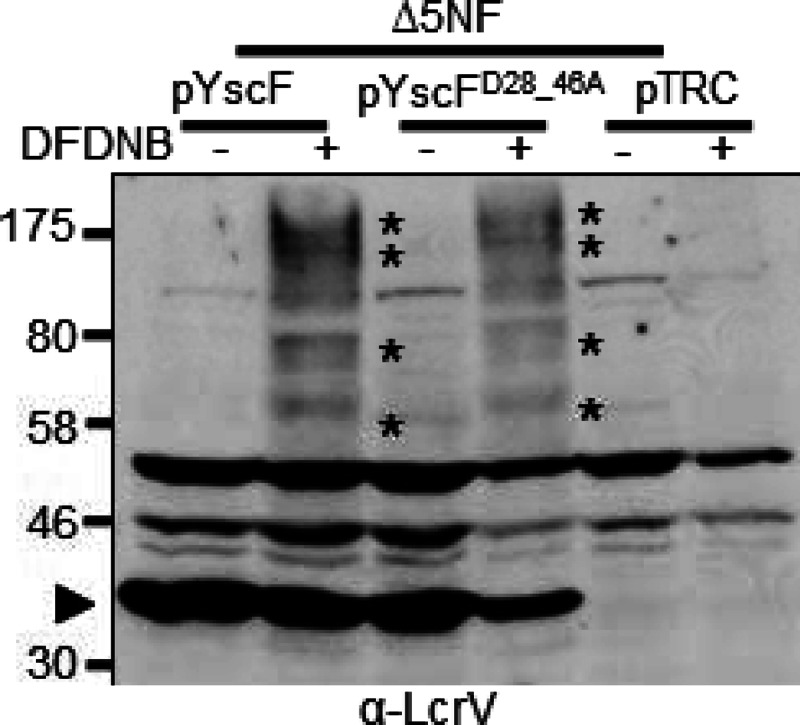
Membrane-associated LcrV forms oligomers in both WT YscF- and YscFD28AD46A-expressing strains. Y. pseudotuberculosis ΔyopHEMOJN ΔyscF (Δ5NF) expressing pTRC99A, pTRC99A-yscF, or pTRC99A-yscFD28AD46A was grown at 37°C for 1.5 h with 30 μM IPTG to induce expression of YscF. The bacteria were then used to infect HEp-2 cells at an MOI of 50:1. After a 1-h incubation at 37°C, HEp-2 cells were collected, and the bacteria were removed by centrifugation. Lysates were incubated with 500 μM DFDNB before plasma membrane vesicles were isolated. Proteins were separated by SDS-PAGE and analyzed by Western blotting with anti-LcrV antibody. Asterisks indicate HMW LcrV complexes formed by the addition of DFDNB. The experiment was repeated twice, and a representative blot is shown.
Yop secretion in response to cell contact is triggered in the yscFD28AD46A mutant.
It is currently unknown what signal initiates effector secretion from Y. pseudotuberculosis in the presence of host cells. Possibilities include that the tip complex may directly senses host contact or may interact with the YopB/YopD pore, leading to a conformational shift that is propagated through the needle (1, 9, 30). Since LcrV has an aberrant association with polymers of YscFD28AD46A based on cross-linking results (Fig. 4B), the study of this YscF mutant may indicate whether a normal tip complex, as defined by the association of YscF and LcrV detected by chemical cross-linking, is needed to trigger Yop secretion in the presence of host cells. To determine if the yscFD28AD46A strain initiated Yop secretion after exposure to mammalian cells, HEp-2 cells were infected with Y. pseudotuberculosis expressing either WT yscF or yscFD28AD46A. After 1 h, the proteins secreted into the tissue culture supernatants were collected, and the presence of YopE was detected by Western blot analysis. In the absence of HEp-2 cells, YopE was detected only in the medium containing the constitutively secreting ΔyopN mutant (Fig. 8A and B). All other strains did not secrete YopE, indicating that the calcium in RPMI medium is sufficient to suppress Yop secretion (Fig. 8A and B). Strikingly, in the presence of HEp-2 cells, the yscFD28AD46A-expressing strain secreted high levels of YopE, whereas the WT secreted very low levels of YopE (Fig. 8A). Thus, despite the inability of this YscF mutant to form cross-links with LcrV, this strain sensed cells and secreted Yops in response to cell contact. This suggests that a stable association of the tip complex with YscF is not required to trigger Yop secretion in response to cell contact.
Fig 8.
YscFD28AD46A secretes Yops into the cell supernatant in the presence of HEp-2 cells. A WT, ΔyopN, ΔyopB, or ΔyscF strain expressing pTRC99A, pTRC99A-yscF, or pTRC99A-yscFD28AD46A was grown in 2× YT medium plus 5 mM CaCl2 for 2 h at 26°C and then grown at 37°C for 2 h. Strains carrying pTRC99A or plasmid derivatives were exposed to IPTG to induce expression of YscF. (A) HEp-2 cells were infected at an MOI of 50:1 (+). Equivalent numbers of bacteria were added to wells lacking HEp-2 cells (−). After a 1-h incubation at 37°C, supernatants were collected, and proteins were analyzed by TCA precipitation and separated by SDS-PAGE. Proteins were visualized by Western blot analysis with anti-YopE antibody. (B) After growth at 37°C, bacteria were gently spun and grown for an additional hour in RPMI medium. Supernatants were collected, and proteins were precipitated with TCA, separated by SDS-PAGE, and visualized by Coomassie blue dye. Each experiment was repeated at least twice, and a representative blot is shown.
DISCUSSION
The translocation of effectors across the membrane of target cells during infection is an essential step in Yersinia pathogenesis; however, the mechanisms that drive this process have remained poorly understood (8). One particularly enigmatic question has been whether the tip complex is involved in host cell sensing and acts as the trigger for effector release from the bacteria. A second question is whether contact between the tip complex and the needle is required to form a pore. Continuous contact between the two would support the idea that a conduit is formed through which translocating effectors traverse. In order to answer these questions, assessment of the tip complex is necessary. Previous elegant methods to analyze the tip complex have involved using EM reconstructions of purified needle complexes (11, 13). However, this assay is technically challenging and requires specialized equipment, so it is not amenable to a high-throughput approach necessary to measure the effects of various mutations and/or conditions. In addition, purification of needle complexes may disrupt weak interactions or fail to reveal transient interactions that may occur among components. Therefore, we developed a simple biochemical cross-linking assay to evaluate the state of the LcrV, YscF, YopB, and YopD. With this assay we demonstrated that we could observe cross-links of LcrV to YscF. Furthermore, we found that LcrV formed HMW complexes that are dependent upon YscF and are consistent with multimers of LcrV, suggesting that these HMW LcrV complexes represent the tip complex. In addition, the observation that no YopB-YscF cross-linked complexes were detected indicates that not all TTSS substrates passing through the needle are cross-linked with YscF. This suggests that the interaction between YscF and LcrV is specific and does not arise during its transit through the needle.
Interestingly, HMW YopD-containing complexes formed in an YscF- and LcrV-dependent manner, indicating that they are extracellular but associated with Yersinia. Veenendaal et al. demonstrated that both IpaB and IpaC (orthologues of YopB and YopD, respectively) are localized to the tips of purified Shigella flexneri needles in an IpaD-dependent manner (IpaD is an orthologue of LcrV in Shigella) (40). In contrast, YopB and YopD have not been observed on the Yersinia needle tip. While YopD, but not YopB, has been detected in preparations of purified needle complexes, this copurification may be due to insoluble YopD (11, 12). We were unable to demonstrate a direct interaction between LcrV and YopD even though the YopD HMW complexes were dependent on LcrV. Therefore, LcrV may transiently interact with YopD to facilitate formation of these complexes, and we may have been unable to catch these transient interactions in our cross-linking studies. Alternatively, our inability to detect HMW YopD-containing complexes in the ΔlcrV strain may be due to the fact that less YopD is secreted in the absence of LcrV (41), and, hence, its concentration may not have been sufficient to form multimers. Finally, these YopD HMW complexes may arise from secreted, insoluble forms of YopD that may associate with the bacteria. Since purified YopD and LcrV interact with each other (42), we favor the idea that LcrV directly interacts with YopD extracellularly on Y. pseudotuberculosis cells but in a manner that our cross-linker was unable to capture.
No YopB-associated proteins were detected with the cross-linker used in this study. The lack of cross-linked YopB complexes on the bacterial surface is consistent with the observation that YopB is not associated with purified needles (11). Furthermore, this result suggests that any transient YopB interactions with the needle complex cannot be cross-linked by the amine-to-amine cross-linker used here and that not all putative type III secretion substrates can be cross-linked with YscF and/or LcrV. If YopB is not associated with needle-tip complexes, then YopB acts differently than IpaB from Shigella, which localizes at the tip prior to host cell contact (43). In fact, IpaB is proposed to be a member of the tip complex itself with four monomers of IpaD and one monomer of IpaB (43). This, however, is not the case in Yersinia, where LcrV exists as a homopentamer both by modeling predictions (9) and experimental evaluation (12, 13). On the other hand, since LcrV is involved in the insertion of YopB into membranes (12), it seems plausible that an interaction between YopB and LcrV occurs but that this interaction is unstable and transient, that it occurs only upon contact with the plasma membrane, and/or that it could not be detected with sulfo-EGS. The YscFD28AD46A mutant may be defective in interacting with YopB for several reasons. The polymer itself may be defective in harboring and/or inserting YopB into the membrane, or YopB may require an YscF-LcrV interaction prior to insertion into the membrane. For example, the YscF-LcrV interaction may be critical for a particular YopD interaction, which, in turn, may be necessary for YopB placement in the membrane.
The existence of mutations in yscF that lead to a calcium-blind phenotype in Yersinia and of mutations in MxiH, the Shigella ortholog of YscF, that lead to Congo red insensitivity in Shigella, suggests that the needle is involved in sensing the environment (29, 30, 44). One such mutant in Y. pestis, the yscFD28AD46A strain, was unable to sense calcium, leading to constitutive secretion of effectors into the medium (30). Interestingly, this mutant was unable to translocate Yops, despite YscF polymers that are very similar to WT polymers, suggesting that this mutant could have an unstable interaction with LcrV. Surprisingly, when we expressed this mutation in Y. pseudotuberculosis, we observed that the YscFD28AD46A mutant was now calcium regulated but still defective for translocation. The reason for this difference is unclear but is likely due to differences between regulation of the calcium response between Y. pestis and the enteric Yersinia spp. (45, 46). Evaluation of the tip complex using chemical cross-linking demonstrated that YscFD28AD46A polymers were unable to generate the same HMW LcrV complexes observed in the WT, despite detection of LcrV on the bacterial surface by immunofluorescence. This suggests that although an interaction between LcrV and the YscFD28AD46A needle polymer occurs, these mutant needles do not form an LcrV tip complex. The homopentamer of LcrV may be unable to form, or the conformation of the pentamer may be altered such that it is no longer able to be cross-linked, as demonstrated here. We predict that changes at both D28 and D46 are necessary to prevent insertion of YopB because low levels of translocation have been detected when YscF has changes in only D28 or D46 (29, 30).
Deane et al. (9) proposed a model whereby the needle polymer undergoes a conformational shift upon induction of secretion. In support of this model, they showed that mutations that interfere with regulatory mechanisms governing secretion lie in regions of MxiH required for monomer-monomer interactions (9). It has been hypothesized that the trigger for a conformational change in the needle polymer comes from a conformational change that occurs in the IpaB protein, a component of the Shigella tip complex, presumably in response to cell contact (47). Roehrich et al. demonstrated that a small deletion mutation in the C terminus of IpaB (ipaBΔ3) leads to constitutive secretion and recruitment of IpaC to the tip upon induction of secretion (47). These observations led them to propose that the ipaBΔ3 mutant mimics a “secretion-on” conformation (47). We demonstrate here that the YscFD28AD46A mutant cannot direct the insertion of YopB into membranes, suggesting that LcrV and YopB do not interact productively in the context of this polymer. In addition, the YscFD28AD46A mutant fails to interact with the tip complex in a manner that can be detected by cross-linking with sulfo-EGS. If sensing cell contact occurs by the tip complex or by YopB, then we would predict that this mutant may be unable to secrete Yops in response to cells. Astonishingly, we found that this mutant readily secretes Yops in response to cell contact, indicating that neither a normal tip complex nor YopB insertion into the plasma membrane is required for sensing host cells. The LcrV at the YscFD28AD46A polymer tip may still retain the ability to sense cell contact and propagate this message, even though it no longer retains the ability to facilitate YopB insertion. Alternatively, the mechanism for sensing cell-contact may be LcrV independent in Yersinia.
One critical function of LcrV is to mediate organization of the pore (12, 25, 27). We observed that LcrV secreted by the yscFD28AD46A mutant also associates with the plasma membrane in multimeric structures that are indistinguishable from those made by the WT. However, functional pores were not generated by LcrV secreted from YscFD28AD46A, indicating that these plasma membrane-associated HMW LcrV complexes do not facilitate formation of a functional pore. This could be because the HMW LcrV complexes from YscFD28AD46A mutants were ill formed due to an aberrant needle and that these defects were not detectable with the cross-linker used. Alternatively, secreted LcrV may form HMW LcrV complexes in the presence of membranes which are not capable of regulating YopB and YopD pore formation. Regardless, our results are consistent with a model that contact between the LcrV tip complex and the YscF polymer is required for insertion of YopB and formation of a functional pore. Whether this tip complex stably associates with the pore throughout the process of effector translocation, however, is still unknown. Recently, another model for Yop translocation has been proposed in which effector Yops localized to the outer membrane are translocated from Y. pseudotuberculosis into the cytoplasm of target cells (48). If these extracellular Yops prove to be a major reservoir of Yops that are delivered to host cells, the roles of the needle and tip complex may primarily be to sense contact with host cells and/or to form the translocon.
In summary, Yersinia expressing a needle polymer that is unable to form an interaction with the tip complex and form HMW LcrV complexes detectable by chemical cross-linking still retains the ability to sense contact with host cells. However, it cannot insert YopB into the membrane and generate a functional translocon. Therefore, a normal tip complex appears to be critical for pore formation. Additional work will elucidate the mechanism by which Yersinia senses contact with host cells. However, whereas IpaB is required for sensing cells in Shigella, insertion of the Yersinia ortholog, YopB, is not required for sensing of cells by Yersinia, suggesting that the cell-sensing mechanisms used by these two bacteria may differ.
ACKNOWLEDGMENTS
We thank James Bliska for his gift of the YopB antibody, Joan-Miquel Balada-Llasat for construction of plasmids, and Perry Riggle for advice about various bacterial growth mediums. We also thank members of the Mecsas lab for critical discussions and review of the manuscript.
This work was supported by NIH grants AI073759 to J.M., T32AI007422 to D.E.H. and J.L.M., and DK075720 to A.J.D.
Footnotes
Published ahead of print 8 March 2013
REFERENCES
- 1. Mueller CA, Broz P, Cornelis GR. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085–1095 [DOI] [PubMed] [Google Scholar]
- 2. Putzker M, Sauer H, Sobe D. 2001. Plague and other human infections caused by Yersinia species. Clin. Lab. 47:453–466 [PubMed] [Google Scholar]
- 3. Huang XZ, Nikolich MP, Lindler LE. 2006. Current trends in plague research: from genomics to virulence. Clin. Med. Res. 4:189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornelis GR, Boland A, Boyd AP, Geuijen C, Iriarte M, Neyt C, Sory MP, Stainier I. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durand EA, Maldonado-Arocho FJ, Castillo C, Walsh RL, Mecsas J. 2010. The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell Microbiol. 12:1064–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. 2005. Plague bacteria target immune cells during infection. Science 309:1739–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koberle M, Klein-Gunther A, Schutz M, Fritz M, Berchtold S, Tolosa E, Autenrieth IB, Bohn E. 2009. Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 5:e1000551 doi:10.1371/journal.ppat.1000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornelis GR. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811–825 [DOI] [PubMed] [Google Scholar]
- 9. Deane JE, Roversi P, Cordes FS, Johnson S, Kenjale R, Daniell S, Booy F, Picking WD, Picking WL, Blocker AJ, Lea SM. 2006. Molecular model of a type III secretion system needle: implications for host-cell sensing. Proc. Natl. Acad. Sci. U. S. A. 103:12529–12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ligtenberg KG, Miller NC, Mitchell A, Plano GV, Schneewind O. 2013. LcrV mutants that abolish Yersinia type III injectisome function. J. Bacteriol. 195:777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, Kuhn M, Engel A, Cornelis GR. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674–676 [DOI] [PubMed] [Google Scholar]
- 12. Broz P, Mueller CA, Muller SA, Philippsen A, Sorg I, Engel A, Cornelis GR. 2007. Function and molecular architecture of the Yersinia injectisome tip complex. Mol. Microbiol. 65:1311–1320 [DOI] [PubMed] [Google Scholar]
- 13. Gebus C, Faudry E, Bohn YS, Elsen S, Attree I. 2008. Oligomerization of PcrV and LcrV, protective antigens of Pseudomonas aeruginosa and Yersinia pestis. J. Biol. Chem. 283:23940–23949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price SB, Cowan C, Perry RD, Straley SC. 1991. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J. Bacteriol. 173:2649–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nilles ML, Williams AW, Skrzypek E, Straley SC. 1997. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skrzypek E, Straley SC. 1995. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177:2530–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hakansson S, Schesser K, Persson C, Galyov EE, Rosqvist R, Homble F, Wolf-Watz H. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812–5823 [PMC free article] [PubMed] [Google Scholar]
- 18. Pettersson J, Holmstrom A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961–976 [DOI] [PubMed] [Google Scholar]
- 19. Rosqvist R, Persson C, Hakansson S, Nordfeldt R, Wolf-Watz H. 1995. Translocation of the Yersinia YopE and YopH virulence proteins into target cells is mediated by YopB and YopD. Contrib. Microbiol. Immunol. 13:230–234 [PubMed] [Google Scholar]
- 20. Perry RD, Harmon PA, Bowmer WS, Straley SC. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamad MA., Nilles ML. 2007. Roles of YopN, LcrG and LcrV in controlling Yops secretion by Yersinia pestis. Adv. Exp. Med. Biol. 603:225–234 [DOI] [PubMed] [Google Scholar]
- 22. Montagner C, Arquint C, Cornelis GR. 2011. Translocators YopB and YopD from Yersinia enterocolitica form a multimeric integral membrane complex in eukaryotic cell membranes. J. Bacteriol. 193:6923–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marenne MN, Journet L, Mota LJ, Cornelis GR. 2003. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35:243–258 [DOI] [PubMed] [Google Scholar]
- 24. Tardy F, Homble F, Neyt C, Wattiez R, Cornelis GR, Ruysschaert JM, Cabiaux V. 1999. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 18:6793–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmstrom A, Olsson J, Cherepanov P, Maier E, Nordfelth R, Pettersson J, Benz R, Wolf-Watz H, Forsberg A. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620–632 [DOI] [PubMed] [Google Scholar]
- 26. Lee VT, Tam C, Schneewind O. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275:36869–36875 [DOI] [PubMed] [Google Scholar]
- 27. Goure J, Broz P, Attree O, Cornelis GR, Attree I. 2005. Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J. Infect. Dis. 192:218–225 [DOI] [PubMed] [Google Scholar]
- 28. Hoiczyk E, Blobel G. 2001. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 98:4669–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis AJ, Mecsas J. 2007. Mutations in the Yersinia pseudotuberculosis type III secretion system needle protein, YscF, that specifically abrogate effector translocation into host cells. J. Bacteriol. 189:83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torruellas J, Jackson MW, Pennock JW, Plano GV. 2005. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol. Microbiol. 57:1719–1733 [DOI] [PubMed] [Google Scholar]
- 31. Straley SC, Bowmer WS. 1986. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pujol C, Bliska JB. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71:5892–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher ML, Castillo C, Mecsas J. 2007. Intranasal inoculation of mice with Yersinia pseudotuberculosis causes a lethal lung infection that is dependent on Yersinia outer proteins and PhoP. Infect. Immun. 75:429–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Logsdon LK, Mecsas J. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 71:4595–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donnenberg MS, Kaper JB. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amann E, Ochs B, Abel KJ. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 69:301–315 [DOI] [PubMed] [Google Scholar]
- 37. Davis AJ, Diaz DA, Mecsas J. 2010. A dominant-negative needle mutant blocks type III secretion of early but not late substrates in Yersinia. Mol. Microbiol. 76:236–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harmon DE, Davis AJ, Castillo C, Mecsas J. 2010. Identification and characterization of small-molecule inhibitors of Yop translocation in Yersinia pseudotuberculosis. Antimicrob. Agents Chemother. 54:3241–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Black DS, Bliska JB. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515–527 [DOI] [PubMed] [Google Scholar]
- 40. Veenendaal AK, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. 2007. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 63:1719–1730 [DOI] [PubMed] [Google Scholar]
- 41. Sarker MR, Neyt C, Stainier I, Cornelis GR. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Costa TR, Edqvist PJ, Broms JE, Ahlund MK, Forsberg A, Francis MS. 2010. YopD self-assembly and binding to LcrV facilitate type III secretion activity by Yersinia pseudotuberculosis. J. Biol. Chem. 285:25269–25284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson S, Roversi P, Espina M, Olive A, Deane JE, Birket S, Field T, Picking WD, Blocker AJ, Galyov EE, Picking WL, Lea SM. 2007. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J. Biol. Chem. 282:4035–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kenjale R, Wilson J, Zenk SF, Saurya S, Picking WL, Picking WD, Blocker A. 2005. The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J. Biol. Chem. 280:42929–42937 [DOI] [PubMed] [Google Scholar]
- 45. Chauvaux S, Dillies MA, Marceau M, Rosso ML, Rousseau S, Moszer I, Simonet M, Carniel E. 2011. In silico comparison of Yersinia pestis and Yersinia pseudotuberculosis transcriptomes reveals a higher expression level of crucial virulence determinants in the plague bacillus. Int. J. Med. Microbiol. 301:105–116 [DOI] [PubMed] [Google Scholar]
- 46. Hinchliffe SJ, Isherwood KE, Stabler RA, Prentice MB, Rakin A, Nichols RA, Oyston PC, Hinds J, Titball RW, Wren BW. 2003. Application of DNA microarrays to study the evolutionary genomics of Yersinia pestis and Yersinia pseudotuberculosis. Genome Res. 13:2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roehrich AD, Martinez-Argudo I, Johnson S, Blocker AJ, Veenendaal AK. 2010. The extreme C terminus of Shigella flexneri IpaB is required for regulation of type III secretion, needle tip composition, and binding. Infect. Immun. 78:1682–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Akopyan K, Edgren T, Wang-Edgren H, Rosqvist R, Fahlgren A, Wolf-Watz H, Fallman M. 2011. Translocation of surface-localized effectors in type III secretion. Proc. Natl. Acad. Sci. U. S. A. 108:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]