Abstract
Many bacteria can accumulate glycine betaine for osmoprotection and catabolize it as a growth substrate, but how they regulate these opposing roles is poorly understood. In Pseudomonas syringae B728a, expression of the betaine catabolism genes was reduced by an osmotic upshift to an intermediate stress level, consistent with betaine accumulation, but was increased by an upshift to a high stress level, as confirmed by an accompanying increase in degradation of radiolabeled betaine. Deletion of the gbcAB betaine catabolism genes reduced osmotolerance at a high osmolarity, and this reduction was due to the relief of betaine-mediated suppression of compatible solute synthesis. This conclusion was supported by the findings that, at high osmolarity, the ΔgbcAB mutant accumulated high betaine levels and low endogenous solutes and exhibited reduced expression of the solute synthesis genes. Moreover, the ΔgbcAB mutant and a mutant deficient in the synthesis of the compatible solutes NAGGN and trehalose exhibited similar reductions in osmotolerance and also in fitness on bean leaves. Activation of betaine catabolism at high osmotic stress resulted, in part, from induction of gbdR, which encodes the transcriptional activator GbdR. Betaine catabolism was subject to partial repression by succinate under hyperosmotic stress conditions, in contrast to strong repression in the absence of stress, suggesting that betaine functions both in nutrition and as an intracellular signal modulating solute synthesis under hyperosmotic stress conditions. Collectively, these results begin to provide a detailed mechanistic understanding of how P. syringae transitions from reliance on exogenously derived betaine to the use of endogenous solutes during adaptation to hyperosmotic conditions.
INTRODUCTION
Pseudomonas syringae pv. syringae is a foliar pathogen of common bean (Phaseolus vulgaris L.). It has been used as a model organism for understanding the cellular and molecular biology, ecology, and epidemiology of pathogen interactions with plants (1, 2). Due to the highly exposed nature of aerial plant leaves, P. syringae must confront rapid fluctuations in water availability during leaf colonization (3–5). One P. syringae strategy to tolerate limited water availability on leaves is to form aggregates; such aggregation has been associated with survival superior to that seen with solitary cells under dry conditions (5). Like other bacteria, P. syringae also accumulates soluble organic compounds, designated compatible solutes or osmolytes, to alleviate the deleterious effects of water limitation. P. syringae can synthesize several endogenous osmolytes, namely, the disaccharide trehalose, the dipeptide N-acetylglutaminylglutamine amide (NAGGN), and l-glutamate, in response to water limitation imposed by osmotic stress (6–8).
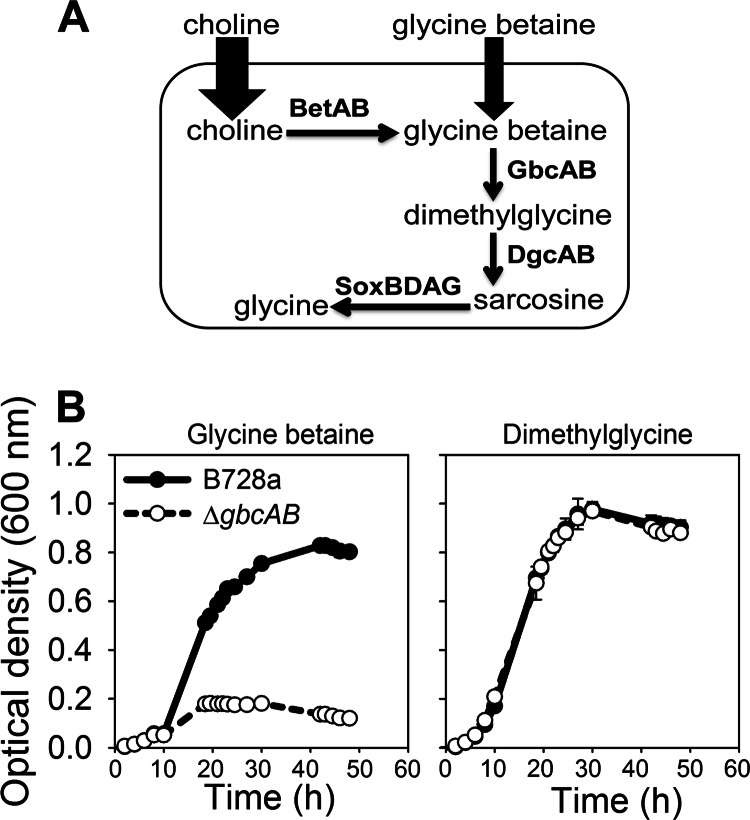
P. syringae can also benefit from the uptake of exogenous compounds for osmoprotection, with this uptake being energetically favored over de novo solute synthesis. The quaternary ammonium compound glycine betaine (here referred to as betaine) functions as a particularly effective protectant against osmotic stress in many organisms (9, 10). Although betaine is not synthesized de novo in P. syringae (7), it can accumulate following uptake of betaine or a betaine precursor (Fig. 1A). Betaine accumulation under conditions of high osmotic stress has been associated with a low accumulation of the endogenous osmolytes that are synthesized de novo (11–13). P. syringae has multiple transporters for the uptake of betaine and its precursors choline and phosphocholine (10, 14, 15), with a particularly high capacity for choline uptake (14). A primary source of these compounds is likely to be the major plant membrane lipid phosphatidylcholine, which may be degraded to choline and phosphocholine by plant and bacterial phospholipases (16, 17).
Fig 1.
GbcAB is involved in the betaine catabolism pathway in P. syringae B728a. (A) Choline and betaine degradation pathway and the enzymes that are involved; B728a has a higher uptake capacity for choline than for betaine. (B) Growth of the ΔgbcAB mutant and B728a in MinA medium with 20 mM betaine or dimethylglycine based on optical density of cultures in test tubes. Values are means ± standard errors of the means (SEM) (n = 3).
Whereas many microbes use betaine solely as a compatible solute, the pseudomonads P. syringae and P. aeruginosa can additionally use it for nutrition and energy under water-replete conditions (10, 18). P. aeruginosa, an animal pathogen, catabolizes it to glycine through the sequential action of enzymes encoded by gbcAB, dgcAB, and soxBDAG. The transcription of these genes requires the AraC family transcription activator GbdR with betaine or its downstream catabolite dimethylglycine as a coinducer (18, 19). Betaine catabolism appeared to be inhibited by hyperosmolarity in P. aeruginosa (12, 20), as well as in the plant symbiont Sinorhizobium meliloti (21), consistent with its accumulation as a compatible solute. This inhibition can be quite rapid, as illustrated by the suppression of betaine catabolism within only 10 min following an osmotic upshift of S. meliloti (21). Moreover, strong P. aeruginosa growth under conditions of high osmolarity required a large intracellular betaine pool, as indicated by the reduced growth rate when the pool was depleted by overexpressing gbcAB (20).
P. aeruginosa and S. meliloti have both been reported to catabolize betaine under hyperosmotic conditions, although the ramifications of this catabolism for betaine accumulation and osmotolerance were not explored. P. aeruginosa catabolism of betaine under hyperosmotic stress conditions is suppressed by succinate (11), suggesting that betaine catabolism is subject to catabolite repression and thus that betaine could be used as a nutrient source under these conditions. S. meliloti catabolism of betaine may be coordinated with endogenous osmolyte synthesis based on the preferential accumulation of betaine over endogenous osmolytes during early exponential growth but the transition to a predominance of endogenous osmolytes during late exponential growth and stationary phase (13).
In this work, we characterized the conditions under which P. syringae strain B728a catabolizes betaine in the presence of osmotic stress, the contribution of this catabolism to osmoadaptation, and the molecular mechanisms by which betaine catabolism influences B728a osmotolerance. Collectively, our data support a model in which maximal osmotolerance of P. syringae at high osmotic stress requires the upregulation of betaine catabolism to reduce the suppressive effect of betaine on endogenous compatible solute synthesis. Moreover, our data identify some of the molecular mechanisms underlying this upregulation, including the finding that suppression of compatible solute synthesis, activation of betaine catabolism, and production of a transcriptional activator of betaine catabolism are all regulated at the transcriptional level in response to hyperosmotic stress. Lastly, we show that betaine catabolism in B728a is subject to succinate-mediated repression at low osmotic stress, consistent with the use of betaine for nutrition, but is only partially repressed at high osmotic stress, consistent with a function for betaine as both an energy source and an intracellular signal modulating the accumulation of compatible solutes under hyperosmotic stress conditions.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. The P. syringae strains were grown on solid King's B medium (22) before inoculation into liquid MinA medium (23) which contained 10 mM pyruvate (MinA-pyruvate) or succinate (MinA-succinate) as a carbon source unless otherwise indicated. NaCl amendments were added to reach the stated final concentrations, and, unless otherwise indicated, betaine amendments were added to reach a final concentration of 1 mM. Cells were grown at 25°C with shaking. Antibiotics were added to the growth media as needed at the following concentrations (μg ml−1): kanamycin (Km), 50; rifampin (Rif), 100; tetracycline (Tet), 20; spectinomycin (Spc), 60; and cycloheximide (Cm), 100. Cell growth was monitored either in test tubes based on the optical density at 600 nm (OD600) or in microtiter plates based on measurements at both 630 nm and 450 nm to compensate for the optical interference of water condensation within the wells, with the OD630/OD450 ratios subsequently converted to OD600 values using a standard curve.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description or relevant genotypea | Reference or source |
|---|---|---|
| P. syringae strain | ||
| B728a | Wild type; Rifr | 36 |
| B728a ΔgbcAB | B728a ΔgbcAB; Rifr | This work |
| B728a ΔgbdR | B728a ΔgbdR; Rifr | This work |
| B728a Δggn | B728a ΔggnAB; Rifr | C. Chen and G. A. Beattie, unpublished data |
| B728a Δggn Δtre | B728a ΔggnAB ΔPsyr_2489-Psyr_2491 ΔPsyr_2992-Psyr_3001; Rifr | This work |
| Plasmid | ||
| pTOK2T | pTOK2 with restored lacZ activity; Tetr | 15 |
| pKD13 | Template for kan cassette flanked by FLP recombination target sites; Apr Kmr | 24 |
| pFlp2Ω | Encodes Flp recombinase, suicide vector in P. syringae derived from pFlp2 (37); Apr Spcr | C. Chen and G. A. Beattie, unpublished data |
| pRK2013 | RP4 transfer functions for mobilization; Kmr | 38 |
Ap, ampicillin.
Construction of mutant strains.
Unmarked deletion mutants of B728a were generated as described previously (15). Briefly, two 1-kb fragments flanking the target locus were PCR amplified from B728a genomic DNA and a kanamycin (kan) cassette containing flanking FLP recombination target (FRT) sites was PCR amplified from pKD13 (24) using the primer pairs listed in Table S1 in the supplemental material. The three fragments were ligated together by splice-overlap-extension PCR using the original F1 and R2 primers to produce a single 3.5-kb fusion product consisting of the kan cassette and the flanking sequences. This product was cloned into SmaI-digested pTOK2T, which was then introduced into B728a cells by a triparental mating with pRK2013. The deletion mutants were identified as Rifr Kmr Tets and confirmed by PCR. The kan cassette was excised by introducing pFlp2Ω, which was later cured using sucrose (20%) counterselection, and excision was confirmed by PCR and DNA sequencing. To construct the Δggn Δtre mutant, the gene loci Psyr_2489 to Psyr_2491 and Psyr_2992 to Psyr_3001 were sequentially deleted in the Δggn mutant. The NAGGN deficiency of the Δggn mutant was confirmed by 13C nuclear magnetic resonance (13C-NMR) spectroscopy (see Fig. S1A in the supplemental material). The trehalose deficiency of the Δggn Δtre mutant was confirmed based on the lack of detectable trehalose using a trehalose assay kit (Megazyme, Ireland) (see Fig. S1B in the supplemental material).
Quantitative real-time PCR.
Cells were grown in MinA medium containing pyruvate to an OD600 of 0.5, amended with betaine to 1 mM and NaCl to the indicated concentrations, and incubated with shaking in test tubes for the time indicated. Cells were diluted with RNAprotect Bacteria Reagent (Qiagen, Inc., Valencia, CA) before harvest, and RNA was purified using an RNeasy kit and on-column DNase I digestion to remove DNA (Qiagen, Inc., Valencia, CA). One-step reverse transcription (RT) conversion of RNA to cDNA and amplification was performed using a qScript 1-Step SYBR green quantitative RT-PCR (qRT-PCR) kit (Quanta BioSciences, Inc., Gaithersburg, MD). The PCR program was 50°C for 30 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 30 s. The housekeeping gene hemD (Psyr_0061) was used as an internal control to normalize induction values for all genes. The fold change for each open reading frame (ORF) was calculated using 2(−ΔΔCT), where ΔΔCT = (CT [target] − CT [hemD]) for the treatment − (CT [target] − CT [hemD]) for the control, where CT is threshold cycle. The primers used are shown in Table S2 in the supplemental material. The specificity of the PCR amplification was examined based on the melting curve.
Determination of intracellular compatible solutes by 13C-NMR spectroscopy.
Cells were grown to the log phase in MinA medium containing pyruvate, and the reaction mixture was then amended with 0.6 M NaCl and 1 mM betaine and incubated for 5 and 30 h, and similar numbers of cells, as estimated based on optical density, were harvested from all cultures. The cells were washed twice in MinA medium containing 0.6 M NaCl and pyruvate and extracted in 80% ethanol. After centrifugation, the supernatant was evaporated to dryness in a Speed-Vac concentrator and dissolved in 0.8 ml D2O. 13C-NMR spectra were recorded with a Bruker Avance 700 MHz NMR spectrometer. DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) was added to each sample to adjust the chemical shift (indicated by peaks at 0 ppm).
Radiotracer assays.
[methyl-14C]glycine betaine was prepared enzymatically from [methyl-14C] choline as previously described (10). Cells were grown in MinA medium with pyruvate or succinate to the log phase, amended with 0.6 M NaCl and 1 mM [methyl-14C]glycine betaine, and grown in 250-ml sidearm flasks with shaking and with filter paper moistened with 5 M KOH in the side arm. Replicate flasks were prepared for sampling after 5 h and 30 h. At those times, the filter papers were removed and the 14CO2 that was trapped was determined by measuring the radiolabel using a liquid scintillation counter as described in previous studies (11, 13). Measurements were expressed as counts per minute and were normalized to the OD600.
Characterization of bacterial survival and growth on plants.
Bacterial survival following inoculation onto plant surfaces was evaluated by growing bacteria on solid King's B medium, diluting a cell suspension to a density of 106 cells ml−1 in sterile H2O containing 0.01% Silwet L-77 (Lehle Seeds, Round Rock, TX), and inoculating bean leaves (Phaseolus vulgaris cultivar Bush Blue Lake 274) by leaf immersion in the bacterial suspension. At various times after inoculation, the epiphytic bacterial populations were recovered from each of 4 to 8 leaves by gentle sonication for 7 min in phosphate buffer (10 mM, pH 7) containing 0.1% peptone and enumerated on King's B medium containing rifampin and cycloheximide.
Statistics.
All P values reported are for comparisons between the B728a and a mutant using a two-tailed Student's t test or a Fisher's least significant difference test.
RESULTS
B728a exhibits differential responses to distinct levels of osmotic stress.
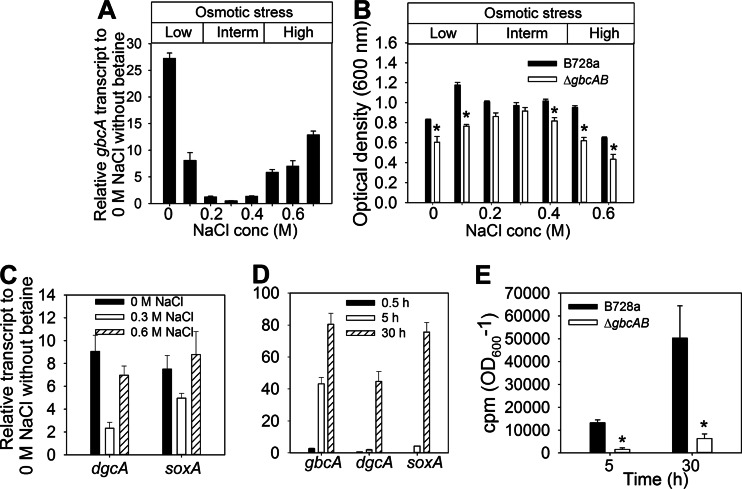
The glycine betaine catabolism genes gbcA and gbcB, which encode products that catalyze the conversion of betaine to dimethylglycine in P. aeruginosa (18), were also required for the first step of betaine catabolism in P. syringae B728a, as evidenced by the poor growth of the ΔgbcAB mutant with betaine but not dimethylglycine (Fig. 1B). The expression levels of gbcA were distinct at different levels of NaCl in the presence of betaine (Fig. 2A): high expression at a low osmotic stress level (≤0.1 M NaCl), low expression at an intermediate stress level (0.2 to 0.4 M NaCl), and high expression at a high osmotic stress level (≥0.5 M NaCl). This osmoregulated expression was relevant to B728a growth at each osmotic stress level based on the greater differences in growth between the wild type and the ΔgbcAB mutant at the low and high osmotic stress levels than at the intermediate levels (Fig. 2B). Additional genes involved in the downstream steps of the betaine catabolic pathway, namely, dgcA and soxA (Fig. 1A), also showed higher expression at 0 and 0.6 M NaCl than at 0.3 M NaCl (Fig. 2C), implying that activation of betaine catabolism genes occurred primarily at low osmotic stress levels, potentially to promote cellular utilization of betaine for nutrition, and at high osmotic stress levels. The expression of these catabolic genes was low in the first 30 min of exposure to 0.6 M NaCl but was greatly increased by 30 h (Fig. 2D), suggesting that betaine catabolism is initially low after an upshift to a high osmotic stress level, which would allow betaine to accumulate, but that its catabolism eventually increases.
Fig 2.
Effect of osmolarity on betaine catabolism. (A) qRT-PCR analysis of gbcA in B728a at 0.5 h after amending cells with 0 to 0.7 M NaCl and betaine. Values are expressed relative to the gbcA transcript level at 0 M NaCl without betaine. Interm, intermediate; conc, concentration. (B) Optical density of cultures of B728a and the ΔgbcAB mutant grown in microtiter plates in MinA-pyruvate containing 0 to 0.6 M NaCl and betaine for 45 h. (C) qRT-PCR analysis of dgcA and soxA in B728a at 30 min after amending cells with 0, 0.3, or 0.6 M NaCl and betaine. Values are expressed relative to transcript levels at 0 M NaCl without betaine. (D) qRT-PCR analysis of gbcA, dgcA, and soxA in B728a at 0.5, 5, and 30 h after amending cells with 0.6 M NaCl and betaine. (E) 14CO2 released by B728a and the ΔgbcAB mutant during their growth in MinA-pyruvate with 0.6 M NaCl and [methyl-14C]glycine betaine for 5 and 30 h. cpm, counts per minute. Asterisks indicate differences between B728a and the ΔgbcAB mutant at the NaCl concentration or time points indicated (P < 0.05). Values are means ± SEM (n = 3 for panels A to D; n = 2 for panel E).
To directly evaluate if betaine is catabolized at high osmotic stress levels, we provided cells with 1 mM [methyl-14C]glycine betaine and 0.6 M NaCl and measured the resulting 14CO2 after 5 and 30 h. Whereas B728a produced a significant amount of 14CO2 by 5 h and dramatically more after 30 h (Fig. 2E), consistent with the kinetics of the transcription of the betaine catabolic genes (Fig. 2D), the ΔgbcAB mutant released little 14CO2, even after 30 h of incubation, as expected. This reduction in 14CO2 was not due to a lower rate of betaine uptake (data not shown). Collectively, these data indicate that B728a catabolizes betaine following an upshift to high osmotic stress.
Betaine catabolism contributes to tolerance of high osmotic stress by relieving the betaine-mediated suppression of trehalose and NAGGN synthesis.
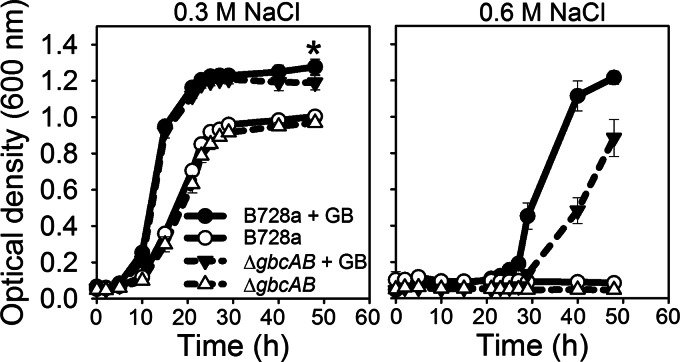
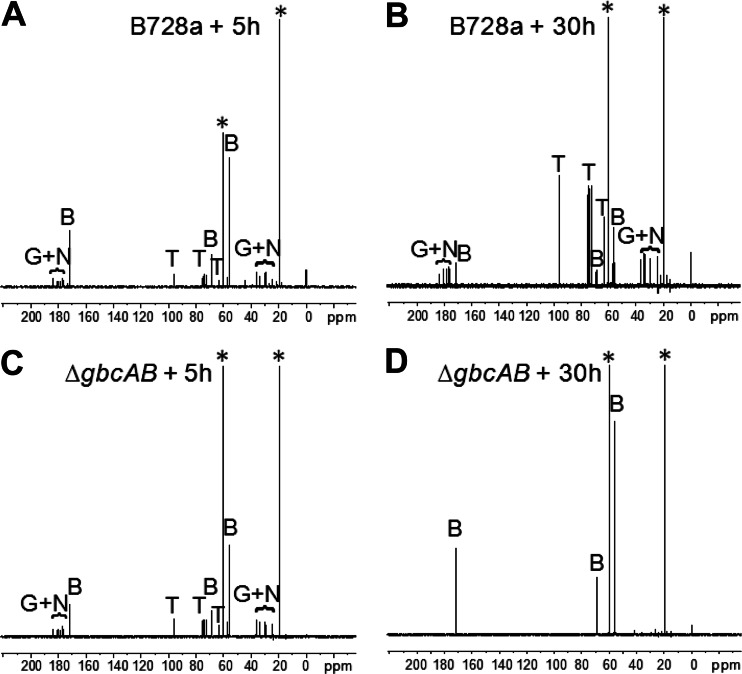
When grown with betaine as an osmoprotectant, the ΔgbcAB mutant showed reduced growth in 0.6 M NaCl, as detected in the early log phase (Fig. 3). In contrast, the ΔgbcAB mutant grew like the wild type in 0.3 M NaCl but exhibited slightly reduced population densities in the stationary phase (Fig. 3). Thus, betaine catabolism contributed to osmotolerance, and this was most dramatic at high stress levels. Based on a previous report that decreased betaine accumulation in S. meliloti was associated with increased endogenous compatible solutes (13), we hypothesized that betaine catabolism is required to enable the accumulation of trehalose, NAGGN, and/or glutamate in P. syringae. To test this, we examined the composition of the organic osmolytes accumulated in osmotically stressed cells using natural-abundance 13C-NMR spectroscopy following exposure for 5 and 30 h to 0.6 M NaCl in the presence of betaine. During the early growth stage (5 h), the B728a and ΔgbcAB strains showed similar compositions of osmolytes, with betaine dominating over the other solutes (Fig. 4A and B). After 30 h, when B728a had reached a higher cell density than the ΔgbcAB mutant, as demonstrated by an optical density at 600 nm of 2.3 for B728a and 1.5 for the ΔgbcAB mutant, the betaine levels in B728a had decreased whereas the trehalose, NAGGN, and glutamate levels had increased (Fig. 4C). These data suggest that B728a catabolized betaine and accumulated endogenous osmolytes over time after the osmotic upshift. In contrast, the ΔgbcAB mutant showed increased betaine levels at 30 h, with no detectable accumulation of the endogenous osmolytes (Fig. 4D). These data support a model in which the high level of betaine accumulated in the ΔgbcAB mutant inhibits the accumulation of the endogenous osmolytes.
Fig 3.
Contribution of betaine catabolism to osmotolerance. B728a and the ΔgbcAB mutant were grown in MinA-succinate with 0.3 M or 0.6 M NaCl and betaine, and growth in microtiter plates was monitored based on optical density. The asterisk indicates differences between B728a and the ΔgbcAB mutant at 0.3 M NaCl (P < 0.05). Values are means ± SEM (n = 4). GB, glycine betaine.
Fig 4.
Effect of betaine catabolism on the composition of the accumulated compatible solutes of B728a (A and C) and the ΔgbcAB mutant (B and D) after 5 h (A and B) and 30 h (C and D) of growth. Cells were collected for 13C-NMR at 5 and 30 h after cultures were amended with 0.6 M NaCl and betaine. Resonances (peaks) corresponding to betaine (B), l-glutamate (G), the dipeptide NAGGN (N), and trehalose (T) are indicated. Asterisks indicate peaks for residual ethanol from sample preparation.
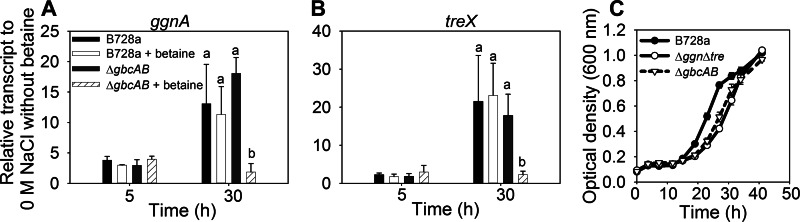
To further explore a possible inhibitory effect of betaine on de novo trehalose and NAGGN synthesis, we examined the transcription of ggnA and treX, which encode enzymes involved in NAGGN and trehalose synthesis, respectively (7). Their levels of transcription were similar in B728a and the ΔgbcAB mutant when cells were grown for up to 5 h at 0.6 M NaCl with or without betaine, but the level was much lower in the ΔgbcAB mutant than in B728a at 30 h in the presence of betaine (Fig. 5A and B). These data support an inhibitory role for accumulated, intracellular betaine in endogenous solute synthesis and indicate that this inhibition occurs, at least in part, at the transcriptional level.
Fig 5.
Role of betaine catabolism in relieving betaine-mediated inhibition of endogenous solute synthesis. (A and B) qRT-PCR analysis of ggnA (A) and treX (B) in B728a and the ΔgbcAB mutant at 5 and 30 h after amending cells with 0.6 M NaCl and betaine. Values are expressed relative to transcript levels at 0 M NaCl without betaine. Values indicated with the same letter do not differ significantly (P < 0.05). (C) B728a and the Δggn Δtre and ΔgbcAB mutants were grown in MinA-succinate with 0.6 M NaCl and betaine, and growth in microtiter plates was monitored based on optical density. Values are means ± SEM (n = 3 for panels A and B; n = 4 for panel C).
Last, we compared the growth of the wild-type B728a strain to that of the Δggn Δtre mutant, which is deficient in NAGGN and trehalose production (see Fig. S1 in the supplemental material), in 0.6 M NaCl amended with betaine. The Δggn Δtre mutant showed a reduction in growth that was similar to that of the ΔgbcAB mutant (Fig. 5C), suggesting that although betaine can protect B728a from the deleterious effects of osmotic stress, it cannot fully compensate for the loss of endogenous osmolyte synthesis during adaptation to high osmolarity.
Betaine catabolism contributes to the fitness of B728a on bean leaves.
In a previous study examining the transcriptome of B728a cells grown on bean leaves for 3 days under controlled conditions (25), the betaine catabolic genes were significantly induced in cells recovered from epiphytic and apoplastic, or intercellular, leaf sites (Table 2). The induction of gbcA was particularly strong. These results suggest that betaine catabolism might contribute to the survival of B728a under conditions of limited water availability in the phyllosphere, particularly on leaf surfaces (Table 2).
Table 2.
The change in the in planta transcript levels of the betaine catabolic genes of P. syringae B728aa
| Locus | Gene | Fold changeb |
|
|---|---|---|---|
| Epiphytic | Apoplastic | ||
| Psyr_4776 | gbcA | 12.6 | 5.2 |
| Psyr_4775 | gbcB | 2.4 | 1.6 |
| Psyr_4782 | dgcA | 5.7 | 4.7 |
| Psyr_4781 | dgcB | 1.7 | 2.7 |
| Psyr_4708 | gbdR | 6.7 | 2.3 |
| Psyr_4715 | soxA | 4.5 | 2.8 |
| Psyr_4713 | soxB | 7.4 | 4.3 |
| Psyr_4714 | soxD | 6.3 | 4.9 |
| Psyr_4716 | soxG | 4.1 | 3.6 |
Data are derived from Gene Expression Omnibus accession no. GSE42544.
The fold change values represent the change in transcript abundance in leaf surface (epiphytic) sites and in intercellular (apoplastic) sites relative to the transcript abundance in a defined medium in culture. Data are adapted from reference 25.
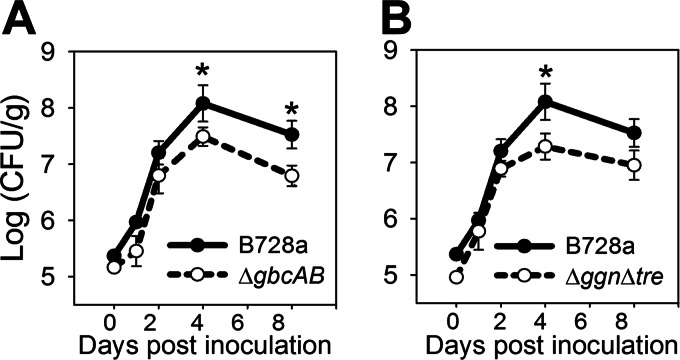
We evaluated the impact of the gbcAB deletion on B728a fitness on bean leaves based on enumeration of cells recovered from leaf surfaces by viable plate counts. The populations of the ΔgbcAB mutant were significantly smaller than those of B728a at 4 and 8 days postinoculation (Fig. 6A), demonstrating that betaine catabolism contributed to epiphytic fitness under these conditions. This contribution could have been due to nutrition and/or tolerance of water limitation. We therefore evaluated the fitness of the Δggn Δtre mutant under the same conditions. Populations of this mutant were also significantly smaller than those of the B728a at 4 days postinoculation (Fig. 6B). Thus, B728a likely experienced water limitation on leaves under these conditions, and the contribution of betaine catabolism to epiphytic fitness was probably due, at least in part, to its contribution to tolerating water limitation.
Fig 6.
Contribution of betaine catabolism to the epiphytic fitness of B728a. Bean leaves were inoculated with B728a and the ΔgbcAB (A) and Δggn Δtre (B) mutants, and the population sizes were estimated at 0, 1, 2, 4, and 8 days postinoculation based on viable plate counts. Values are means ± SEM (n = 4 for days 0 and 1 and n = 8 for days 2, 4, and 8). Asterisks indicate differences between B728a and the mutant at the indicated times (P < 0.05).
The transcriptional regulator GbdR is responsible for the differential expression of betaine catabolic genes at distinct osmotic stress levels.
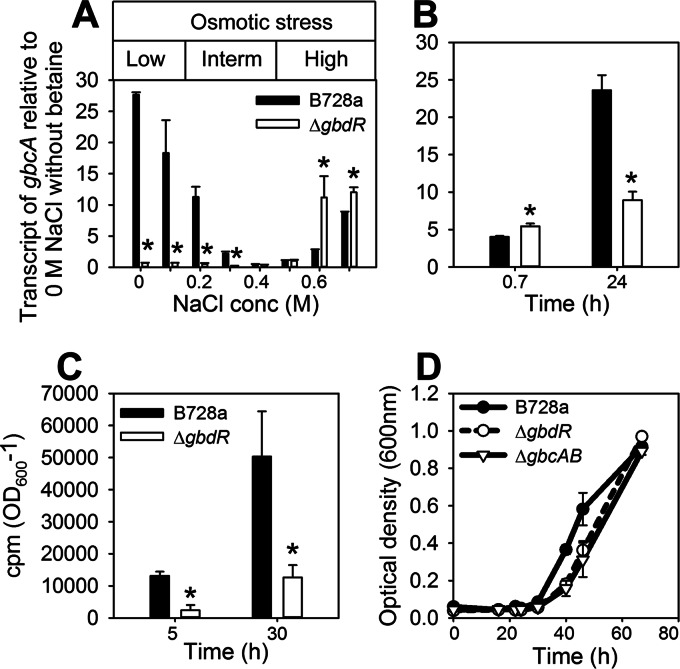
To begin to understand how betaine catabolism is differentially regulated at low versus high osmotic stress levels, we investigated its regulation by GbdR, a transcriptional regulator that induces the betaine catabolic genes in P. aeruginosa (18). Deletion of the gbdR homolog in B728a greatly reduced its growth on betaine and dimethylglycine, consistent with the role of these compounds as coinducers for GbdR activation of gbcA and dgcA (see Fig. S2 in the supplemental material). The ΔgbdR mutant was also reduced in growth on choline (see Fig. S2 in the supplemental material), since growth on choline requires the betaine catabolic pathway. We also evaluated the influence of osmotic stress on GbdR regulation. Differences in gbcA transcript levels between B728a and the ΔgbdR mutant at various NaCl concentrations showed that GbdR positively regulated gbcA transcription at both low and intermediate osmotic stress levels (0 to 0.3 M NaCl) but not at high stress levels (0.6 to 0.7 M NaCl) following a 40-min exposure period (Fig. 7A). After 24 h of exposure to a high stress level, however, GbdR functioned as a positive regulator (Fig. 7B). Positive regulation at high osmotic stress was confirmed based on a significantly reduced liberation of 14CO2 from [methyl-14C]glycine betaine in the ΔgbdR mutant compared to B728a (Fig. 7C). The importance of this catabolism for osmoadaptation was again illustrated by the reduced growth of the ΔgbdR mutant under conditions of high osmotic stress, similar to the reduced growth initially observed for the ΔgbcAB mutant (Fig. 3 and 7D).
Fig 7.
GbdR regulates betaine catabolism under conditions of low and high osmotic stress. (A) qRT-PCR analysis of gbcA in B728a and the ΔgbdR mutant at 40 min (0.7 h) after amending cells with 0 to 0.7 M NaCl and betaine. Values are expressed relative to gbcA transcript levels in B728a at 0 M NaCl without betaine. (B) qRT-PCR analysis of gbcA in B728a and the ΔgbdR mutant at 0.7 and 24 h after amending cells with 0.6 M NaCl and betaine. (C) 14CO2 released by B728a and the ΔgbdR mutant during their growth in MinA-pyruvate with 0.6 M NaCl and [methyl-14C]glycine betaine for 5 and 30 h. (D) B728a and the ΔgbcAB and ΔgbdR mutants were grown in MinA-succinate with 0.6 M NaCl and betaine, and growth in microtiter plates was monitored based on optical density. Asterisks indicate differences between B728a and the ΔgbdR mutant at the indicated concentrations or times (P < 0.05). Values are means ± SEM (n = 3 for panels A to C; n = 4 for panel D).
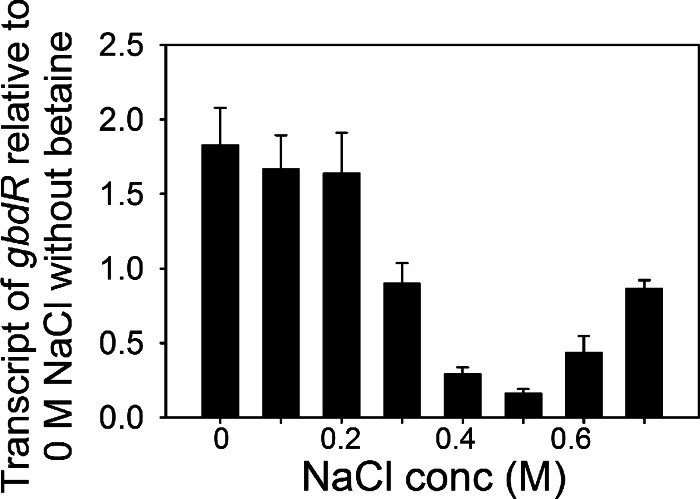
The expression of gbdR under various osmotic stress conditions in the presence of betaine showed that gbdR was subject to an expression pattern similar to that of gbcA, although the absolute expression levels were much lower for gbdR than gbcA (Fig. 2A and 8). This result indicates that the osmotic stress-dependent changes in gbcA transcription are probably due, at least in part, to osmotic stress-dependent changes in the transcription of the activator gene gbdR. Thus, activation of betaine catabolism at high osmotic stress likely resulted, in part, from transcriptional activation of gbdR. We used qRT-PCR to evaluate if GbdR directly regulates the endogenous solute synthesis genes in addition to betaine catabolism and found that the ΔgbdR mutant exhibited a betaine-mediated reduction of the expression of the trehalose and NAGGN synthesis genes (see Fig. S3 in the supplemental material), indicating that GbdR is not required for this regulation.
Fig 8.
Effect of osmolarity on gbdR expression. qRT-PCR analysis of gbdR in B728a at 40 min after amending cells with 0 to 0.7 M NaCl and betaine. Values are expressed relative to transcript levels in B728a at 0 M NaCl without betaine. Values are means ± SEM (n = 3).
Succinate mediates partial repression of betaine catabolism at a high osmotic stress level.
P. syringae B728a grew better than the ΔgbcAB mutant in a succinate-containing high osmotic stress medium (Fig. 3), suggesting that succinate does not fully repress betaine catabolism at a high osmotic stress level, as it does in P. aeruginosa (11, 26). P. syringae, however, was similar to P. aeruginosa in exhibiting succinate-mediated repression of betaine catabolism in the absence of NaCl amendment, as illustrated by the reduced transcription of the betaine-dependent GbdR-regulated genes gbcA, plcA, and pchP in succinate- versus pyruvate-containing media (see Fig. S4 in the supplemental material). In a direct comparison of the impact of succinate to that of pyruvate on growth at high osmolarity, B728a and the ΔgbcAB mutant each grew better with succinate than with pyruvate (Fig. 9A), probably due to a higher energy yield or better uptake of succinate than pyruvate, but the difference in growth between B728a and the ΔgbcAB mutant was much larger when cells were provided with pyruvate than when they were provided with succinate (Fig. 9A), suggesting that succinate partially represses betaine catabolism. The fact that B728a grew better than the ΔgbcAB mutant with succinate indicated that succinate did not fully repress betaine catabolism, and this catabolic activity was confirmed by the liberation of significantly more 14CO2 from [methyl-14C]glycine betaine by B728a than by the ΔgbcAB mutant in a succinate-containing high-osmotic-stress medium (Fig. 9B). The contrast between the strong succinate-mediated repression of betaine catabolism in the absence of osmotic stress and the partial repression in its presence suggests that, under high-osmotic-stress conditions, betaine serves not only as a source of energy or nutrition but also as an intracellular signal to regulate endogenous solute synthesis.
Fig 9.
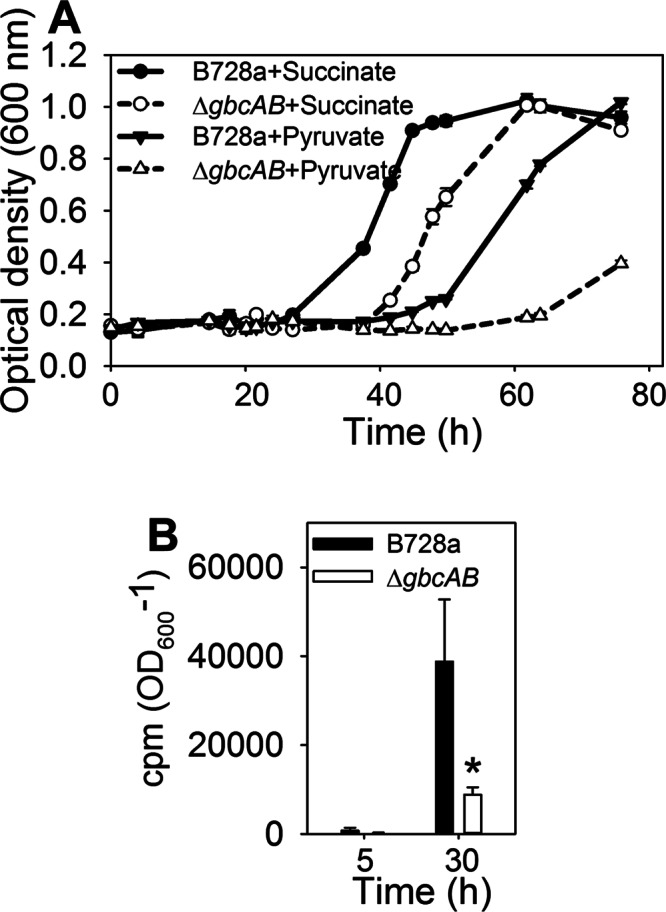
Evaluation of succinate repression of betaine catabolism at high osmolarity. (A) B728a and the ΔgbcAB mutant were grown in MinA-succinate or MinA-pyruvate with 0.6 M NaCl and betaine, and growth in microtiter plates was monitored based on optical density. (B) 14CO2 released by B728a and the ΔgbcAB mutant during their growth in MinA with 20 mM succinate and 0.6 M NaCl and [methyl-14C]glycine betaine for 5 and 30 h. Asterisks indicate differences between B728a and the ΔgbcAB mutant at the indicated times (P < 0.05). Values are means ± SEM (n = 4 for panel A; n = 2 for panel B).
DISCUSSION
The mechanisms by which bacteria change their intracellular osmolyte pools during osmoadaptation are not well understood. Following an osmotic upshift in the presence of betaine, many proteobacteria and other prokaryotes rapidly import betaine and restore cellular homeostasis. Our data are consistent with a model in which, following an osmotic upshift large enough to induce betaine uptake, cells eventually transit from a dependence on betaine to a dependence on endogenous compatible solutes. Most importantly, our data identify some of the molecular mechanisms underlying this transition in P. syringae. These mechanisms include upregulation of the transcriptional activator gene gbdR, resulting in a GbdR-mediated increase in the expression of the betaine catabolism genes and therefore in activation of betaine catabolism. They also include the relief of betaine-mediated suppression of genes required for the synthesis of the endogenous solutes trehalose and NAGGN. This betaine catabolism-driven transition was required for maximal osmotolerance in cells exposed to a high osmotic stress level, as detected by the reduced growth of early log-phase cells of the betaine catabolic ΔgbcAB mutant, but also contributed to osmotolerance at an intermediate osmotic stress level, as detected by slightly reduced growth at a later growth phase (Fig. 3). This observation suggests an expansion of the model of cellular adaption following an osmotic upshift in the presence of betaine to include the provision that the larger the osmotic upshift, the earlier the transition from a dependence on betaine to a dependence on endogenous osmolytes.
For bacteria that import betaine under hyperosmotic conditions, the size of the intracellular betaine pool is regulated by balancing this import with catabolism or export. Our work provides evidence that P. syringae regulates its betaine pools, at least in part, via catabolism based on the higher betaine levels in the gbcAB catabolic mutant. Previous studies with P. aeruginosa and S. meliloti have shown that these organisms also catabolize betaine under conditions of high osmotic stress (11, 13), with the S. meliloti studies documenting a steady depletion of betaine due to catabolism during log-phase growth. Interestingly, upon reaching stationary phase, the intracellular betaine pools in S. meliloti rapidly decreased, likely due to export, consistent with the need for a lower level of osmolytes to maintain turgor in stationary-phase cells (13). Our data showing a difference between the ΔgbcAB mutant and B728a during the stationary phase under conditions of an intermediate osmotic stress (Fig. 3) suggest that catabolism continued to be important into the stationary phase for P. syringae, but we cannot exclude the possibility of additional loss through export. Organisms that cannot catabolize betaine, including Escherichia coli, Bacillus subtilis, and Corynebacterium glutamicum, regulate intracellular betaine pools by attenuating import, such as via the BetP transporter of C. glutamicum (27), or efflux, as shown for the mechanosensitive channels MscCG (28) and the transporter protein EmrE (29) in E. coli. The versatility of these balancing mechanisms is further illustrated by the recent finding that B. subtilis regulates intracellular pools of the compatible solute proline and betaine using a mechanism that involves both release and recapture (30).
Few studies have addressed how organisms that can catabolize betaine at high osmotic stress levels actually regulate this catabolism. Smith and colleagues (1988) found that the activity of the betaine-catabolic enzymes in S. meliloti decreased following a 2-h exposure to osmotic stress and concluded that this decreased activity contributes to the accumulation of betaine for osmoprotection. Moreover, using in vitro enzyme assays, they showed that this inhibition was not due to a direct effect of salt on the enzymes (21). Such inhibition has not been studied in P. aeruginosa or P. syringae, and the enzyme catalyzing the putative rate-limiting step in betaine catabolism is different in Pseudomonas spp. from that in S. meliloti (20, 21). Our work is the first to provide evidence for osmotic regulation of betaine catabolism at the transcriptional level. We demonstrated not only osmotic regulation of the gbcA, dgcA, and soxA genes, which encode enzymes involved in all three steps converting betaine to glycine, but also osmotic regulation of the gbdR gene, which encodes the GbdR transcriptional activator. The dependence of gbcA, dgcA, and soxA on GbdR for expression (18) suggests that their osmoregulation is probably due to the osmoregulated availability of GbdR. The osmotic regulation of gbdR expression itself could result, in part, from autoregulation by GbdR under hyperosmotic conditions, although such autoregulation was not observed under nonstressful conditions (18). Cellular factors that influence the osmoregulation of gbdR expression have not yet been identified.
Analyses of the ΔgbcAB mutant and the wild-type strain indicated an inverse association between durable accumulation of cytosolic betaine and endogenous osmolyte accumulation, consistent with observations in P. aeruginosa (11, 12), S. meliloti (13), and Salmonella enterica serovar Typhimurium (31). These observations have repeatedly led to the prediction that betaine inhibits endogenous solute synthesis. We provided experimental evidence supporting this as-yet-untested prediction. Using the ΔgbcAB mutant to achieve elevated cytosolic betaine levels, we demonstrated that betaine accumulation inhibited the transcription of the biosynthetic genes ggnA and treX required for NAGGN and trehalose synthesis, respectively, under hyperosmotic conditions (Fig. 5A and B). Furthermore, the loss of NAGGN and trehalose synthesis recapitulated the loss of betaine catabolism in reduced osmotolerance in culture and reduced fitness on leaves (Fig. 5C and 6), supporting our model positing that a major consequence of betaine catabolism is relieving the repression of endogenous compatible solute synthesis. We focused on only two of the three known endogenous compatible solutes in P. syringae, NAGGN and trehalose, because their biosynthetic loci are known. Nevertheless, our 13C-NMR analysis indicated that durable accumulation of betaine also inhibited the third solute, l-glutamate, but the mechanisms underlying osmoinduced l-glutamate accumulation are not known. The similarity of the Δggn Δtre mutant to the ΔgbcAB mutant in osmotolerance and fitness on leaves, however, suggests that NAGGN and trehalose were the primary contributors to osmotolerance under these conditions.
GbdR was previously identified as a transcriptional activator that enables P. aeruginosa to grow on betaine and its derivatives as sole carbon or nitrogen sources in the absence of osmotic stress (18). Here, we expanded the role for GbdR to include transcriptional activation of the betaine catabolic genes under hyperosmotic conditions. Unlike the rapid activation of catabolic genes such as gbcA at low or intermediate osmolarity, however, GbdR functioned as a repressor of gbcA at 40 min (0.7 h) after an upshift to high osmolarity but, by 24 h, had resumed activity as an activator (Fig. 7A and B). When the cells were grown in the presence of betaine before the upshift, GbdR did not exhibit repressor activity (data not shown). This finding suggests that intracellular betaine is required for GbdR activator activity, consistent with the known requirement for betaine and its degradation products as coinducers of GbdR (18), and that 40 min was insufficient for establishing an intracellular betaine pool. The repressor activity of GbdR in the absence of its coinducer may be similar to that of AraC in the absence of arabinose (32), which was suggested to result from the formation of a repressive DNA loop structure upstream of the regulated genes. The increased gbcA expression under high-salt conditions in the absence of GbdR (Fig. 7A) may result from a salt-mediated change in supercoiling (33).
Whereas betaine catabolism in P. aeruginosa was subject to complete repression by succinate in the absence (26) and presence (11) of osmotic stress, betaine catabolism in P. syringae was subject to only partial repression under hyperosmotic conditions (Fig. 9). The difference between these findings could reflect differences in the levels of stress used in the assays. Specifically, if succinate-mediated repression is relieved under highly stressful conditions, consistent with the need for betaine to serve a dual role in energy generation and as an intracellular signal, then the 0.5 M NaCl amendment used when evaluating catabolite repression in P. aeruginosa may not have conferred a sufficiently high level of stress to attenuate the succinate-mediated repression (11); in contrast, the 0.6 M NaCl amendment used for P. syringae may have been sufficiently stressful. These findings support the notion of the presence of an osmotically responsive regulator of succinate-mediated catabolite repression in P. syringae.
This report provides evidence that betaine catabolism is relevant to the biology of P. syringae during its interactions with plants. Previously, we obtained transcriptome data (25) showing the expression of genes for betaine catabolism in planta (Table 2). Here, we showed that betaine catabolism contributes to the ability of B728a to establish large populations on leaves of its host plant, bean (Fig. 6A). The reduced epiphytic fitness of the Δggn Δtre mutant on leaves under the same conditions provides strong evidence that B728a is water limited during its growth on leaf surfaces, as we recently concluded based on our transcriptome data (25). Moreover, the similarity of the reduced fitness of the Δggn Δtre mutant to that of the ΔgbcAB mutant strongly supports the hypothesis that a major role of the betaine catabolism on leaves is to relieve the repression of the synthesis of trehalose and NAGGN, although we cannot exclude the possibility that betaine also provides a nutritional and energy benefit to these bacteria in planta. The results strongly support the prediction that B728a can access available, exogenous pools of betaine or a betaine precursor on leaves. We predict that these pools consist primarily of choline and its precursors based on previous studies on B728a transporters that suggested its adaptation to life in choline-rich environments (10, 14), the presence of phosphatidylcholine as a major membrane lipid in plants, and the demonstrated abundance of choline in plants (34).
An issue that remains unresolved by this work is why cells transition from reliance on a continuing supply of exogenous betaine to reliance on endogenous osmolytes. From an ecological perspective, perhaps this transition is a strategy to ensure self-reliance. From a cellular perspective, at least in the case of high osmotic stress levels, perhaps the levels of betaine required to restore cellular homeostasis are toxic to the cells, similar to the reported toxicity of betaine for Salmonella enterica serovar Typhimurium (35) and toxicity of glycine for S. meliloti (21). And last, from a molecular perspective, perhaps the osmoprotection conferred by the endogenous solutes, either singly or as a set, is superior to the osmoprotection conferred by betaine. That last theory is supported by the finding that, in many bacteria, trehalose becomes an increasingly abundant osmolyte over time (11–13), consistent with the particularly potent protection it provides to proteins and membranes under environmentally stressful conditions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (no. MCB-0920156).
We thank Chiliang Chen for comments on the manuscript.
Footnotes
Published ahead of print 22 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00094-13.
REFERENCES
- 1. Quirino BF, Bent AF. 2003. Deciphering host resistance and pathogen virulence: the Arabidopsis/Pseudomonas interaction as a model. Mol. Plant Pathol. 4:517–530 [DOI] [PubMed] [Google Scholar]
- 2. Vinatzer BA, Teitzel GM, Lee MW, Jelenska J, Hotton S, Fairfax K, Jenrette J, Greenberg JT. 2006. The type III effector repertoire of Pseudomonas syringae pv. syringae B728a and its role in survival and disease on host and non-host plants. Mol. Microbiol. 62:26–44 [DOI] [PubMed] [Google Scholar]
- 3. Axtell CA, Beattie GA. 2002. Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat. Appl. Environ. Microbiol. 68:4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beattie GA. 2011. Water relations in the interaction of foliar bacterial pathogens with plants. Annu. Rev. Phytopathol. 49:533–555 [DOI] [PubMed] [Google Scholar]
- 5. Monier JM, Lindow SE. 2005. Aggregates of resident bacteria facilitate survival of immigrant bacteria on leaf surfaces. Microb. Ecol. 49:343–352 [DOI] [PubMed] [Google Scholar]
- 6. Freeman BC, Chen C, Beattie GA. 2010. Identification of the trehalose biosynthetic loci of Pseudomonas syringae and their contribution to fitness in the phyllosphere. Environ. Microbiol. 12:1486–1497 [DOI] [PubMed] [Google Scholar]
- 7. Kurz M, Burch AY, Seip B, Lindow SE, Gross H. 2010. Genome-driven investigation of compatible solute biosynthesis pathways of Pseudomonas syringae pv. syringae and their contribution to water stress tolerance. Appl. Environ. Microbiol. 76:5452–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sagot B, Gaysinski M, Mehiri M, Guigonis JM, Le Rudulier D, Alloing G. 2010. Osmotically induced synthesis of the dipeptide N-acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria. Proc. Natl. Acad. Sci. U. S. A. 107:12652–12657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert H, Le Marrec C, Blanco C, Jebbar M. 2000. Glycine betaine, carnitine, and choline enhance salinity tolerance and prevent the accumulation of sodium to a level inhibiting growth of Tetragenococcus halophila. Appl. Environ. Microbiol. 66:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen C, Beattie GA. 2007. Characterization of the osmoprotectant transporter OpuC from Pseudomonas syringae and demonstration that cystathionine-beta-synthase domains are required for its osmoregulatory function. J. Bacteriol. 189:6901–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diab F, Bernard T, Bazire A, Haras D, Blanco C, Jebbar M. 2006. Succinate-mediated catabolite repression control on the production of glycine betaine catabolic enzymes in Pseudomonas aeruginosa PAO1 under low and elevated salinities. Microbiology 152:1395–1406 [DOI] [PubMed] [Google Scholar]
- 12. D'Souza-Ault MR, Smith LT, Smith GM. 1993. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl. Environ. Microbiol. 59:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Talibart R, Jebbar M, Gouffi K, Pichereau V, Gouesbet G, Blanco C, Bernard T, Pocard J. 1997. Transient accumulation of glycine betaine and dynamics of endogenous osmolytes in salt-stressed cultures of Sinorhizobium meliloti. Appl. Environ. Microbiol. 63:4657–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen C, Beattie GA. 2008. Pseudomonas syringae BetT is a low-affinity choline transporter that is responsible for superior osmoprotection by choline over glycine betaine. J. Bacteriol. 190:2717–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C, Malek AA, Wargo MJ, Hogan DA, Beattie GA. 2010. The ATP-binding cassette transporter Cbc (choline/betaine/carnitine) recruits multiple substrate-binding proteins with strong specificity for distinct quaternary ammonium compounds. Mol. Microbiol. 75:29–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J, Zhou D, Zhang Q, Zhang W. 2012. Genomic analysis of phospholipase D family and characterization of GmPLDαs in soybean (Glycine max). J. Plant Res. 125:569–578 [DOI] [PubMed] [Google Scholar]
- 17. Gaude N, Nakamura Y, Scheible WR, Ohta H, Dormann P. 2008. Phospholipase C5 (NPC5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J. 56:28–39 [DOI] [PubMed] [Google Scholar]
- 18. Wargo MJ, Szwergold BS, Hogan DA. 2008. Identification of two gene clusters and a transcriptional regulator required for Pseudomonas aeruginosa glycine betaine catabolism. J. Bacteriol. 190:2690–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wargo MJ, Ho TC, Gross MJ, Whittaker LA, Hogan DA. 2009. GbdR regulates Pseudomonas aeruginosa plcH and pchP transcription in response to choline catabolites. Infect. Immun. 77:1103–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzsimmons LF, Hampel KJ, Wargo MJ. 2012. Intracellular choline and glycine betaine pools impact osmoprotection and phospholipase C production in Pseudomonas aeruginosa. J. Bacteriol. 194:4718–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith LT, Pocard JA, Bernard T, Le Rudulier D. 1988. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J. Bacteriol. 170:3142–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 23. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 24. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu X, Lund SP, Scott RA, Greenwald JW, Records AH, Nettleton D, Lindow SE, Gross DC, Beattie GA. 2013. Transcriptional responses of Pseudomonas syringae to growth in epiphytc versus apoplastic leaf sites. Proc. Natl. Acad. Sci. U. S. A. 110:E425–E434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sage AE, Vasil ML. 1997. Osmoprotectant-dependent expression of plcH, encoding the hemolytic phospholipase C, is subject to novel catabolite repression control in Pseudomonas aeruginosa PAO1. J. Bacteriol. 179:4874–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Botzenhardt J, Morbach S, Kramer R. 2004. Activity regulation of the betaine transporter BetP of Corynebacterium glutamicum in response to osmotic compensation. Biochim. Biophys. Acta 1667:229–240 [DOI] [PubMed] [Google Scholar]
- 28. Börngen K, Battle AR, Moker N, Morbach S, Marin K, Martinac B, Kramer R. 2010. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim. Biophys. Acta 1798:2141–2149 [DOI] [PubMed] [Google Scholar]
- 29. Bay DC, Turner RJ. 2012. Small multidrug resistance protein EmrE reduces host pH and osmotic tolerance to metabolic quaternary cation osmoprotectants. J. Bacteriol. 194:5941–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoffmann T, von Blohn C, Stanek A, Moses S, Barzantny H, Bremer E. 2012. Synthesis, release, and recapture of compatible solute proline by osmotically stressed Bacillus subtilis cells. Appl. Environ. Microbiol. 78:5753–5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pilonieta MC, Nagy TA, Jorgensen DR, Detweiler CS. 2012. A glycine betaine importer limits Salmonella stress resistance and tissue colonization by reducing trehalose production. Mol. Microbiol. 84:296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schleif R. 2003. AraC protein: a love-hate relationship. Bioessays 25:274–282 [DOI] [PubMed] [Google Scholar]
- 33. Higgins CF, Dorman CJ, Stirling DA, Waddell L, Booth IR, May G, Bremer E. 1988. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell 52:569–584 [DOI] [PubMed] [Google Scholar]
- 34. McNeil SD, Nuccio ML, Ziemak MJ, Hanson AD. 2001. Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc. Natl. Acad. Sci. U. S. A. 98:10001–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jovanovich SB, Martinell M, Record MT, Jr, Burgess RR. 1988. Rapid response to osmotic upshift by osmoregulated genes in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 170:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loper JE, Lindow SE. 1987. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology 77:1449–1454 [Google Scholar]
- 37. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 38. Ditta G, Stanfield S, Corbin D, Helinski DR. 1980. Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. U. S. A. 77:7347–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.