Abstract
Background:
Different stressors induce learning and memory impairment and physical activity influence learning and memory enhancement. In this research, we investigated the effect of synchronized running activity with stress on acquisition and retention time of passive avoidance test.
Methods:
Male Wistar rats were randomly divided into five groups as follows: Control (Co), Sham (Sh), Exercise (Ex), Stress (St), synchronized exercise with stress (St and Ex) groups. Chronic restraint stress was applied by 6 h/day for 21 days and treadmill running 1 h/day for 21 days. For evaluation of learning and memory, initial and step-through latency were determined at the end of study by using passive avoidance learning test.
Results:
Our results showed that: (1) Exercise under no stress provides beneficial effects on memory acquisition and retention time compared to Control group; especially retention time had significantly (P < 0.05) increased in exercised group. (2) Chronic stress with and without synchronized exercise significantly (P < 0.01, P < 0.05, respectively) impaired acquisition and retention time. (3) Body weight differences were significantly (P < 0.01, P < 0.001 and P < 0.001) lower than Control group in exercise, stress and synchronized exercise with stress groups, respectively. (4) Adverse effects of restraint stress (psychical stress) were probably greater than physical activity effects on learning, memory and weight loss.
Conclusions:
The data confirmed that synchronized exercise with stress had not significantly protective role in improvement of passive avoidance acquisition and retention time; hence it did not significantly improve learning and memory deficit in stressed rats; whereas exercise alone could improve memory deficit in rats.
Keywords: Body weight, learning, passive avoidance, physical activity, stress
INTRODUCTION
Stress is an important factor that influences learning and memory processes, and may result in psychological disorders,[1] especially when it is prolonged and uncontrollable.[2,3] Accordingly, a few studies have investigated possible ways of eliminating stress deleterious effects.[4] Physical activity is one of these strategies that is proposed to enhance cognitive functions.[5] Previous studies indicated that exercise can facilitate acquisition and/or retention in various hippocampal-dependent behavioral tasks including the passive avoidance,[6,7] active avoidance,[8] Morris water maze,[9,10] radial arm maze,[11] radial arm water maze[12,13] and object recognition.[14] It is documented that such kind of physical activity improve cognitive and spatial learning;[15–17] hence, exercise probably enhances them via different mechanisms such as changes of neuronal activity, synaptic structure and the neurotransmitters synthesis that are important in learning and memory processing.[18]
Given that humans cannot spend much time during the day for performing running activity;[6] in present study, we used treadmill running since it is more similar to human exercise training, and allows to animals to run only for a limited time per day. Therefore, we are able to truly estimate protective effects of running activity. In other word, using a forced running paradigms (e.g., treadmill) are less well demonstrated on learning and memory. The goal of the present study was to determine the effects of synchronized running activity with stress on acquisition, consolidation and retrieval phases in cognitive function induced by chronic restraint stress. Therefore, in this study, we examined whether synchronized forced exercise with stress may in fact help to moderate learning impairment in stressed rats and to alter the behavioral response and learning performance.
METHODS
Experimental animals
Experiments were performed on 50 male Wistar rats, with an initial weight of 250-300 g that were obtained from Jondishapour Institute, Ahwaz, Iran. All of the experimental protocols were approved by the Committee of Ethics of the Isfahan University of Medical Science (Isfahan, Iran), followed the “Principles of Laboratory Animal Care” and carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). Rats were housed five per each cage; under light-controlled condition (12 h light/dark cycle; lights on at 07:00 am -19:00 pm). The room temperature was 22 ± 2°C. Food and water were available ad libitum, except during the stressing procedure. All behavioral experiments were carried out at 13:00 pm -14:00 pm. A two weeks period were allowed to help animals adapt themselves to environment. Rats were randomly divided into five groups (n = 10 in each) as follows:
Control group (Co); rats were transported to the laboratory room and handled the same as the experimental animal throughout the study period and had no special treatment.
Sham group (Sh); rats were put on the treadmill without running during 1 h/day for 21 days.
Exercise group (Ex); rats ran during 1 h/day for 21 days on the treadmill.
Under stress group (St); rats were under restraint stress during 6 h/day for 21 days.
Synchronized exercise with stress group (St and Ex); per each day, exercise and stress were synchronically induced and with the same as above protocol for 21 days.
Experimental procedures
Experiments were performed in light cycle. Body weight was measured in days 1 and 21 of the experiment and body weight differences (BWD = BWFinal-BWInitial) were evaluated; then at the end of the experiments; all rats were subjected to passive avoidance learning (PAL) test.
Stress paradigms
In current study, rats were placed in Plexiglas cylindrical restrainers and fit tightly into them during 6 h/day for 21 days in the chronic stress model. It was not possible for them to move or turn around.[19] Hence, restraint was a powerful stress in rats,[20] the stress procedure was carried out in the institutional animal facility throughout the experimental period at 8:00 am-14:00 pm each day. The animals from each group were randomly assigned to one apparatus.[21,22]
Exercise paradigms
The exercise protocol consisted of 1 h/day/6 consecutive days at 20-21 m/min, ° slope, for 21 days running. Adaptation to treadmill running was performed before the experiments. Rats ran on the treadmill at 07:00 am -8:00 am. They were forced to run at the speed of the treadmill and received a mild electric shock from the grid, located just behind the treadmill. Electric shocks were used sparingly to motivate the animals to run. The stress synchronized with the likelihood of getting shock was controlled by exposing the Sham groups to the treadmill apparatus without switching on the treadmill. These rats would receive the same electric shock when they stepped onto the grid.
Behavioral apparatus and method
The passive avoidance (PA) apparatus (Shuttle box 75 × 20 × 15 cm) was divided into two compartments that had grid floor and wooden walls. It consisted of a small light compartment (25 × 25 × 20 cm) and a larger dark room (50 × 25 × 20 cm). The two compartments were separated by a sliding guillotine door. The habituation trial was performed 1 day before the acquisition trial. Each rat was placed in the apparatus without electric shock for 5 min and the animal was allowed to explore the apparatus freely.
The acquisition trial was performed on the first experimental day; rats were placed individually in the light room for 1 min and then the guillotine door was raised, when the rat entered the dark room, the door was closed and an inescapable scrambled single foot electric shock (50 Hz, 0.2 mA, 3 s; once) was delivered through the grid floor by an isolated stimulator and the initial latency (IL) of entrance into the dark room was recorded. Rats with initial latency greater than 60 s were excluded from the study. Then the rat was removed from the PA apparatus to its home cage. The animals were tested for retention of passive avoidance response only once, 24 h later. The rat was placed in the light room again with access to the dark room without any shock for retention. The delay of entering to the dark room from light room was measured as step-through latency (STL) (up to a maximum of 300 s). If an animal did not enter the dark room within 300 s, the trial was terminated.[23] Absence of entry to the dark room or a longer duration in the light room indicated a positive response.[4] The passive avoidance task determines the ability of a rat to remember a delivered foot shock.
Statistical analysis
The latency of the passive avoidance test was analyzed using a Kruskal-Wallis nonparametric one-way analysis of variance corrected for ties, followed by a two-tailed Mann-Whitney U test. The comparisons of acquisition and retention time 24 h afterwards (within groups) were analyzed by Friedman test, followed by a Wilcoxon signed ranks test. Body weight differences were analyzed by ANOVA followed by Tukey's post hoc test for multiple groups.
All data were reported as the mean ± SEM in spite of the probable no normality of the distribution of scores, because it seems these parameters provide a clearer indication for most investigators. A P value less than 0.05 (P < 0.05) was considered as significant.
RESULTS
Passive avoidance learning test
Figures 1 and 2 respectively shows the initial latency (IL; acquisition latency time) and step-through latency (STL; retention latency time) of all groups in a single trial passive avoidance test, respectively.
Figure 1.

Initial latency to enter the dark room of passive avoidance apparatus during acquisition test for all groups before receiving foot shock. Data represent mean±SEM (n=10). There was no significant difference between Co and Sh groups; The IL had significant (**P<0.01, ##P<0.01, respectively) decreases in St and St and Ex groups compared to Co and Sh groups; In St and St and Ex groups, the IL was significantly (in both them, ΔΔP<0.01) lower than Ex group; The IL showed nonsignificant (P>0.05) increase in St and Ex group compared to St group; Kruskal Wallis test, Mann-Whitney U test
Figure 2.

Step through latency to enter the dark room of passive avoidance apparatus during retention test for all groups 1 day after receiving foot shock. Data represent mean±SEM (n=10). There was no significant difference between Co and Sh groups; The STL had significant (*P<0.05, #P<0.05, respectively) decreases in Ex, St, and St and Ex groups compared to Co and Sh groups; In St and St and Ex groups, the STL was significantly (in both them, ΔΔΔP<0.001) lower than Ex group; The STL showed nonsignificant (P>0.05) increase in St and Ex group compared to St group; Kruskal-Wallis test, Mann-Whitney U test
Results indicated that there were not significant differences between Control and Sham groups in IL and STL, indicating that the treadmill electric shock had no significant effect in these parameters [Figures 1 and 2]. In Exercise (Ex) group, only STL was significantly (P < 0.05) higher (13.95%) than Control group [Figure 2].
In Stress group, both IL and STL were significantly (P < 0.01, P < 0.05; respectively) lower (55.95% and 24.33%; respectively) than Control group [Figures 1 and 2]. Therefore, stress obviously decreased acquisition and recall of passive avoidance response in this group. Also in St group, both IL and STL were significantly (P < 0.01, P < 0.001; respectively) lower (59.21% and 33.59%; respectively) than Ex group [Figures 1 and 2].
In synchronized exercise with stress group (St and Ex group), both IL and STL showed significant (P < 0.01, P < 0.05; respectively) decreases (52.28% and 21.03%; respectively) from Control group [Figures 1 and 2]. In addition, in St and Ex group, both IL and STL were significantly (P < 0.01, P < 0.001; respectively) lower than Ex (55.80% and 30.75%; respectively) group [Figures 1 and 2].
In St and Ex groups, IL and STL had no significant differences from St group [Figures 1 and 2]; indicating that synchronized exercise with stress could not significantly increases IL and STL.
The results of initial and step-through latency (IL and STL, respectively) were analyzed by related sample to evaluate within group latency changes. Our data showed that there were significant (P < 0.01) differences between IL and STL in all groups [Figure 3]. In overall, learning happened in all groups.
Figure 3.
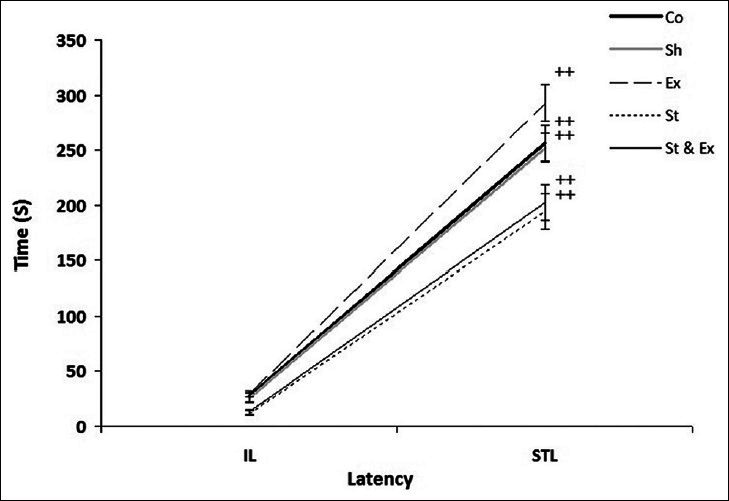
Initial and step through latency (IL and STL, respectively) to enter the dark room of passive avoidance apparatus during acquisition and retention test before and after receiving foot shock (within groups). Data represent mean±SEM (n=10). Comparison of IL with STL (and/or pre vs. post foot shock) in all groups showed a significant (++P<0.01) increase; by Friedman test, Wilcoxon signed ranks test
Body weight difference
Results indicated that there were not significant differences between Control and Sham groups in body weight difference (BWD; differences between final and initial weights), indicating that the treadmill electric shock had no significant effect in this parameter [Figure 4].
Figure 4.

Comparison of body weight differences (BWD = BWFinal-BWInitial) in all groups. Data represent mean±SEM (n=10). There was no significant difference between Co and Sh groups; The BWDs had significant (***P<0.001, ###P<0.001, respectively) decreases in St and St and Ex groups compared to Co and Sh groups; In Ex group, the BWD was significantly (**P<0.01, #P<0.05, respectively) lower than Co and sh group; The BWD showed nonsignificant (P>0.05) decrease in St and St and Ex groups compared to Ex group; The BWD showed nonsignificant (P>0.05) decrease in St and Ex group compared to St group; ANOVA test
In St and St and Ex groups, the BWDs significantly (ANOVA, Tukey: P < 0.001) were lower than Control (90.05% and 92.27%; respectively) and Sham (89.35 % and 91.73%; respectively) group [Figure 4].
In Ex group, BWD showed significant (P < 0.01) decrease (69.97%) from Control group [Figure 4].
The BWD in synchronized exercise with stress group (St and Ex group) had not significant differences from stressed group [Figure 4], indicating lower effect of running activity than chronic psychical stress on body weight loss.
DISCUSSION
The present results showed that although learning happened in all groups and had progressive trend, but it was different for each group [Figure 3]. Our results clearly confirmed that stress is accompanied by disturbance in the initial latency (IL; acquisition latency time) and step-through latency (STL; retention latency time) of animal performance in PAL [Figure 3]. In support of our finding, several studies have shown that chronic restraint stress impairs acquisition and retention of spatial memory tasks in rats.[24] Indeed, chronic stress is an unavoidable condition and a negative modulator of learning and memory process.[2,25] Since multiple transmitter systems interact extensively in the stressed rat brain, it can accelerate the onset and severity of cognitive dysfunctions.[26] Several researches on rodents and humans have indicated that stress is an important factor that can alter brain cell properties[27,28] and release of some neurotransmitters such as acetylcholine (Ach) that is important for learning.[29] Therefore, stress can probably impair acquisition and retention of passive avoidance learning and/or disturb cognitive processes such as, learning and memory via disturbances of neurotransmitters release.
Present study also showed that exercise has beneficial effects on learning and memory. Animal studies on rats and mice reported better cognitive performance as a result of physical activities.[30–32] Mechanisms of exercise effects on brain function are vary. It may result from structural and biological changes in the brain.[33] Similarly, several studies have indicated that exercise can increase the speed of learning and establishment of memory and improve cognitive performance.[32,34–36] Chen and et al. showed that treadmill exercise training facilitated PA aversive learning.[37] There are various finding on improvements in both acquisition and retention, suggesting that exercise effects on different aspects of cognition may depend on factors such as the duration of exercise exposure, type of exercise performed (e.g. forced vs. voluntary), task difficulty, or other variables that have not yet been defined.[38]
The STL of synchronized exercise with stress (St and Ex group) had no significant improvement compared to stress group [Figure 2]. On the other hand, similar comparison of STL between St and Ex group and Control group showed significant (P < 0.01) decrease [Figure 2]. Therefore, synchronized running activity with stress did not significantly eliminate negative effects of stress on learning. It seems that chronic stress has probably a much greater effect than running activity on retention trial with this exercise protocol. Similarly, Barnes et al. also did not observe beneficial effects of exercise on spatial memory.[39] The mechanism of synchronized exercise with stress may be attributed to increases of oxidant status of the body by forced synchronized exercise with stress. Other possibility is that since chronic stress effects were stronger than running activity in this study, therefore stress deleterious effects do not allow the positive effects of exercise become apparent. On the contrary, several researchers have argued that physical activity may modify and reduce the physiological effects of stress.[40] Zheng and et al.[41] indicated protective properties of exercise against depressive behavior (psychologic behavior) but these were not surprisingly agree to synchronized exercise with stress.[41] Therefore, it seems that the observed differences in associated physical activity with psychological behavior may be due to involvement of different mechanisms.
Since in our study, regular treadmill running was performed with constant velocity or intensity, we suggest that if exercise must be performed progressively (it should begin from low to high duration and velocity) in synchronized running activity with stress, perhaps it can overcome stress deleterious effects. Other studies have also found that regular treadmill running, a mandatory exercise paradigm with defined exercise intensity and duration, had beneficial effects on neural health and function, and it can protect neurons from various brain insults[42,43] and also, improved PA retention and spatial learning.[15,16,34] Therefore, it is noteworthy to consider emotional stress during exercise results in diminishing beneficial effect of exercise, so the results fully corroborated that exercise is presumably time dependent respect to stress.
According to our findings, body weight decreased in emotional stressed and exercised group [Figure 4] which was in conformity with previous reports.[44,45] Contrary to our finding, Marin and et al. reported that weight loss was not observed by restraint stress.[22] Mechanism of restraint stress and exercise may respectively be due to decreasing food intake and decline of fatty mass. Also, it is possible that other factors such as leptin involve in body weight changes. In the current study, also weight loss in St and Ex group was not significantly higher than St group. It indicates that psychical and/or emotional stress has probably greater effect than physical activity on weight loss.
CONCLUSIONS
Our results for the passive avoidance learning test indicated synchronized running activity with stress has little beneficial effects in improvement of acquisition and retention time of passive avoidance due to stress. Therefore, this kind of exercise did not significantly improve both acquisition and retention for under stress rats. Further research needs to be conducted to identify these mechanisms in animal, hence it is better that some neurotransmitters and other factors such as leptin that involve in these variables are assayed.
ACKNOWLEDGMENTS
This research was supported by Isfahan University of Medical Sciences, Isfahan, Iran
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: Sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–16. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Yoon KS. Stress: Metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–9. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 4.Kumar RS, Narayanan SN, Nayak S. Ascorbic acid protects against restraint stress-induced memory deficits in Wistar rats. Clinics (Sao Paulo) 2009;64:1211–7. doi: 10.1590/S1807-59322009001200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pluncevic J. Influence of the physical activity on the cognitive functions with people depending on their age. Med Arh. 2012;66:271–5. [PubMed] [Google Scholar]
- 6.Radak Z, Toldy A, Szabo Z, Siamilis S, Nyakas C, Silye G, et al. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int. 2006;49:387–92. doi: 10.1016/j.neuint.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Saadipour K, Sarkaki A, Alaei H, Badavi M, Rahim F. Forced exercise improves passive avoidance memory in morphine-exposed rats. Pak J Biol Sci. 2009;12:1206–11. doi: 10.3923/pjbs.2009.1206.1211. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 9.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE. Exercise-induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochem Int. 2006;48:9–16. doi: 10.1016/j.neuint.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2007;184:124–32. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus. 2010;20:637–45. doi: 10.1002/hipo.20657. [DOI] [PubMed] [Google Scholar]
- 14.O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Alaei H, Borjeian L, Azizi M, Orian S, Pourshanazari A, Hanninen O. Treadmill running reverses retention deficit induced by morphine. Eur J Pharmacol. 2006;536:138–41. doi: 10.1016/j.ejphar.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113:803–11. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Shen YJ. Effects of brain histamine on memory deficit induced by nucleus basalis-lesion in rats. Acta Pharmacol Sin. 2002;23:66–70. [PubMed] [Google Scholar]
- 18.Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–29. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 19.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–57. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 20.Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Wang GW, Cai JX. Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats. Neurobiol Learn Mem. 2008;90:365–73. doi: 10.1016/j.nlm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Rao BS, Raju TR. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Curr Sci. 2000;79:1581–4. [Google Scholar]
- 25.Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, et al. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer's disease model. FASEB J. 2006;20:729–31. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- 27.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 28.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–74. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem. 2007;87:257–63. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- 31.Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46:123–33. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 32.Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–9. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- 33.Uysal N, Tugyan K, Kayatekin BM, Acikgoz O, Bagriyanik HA, Gonenc S, et al. The effects of regular aerobic exercise in adolescent period on hippocampal neuron density, apoptosis and spatial memory. Neurosci Lett. 2005;383:241–5. doi: 10.1016/j.neulet.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 34.Ang ET, Dawe GS, Wong PT, Moochhala S, Ng YK. Alterations in spatial learning and memory after forced exercise. Brain Res. 2006;1113:186–93. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002;23:415–21. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- 36.Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, et al. Treadmill exercise enhances passive avoidance learning in rats: The role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem. 2008;89:489–96. doi: 10.1016/j.nlm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes CA, Forster MJ, Fleshner M, Ahanotu EN, Laudenslager ML, Mazzeo RS, et al. Exercise does not modify spatial memory, brain autoimmunity, or antibody response in aged F-344 rats. Neurobiol Aging. 1991;12:47–53. doi: 10.1016/0197-4580(91)90038-l. [DOI] [PubMed] [Google Scholar]
- 40.Starzec JJ, Berger DF, Hesse R. Effects of stress and exercise on plasma corticosterone, plasma cholesterol, and aortic cholesterol levels in rats. Psychosom Med. 1983;45:219–26. doi: 10.1097/00006842-198306000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, et al. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–84. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 44.Radahmadi M, Shadan F, Karimian SM, Sadr SS, Nasimi A. Effects of stress on exacerbation of diabetes mellitus, serum glucose and cortisol levels and body weight in rats. Pathophysiology. 2006;13:51–5. doi: 10.1016/j.pathophys.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]


