Abstract
The strict human pathogen Neisseria gonorrhoeae utilizes homologous recombination to antigenically vary the pilus, thus evading the host immune response. High-frequency gene conversion reactions between many silent pilin loci and the expressed pilin locus (pilE) allow for numerous pilus variants per strain to be produced from a single strain. For pilin antigenic variation (Av) to occur, a guanine quartet (G4) structure must form upstream of pilE. The RecQ helicase is one of several recombination or repair enzymes required for efficient levels of pilin Av, and RecQ family members have been shown to bind to and unwind G4 structures. Additionally, the vast majority of RecQ helicase family members encode one “helicase and RNase D C-terminal” (HRDC) domain, whereas the N. gonorrhoeae RecQ helicase gene encodes three HRDC domains, which are critical for pilin Av. Here, we confirm that deletion of RecQ HRDC domains 2 and 3 causes a decrease in the frequency of pilin Av comparable to that obtained with a functional knockout. We demonstrate that the N. gonorrhoeae RecQ helicase can bind and unwind the pilE G4 structure. Deletion of the RecQ HRDC domains 2 and 3 resulted in a decrease in G4 structure binding and unwinding. These data suggest that the decrease in pilin Av observed in the RecQ HRDC domain 2 and 3 deletion mutant is a result of the enzyme's inability to efficiently bind and unwind the pilE G4 structure.
INTRODUCTION
Neisseria gonorrhoeae is an obligate human pathogen and the causative agent of the sexually transmitted infection gonorrhea. Gonococci generally infect the urogenital tract, and the infection typically presents as urethritis in men and cervicitis in women, but many women can be asymptomatic carriers (1). The N. gonorrhoeae type IV pilus is essential for establishing infection (2). Pili assist in epithelial adherence and gonococcal cell aggregation and also mediate twitching motility (3–5). Protective immunity never develops, partially because the bacterium can evade host immune selection by antigenically varying surface antigens, including lipooligosaccharides, the opacity family of outer membrane proteins, and the type IV pilus (6–10).
N. gonorrhoeae possesses one pilin expression locus (pilE) and up to 19 silent pilin storage copies, which reside in up to 6 discrete loci (pilS) in the genome (11). Pilin antigenic variation (Av) occurs as a result of nonreciprocal DNA recombination between any pilS copy and pilE, leading to the expression of a new variant (7). N. gonorrhoeae pilin Av is a specialized high-frequency recombination system that occurs via a RecF-like pathway of homologous recombination whereby a functional knockout of the recQ gene, which encodes the RecQ DNA helicase, results in an Av deficiency (12–15). In addition, pilin Av requires the formation of a pilE guanine quartet/quadruplex (G4) structure.
The pilE G4-forming sequence was identified using a targeted genetic screen in a DNA region upstream of pilE, where insertions were found to block pilin Av but not alter pilin expression (16, 17). In this region, individual mutation of 11 GC base pairs completely blocked pilin Av, and mutation of a 12th GC base pair produced reduced but residual pilin Av frequency (14, 16). Mutation of an adjacent 13th GC base pair did not affect pilin Av, but mutation of this GC base pair in addition to the 12th resulted in a complete loss of function, suggesting that this 13th GC base pair could partially substitute for the mutated 12th GC base pair (14, 16). The organization of these GC base pairs conforms to a G4 motif (16). Biophysical studies showed that this G-rich sequence formed a G4 structure in which mutations that block pilin Av also inhibited structure formation (16). N. gonorrhoeae grown on N-methyl mesoporphyrin IX, a compound that specifically binds and stabilizes G4 structures but not double- or single-stranded DNA (ssDNA) (18), decreased the frequency of pilin Av (16). Moreover, point mutations in N. gonorrhoeae that block pilin Av and G4 structure formation prevented single-stranded nicks from being detected in the G4-forming sequence and complement strand (16).
Pilin Av also requires transcription of a small RNA (sRNA) molecule that initiates within the G4-forming sequence (19). Mutation of the promoter of this sRNA prevents detectable pilin Av from occurring, and replacement of the normal promoter with a phage-specific promoter allows restoration of pilin Av only when the phage polymerase is expressed (19). However, expression of this sRNA from an ectopic locus does not rescue a promoter mutation, showing that this sRNA must be expressed in its normal location to function (19). We postulate that transcription is required to open the DNA duplex, but further roles for this sRNA in the process of pilin Av remain to be determined.
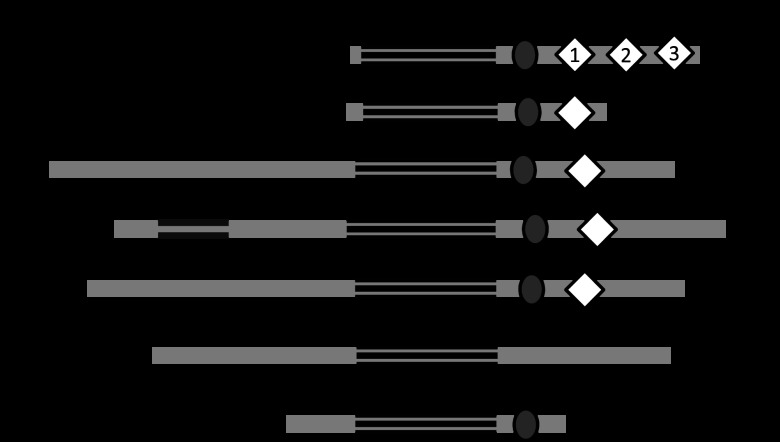
The pathogenic Neisseria RecQ helicase is unusual among RecQ family members, having three “helicase and RNase D C-terminal” (HRDC) domains (Fig. 1). Only one other organism, Deinococcus radiodurans, has been reported to possess this unusual RecQ triple-HRDC domain structure (20), while most RecQs contain one HRDC domain and a few RecQ orthologs have no HRDC repeats (Fig. 1). Previously, it was shown that deletion of the N. gonorrhoeae HRDC domains inhibited pilin Av (21). Since RecQ HRDC domains have been shown to provide additional DNA specificity and modulate the affinity of the enzyme for single-stranded, duplex, and Holliday junction DNA structures (20, 22–24) and pilin Av depends on a G4 structure, it was possible that the RecQ HRDC domains might play a role in binding and unwinding the pilE G4 structure required for pilin Av (16). Moreover, some RecQ helicase enzymes have been shown to unwind G4 structures (25–28). In addition, only RecQ helicase family members that have an HRDC domain, i.e., Saccharomyces cerevisiae Sgs1, human WRN and BLM, and Escherichia coli RecQ, have been shown to process G4 structures (25–28), whereas RecQ family members human RecQL1 and RecQL4, which do not have an HRDC domain, do not interact with G4 structures (29, 30) (Fig. 1). Since the N. gonorrhoeae RecQ helicase HRDC domains are required for efficient levels of pilin Av, we determined whether these domains facilitate the interaction of RecQ helicase with the pilE G4.
Fig 1.
RecQ helicase family members. Schematic representation of the relative location of conserved domains in selected RecQ family members including N. gonorrhoeae, E. coli, S. cerevisiae Sgs1, human WRN, BLM, RecQL4, and RecQL1. If applicable, the exonuclease, helicase, RecQ C-terminal (RQCt), and helicase and RNase D C-terminal (HRDC) domains are designated. HRDC domains 1, 2, and 3 are labeled for N. gonorrhoeae. The numbers of amino acid residues in the protein are indicated on the right.
MATERIALS AND METHODS
Bacterial growth conditions.
E. coli One Shot TOP10 competent cells (Invitrogen) were grown in Luria-Bertani (LB) broth or on solid medium containing 15 g/liter agar at 37°C and used to propagate plasmids. E. coli strains with plasmids containing kanamycin or erythromycin resistance were selected on media containing 50 or 100 μg/ml of the respective antibiotic. Gonococcal strains were grown on GC Medium Base (Difco) plus Kellogg supplements (GCB) [22.2 mM glucose, 0.68 mM glutamine, 0.45 mM cocarboxylase, 1.23 mM Fe(NO3)3; all from Sigma] at 37°C in 5% CO2; when applicable, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added for induction. Gonococcal transformants were selected on media containing 80 μg/ml kanamycin, 10 μg/ml erythromycin, or 2 μg/ml tetracycline.
Construction of strains.
Gonococcal strains VD300, PK133, PK134, PK131, and recQ::ermC (21) were transformed with chromosomal DNA from a strain containing the recA6 construct, where recA is IPTG inducible and linked to tetracycline resistance (recA6) (31). The VD300 recA6 strain was transformed with a plasmid containing a kanamycin cassette insertion within repeat sequence 1 (RS1) that has no effect on pilin Av (pilE::mTn#9) (16), which links a specific pilE sequence to kanamycin resistance. Then PK133, PK134, PK131, and recQ::ermC were transformed with chromosomal DNA from VD300 pilE::mTn#9 recA6 selecting for kanamycin resistance and the presence of specific RecQ mutations, and matching pilE genes for all strains were verified by sequence analysis.
Pilin Av assays.
Kinetic pilus-dependent colony phase variation assays were performed as previously described (13). The pilin Av sequencing assay (15) was performed by selection and disruption of P+ progenitors in GCB liquid medium (GCBL), which were spread on solid medium with IPTG for RecA induction. After 22 h, the pilE genes from 95 piliated colonies from each progenitor were sequenced. The parental strain had 2 progenitors, while the recQ::ermC mutant and the RecQ mutant with deletion of HRDC 2 and 3 (RecQ HRDC Δ2&3) had 3 progenitors.
Circular dichroism (CD) spectroscopy.
CD spectra were recorded at the Northwestern University Keck Biophysics Core Facility on a Jasco-815 CD spectrometer. Spectra were recorded at room temperature in a 1-mm-path-length quartz cuvette as an average of 3 scans over 200 to 400 nm, with a scan rate of 100 nm m−1, a bandwidth of 2.0 nm, and a response time of 2 s. The spectra were normalized by subtraction scan obtained with a buffer solution. Oligonucleotides were measured at a concentration of 2 μM under G4-forming conditions (100 mM KCl and 50 mM Tris [pH 7.5]) or ssDNA conditions (50 mM Tris, pH 7.5).
Fluorescence anisotropy.
Protein purification was performed as previously described (21). Concentrated protein stocks of RecQ or RecQ HRDC Δ2&3 mutant in storage buffer were serially diluted in DNA binding buffer (20 mM Tris [pH 8], 100 mM NaCl, 1 mM β-mercaptoethanol, 1 mM MgCl2, 0.1 g/liter bovine serum albumin [BSA], 4% glycerol) and incubated with 10 nM 3′ 6-carboxyfluorescein (FAM)-pilE G4 (ssDNA or structured) at room temperature for 30 min. Then, the fluorescence anisotropy for each sample was measured on a Molecular Devices SpectroMax M5 plate reader at 490-nm excitation and 535-nm emission wavelengths. Apparent Kd (dissociation constant), average, and standard error values were calculated using Origin software. Briefly, a sigmoidal fit was found using the equation y = A2 + (A1 − A2)/[1 + (x/x0)p], where A1 is the initial value, A2 the final value, x0 is the center, and p is the power.
pilE G4 unwinding.
The RecQ or RecQ HRDC Δ2&3 mutant proteins were preincubated with the 5′FAM-3′ Black Hole Quencher (BHQ)-labeled pilE G4 structure for 20 min at a 1:1 ratio in unwinding buffer (25 mM Tris [pH 7.5], 50 mM NaCl, 3 mM MgCl2, 0.1 mM dithiothreitol [DTT]). Then, fluorescence intensity was measured over time immediately after the addition of 1 mM ATP. Unwinding efficiency and standard error values were calculated using Origin software, and a sigmoidal fit was determined as described above.
RESULTS
Pilin antigenic variation and the N. gonorrhoeae RecQ helicase.
During pilin Av, nonpiliated (P−) colonies can arise from piliated (P+) progenitors from a variety of mechanisms such as the introduction of a stop codon or a pilE sequence that interferes with pilus assembly (32, 33). Previously, deletion of N. gonorrhoeae HRDC domains 2 and 3 or 1 to 3 was shown to decrease the percentage of P− colonies that arose from a P+ progenitor, indicating that these domains are required for pilin Av (21). In contrast, deletion of N. gonorrhoeae HRDC domain 3 had no effect on the frequency of pilin Av (21).
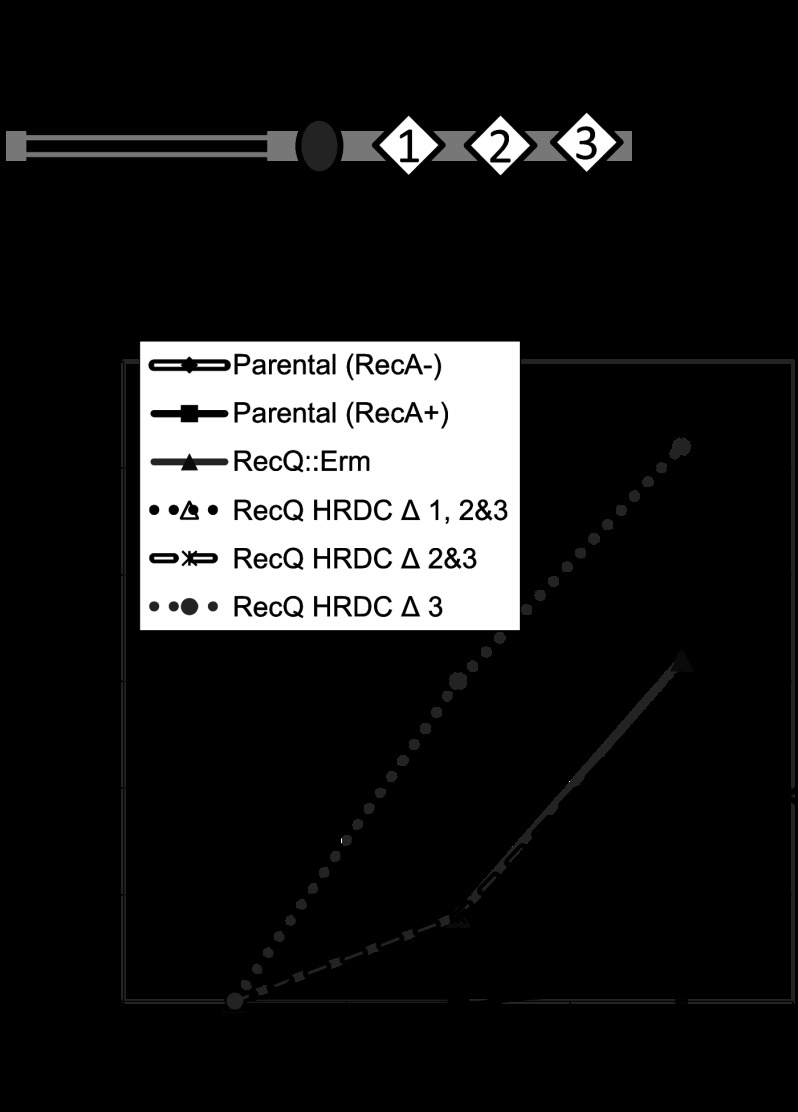
Since the starting pilE sequence can influence the frequency of pilin Av (34) and there is some variability in measuring P− colonies that arise from P+ progenitors, we constructed the previously reported RecQ HRDC mutant strains (Fig. 2A) but made sure that they all had the same pilE sequence, and these assays were performed in a recA6 genetic background, where recA is under the control of IPTG, so that the pilE sequence was stable until RecA induction (31, 35). None of the recQ mutant strains showed a difference in growth rate, which can influence the frequency of pilin Av (data not shown). The kinetic pilus-dependent colony phase variation assay was used as a surrogate measure for pilin Av in the recQ mutants (13) (Fig. 2B). In this assay, we measured P− sectors arising from individual P+ colonies over time after RecA induction. The RecQ HRDC Δ3 mutant had no pilin Av defect, whereas the RecQ HRDC Δ2&3 or RecQ HRDC Δ1, 2&3 mutants both showed an Av defect similar to that seen in the recQ null strain (Fig. 2B). These results are consistent with the previously reported phenotypes for these strains (21). Next, we directly determined the frequency of pilin Av by DNA sequencing (15). The pilE gene was sequenced from 95 colonies arising from two or three progenitor colonies grown on media containing IPTG. We analyzed two progenitors from the parental strain and three progenitors for the RecQ null and RecQ HRDC Δ2&3 mutant. The frequency of pilin Av for the parental strain was 11.6%, while the RecQ null and the RecQ HRDC Δ2&3 mutants showed decreased frequencies of 0.7% and 2.1%, respectively (Table 1). These results suggest that RecQ HRDC domain 2 or the maintenance of two HRDC domains is required for pilin Av.
Fig 2.
N. gonorrhoeae RecQ HRDC domains and pilin antigenic variation. (A) Schematic of the N. gonorrhoeae RecQ helicase, in which stop codon insertions are indicated by arrows. Residues are numbered, with the corresponding nucleotide positions in parentheses. (B) Kinetic pilus-dependent colony phase variation assay of the RecQ deletion and HRDC mutants. This standard assay measures the average number of visible pilus-dependent colony morphology changes occurring over time and is a surrogate measure for antigenic variation (Av) (13). RecQ HRDC Δ3 has no pilin Av defect, whereas RecQ HRDC Δ1, 2&3 and HRDC Δ 2&3 show a significant decrease in pilin Av, similar to that of the RecQ null strain. *, P < 0.05 as determined by two-tailed Student's t test. Error bars represent the standard errors of the means for 10 colonies; shown is a representative of 5 replicates.
Table 1.
Frequency of recombination for the parental, RecQ null mutant, and RecQ HRDC Δ2&3 mutant strains
| Straina | Progenitorb | No. of recombination eventsc/no. of sequenced colonies | Total no. of recombinants/total no. sequenced | Frequency of recombination (%) |
|---|---|---|---|---|
| Parental | A | 11/95 | 22/190 | 11.60 |
| B | 11/95 | |||
| RecQ null mutant | A | 1/95 | 2/285 | 0.70 |
| B | 0/95 | |||
| C | 1/95 | |||
| RecQ HRDC Δ2&3 mutant | A | 0/95 | 6/285 | 2.10 |
| B | 5/95 | |||
| C | 1/95 |
All strains are derivatives of N. gonorrhoeae isolate VD300 carrying the IPTG-regulatable recA allele.
Single-colony progenitors were grown with IPTG in the medium for 22 h (about 20 generations), the progeny were propagated without IPTG, and the pilE genes from the indicated number of progeny colonies were amplified and sequenced.
Number of independent recombination events in the sequenced pilE genes. Multiple events in a single pilE are recorded as separate events.
N. gonorrhoeae RecQ binding to the pilE G4 structure and sequence.
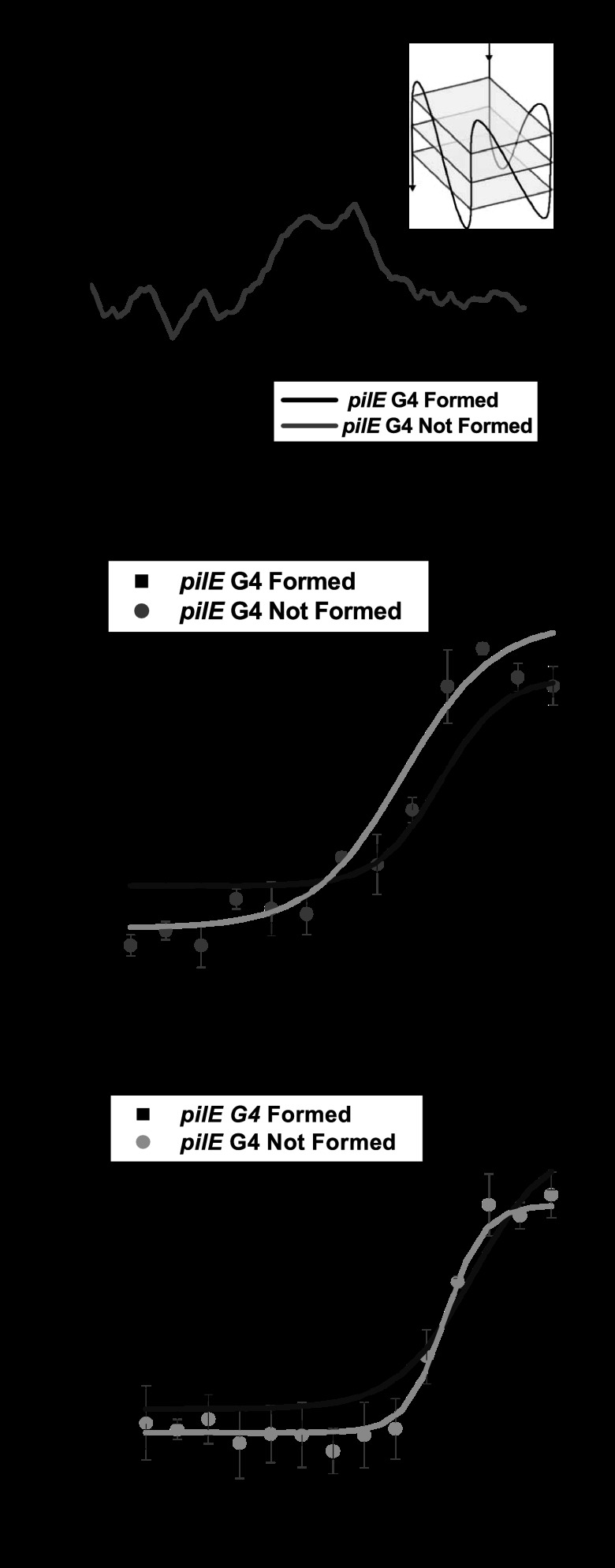
G4 structures have been implicated in many biological processes (36–38), and some RecQ helicase family members can process these structures (25–28). Since a RecQ deletion mutant causes an Av deficiency, we tested whether the N. gonorrhoeae RecQ helicase could bind the pilE G4 sequence or structure by fluorescence anisotropy. First, we determined that the pilE G4 3′FAM-labeled oligonucleotide formed the same parallel G4 structure as the unlabeled oligonucleotide (forming conditions) (Fig. 3A). The CD spectra confirm that the pilE G4 3′FAM-labeled oligonucleotide forms a parallel G4 structure upon addition of potassium (Fig. 3A), which is the same structure that forms when the oligonucleotide is unlabeled (16). Next, we compared the binding of gonococcal RecQ to the unfolded or folded oligonucleotide (pilE G4 structure) by measuring changes in fluorescence anisotropy (Fig. 3B). The apparent Kd of RecQ for the unfolded oligonucleotide was 25.7 ± 5.9 nM, whereas the apparent Kd of RecQ for the formed structure was 55.1 ± 10.5 nM. The apparent Kd of N. gonorrhoeae RecQ helicase for the pilE G4 structure is comparable to its affinity for duplex DNA, which has an apparent Kd of 58.2 ± 3.9 nM (21). These results confirm that the purified N. gonorrhoeae RecQ helicase can bind the G4 structure with relevant affinity to other known substrates.
Fig 3.
The RecQ HRDCΔ 2&3 mutant is defective for binding the pilE G4 structure and sequence. (A) CD spectroscopy of a 3′FAM-labeled pilE G4 oligonucleotide under conditions that allow or do not allow formation of the structure. The sequence of the oligonucleotide and a representation of the G4 structure are shown. (B) RecQ binding measured by fluorescence anisotropy of the pilE G4 3′FAM-labeled oligonucleotide. The fluorescence anisotropy is plotted against increasing protein concentration per 1 nM DNA; symbols represent RecQ binding to either a formed (black) or unformed (gray) pilE G4 3′FAM-labeled oligonucleotide. Error bars represent the standard errors of the means for 3 independent experiments with a total of 6 replicates and for 6 independent experiments with a total of 12 replicates for the formed and unformed oligonucleotides, respectively. (C) RecQ HRDC Δ2&3 binding measured by fluorescence anisotropy of the pilE G4 3′FAM-labeled oligonucleotide. The fluorescence anisotropy of RecQ HRDC Δ2&3 is plotted as described above. Error bars represent the standard errors of the means for 3 independent experiments with a total of 6 replicates for the formed and unformed oligonucleotides.
We then determined that the apparent Kd of the RecQ HRDC Δ2&3 mutant for the single-stranded pilE G4 sequence was 46.7 ± 2.5 nM whereas the apparent Kd for the pilE G4 structure was 85.6 ± 33.4 nM (Fig. 3C). These results demonstrate that the RecQ HRDC Δ2&3 mutant is approximately 2 times less efficient at binding the pilE G4 sequence and structure. Therefore, the N. gonorrhoeae RecQ HRDC domains provide some specificity or modulate the affinity of the enzyme for the pilE G4 sequence and structure but do not differentially alter DNA recognition.
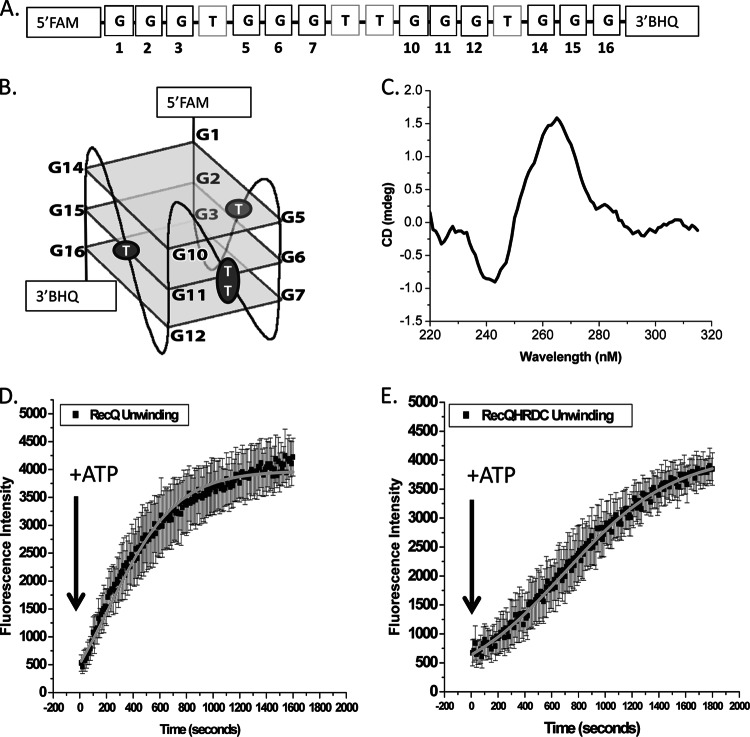
The Neisseria gonorrhoeae RecQ HRDC domains are required for efficient unwinding of the pilE guanine G4 structure.
Since RecQ family members have been shown to unwind G4 structures (25–28), we tested whether the gonococcal RecQ and HRDC Δ2&3 mutant could also unwind the pilE G4 structure. To determine helicase unwinding efficiency, we used a pilE G4 oligonucleotide with a 5′FAM and a 3′BHQ (Fig. 4A). Upon G4 structure formation, the FAM and BHQ molecules are in closer proximity, which enables quenching of the fluorophore (Fig. 4B). As the structure is processed or unwound, the increase in fluorescence intensity can be measured over time to calculate the apparent percentage of processed or unwound DNA. The ability of the pilE G4 5′FAM-3′BHQ-labeled oligonucleotide to form a parallel G4 structure was confirmed by CD spectroscopy (Fig. 4C) (16). Incubation of the pilE G4 5′FAM-3′BHQ-labeled structure with RecQ at a 1:1 ratio without ATP allowed binding, and the change in fluorescence intensity over time after the addition of ATP was recorded as unwinding. It took RecQ 211.4 ± 12.9 s to unwind 50% of the pilE G4 5′FAM-3′BHQ-labeled structure (Fig. 4D). Previously, the RecQ HRDC Δ2&3 mutant was reported to have the same unwinding efficiency on duplex DNA as the wild-type enzyme and did not have an altered ATP hydrolysis rate (21). However, the RecQ HRDC Δ2&3 mutant had a defect in unwinding G4 DNA, taking 692.5 ± 45.7 s to unwind 50% of the pilE G4 5′FAM-3′BHQ-labeled structure (Fig. 4E). Therefore, the RecQ HRDC Δ2&3 mutant enzyme is 3.5 times less efficient at unwinding the pilE G4 than the wild-type enzyme. These data suggest that the phenotype of the RecQ Δ2&3 mutation on pilin Av may be due, at least in part, to its inability to efficiently process the pilE G4 structure in the chromosome.
Fig 4.
The RecQ HRDC Δ2&3 mutant is defective for unwinding the pilE G4 structure. (A) 5′FAM-3′Black Hole Quencher (BHQ)-labeled pilE G4 oligonucleotide. (B) pilE G4 structured oligonucleotide. The 5′FAM and 3′BHQ labels are indicated, where guanines are numbered and correspond to those shown in panel A. (C) CD spectrum of the 5′FAM-3′BHQ-labeled pilE G4 oligonucleotide under conditions that allow G4 formation. (D) RecQ unwinding measured by the change in fluorescence of the 5′FAM-3′BHQ-labeled pilE G4 oligonucleotide. Shown is the fluorescence intensity over time of a 1:1 ratio of RecQ to DNA immediately after the addition of ATP. Symbols represent the interaction of RecQ with the 5′FAM-3′BHQ-labeled pilE G4 oligonucleotide. Error bars represent the standard errors of the means for 3 independent experiments. (E) RecQ HRDC Δ2&3 unwinding measured by change in fluorescence of the 5′FAM-3′BHQ-labeled pilE G4 oligonucleotide. Shown is the fluorescence intensity over time of a 1:1 ratio of RecQ HRDC Δ2&3 to DNA immediately after the addition of ATP. Error bars represent the standard errors of the means for 3 independent experiments.
DISCUSSION
The Neisseria RecQ helicase is unusual among RecQ family members, having three HRDC domains. Only the RecQ from one other organism, D. radiodurans, has a similar triple-HRDC arrangement. There are some parallels that can be drawn between pathogenic Neisseria and D. radiodurans. The pathogenic Neisseria strains exist only in humans, and therefore the Neisseria repair capabilities are predicted to be specialized for damage that might occur within the host. For example, the pathogenic Neisseria strains possess a diverse range of mechanisms for coping with the reactive oxygen species (ROS) that are encountered during aerobic respiration and interactions with phagocytic cells (39, 40), in addition to evasion of the human immune response by high-frequency recombination at pilE (15). D. radiodurans is an extremophile that can survive massive levels of DNA-damaging radiation. Both the pathogenic Neisseria strains and D. radiodurans have a high demand for DNA repair and recombination for survival, and therefore the maintenance of three RecQ HRDC domains suggests that they may utilize similar RecQ-dependent strategies of DNA recombinatorial repair. In addition, these organisms have G4 structures in their genomes (16, 41) suggesting that RecQ may process these structures.
In this work, we confirm that deleting HRDC domains 2 and 3 of N. gonorrhoeae RecQ helicase causes a decrease in the frequency of pilin Av that is comparable to that of functional knockout (Table 1). Only RecQ helicase family members that have an HRDC domain can unwind G4 structures (25–28), whereas RecQL1 and RecQL4, which do not have an HRDC domain, do not interact with G4 structures (29, 30). We determined that the N. gonorrhoeae RecQ helicase HRDC Δ2&3 mutant is deficient for binding and unwinding the pilE G4 structure required for pilin Av (16). Genetic evidence supports a model whereby the initiation of pilin Av occurs as a result of the formation of the pilE G4 structure (14, 16), which occurs upstream of RecQ helicase (42), and therefore RecQ may be required to unwind the structure, which is facilitated by its HRDC domains.
The RecQ HRDC Δ2&3 mutant's loss of binding to the pilE G4 may be a general loss of binding to all G4 structures. We tested RecQ binding to another G4 sequence found in the gonococcal chromosome that forms a parallel G4 structure in vitro and determined that the RecQ HRDC Δ2&3 mutant was 2 times less efficient at binding this structure (data not shown). These results are similar to those observed for the pilE G4 structure, which suggests that the RecQ HRDC domains provide some general specificity to G4 structures. Even though deletion of RecQ HRDC 2&3 decreased binding efficiency and unwinding, we did not observe any gonococcal growth defects, suggesting that deletion of the RecQ HRDC domains does not cause pleotropic effects in the cell (21), and the first RecQ HRDC domain may be sufficient to process other RecQ targets or other helicases may compensate for partial RecQ function.
Since the pilE G4 structure is located on the lagging stand during DNA replication (14, 16), we hypothesize that the structure stalls replication, causing a nick on the leading strand, which initiates pilin Av. The pilE G4 structure then remains on the lagging strand and must be unwound by RecQ helicase lest the structure halt replication, which would be detrimental to the cell. Other helicase families, such as Pif1, FANCJ (nematode DOG-1), and DNA2, have also been shown to unwind G4 structures (43–45). N. gonorrhoeae does not encode a Pif1 helicase. However, the pathogen does encode a DinG helicase that shares a domain with DOG-1. Previously, we created a functional deletion of the N. gonorrhoeae DinG helicase, but the mutant did not have a pilin Av defect (data not shown). Although we cannot rule out a role for DinG unwinding G4 structures in the gonococcal cell, the helicase does not function during pilin Av and must not act on the pilE G4. N. gonorrhoeae also encodes UvrD and Rep helicases, which share a domain with DNA2 helicase. UvrD does not function during pilin Av (46); however, Rep does have a role (47). Further investigation will determine whether Rep helicase unwinds the pilE G4 structure. Since pilin Av is not completely blocked upon RecQ deletion, it is possible that there are redundant helicases such as Rep or an as-yet-unidentified helicase that may act at the pilE structure.
ACKNOWLEDGMENTS
We thank all the Seifert lab members for suggestions into this work. We thank Mike Killoran for initiation of the collaboration, Kari Barlan for creating the DinG deletion mutant, and Vitaly Kuryavyi for modeling the pilE G4 structure.
This work was supported by NIH grants R37AI033493, R01AI055977, and R01AI044239 to H.S.S. and R01GM068061 to J.L.K. L.A.C. was partially supported by NIH training grant T32GM08061 and NIH Diversity Supplement for R37AI033493, and E.R. was partially supported by NIH NSRA F32 AI094945.
Footnotes
Published ahead of print 8 March 2013
REFERENCES
- 1. Edwards JL, Apicella MA. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965–981, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen MS, Cannon JG. 1999. Human experimentation with Neisseria gonorrhoeae: progress and goals. J. Infect. Dis. 179(Suppl 2):S375–S379 [DOI] [PubMed] [Google Scholar]
- 3. Park HS, Wolfgang M, van Putten JP, Dorward D, Hayes SF, Koomey M. 2001. Structural alterations in a type IV pilus subunit protein result in concurrent defects in multicellular behaviour and adherence to host tissue. Mol. Microbiol. 42:293–307 [DOI] [PubMed] [Google Scholar]
- 4. Rudel T, van Putten JP, Gibbs CP, Haas R, Meyer TF. 1992. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol. Microbiol. 6:3439–3450 [DOI] [PubMed] [Google Scholar]
- 5. Wolfgang M, Lauer P, Park HS, Brossay L, Hebert J, Koomey M. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321–330 [DOI] [PubMed] [Google Scholar]
- 6. Danaher RJ, Levin JC, Arking D, Burch CL, Sandlin R, Stein DC. 1995. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J. Bacteriol. 177:7275–7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagblom P, Segal E, Billyard E, So M. 1985. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature 315:156–158 [DOI] [PubMed] [Google Scholar]
- 8. Jennings MP, Hood DW, Peak IR, Virji M, Moxon ER. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18:729–740 [DOI] [PubMed] [Google Scholar]
- 9. Stern A, Brown M, Nickel P, Meyer TF. 1986. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47:61–71 [DOI] [PubMed] [Google Scholar]
- 10. Boslego JW, Tramont EC, Chung RC, McChesney DG, Ciak J, Sadoff JC, Piziak MV, Brown JD, Brinton CC, Jr, Wood SW, Bryan JR. 1991. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9:154–162 [DOI] [PubMed] [Google Scholar]
- 11. Hamrick TS, Dempsey JA, Cohen MS, Cannon JG. 2001. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology 147:839–849 [DOI] [PubMed] [Google Scholar]
- 12. Mehr IJ, Seifert HS. 1998. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair. Mol. Microbiol. 30:697–710 [DOI] [PubMed] [Google Scholar]
- 13. Sechman EV, Rohrer MS, Seifert HS. 2005. A genetic screen identifies genes and sites involved in pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 57:468–483 [DOI] [PubMed] [Google Scholar]
- 14. Cahoon LA, Seifert HS. 2011. Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria. Mol. Microbiol. 81:1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Criss AK, Kline KA, Seifert HS. 2005. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Mol. Microbiol. 58:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325:764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kline KA, Criss AK, Wallace A, Seifert HS. 2007. Transposon mutagenesis identifies sites upstream of the Neisseria gonorrhoeae pilE gene that modulate pilin antigenic variation. J. Bacteriol. 189:3462–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ren J, Chaires JB. 1999. Sequence and structural selectivity of nucleic acid binding ligands. Biochemistry 38:16067–16075 [DOI] [PubMed] [Google Scholar]
- 19. Cahoon LA, Seifert HS. 2013. Transcription of a cis-acting, noncoding, small RNA is required for pilin antigenic variation in Neisseria gonorrhoeae. PLoS Pathog. 9:e1003074 doi:10.1371/journal.ppat.1003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Killoran MP, Keck JL. 2006. Three HRDC domains differentially modulate Deinococcus radiodurans RecQ DNA helicase biochemical activity. J. Biol. Chem. 281:12849–12857 [DOI] [PubMed] [Google Scholar]
- 21. Killoran MP, Kohler PL, Dillard JP, Keck JL. 2009. RecQ DNA helicase HRDC domains are critical determinants in Neisseria gonorrhoeae pilin antigenic variation and DNA repair. Mol. Microbiol. 71:158–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernstein DA, Keck JL. 2003. Domain mapping of Escherichia coli RecQ defines the roles of conserved N- and C-terminal regions in the RecQ family. Nucleic Acids Res. 31:2778–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernstein DA, Keck JL. 2005. Conferring substrate specificity to DNA helicases: role of the RecQ HRDC domain. Structure 13:1173–1182 [DOI] [PubMed] [Google Scholar]
- 24. Bennett RJ, Keck JL. 2004. Structure and function of RecQ DNA helicases. Crit. Rev. Biochem. Mol. Biol. 39:79–97 [DOI] [PubMed] [Google Scholar]
- 25. Sun H, Karow JK, Hickson ID, Maizels N. 1998. The Bloom's syndrome helicase unwinds G4 DNA. J. Biol. Chem. 273:27587–27592 [DOI] [PubMed] [Google Scholar]
- 26. Sun H, Bennett RJ, Maizels N. 1999. The Saccharomyces cerevisiae Sgs1 helicase efficiently unwinds G-G paired DNAs. Nucleic Acids Res. 27:1978–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fry M, Loeb LA. 1999. Human Werner syndrome DNA helicase unwinds tetrahelical structures of the fragile X syndrome repeat sequence d(CGG)n. J. Biol. Chem. 274:12797–12802 [DOI] [PubMed] [Google Scholar]
- 28. Wu X, Maizels N. 2001. Substrate-specific inhibition of RecQ helicase. Nucleic Acids Res. 29:1765–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Popuri V, Bachrati CZ, Muzzolini L, Mosedale G, Costantini S, Giacomini E, Hickson ID, Vindigni A. 2008. The human RecQ helicases, BLM and RECQ1, display distinct DNA substrate specificities. J. Biol. Chem. 283:17766–17776 [DOI] [PubMed] [Google Scholar]
- 30. Singh DK, Popuri V, Kulikowicz T, Shevelev I, Ghosh AK, Ramamoorthy M, Rossi ML, Janscak P, Croteau DL, Bohr VA. 2012. The human RecQ helicases BLM and RECQL4 cooperate to preserve genome stability. Nucleic Acids Res. 40:6632–6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seifert HS. 1997. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene 188:215–220 [DOI] [PubMed] [Google Scholar]
- 32. Gibbs CP, Reimann BY, Schultz E, Kaufmann A, Haas R, Meyer TF. 1989. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature 338:651–652 [DOI] [PubMed] [Google Scholar]
- 33. Haas R, Meyer TF. 1986. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell 44:107–115 [DOI] [PubMed] [Google Scholar]
- 34. Rohrer MS, Lazio MP, Seifert HS. 2005. A real-time semi-quantitative RT-PCR assay demonstrates that the pilE sequence dictates the frequency and characteristics of pilin antigenic variation in Neisseria gonorrhoeae. Nucleic Acids Res. 33:3363–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koomey M, Gotschlich EC, Robbins K, Bergstrom S, Swanson J. 1987. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics 117:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fry M. 2007. Tetraplex DNA and its interacting proteins. Front. Biosci. 12:4336–4351 [DOI] [PubMed] [Google Scholar]
- 37. Paeschke K, Juranek S, Simonsson T, Hempel A, Rhodes D, Lipps HJ. 2008. Telomerase recruitment by the telomere end binding protein-beta facilitates G-quadruplex DNA unfolding in ciliates. Nat. Struct. Mol. Biol. 15:598–604 [DOI] [PubMed] [Google Scholar]
- 38. Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. 2002. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl. Acad. Sci. U. S. A. 99:11593–11598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190:136–147 [DOI] [PubMed] [Google Scholar]
- 40. Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 70:344–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beaume N, Pathak R, Yadav VK, Kota S, Misra HS, Gautam HK, Chowdhury S. 2013. Genome-wide study predicts promoter-G4 DNA motifs regulate selective functions in bacteria: radioresistance of D. radiodurans involves G4 DNA-mediated regulation. Nucleic Acids Res. 41:76–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sechman EV, Kline KA, Seifert HS. 2006. Loss of both Holliday junction processing pathways is synthetically lethal in the presence of gonococcal pilin antigenic variation. Mol. Microbiol. 61:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu Y, Shin-ya K, Brosh RM., Jr 2008. FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol. Cell. Biol. 28:4116–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paeschke K, Capra JA, Zakian VA. 2011. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell 145:678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Masuda-Sasa T, Polaczek P, Peng XP, Chen L, Campbell JL. 2008. Processing of G4 DNA by Dna2 helicase/nuclease and replication protein A (RPA) provides insights into the mechanism of Dna2/RPA substrate recognition. J. Biol. Chem. 283:24359–24373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. LeCuyer BE, Criss AK, Seifert HS. 2010. Genetic characterization of the nucleotide excision repair system of Neisseria gonorrhoeae. J. Bacteriol. 192:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kline KA, Seifert HS. 2005. Role of the Rep helicase gene in homologous recombination in Neisseria gonorrhoeae. J. Bacteriol. 187:2903–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]