Abstract
Background:
Based on literature, in vitro cholesterol removal of lactic acid bacteria has been accounted for their in vivo cholesterol reduction. But recently it has been proposed that such in vitro characteristic may not be directly relevant to their in vivo activity. The objective of this study was to find how much in vitro cholesterol reducing potential of Lactobacillus plantarum A7 (LA7), a native strain isolated from an infant fecal flora, reflects its in vivo efficiency. LA7 previously showed serum cholesterol reducing capability in mice subjected to fatty diet. Here, we investigate whether the given strain is capable of in vitro cholesterol assimilation or consumption.
Method:
LA7 was cultured in whole milk and de-Man–Rogosa–Sharpe (MRS) added with water-soluble cholesterol. Colorimetric method was adopted for cholesterol determination in both cultured media during incubation period.
Results:
No cholesterol assimilation was detected by growth and incubation of the active culture in either of the medium. Thus, in vivo cholesterol function of LA7 was not caused by cholesterol consumption. A comprehensive review of literature on the related studies also showed that there are other documented studies which evidenced the uncertainty of the direct relation between in vitro and in vivo studies.
Conclusion:
Cholesterol removal from the cultured media may not be considered as an appropriate integral index for selection of Lactobacillus strains with cholesterol-lowering activity.
Keywords: Cholesterol, in vitro, in vivo, Lactobacillus plantarum, milk
INTRODUCTION
Alleviation of hypercholesterolemia by introduction of high population of lactic acid bacteria in the diet has remained a controversial subject.[1–3] Some strains of Lactobacillus genera have been identified to exhibit cholesterol-reducing capability through in vitro or/and in vivo examinations.[4–11] The mechanisms underlying this activity have been proposed to involve assimilation of cholesterol, cholesterol adherence to the bacterial cell wall or its incorporation into bacterial cells, physiological action of the end products of short chain fatty acids by fermentation, destabilization and co-precipitation of the cholesterol micelles, bile salt hydrolase activity of the lactobacilli,[8,11] cholesterol oxidize activity,[12] and finally production of some functional peptides.[4]
Since lowering the serum cholesterol is a health promoting characteristic, the idea of selection of microbial strains with cholesterol-reducing effect has been developed as a tool in order to introduce new probiotic microorganisms.[9] Some studies looked to the cholesterol-reducing activity of some Lactobacillus strains for providing healthier cholesterol-reduced fermented products.[10,12] Probiotics are viable microbial supplements that beneficially affect the host through their effects in the intestinal tract.[8] Despite many mechanisms involved, strain dependency is the general concept emphasized in most of the related studies.
To find new probiotics with cholesterol-reducing capability, many Lactobacillus strains have been examined using in vivo and/or in vitro tests. Generally, application of in vitro tests precedes the in vivo trials. However, some positive in vivo studies used Lactobacillus strains lacking any history of in vitro cholesterol activity. Considering the in vitro studies, most of them proposed one or more mechanisms mentioned above to be responsible for their observed results. In Table 1, 16 in vitro studies are summarized.[4,8,10,13–25] Cholesterol assimilation or reduction in the culture media is one of the most referred mechanisms among the in vitro experiments, which is measured by determination of cholesterol in the cholesterol added de-Man–Rogosa–Sharpe (MRS) medium before and after the complete growth of the examined Lactobacillus strains.[8–11] In addition, full fat milk is occasionally used as the culture media.[10] The more assimilated cholesterol in the spent culture broth, the more cholesterol-reducing activity is attributed to the examined strain. The bacterial strains with the potential cholesterol assimilation or cholesterol adherence display a positive reaction in the test tube.
Table 1.
In vitro studies on the cholesterol reduction capability of Lactobacillus strains in MRS broth or in milk
Recently, in an animal study in Isfahan University of Medical Science, Lactobacillus plantarum A7 (LA7) effectively changed the serum lipid profile of the tested animal. LA7 is a native strain, isolated from an infant fecal flora and was characterized as probiotic potential.[26,27] Administration of 108 cells of LA7 through milk-based formula led to lower plasma cholesterol in a group of mice, under fatty diet.[27] At the end of the study, low-density lipoprotein (LDL) cholesterol and total triglycerides in the treated group of mice were shown to be level off by 1.73% and 7.77% than that of the original concentration, respectively, demonstrating 28-30% reduction in both parameters in the treated group. Here in the present study, the objective was to investigate the reaction of LA7 in the Lactobacillus-specific media enriched with cholesterol and milk. The expectation was to observe a noticeable in vitro cholesterol-reducing activity.
METHODS
Bacterial strain and culture media
LA7 was provided by microbial collection of Food Microbiology and Biotechnology of Isfahan University of Technology, Isfahan, Iran. Water-soluble cholesterol; polyoxyethanyl cholesteryl sebacate (Sigma Chemical Co., St. Louis, MO, USA) was used as a source of cholesterol to be added (100 mg/l) into the sterilized de-Mans-Rogosa broth (Merck-Germany). One hundred milliliter of commercial ultra high temperature (UHT) sterilized milk (Mihan, Iran) 30 g/l fat and MRS added cholesterol were inoculated with the 1% overnight culture of LA7 and incubated at 37°C aerobically for 24 and 72 hours, respectively. Viable plate count and change in pH was performed for bacterial growth monitoring in both media.
Cholesterol determination
Five milliliter samples of the cultured media were removed at the time intervals of 3, 5, 7, 12, and 24 hours incubation of both media and continued up to 72 hours for cultured UHT milk. Samples were centrifuged and spent broth as well as the cell pellet fractions were used individually for cholesterol measurement. Lipid extract from milk and cultured milk was prepared using the Folch method; briefly 5 ml of milk were mixed by 50 ml chloroform-methanol solvent (2:1, v/v) and lipid extract was washed by 20% of its volume ratio with distilled water, chloroform layer was dried under nitrogen.[28] The modified colorimetric method[8,29] was adopted in order to determine the water-soluble cholesterol and natural milk cholesterol. One milliliter of the tested solution was added to 1 ml of 33% w/v potassium hydroxide and 2 ml of absolute ethanol, mixed for 1 min and incubated at 37°C for 15 min. After cooling, 2 ml of distilled water and 3 ml of hexane layer were removed and transferred into a test tube and evaporated under nitrogen. The dried material was dissolved in 2 ml of o-phthalaldehyed reagent and mixed thoroughly. Then, 0.5 ml of sulfuric acid (12 N, Merck), was added and the mixture was mixed for 1 min. After 10 min, absorbance was read at 550 nm (Genway–model 6800). Cell pellet fraction was resolved in 1 m of phosphate buffer (pH = 7) and processed as mentioned above. Determination of cholesterol in milk and cultured milk were carried out in the same manner as cultured media with the exception of applying 1 ml lipid extract of milk instead of 1 ml MRS as the sample. The milk lipid extract was obtained as described elsewhere.[28,30]
Preparation of standard curve
Several preparations of the standard solutions at the concentrations of 1, 2, 5, 7, 10, 50, 100, and 200 mg/l of polyoxyethanyl cholesteryl sebacate in sterilized MRS (Merck) were prepared. Each standard solution was treated as a sample and underwent the whole procedure of the experiment.
Evaluation of reproducibility
For the evaluation of reproducibility of the method, inter assay coefficient of variation (Cv) for the measurement of 50 mg/l solution of polyoxyethanyl cholesteryl sebacate in MRS during four consequent days (n = 2 × 4) was determined. Intra assay Cv for the measurement of the given concentration of polyoxyethanyl cholesteryl sebacate was investigated by ten 6 replicate of the measurement through (n = 6 × 1) a day.
Statistical analysis
Data was analyzed using Minitab statistical software version 16 (Minitab Inc, State College, PA, USA), diagrams were drawn using Excel 2007.
RESULTS
The growth of LA7 was not restricted or enhanced by the presence of water-soluble cholesterol in MRS. In MRS containing 100 mg/l water-soluble cholesterol, LA7 showed a typical growth pattern of L. plantarum, producing the maximum optical density of 3.5 at 620 nm corresponding to 1010 colony forming unit (CFU/ml) after 20 hours of incubation. Cells of LA7 showed slow growth in milk, however, after 72 hours, total count reached at 108 CFU/ml.
Quality parameters
Standard curve for water-soluble cholesterol showed linearity over the range of tested concentrations (R2= 0.98.5, a = 1748, b = –3.8). Inter and intra assay Cv for cholesterol determination experiment were obtained as 26% and 11.21%, respectively, and the analytical recovery was determined as 86% using 50 mg/l standard sample as spiked.
The results of cholesterol measurement
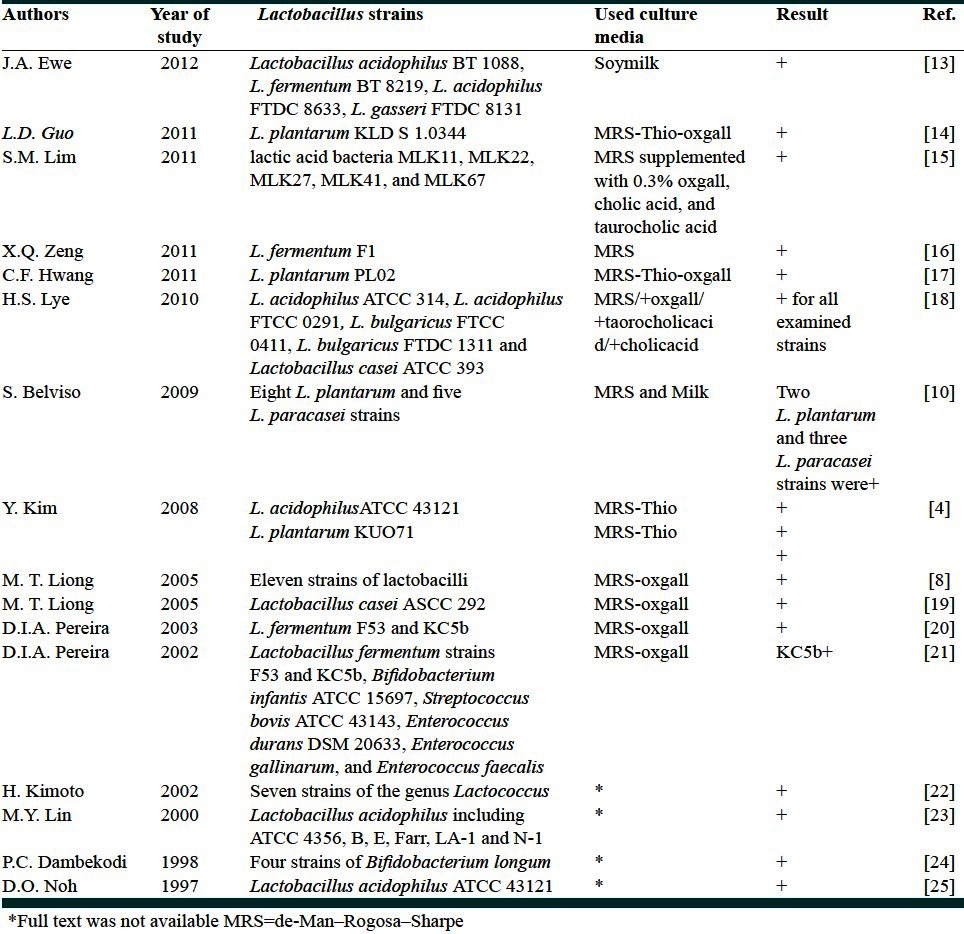
Figure 1 illustrates the cholesterol concentration measured in the spent MRS broth and in the corresponding precipitate fraction, a variation of about 10 mg/l cholesterol from that of the adjusted concentration was seen in the supernatant during the incubation period. However, the final cholesterol concentration remained stable during the growth of LA7 strain in MRS. In addition, a slight increase in the cholesterol content of the precipitate fraction was observed at the end stage of incubation. Cholesterol concentration of the whole milk during 72 hours of LA7 growth was not reduced [Figure 2]. The synthetic water-soluble cholesterol was not assimilated by the given strain nor the milk lipoprotein-bounded cholesterol concentration was affected during the growth in milk; therefore the serum cholesterol reduction capability, which was observed in mice, was not associated with the in vitro cholesterol assimilation and consumption.
Figure 1.
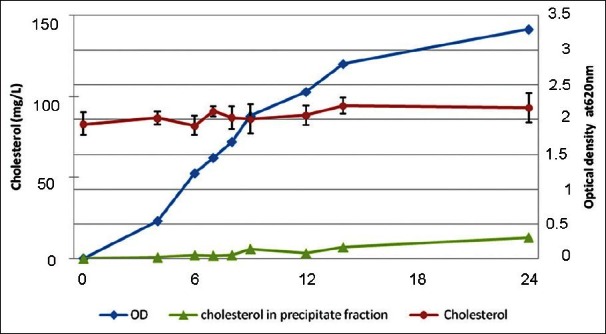
Cholesterol in the cell free supernatant of MRS broth and in corresponding precipitate fraction measured during the growth of L. plantarum A7
Figure 2.
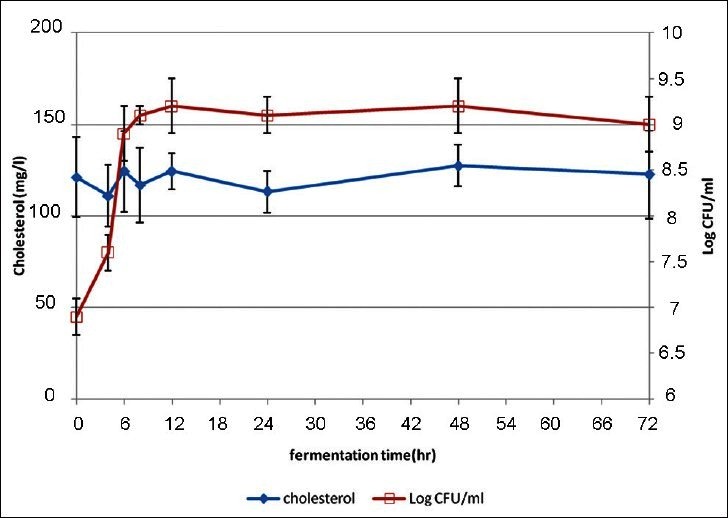
Measured cholesterol in whole milk during the growth of L. plantarum A7
DISCUSSION
The in vitro study of cholesterol removal of Lactobacilli has been consistently used as a screening tool for selection of probiotic stains with diverse health promoting characteristics.[9] Gilliland et al. were the first to show that in vivo efficiency of Lactobacilli could be directly associated with the strains’ cholesterol-removal capability in the cholesterol-enriched media. In their studies, only 2 μg/ml difference in cholesterol removal between Lactobacillus strains resulted in significant different responses in reducing the pig's plasma cholesterol, they emphasized on the impact of anaerobic condition as well as presence of bile in the medium, as the intransitive factors, affecting the results of in vitro cholesterol removal or uptake by Lactobacilli as if, in the absence of bile salts, no cholesterol reduction has taken place.[31] Cholesterol removal from the culture was attributed to the following mechanisms: Cholesterol assimilation, incorporation to cell membrane or attachment to the bacterial cell surfaces, and destabilization of cholesterol micelles resulting in the co-precipitation of cholesterol with bile salts. Presence of bile salts in the cholesterol containing test media is needed to mimic the human gut, however, the role of the bile in the uptake of cholesterol by Lactobacilli is explained by the co-precipitation of bile acid with cholesterol in low pH environment and more likely to occur at the end of fermentation process. Moreover, it was stated that due to the impact of bacterial bile salt hydrolysis, conjugated bile acids turn to be less soluble de-conjugated counterparts leading to more precipitating of cholesterol and bile acids. Accordingly, lack of cholesterol removal capability of LA7 in the present study may be due to the absence of bile in the medium. Note that, reviewing the literature showed that having a media free of bile could not be very restrictive; in Table 1, in vitro studies on the cholesterol removal properties of lactic strains are summarized; based on the result of the studies presented in this table, positive in vitro studies without using bile salts are evidenced.[8,32] The highest in vitro cholesterol reduction of nearly 310-490 mg/l was reported for L. plantarum strains in the absence of bile.[32] When milk was used as the culture media in some studies, again, bile component was not added to the milk.[12] Moreover, it was shown that cheese isolated L. plantarum strains in an aerobic condition can remove off 8% of the whole homogenized milk cholesterol after complete growth. Cell wall binding capacity was stated to be the major mechanism involved in the latter study.[10] In another study, 100% of milk cholesterol reduction of L. helveticus cultured in aerobic, bile free condition was attributed to the production of cholesterol oxidase.[12] Cholesterol reducing capability of 3-100 mg/l from the media or milk is reported by other studies as presented in Table 1.[4,8,10,13–25] In this study, slight increase in the cholesterol content of growing cell precipitates unlikely explains the L. plantarum in vivo cholesterol activity and our final conclusion was that, despite the effectiveness of LA7 for reduction of lipid parameters in mice, it was unable to remove cholesterol from culture media or milk. Since this result sound not to be in accordance with the general belief that “in vitro cholesterol removal of a lactobacillus strain predicts it is in vivo action”; a comprehensive review literature was carried out in order to explain to what extent this relation exists. The results are shown in Tables 2 and 3.
Table 2.
In vivo studies on the cholesterol reduction capability of Lactobacillus strains in MRS broth or in milk without history of in vitro cholesterol reduction capability
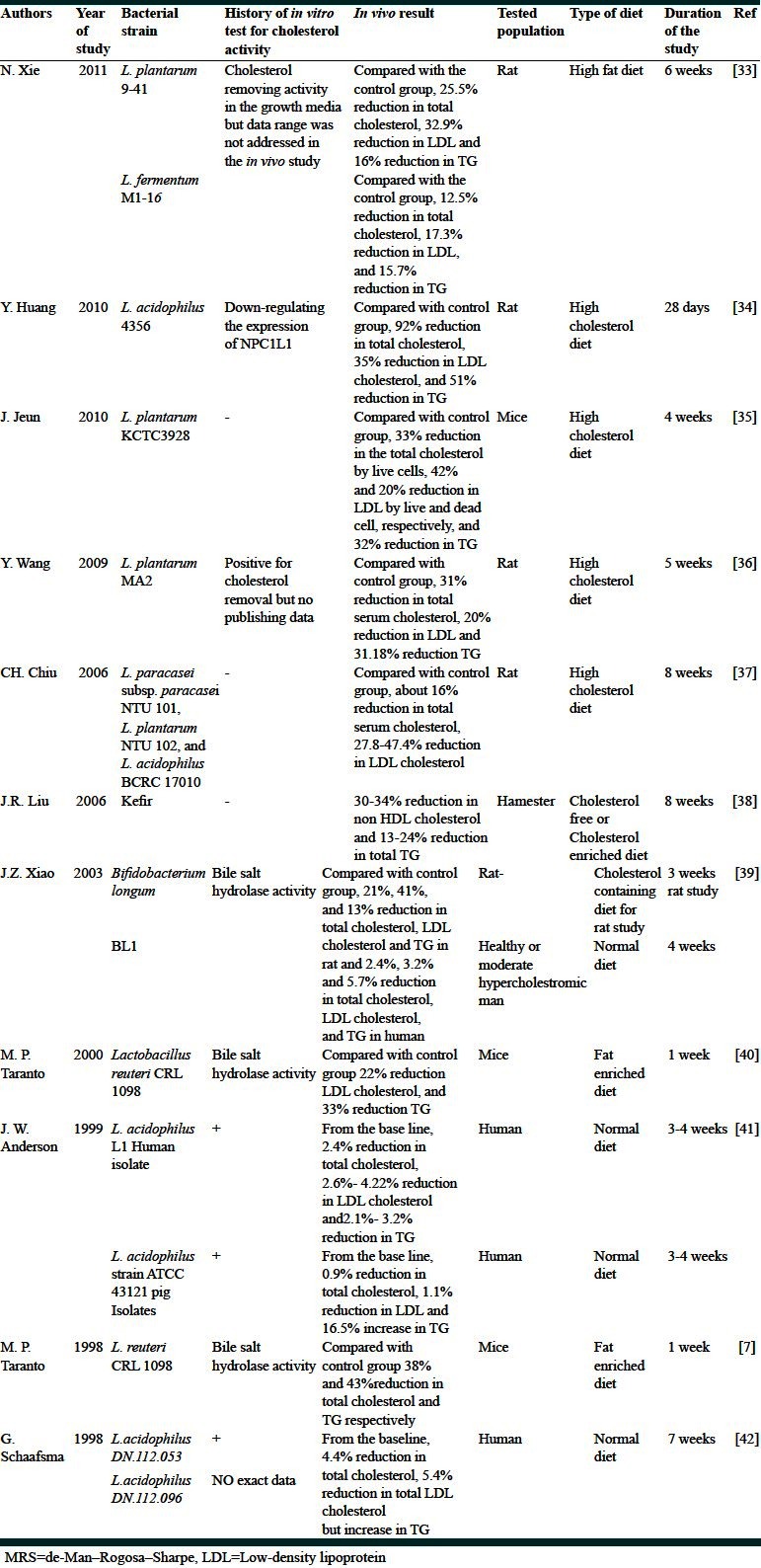
Table 3.
The studies included both in vitro and in vivo experiments
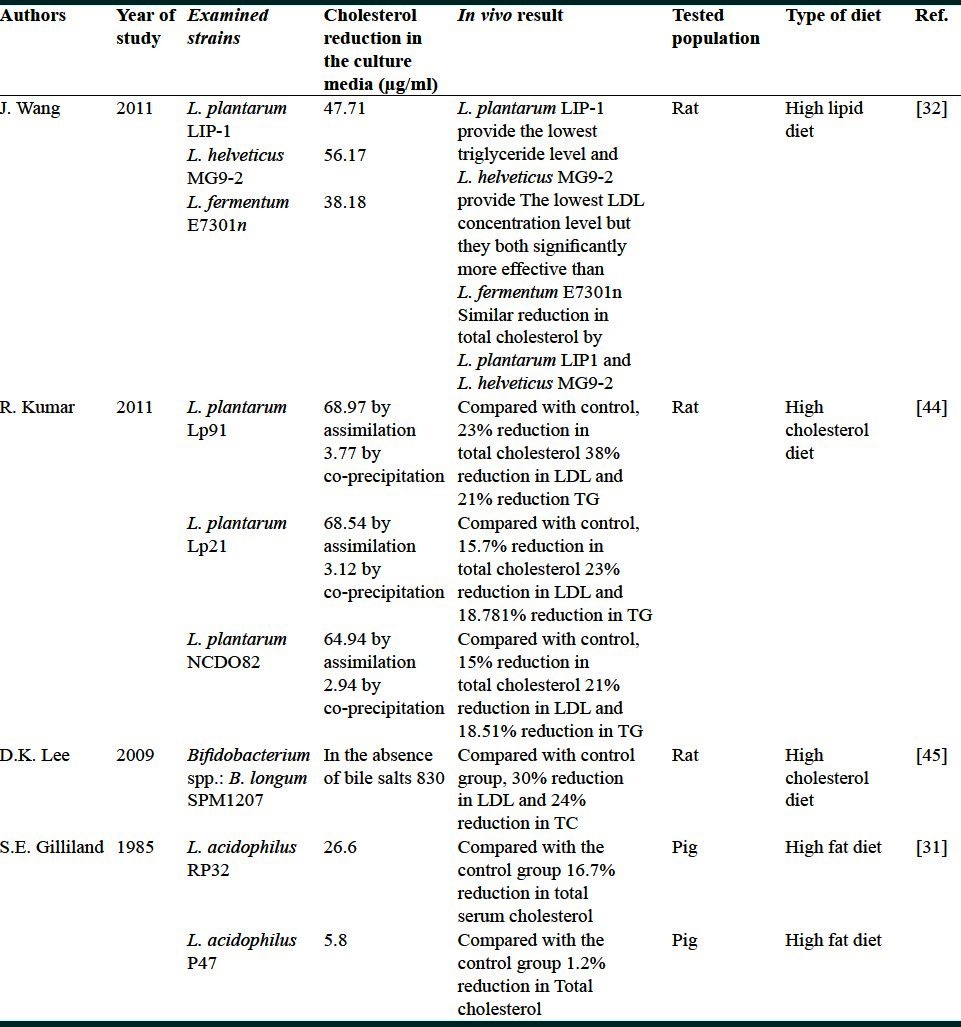
Table 2 presents the summary of 11 rat or human studies[7,33–40] in which different lactic strains administered to the subjects to investigate the serum cholesterol properties of the strains. Some of these studies lacked information on the in vitro cholesterol removal of the used bacterial strains.[35,37,38] Others pointed to this property but they did not mention how much the tested strains were capable of cholesterol removal in the primary in vitro experiment.[7,33,34,36,39–42] Among these studies, four studies relied on cholesterol-reducing activity of the tested strains that had been observed primarily in their in vitro test.[33,36,41,42] However, there was a great difference in the efficacy of the lactic strains between animal and human studies. Among the animal studies, 12-92.5% reduction in total cholesterol was observed, as if, the reduction rate of 20-40% was prevalent. While in the human studies, reduction rate of serum cholesterol did not exceeds 4.4%. Reduction rate of LDL cholesterol and serum triglyceride was 17-47% and 15.7-51% among the animal studies but 3.4% and 5.7% for the human trials. Considering the vast number of positive in vivo studies, general agreement on probiotic Lactobacillus intervention for serum cholesterol reduction exists; however, there is controversy over the effectiveness of such intervention particularly in human studies.[2,32] It was known that the outcomes of in vivo experiments can be affected by some identified reasons. One of the most important challenges is the absence of proper placebo. Milk was used in many of these studies as placebo, whereas it has been identified to pose hypocholesterolemic effect and not to be a proper placebo. In some studies yogurt was considered as placebo. Traditional yogurt starter culture is composed of L. bulgaricus and Sterptoccocus thermophilus. Alkalin[43] and Schafsms[42] in two individual studies concluded that probiotic L. acidophilus strain could be more effective in reducing human serum cholesterol concentrations than that of normal yogurt. It is interesting to know that in the recent in vitro study performed by Ooi et al.[2] L. bulgaricus strains showed more cholesterol-reducing activity from the culture medium than that of Lactobacillus strains belonging to the common probiotic species like L. casei. Therefore, the latter study can be regarded as a complete controversy if in vitro cholesterol reduction is believed to be a reliable index for in situ condition.
Table 3 presents the results of the studies including both in vitro and in vivo animal experiments.[31,32,41,42,44,45] In vitro experiments on the tested strains resulted in 5.8-71 mg/l cholesterol removal and subsequent animal studies using the given strains resulted in 1.2-24% reduction in the total serum cholesterol in the examined animals.
Comparing the results of the studies which are presented in Tables 2 and Table 3 regarding cholesterol reduction efficacy, it was revealed that the diversity of serum cholesterol reduction resulted by the in vivo studies without any in vitro background (Table 2) was in the range of 16–33%, not much different than that of the studies presented in Table 3 (1.2–24%). While the latter studies used bacterial strains which demonstrated remarkable serum cholesterol reduction in the culture media before in vivo trial. Such finding is in agreement with a very recent report by Wang et al., who concluded that in vitro efficacy of Lactobacilli for cholesterol reduction in media was not consistent with their results in the in vivo condition.[32]
CONCLUSIONS
The capability of LA7 in serum lipid cholesterol reduction was not associated by cholesterol assimilation in either Lactobacilli specific medium or whole milk, at least in aerobic and bile free condition. Other mechanisms, for example, production of active metabolites may be involved in bioactivity of this strain. Evaluation of short chain fatty acids production is a characteristic which is overlooked when selecting a probiotic culture for cholesterol-reducing ability. Thus, cholesterol consumption or its removal from the cultured media may not be an integral, reliable index for selection of Lactobacillus strains with cholesterol-lowering activity.
ACKNOWLEDGMENTS
The authors wish to acknowledge the Food Security Research Center for the financial support.
Footnotes
Source of Support: Food Security Research Center in Isfahan University of Medical Science
Conflict of Interest: We certify that there is no conflict of interest with any financial organization regarding the material discussed in this article
REFERENCES
- 1.Lichtenstein AH, Goldin B. Lactic acid bacteria and intestinal drug and cholesterol metabolism. In: Salminen S, Wright AV, Ouwehand A, editors. Lactic acid bacteria microbial and functional aspects. 3th ed. New York: Marcel Dekker; 2004. pp. 507–14. [Google Scholar]
- 2.Ooi L, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St-Onge M, Farnworth EF, Jones P. Consumption of fermented and nonfermented dairy products: Effects on cholesterol concentrations and metabolism. Am J Clin Nutr. 2000;71:674–81. doi: 10.1093/ajcn/71.3.674. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, Whang JY, Whang KY, Oh S, Kim SH. Characterization of the cholesterol reducing activity in a cell-free supernatate of Lactobacillus acidophilus ATCC43121. Biosci Biotechnol Biochem. 2008;72:1483–90. doi: 10.1271/bbb.70802. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TD, Kang JH, Lee MS. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowereing effect. Int J Food Microbiol. 2007;113:358–61. doi: 10.1016/j.ijfoodmicro.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Brashears MM, Gilliand SE, Buck LM. Bile salt deconjucation and cholesterol removal from media by Lactobacillus casei. J Dairy Sci. 1998;81:2103–10. doi: 10.3168/jds.S0022-0302(98)75785-6. [DOI] [PubMed] [Google Scholar]
- 7.Tananto MP, Medici M, Perdigon G, Holgado Ruiz AP, Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci. 1998;81:2336–40. doi: 10.3168/jds.S0022-0302(98)70123-7. [DOI] [PubMed] [Google Scholar]
- 8.Liong MT, Shah NP. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J Dairy Sci. 2005;88:55–66. doi: 10.3168/jds.S0022-0302(05)72662-X. [DOI] [PubMed] [Google Scholar]
- 9.Gilliand SE, Walker DK. Factors to consider when selecting a culture of L. acidophilus as a dietary adjunct to produce a hypercholesterolemic effect in human. J Dairy Sci. 1990;73:905–9. doi: 10.3168/jds.S0022-0302(90)78747-4. [DOI] [PubMed] [Google Scholar]
- 10.Belviso S, Giordano M, Dolci P, Zeppa G. In vitro cholesterol- lowering activity of Lactobacillus plantarum and Lactobacillus paracasei strains isolated from the Italian Castelmango PDO cheese. Dairy Sci Technol. 2009;89:169–76. [Google Scholar]
- 11.Sirilun S, Chaiyasut Ch, Kantachote D, Luxananil P. Characterization of non-human origin probiotic Lactobacillus plantarum with cholesterol-lowering property. Afr J Microbiol Res. 2010;10:997–1000. [Google Scholar]
- 12.Ahire JJ, Bhat AA, Thakare JM, Pawar PB, Zope DG, Jain RM, et al. Cholesterol assimilation and biotransformation by Lactobacillus helveticus. Biotechnol Lett. 2012;34:103–7. doi: 10.1007/s10529-011-0733-2. [DOI] [PubMed] [Google Scholar]
- 13.Ewe JA, Karim AA, Bhat R, Abdullah Wan WN, Liong MT. Enhanced growth of lactobacilli and bioconversion of isoflavones in biotin-supplemented soymilk upon ultrasound-treatment. Ultrason Sonochem. 2012;19:160–73. doi: 10.1016/j.ultsonch.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Guo LD, Yang L, Huo G. Cholesterol removal by Lactobacillus plantarum isolated from homemade fermented cream in Inner Mongolia of China. Czech J Food Sci. 2011;29:219–25. [Google Scholar]
- 15.Lim SM. Bile salts degradation and cholesterol assimilation ability of Pediococcus pentosaceus MLK67 isolated from mustard leaf Kimchi. Korean J Microbiol. 2011;47:231–40. [Google Scholar]
- 16.Zeng XQ, Pan DD, Zhou PD. Functional characteristics of Lactobacillus fermentum F1. Curr Microbiol. 2011;62:27–31. doi: 10.1007/s00284-010-9669-3. [DOI] [PubMed] [Google Scholar]
- 17.Hwang CF, Chen J, Huang Y, Mao Z. Biomass production of Lactobacillus plantarum LP02 isolated from infant feces with potential cholesterollowering ability. Afr J Biotechnol. 2011;10:7010–20. [Google Scholar]
- 18.Lye HS, Rahmat-Ali GR, Liong M. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J. 2010;20:169–75. [Google Scholar]
- 19.Liong MT, Shah NP. Optimization of cholesterol removal by probiotics in the presence of prebiotics by using a response surface method. Appl Environ Microbiol. 2005;71:1745–53. doi: 10.1128/AEM.71.4.1745-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira DI, McCartney AL, Gibson GR. An In vitro study of the probiotic potential of a bile-salt-hydrolyzing lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl Environ Microbiol. 2003;69:4743–52. doi: 10.1128/AEM.69.8.4743-4752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira DI, Gibson GR. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl Environ Microbiol. 2002;68:4689–93. doi: 10.1128/AEM.68.9.4689-4693.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimoto H, Ohmomo S, Okamoto T. Cholesterol removal from media by Lactococci. J Dairy Sci. 2002;85:3182–8. doi: 10.3168/jds.S0022-0302(02)74406-8. [DOI] [PubMed] [Google Scholar]
- 23.Lin MY, Chen TW. Reduction of cholesterol by lactobacillus acidophilus in culture broth. J Food Drug Anal. 2000;8:97–102. [Google Scholar]
- 24.Dambekodi PC, Gilliland SE. Incorporation of cholesterol into the cellular membrane of bifidobacterium longum. J Dairy Sci. 1998;81:1818–24. doi: 10.3168/jds.S0022-0302(98)75751-0. [DOI] [PubMed] [Google Scholar]
- 25.Noh DO, Kim SH, Gilliland SE. Incorporation of cholesterol into the cellular membrane of lactobacillus acidophilus ATCC 43121. J Dairy Sci. 1997;80:3107–13. doi: 10.3168/jds.S0022-0302(97)76281-7. [DOI] [PubMed] [Google Scholar]
- 26.Mirlohi M, Soleimanian-Zad S, Dokhani SH, Sheikh-Zeinodin M. Investigation of bile and acid tolerance of native lactobacilli from fecal samples and commercial probiotics through growth and survival studies. Iran J Biotechnol. 2009;7:233–40. [Google Scholar]
- 27.Fazeli H, Moshtaghian J, Mirlohi M, Shirzadi M. Reduction in lipid serum parameter by incorporation of a native strain of Lactobacillus plantarum A7 in mice. IJDLD. 2010;9:1–7. [Google Scholar]
- 28.Park YW. Cholesterol contents of U.S. and imported goat milk cheeses as quantified by different colorimetric methods. Small Rumin Res. 1999;32:77–82. [Google Scholar]
- 29.Rudel LL, Morris MD. Determination of cholesterol using O- phthalaldehyde. J Lipid Res. 1973;14:364–6. [PubMed] [Google Scholar]
- 30.Mirlohi M, Madani G, Hassanzadeh A, Yahay M. On the colorimetric method for cholesterol determination in the laboratory media. Int J Biol Chem. 2012:637–42. [Google Scholar]
- 31.Gilliland SE, Nelson CR, Maxwell C. Assimilation of cholesterol by lactobacillus acidophilus. Appl Environ Microbiol. 1985;49:377–81. doi: 10.1128/aem.49.2.377-381.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Zhang H, Chen X, Chen Y, Menghebilige, Bao Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J Dairy Sci. 2011;95:1645–54. doi: 10.3168/jds.2011-4768. [DOI] [PubMed] [Google Scholar]
- 33.Xie N, Cui Y, Yin Y, Zhao X, Yang J, Wang Z, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement Altern Med. 2011;11:1–11. doi: 10.1186/1472-6882-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Wang J, Cheng Y, Zheng Y. The hypocholesterolaemic effects of Lactobacillus acidophilus American Type Culture Collection 4356 in rats are mediated by the down-regulation of Niemann-Pick C1-Like 1. Br J Nutr. 2010;104:807–12. doi: 10.1017/S0007114510001285. [DOI] [PubMed] [Google Scholar]
- 35.Jeun J, Sukyung K, Sung-Yun C, Hee-jin J, Hyun-Jin P, Jae-Gu S, et al. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition. 2010;26:321–30. doi: 10.1016/j.nut.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Xu N, Xi A, Ahmed Z, Zhang B, Bai X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol. 2009;84:341–7. doi: 10.1007/s00253-009-2012-x. [DOI] [PubMed] [Google Scholar]
- 37.Chiu CH, Tzu-Yu L, Yun-Yu T, Tzu-Ming P. The effects of Lactobacillus-fermented milk on lipid metabolism in hamsters fed on high-cholesterol diet. Appl Microbiol Biotechnol. 2006;71:238–45. doi: 10.1007/s00253-005-0145-0. [DOI] [PubMed] [Google Scholar]
- 38.Liu JR, Wang S, Chen M, Chen H, Yueh P, Lin C. Hypocholesterolaemic effects of milk-kefir and soyamilk-kefir in cholesterol-fed hamsters. Br J Nutr. 2006;95:939–46. doi: 10.1079/bjn20061752. [DOI] [PubMed] [Google Scholar]
- 39.Xiao JZ, Kondo S, Takahashi N, Miyaji K, Oshida K, Hiramatsu A, et al. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci. 2003;86:2452–61. doi: 10.3168/jds.S0022-0302(03)73839-9. [DOI] [PubMed] [Google Scholar]
- 40.Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in mice. J Dairy Sci. 2000;83:401–3. doi: 10.3168/jds.S0022-0302(00)74895-8. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JW, Gilliland SE. Effect of fermented milk (Yogurt) containing lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J Am Coll Nutr. 1999;18:43–50. doi: 10.1080/07315724.1999.10718826. [DOI] [PubMed] [Google Scholar]
- 42.Schaafsma G, Meuling WJ, Dokkum W, Bouley C. Effects of a milk product, fermented by Lactobacillus acidophilus and with fructo-oligosaccharides added, on blood lipids in male volunteers. Eur J Clin Nutr. 1998;52:436–40. doi: 10.1038/sj.ejcn.1600583. [DOI] [PubMed] [Google Scholar]
- 43.Akalin AS, Gönç S, Düzel S. Influence of yogurt and acidophilus yogurt on serum cholesterol levels in mice. J Dairy Sci. 1997;80:2721–5. doi: 10.3168/jds.s0022-0302(97)76233-7. [DOI] [PubMed] [Google Scholar]
- 44.Kumar R, Grover S, Batish VK. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague–Dawley rats. Br J Nutr. 2011;105:561–73. doi: 10.1017/S0007114510003740. [DOI] [PubMed] [Google Scholar]
- 45.Lee DK, Jang S, Baek EH, Kim MJ, Lee KS, Shin HS, et al. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009;8:1–8. doi: 10.1186/1476-511X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


