Abstract
Progressive microglial accumulation at amyloid-β (Aβ) plaques is a well-established signature of the pathology of Alzheimer's disease, but how and why microglia accumulate in the vicinity of Aβ plaques is unknown. To understand the distinct roles of Aβ on microglial accumulation, we quantified microglial responses to week-long lasting gradients of soluble Aβ and patterns of surface-bound Aβ in microfluidic chemotaxis platforms. We found that human microglia chemotaxis in gradients of soluble Aβ42 was most effective at two distinct concentrations of 23 pg.mL−1 and 23 ng.mL−1 Aβ42 in monomers and oligomers. We uncovered that while the chemotaxis at higher Aβ concentrations was exclusively due to Aβ gradients, chemotaxis at lower concentrations was enhanced by Aβ-induced microglial production of MCP-1. Microglial migration was inhibited by surface-bound Aβ42 in oligomers and fibrils above 45 pg.mm−2. Better understanding of microglial migration can provide insights into the pathophysiology of senile plaques in AD.
Microglial cells are resident macrophages in the central neural system (CNS) and have multiple functions in normal and pathological brains1,2,3,4,5,6. Ramified microglia are believed to constantly scan the brain and clear the CNS of toxic agents and debris7,8. In the context of Alzheimer's disease (AD), diminished clearance of Aβ due to impaired microglia is postulated to be one of the mechanisms of Aβ plaque formation9. Once Aβ forms plaques, microglia become hyper-activated10, accumulate around the plaques, and consequently secrete neurotoxic agents including neuroinflammatory mediators, reactive oxygen species, and free radicals11,12,13,14. Understanding the mechanisms that lead to microglial accumulation and inflammatory responses may provide useful insights into developing strategies for AD treatment. However, several studies report different interactions between microglia and other factors15,16,17. Previous in vivo studies of microglia migration were hampered by both the inability to observe long-term effects of Aβ on microglia18 and the complexity of senile plaques, which are deposits of oligomeric and fibrillar Aβ, surrounded by a “halo” of soluble oligomeric Aβ19, activated glia, and dystrophic neurites12. Activated microglial cells take on various morphologies: rounded, ramified shapes, rods to amoeboids, which complicate the visual tracking of these cells individually. Furthermore, previous in vitro attempts to study rat microglia migration in the presence of short lived, damaged axons20, could not establish long-lasting gradients and were not conclusive for differentiating slow accumulation of microglia from random navigation21,22.
Here, we have developed a novel microfluidic chemotaxis platform to study the motility of microglia responding to various types of Aβ in a regulated manner. The new platform models soluble and bound Aβ environments in AD brains and enables us to measure the distinct effects of both gradients of soluble Aβ, lasting longer than a week and surface-bound patterns of Aβ on the migration of microglia.
Results
A microfluidic platform provides a gradient of soluble Aβ and allows monitoring of microglial motility
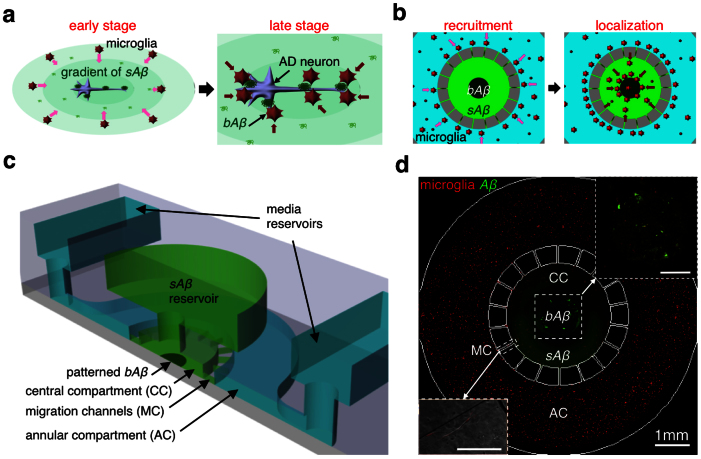
Based on in vivo observation of microglial accumulation on Aβ plaques, we hypothesize that soluble Aβ is an effective stimulus in recruiting microglia, while insoluble, surface-bound Aβ can capture the attracted microglia at the sites of surface-bound Aβ (Fig. 1a). To understand how microglial migration is regulated by Aβ in AD, we designed a unique microfluidic chemotactic platform composed of a large central reservoir (100 μL) supplying various types of soluble factors and two side reservoirs (100 μL each) containing medium. Human microglia were loaded in the annular (4 μL) compartment and their migration was observed towards the central (1 μL) compartment (Fig. 1b–c). The central and annular compartments were linked by migration channels (10 × 50 × 500 μm3 in height, width, and length) forming gradients of soluble factors. Compared to a parallel configuration having equal surface areas for cell loading and cell collection, our axisymmetric configuration has a small central cell collecting compartment, which is smaller than a large annular cell loading compartment by a factor of 6. This enhances detection of recruitment. The long and thin migration channels can screen out unwanted entrance of inactivated microglia through mechanical constraints. Using this platform, we observed the migration of microglial cells, at single cell resolution, in real-time, for extended periods (Fig. 1d). We verified that refreshing media every two days allowed our experimental conditions to have a reasonable degree of physical and chemical stability of soluble Aβ forms: 90 and 95% of original Aβ gradients of monomeric and oligomeric soluble Aβ for up to nine days (Supplementary Fig. S1), 74 and 84% of the total amounts of monomeric and oligomeric Aβ solutions for two days (Supplementary Fig. S2). Thus, for most experiments, we refreshed media including soluble factors every two days. To evaluate the response of microglia to various types of Aβ, we tested 200 independent conditions at the same time, by imaging microglia in 8 plates, each with twenty-five platforms in an array format (Supplementary Fig. S3).
Figure 1. Replication of AD-Aβ environment in a microfluidic chemotaxis platform.
(a) We hypothesize that at early stages microglia are recruited by soluble Aβ (sAβ) (left) and at late stages co-localized with surface-bound Aβ (bAβ) near Aβ plaques and neurons in AD brains (right). (b), (c) A microfluidic chemotaxis platform models the pathological Aβ environments in AD brains and provides surface-bound Aβ inside the central compartment (CC) and gradients of soluble Aβ between the CC and the annular compartment (AC), along migration channels (MC). The axisymmetric configuration enhances the microglia counting during Aβ recruitment and the long and thin migration channels help avoid counting errors due to spontaneous migration. (d) Fluorescent images show human microglia (red) inside the AC and soluble Aβ (light green) and patterned bAβ (dark green) inside the CC. Microglia move in a gradient of sAβ between AC and CC, along the MC (left-lower inset). Microglia and bAβ co-localize in the CC after six days (right-upper inset). Scale bars, 200 μm.
Directional migration of microglia towards soluble Aβ
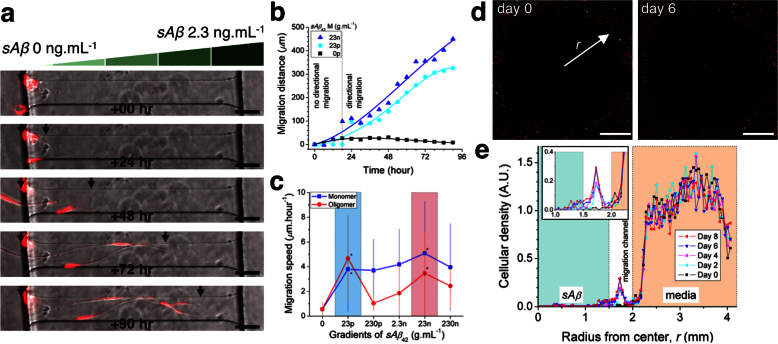
To characterize the kinetics of microglial migration driven by soluble Aβ, we monitored individual microglial cells exposed to stable gradients of soluble Aβ for four days using time-lapse microscopy. We observed that in the first 24 hours after introducing soluble Aβ42 into a reservoir, microglia changed shape, became more elongated, and produced filopodia in random directions (Fig. 2a, Supplementary Movies S1). However, most of the cells remained stationary for the first 24 hours. Microglia started migrating on the second day of exposure to soluble Aβ42 and migration was directional towards the soluble Aβ42 reservoir (Fig. 2b). In the absence of soluble Aβ42, microglia expanded their bodies but did not migrate during four-day experiments (Supplementary Movies S2). We measured comparable effects of monomeric and oligomeric forms, and measured a maximal migration speed of 5.1 ± 4.2 μm.hr−1 at 23 ng.mL−1 soluble monomeric Aβ42 (Fig. 2c). We observed that microglia also entered the central compartment, the source of 2.3 ng.mL−1 soluble Aβ42 monomers (Fig. 2d). Additional quantification of the effect of soluble Aβ on cellular distribution was performed by analyzing the changes in cellular density profile over time (Fig. 2e).
Figure 2. Inducement of microglial directional migration by gradients of soluble Aβ.
(a) Individual microglia migrate directionally along the gradient of sAβ monomers formed in migration channels. (b) Activation of directional motility can be discerned after 24 hours-exposure to gradients of sAβ42 in monomers at 23 pg.mL−1 and 23 ng.mL−1. (c) Dose-dependence of microglia migration speed during 4 days observations reveal two peak activities under gradients of soluble Aβ42 monomers and oligomers at concentrations of 23 pg.mL−1 and 23 ng.mL−1 (ranges highlighted in cyan and pink colors, respectively). (d) Fluorescence images present detectable microglia accumulation toward the source of soluble Aβ42 at day 6 compared to day 0. (e) Microglial density profiles at different days quantify the migration of microglia populations toward the source of soluble Aβ42 in monomers at 2.3 ng.mL−1. See the details on the stability and the preparation of soluble monomeric and oligomeric Aβ in Figs. S1, S2, and Supplementary methods. (Student's t-test. * P < 0.01 with respect to no sAβ42). ncell = 18 for each condition. Data represent mean ± s.e.m.
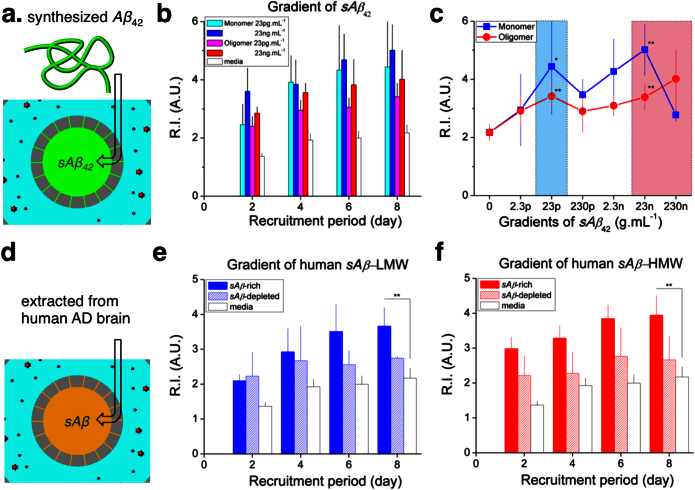
To compare the effect of soluble Aβ in various forms and concentrations on microglial recruitment, we defined a recruitment index, R.I. (as defined in Supplementary Methods, Supplementary Fig. S7), representing the fraction of microglia cells recruited to the central compartment after loading soluble Aβ in the central compartment. We compared the R.I. values under various types of soluble Aβ42 for eight days (Fig. 3a, Supplementary Fig. S4) and found that the R.I. value reached 5.0 ± 0.9 after exposure to monomeric synthetic Aβ42 at 23 ng.mL−1 and 4.0 ± 1.0 after exposure to oligomeric forms at 230 ng.mL−1, on ‘day 8’ (Fig. 3b). Measuring the concentration dependence of directional migration speed and R.I. revealed two peaks of activity at two concentrations of soluble Aβ42 that were three orders of magnitude apart (23 pg.mL−1 and 23 ng.mL−1) (Fig. 2c and Fig. 3c). Interestingly, the low peak concentrations corresponds to the levels in normal and early AD brains, while the high peak to late AD brains23.
Figure 3. Microglial recruitment using various soluble Aβ.
(a) Gradients of synthetic Aβ42 monomers and oligomers are formed between Aβ reservoir and a microglia hosting annular compartment. (b) Significant recruitment of human primary microglia is measured in the presence of gradients of synthetic sAβ42. (c) Dose-dependence experiments quantified with a recruitment index, R.I. on a ‘day 8’, reveal peak activities under gradients of sAβ42 monomers and oligomers at both concentrations of 23 pg.mL−1 and 23 ng.mL−1 compatible with measurement of a migration speed in Figure 2. (d) Human sAβ is extracted from AD brains in low and high molecular weights and forms gradients between Aβ reservoir and a microglia hosting annular compartment. (e), (f) Considerable recruitment of human primary microglia is measured along gradients of human-derived sAβ at a low molecular weight (LMW) (e) and a high molecular weight (HMW) (f) Immune-depletion of sAβ from the same human samples results in reduced microglial recruitment effect. (Student's t-test. * P < 0.1, ** P < 0.05 with respect to no sAβ). nplatform = 4 and ncell ≈ 2,000 for each condition. Data represent mean ± s.e.m.
Microglial recruitment by human-derived soluble Aβ
To validate the biological relevance of our platform, we measured the microglial response to gradients of soluble Aβ enriched or depleted samples derived from tris-buffered saline (TBS) extracts of human AD brains as previously described24. For this purpose, low (8 to 20 kDa) and high molecular weight fractions (more than 100 kDa) were separated by size exclusion chromatography (Fig. 3d). Both low-molecular-weight soluble Aβ (LMW) and high-molecular-weight soluble Aβ (HMW) fractions were similar in recruiting microglia towards the central compartment on the eighth day (R.I. = 3.7 ± 0.5 for LMW and R.I. = 3.9 ± 0.6 for HMW) (Fig. 3e–f). Removing the soluble Aβ by immune-precipitation considerably reduced the microglial accumulation (R.I = 2.7 ± 0.1 for LMW and 2.7 ± 0.7 for HMW), suggesting that a major factor of the TBS extract inducing microglial migration was Aβ.
Self-promoting microglial recruitment by Aβ-induced MCP-1 secretion
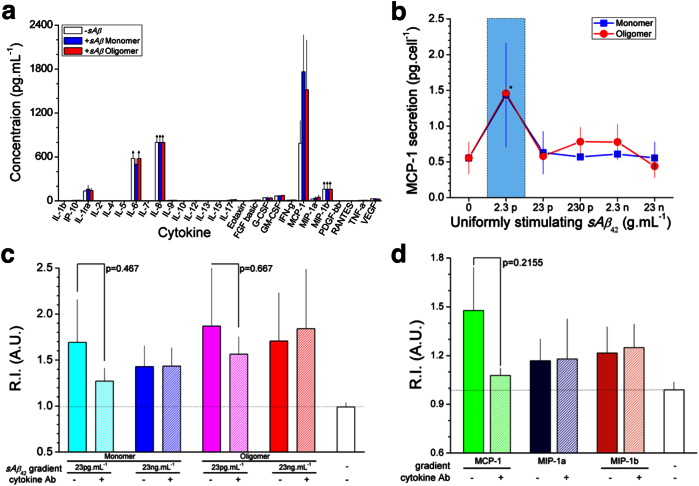
Because typical chemotactic responses of various cells display only one activity peak, we investigated further the mechanisms behind the double peak chemotactic activities of microglia in response to Aβ. To probe if a second chemoattractant may explain the unusual double chemotactic peak, we utilized a multiplexed cytokine assay to examine 27 cytokines expressed into the media collected from microglia cultured in the presence of soluble Aβ42 at 2.3 pg.mL−1 for two days. We identified five cytokines at detectable levels: IL-1ra, IL-6, IL-8, MCP-1, and MIP-1b while the remaining cytokines were either below, or just at the threshold of detectability (Fig. 4a). Arrows indicate saturated measurement of cytokines relative to the standard curve. Because MCP-1, MIP-1a, and MIP-1b are known chemoattractant molecules for macrophages3,7,25, we measured microglial recruitment under gradients of soluble Aβ42 in addition to a mixture of neutralizing antibodies against MCP-1, MIB-1a, and MIB-1b and observed reduced activity at 23 pg.mL−1 but not at 23 ng.mL−1 of soluble Aβ42 (Fig. 4c). In control experiments, we validated that MCP-1 alone can promote microglial recruitment and verified that neutralizing antibodies can reduce microglial recruitment towards the single cytokine (Fig. 4d). We further measured the MCP-1 concentration in media from microglia stimulated with various concentrations of both monomeric and oligomeric soluble Aβ42. We estimated the maximum secretion of MCP-1 at 1,433.9 ± 816.0 and 1,457.3 ± 109.4 fg.cell−1 in the presence of 2.3 pg.mL−1 of soluble monomeric and oligomeric Aβ42, respectively and 554.4 ± 224.3 fg.cell−1 in the absence of soluble Aβ42 (Fig. 4b). Interestingly, the MCP-1 secretion in the presence of 2.3 pg.mL−1 Aβ42 was significantly higher than at other concentrations of Aβ42 oligomers. While the migration of microglia occurs at higher levels of the gradient (23 pg.mL−1) than uniform Aβ42 (2.3 pg.mL−1), the difference could be explained by the lower effective concentration at the initial region of a gradient. It is possible that MCP-1 plays a role in the peak microglia recruitment activity at a gradient of 23 pg.mL−1 soluble Aβ42, in addition to other reported roles in microglia-mediated neurodegeneration26.
Figure 4. MCP-1 secretion from microglia under stimulation of soluble Aβ and self-promoted microglial recruitment.
(a) Microglial cells are cultured in wells containing sAβ42 and discernibly expressed five cytokines (MCP-1, MIP-1b, IL-ra1, IL-6, and IL-8) are measured among tested twenty-seven human cytokines from extracted solutions. (b) Highest levels of a cytokine, MCP-1 are measured when microglia are cultured under sAβ42 in monomers and oligomers at 2.3 pg.mL−1. (c) Microglial cells are cultured in the presence of gradients of cytokine-neutralizing antibody (Ab) combined with sAβ42 or cytokines and reduction of recruitment index is measured in the presence of neutralizing antibody against MCP-1 and sAβ42 at 23 pg.mL−1 but not 23 ng.mL−1. (d) MCP-1 is validated to be a potent chemoattractant for microglia and the recruitment is inhibited by immune-neutralization of MCP-1. However, MIP-1a and MIP-1b have no microglial chemoattractant activity in this assay. (Student's t-test. *P < 0.01 for oligomers with respect to no sAβ42). nwell = 3, ncell ≈ 2,000 in ‘(a)’, ‘(b)’ and nplatform = 4, ncell ≈ 2,500 in ‘(c)’, ‘(d)’ for each condition. Data represent mean ± s.e.m.
Reduced microglial mobility and viability on Aβ fibril-coated surface
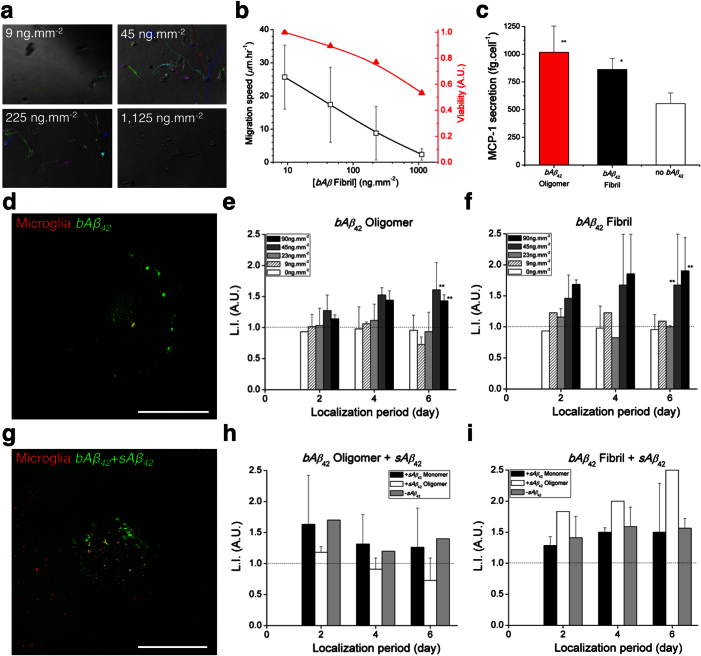
In AD brains, microglia presumably migrate towards plaques, and then remain stably associated with the plaques. To examine the mobility of microglia on surface-bound Aβ, we prepared wells uniformly coated with surface-bound Aβ42 fibrils and tracked individual microglia cells moving on these surfaces (Fig. 5a). We observed that the migration speed of microglia decreased substantially from 26 to 2 μm.hr−1 with an increase of surface-bound Aβ42 concentration from 9 to 1,125 pg.mm−2, consistent with the possibility that surface-bound Aβ42 inhibited microglial migration (Fig. 5b). We also observed some of the microglia internalizing particles formed on Aβ42 fibril–coated surfaces, as well as dead cells (Supplementary Movies S3, S4). At the same time, the viability of microglia decreased substantially on highly concentrated surface-bound Aβ42. Only 50% of microglia remained viable at 1,125 pg.mm−2 after four days, suggesting acute toxicity of surface-bound Aβ fibrils to microglia.
Figure 5. Microglial co-localization on surface-bound Aβ alone and in combination with soluble Aβ.
(a) Microglial cells were cultured in wells uniformly coated with bAβ42 fibrils and tracked individually. (b) The bAβ42 fibrils induce the simultaneous decrease of the mobility and the viability of microglia, proportional to increased concentrations of the bAβ fibrils. (c) The level of secreted MCP-1 is elevated by about two-fold on surfaces coated with bAβ42 oligomers and bAβ42 fibrils of 9 ng.mm−2 compared to an uncoated surface. (d) Fluorescent image visualized co-localized microglia in red on a spot of bAβ42 fibrils at 90 ng.mm−2 in green. Co-localization of microglia on the patterned bAβ42, becomes more effective as the concentration increases with 1.5-fold enrichment on bAβ42 oligomers (e) and 2-fold on bAβ fibrils (f) at 90 ng.mm−2 on a ‘day 6’, respectively. (g) Fluorescent image visualized co-localized microglia on bAβ42 fibrils at 90 ng.mm−2 combined with sAβ42 oligomers at 23 ng.mL−1 in a microfluidic platform. Co-localization is 1.6-fold on bAβ42 fibrils alone (h) compared to 2.5-fold on bAβ42 fibrils at 90 ng.mm−2 in combination with a gradient of sAβ42 oligomers at 23 ng.mL−1 (i). Scale bars, 1 mm. (Student's t-test. * P < 0.1, ** P < 0.05 with respect to no bAβ42). nwell = 2 in ‘(a)’, ‘(b)’, ‘(e)’, ‘(f)’ and nplatform = 2, ncell ≈ 2,000 in ‘(h)’, ‘(i)’ for each condition. Data represent mean ± s.e.m.
MCP-1 secretion stimulated by surface-bound Aβ
The level of secreted MCP-1 was elevated also by bAβ about two-fold (1,128.0 ± 149.8 and 1,088.7 ± 378.2 fg.cell−1 under Aβ oligomers and Aβ fibrils at 9 ng.mm−2, respectively) compared to without Aβ (554.4 ± 96.0 fg.cell−1) (Fig. 5c).
Microglial co-localization with patterned Aβ
To further understand how microglia target surface-bound Aβ–rich plaques, we patterned surface-bound Aβ42 in both oligomeric and fibril forms on wells at various concentrations by using PDMS stencils of 2 mm holes (Fig. 5d, Supplementary Fig. S5). We quantified the co-localization of microglia on the surface-bound Aβ42 patterns by calculating a localization index (L.I., defined in Supplementary Methods, Supplementary Fig. S8), representing the amount of localized cells on the patterned surface-bound Aβ relative to the day of microglia plating. We found that microglia co-localized with surface bound Aβ42 patterns at concentrations higher than 45 pg.mm−2. We measured a L.I. = 1.4 ± 0.1 with oligomers and L.I. = 1.9 ± 0.5 with fibrils at 90 pg.mm−2 on the eighth day (Fig. 5e–f).
Microglial recruitment and accumulation in the presence of soluble and patterned Aβ
To reconstruct the Aβ microenvironment analogous to that encountered by microglia near plaques, a core of fibrillar Aβ surrounded by a halo of soluble Aβ likely oligomers in the AD brains, we combined patterned surface-bound Aβ42 by using PDMS stencils of 1 mm holes and the gradient of soluble Aβ42 in the microfluidic platform (Fig. 5g, Supplementary Fig. S6). The combination of a gradient of soluble Aβ42 oligomers at 5 nM and surface-bound Aβ42 fibrils at 90 pg.mm−2 achieved the most effective localization by 2.5 times compared to 1.6 ± 0.2 times with surface-bound Aβ42 fibrils at 90 pg.mm−2 only (Fig. 5h–i).
Discussion
Our study demonstrates that soluble monomeric and oligomeric Aβ serve as a “recruiting signal” and bound fibrillar and oligomeric surface-bound Aβ acts as a “targeting signal” during microglia recruitment and localization. Together, soluble and insoluble Aβ have synergistic effects on microglial accumulation to sites of Aβ deposits, and could explain microglial accumulation in the vicinity of Aβ plaques in the AD cortex.
Moreover, the MCP-1 dependent mechanism of microglia recruitment in response to lower doses of Aβ may be important during physiological neuroinflammation and could be relevant to the early stages of microglial activation in AD. The microfluidic platform can be extended to the study of migration of other cells relevant to the progression of neurodegenerative diseases27,28, and could help quantify the potency of various cytokines and chemokines, which have been detected in the brain alone and in combinations11, in modulating microglia recruitment and accumulation. With high-throughput capabilities and regulated microenvironments, our platforms can facilitate systematic monitoring of microglial migration and its modulation by compounds for the treatment of neuroinflammation in various disease conditions, including Alzheimer's disease.
Methods
Microfluidic platform fabrication
Negative photoresists, SU-8 50 and SU-8 100 (MicroChem, Newton, MA, USA), were sequentially patterned using standard lithography on a 4” silicon wafer to create a mold for cell migration channels of 50 μm in height and chemokine compartments of 100 μm in height. A mixture of a base and a curing agent with a 10∶1 weight ratio (SYLGARD 184 A/B, Dowcorning, Midland, MI, USA) was poured onto the SU-8 mold and cured for one hour at room temperature under vacuum and, subsequently, cured for more than 3 hours in an oven at 80°C. The cured polydimethyl-siloxane (PDMS) replica was peeled off from the mold and holes were punched for fluid reservoirs. Arrayed holes were also laser-cut (Zing 24, Epilog Laser, Golden, CO, USA) into a thin PDMS membrane of 250 μm in thickness (HT 6240, Bisco Silicones, Elk Grove, IL, USA) and an acrylic plate of 6 mm in thickness. The machined membrane and the plate were glued together using uncured PDMS and incubated at 80°C overnight. This assembly was irreversibly bonded first to the PDMS replica using oxygen plasma at 50 mW, 5 ccm, for 30 seconds (PX-250, March Plasma Systems, Petersburg, FL, USA), and later to a glass-bottomed UniWell plate (MGB001-1-2-LG, Matrical Bioscience, Spokane, WA, USA). Immediately after the bonding, 10 μL of poly (l-lysine) solution (PLL, M.W. 70,000–150,000, 1.0 mg.mL−1, Sigma-Aldrich Co. LLC, St. Louis, MO, USA) was injected into the each platform and incubated for 2 hours at a room temperature to promote cellular adhesion. PLL-treated surface was rinsed with autoclaved and 0.2 μm filtered water (AM9920, Life Technologies, Grand Island, NY, USA) and then the devices were filled with cell culture medium containing 50∶50 of DMEM: F-12 supplemented with 5% FBS (Invitrogen, Grand Island, NY, USA), 10 ng.mL−1 of M-CSF (AF-300-25, PeproTech Inc., Rocky Hill, NJ, USA), 25 μg.mL−1 of gentamicin (G1397, Sigma-Aldrich), and 2.5 μg.mL−1 of amphotericin (A2411, Sigma-Aldrich).
Cell preparation
Human microglial cells (HMG 030, Clonexpress, Inc., Gaithersburg, MD, USA) were isolated initially as a free-floating population of cells from fetal brain tissue samples digested with collagenase and grown in a proprietary medium for 1–2 weeks. Before the experiment, cells were washed using medium without serum and the cell membrane was labeled with red fluorescent dye (PKH26PCL, Sigma-Aldrich). After centrifugation (400 g for 5 minutes), the cell pellet was re-suspended in 1 mL of Diluent C (G8278, Sigma-Aldrich) and immediately mixed with 4 μL of dye solution (P9691, Sigma-Aldrich) in 1 mL of Diluent C. The cell/dye mixture was incubated at room temperature for 4 minutes and periodically mixed by pipetting in order to achieve a bright, uniform, and reproducible labeling. After the incubation, the staining was stopped by adding an equal volume (2 mL) of 1% BSA in PBS and incubating for 1 minute to remove excess dye. Unbound dye was washed by centrifuging and re-suspending the cells in the culture medium. Finally, the stained microglia cells were suspended in the culturing medium at the concentration of 1 M cells.mL−1. Ten μL of cell solution was injected into each platform and 100 μL of a culturing medium was added into side and central extra wells. The loaded cells were incubated at 37°C supplied with 5% CO2 for at least two days before adding the various Aβ solutions. In the experiments, the cell culture media and Aβ solutions were replaced every two days while collecting the used solutions for Aβ analysis.
Sourcing of human brain tissue
Brains from human subjects with a diagnosis of Alzheimer's disease were obtained through the Massachusetts Alzheimer's Disease Research Center. The Massachusetts Alzheimer's Disease Research Center (ADRC) serves, among other clinical and research activities, as a repository of samples from patients with Alzheimer's disease. ADRC clinical and research activities are reviewed among others by the Institutional Review Board at the Massachusetts General Hospital. In addition, the ADRC has a Certificate of Confidentiality issued by the US Department of Health & Human Services/National Institutes of Health to protect the privacy of individuals who are enrolled in this research against forced disclosure in any civil, criminal, administrative, legislative or other proceeding, either at the federal, state of local level. The human samples used in this study were anonymised. The Institutional Review Board at the Massachusetts General Hospital viewed the use of anonymized autopsy material for biochemistry assays as exempt from review.
Aβ extraction from human brain tissues
Cortical gray matter from frontal lobe of AD patient brains was homogenized in 5 volumes of TBSI (Tris-buffered saline with protease inhibitor cocktail (Roche)) with 25 strokes on a mechanical Dounce homogenizer and centrifuged at 260,000 × g for 30 min at 4°C. The supernatant was used as a TBS-soluble fraction28,29. 750 μL of TBS-soluble fraction of human brains was separated by a size exclusion chromatography on double superdex 75 columns (GE Healthcare Life Sciences, Piscataway, NJ, USA) in 50 mM ammonium acetate pH 8.5 with an AKTA purifier 103,30. The individual fractions separated by SEC were analyzed by immunoblotting and Aβ specific sandwich ELISA and fraction 6 to 9 (>100 kDa) was used as a HMW Aβ and fraction 30 to 33 (20 ∼ 8 kDa) was used as a LMW Aβ30. Immediately after the size-exclusion chromatography, we removed soluble Aβ including soluble alpha Aβ precursor protein (APP) from the fractions by immune-depleting with 6E10 antibody (Signet, Dedham, MA, USA).
Time-lapse imaging
For continuous time-lapse imaging to track individual cells, we kept the UniWell plate on a fully automated microscope (Eclipse Ti, Nikon Inc., Melville, NY, USA) integrated with a heated incubating stage (LiveCell 05-11-0032 Rev B, Pathology Devices Inc., Westminster, MD, USA), which was set at 37.7°C, 5% CO2, and 85% humidity. We imaged cells at every 1-hour (NIS Elements, Nikon Inc.) using a bright field and every 6-hour using a TRITC fluorescence microscope for 4 days with a 10× objective lens and a perfect focusing system in a phase contrast mode. For continual time-lapse imaging to count cells, we kept the plate in an incubation chamber set at 37.7°C and 5% CO2 and then imaged it using 4× objective lens in a large-area mode of 8 × 8 mm2 with a 15% stitching, a phase contrast mode, and a TRITC fluorescent microscope every two–three days for seven days. For continual time-lapse imaging to monitor gradient stability, we kept the plate at room temperature in a dark room, and imaged using 4× objective lens in a large-area mode of 12 × 10 mm2 without stitching, a phase contrast mode, and a TRITC fluorescent microscope every two–three days for nine days.
Author Contributions
H.C., L.Z., B.T.H. and D.I. conceived the microfluidic platforms. H.C. and T.H. performed experiments and analysed data. T.H. prepared and analyzed human-derived soluble Aβ. H.C. and E.W. developed the algorithms for analyzing microglial mobility. Y.H. executed ELISA, western blot assays, EM imaging, and analyzed data. L.B.W. and K.M.H. executed a multiple cytokine assays and analyzed data. H.C., L.B.W., B.T.H. and D.I. wrote the manuscript.
Supplementary Material
Supplementary info
Movie S1
Movie S2
Movie S3
Movie S4
Acknowledgments
This work was supported in part by funding from the National Institutes of Health, grants AG005134 – pilot project 27.3, GM092804 and EB002503. We thank the BioMEMS RC for the use of fabrication facilities, Dr. Salil Desai for advise on microscale patterning techniques, and Dr. Ken Arai for discussion of Aβ stability.
References
- Nakajima K. & Kohsaka S. Microglia: activation and their significance in the central nervous system. J. Biochem. 130, 169–175 (2001). [DOI] [PubMed] [Google Scholar]
- Muzio L., Martino G. & Furlan R. Multifaceted aspects of inflammation in multiple sclerosis: the role of microglia. J. Neuroimmunol. 191, 39–44 (2007). [DOI] [PubMed] [Google Scholar]
- Khoury El J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13, 432–438 (2007). [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. & Perry V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009). [DOI] [PubMed] [Google Scholar]
- Milligan E. D. & Watkins L. R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A., Barres B. A. & Bennett M. L. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F. & Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). [DOI] [PubMed] [Google Scholar]
- Hanisch U.-K. & Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 (2007). [DOI] [PubMed] [Google Scholar]
- Mawuenyega K. G. et al. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science 330, 1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs C. K., Karlo J. C., Kao S.-C. & Landreth G. E. β-amyloid stimulation of microglia and monocytes results in TNF α-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 21, 1179–1188 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M. Q. & Hyman B. T. Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J. Neurovirol. 5, 32–41 (1999). [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A. et al. Stable size distribution of amyloid plaques over the course of Alzheimer disease. J. Neuropathol. Exp. Neurol. 71, 694–701 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T. et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 28, 4283–4292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grathwohl S. A. et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nature Neuroscience 12, 1361–1363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M. et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature 451, 720–724 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K. et al. Microglial transplantation increases amyloid-β clearance in Alzheimer model rats. FEBS Lett. 581, 475–478 (2007). [DOI] [PubMed] [Google Scholar]
- Burguillos M. A. et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 472, 319–324 (2011). [DOI] [PubMed] [Google Scholar]
- Grienberger C. et al. Staged decline of neuronal function in vivo in an animal model of Alzheimer's disease. Nat. Comm. 3, 774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie R. M. et al. Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid-β. Brain 135, 2155–2168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane S., Yang I. H., Ruffin A., Thakor N. & Venkatesan A. Circular compartmentalized microfluidic platform: study of axon–glia interactions. Lab on a Chip 10, 741–747 (2010). [DOI] [PubMed] [Google Scholar]
- Taylor A. M. et al. A Microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim H. J. & Jeon N. L. Biological applications of microfluidic gradient devices. Integr Biol (Camb) 2, 584–603 (2010). [DOI] [PubMed] [Google Scholar]
- Fagan A. M. et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Annals of Neurology 65, 176–183 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-Y. et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 30, 2636–2649 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K. et al. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci 51, 135–138 (1997). [DOI] [PubMed] [Google Scholar]
- Yang G. et al. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 21, 279–297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciosi S., Choi H. B., Kim S. U. & McLarnon J. G. IL-8 enhancement of amyloid-beta (Aβ 1-42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J. Neuroimmunol. 159, 66–74 (2005). [DOI] [PubMed] [Google Scholar]
- Fuhrmann M. et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat. Neurosci. 13, 411–413 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T. et al. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 21, 1524–1534 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M., Shankar G. M., Mehta T., Walsh D. M. & Selkoe D. J. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 572, 477–492 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary info
Movie S1
Movie S2
Movie S3
Movie S4