Abstract
The purpose of this study was to determine if recovery of neurologically impaired hand function following isolated motor cortex injury would occur without constraint of the non-impaired limb, and without daily forced use of the impaired limb. Nine monkeys (Macaca mulatta) received neurosurgical lesions of various extents to arm representations of motor cortex in the hemisphere contralateral to the preferred hand. After the lesion, no physical constraints were placed on the ipsilesional arm/hand and motor testing was carried out weekly with a maximum of 40 attempts in two fine motor tasks that required use of the contralesional hand for successful food acquisition. These motor tests were the only “forced use” of the contralesional hand. We also tested regularly for spontaneous use of the contralesional hand in a fine motor task in which either hand could be used for successful performance. This minimal intervention was sufficient to induce recovery of the contralesional hand to such a functional level that eight of the monkeys chose to use that hand on some trials when either hand could be used. Percentage use of the contralesional hand (in the task when either hand could be used) varied considerably among monkeys and was not related to lesion volume or recovery of motor skill. These data demonstrate a remarkable capacity for recovery of spontaneous use of the impaired hand following localized frontal lobe lesions. Clinically, these observations underscore the importance of therapeutic intervention to inhibit the induction of the learned nonuse phenomenon after neurological injury.
Keywords: Brain injury, Hand, Dexterity, Therapeutic intervention, Motor recovery
Introduction
In rehabilitation, the term “learned nonuse” implies that part of the motor deficit following damage to the nervous system is not only a direct result of the tissue damage but also a consequence of learned suppression of affected limb use reinforced over time by the poor quality of its movements (Taub et al. 1977, 2006; Wolf et al. 1989). The most direct experimental evidence of learned nonuse involved unilateral dorsal root deafferentation of the limb in monkeys (for a review, see Taub et al. 2006). However, previous work had shown that monkeys also develop a lasting nonuse of an affected limb following surgically induced lesions of motor cortex (Ogden and Franz 1917). In humans, the learned nonuse phenomenon manifests in a wide variety of neurological conditions including neurodegenerative disorders such as Parkinson's Disease (e.g., Quencer et al. 2007), cerebral palsy (e.g., Crocker et al. 1997), and traumatic brain injury (e.g., Wolf et al. 1989), but most commonly occurs following stroke-induced hemiplegia (Wolf et al. 1989, 2006; Taub et al. 1993, 2006; Winstein et al. 2004). In this circumstance, learned nonuse is typically a direct consequence of the loss of mobility and power of the limb contralateral to supratentorial brain injury.
To overcome learned nonuse of a neurologically impaired limb in monkeys, previous studies suggest that a powerful intervention involving forced use, or training of the affected limb coupled with constraint of the less affected limb is necessary (Ogden and Franz 1917; Knapp et al. 1959; Taub et al. 1966; Murata et al. 2008). In all of these studies, the less affected limb was constrained for a period of at least 2 weeks to force the use of the impaired limb. Interestingly, it has also been shown that subsequent impairment of the originally less affected limb by sequential dorsal root deafferentation reverses learned nonuse and results in recovery of fine hand movement control in the originally affected limb (Knapp et al. 1963; Taub et al. 1966). The experimental induction of this phenomenon and its subsequent reversal has also been demonstrated following serial ablations involving both cerebral hemispheres (Ogden and Franz 1917). Notably, the previous work on effects of forelimb deafferentation involved conditioned-avoidance paradigms in which movement of the impaired limb or hand was required to avoid a painful electric shock, providing a very powerful stimulus to counteract such learned nonuse (Knapp et al. 1959; Taub et al. 1966). However, some purposeful use of the deafferented limb with the less affected limb restrained was also observed outside of conditioned avoidance paradigms (Knapp et al. 1963). Other work has also shown that the monkeys could learn to use the deafferented limb for precise pointing at visual targets with and without vision of the limb (Taub et al. 1975).
It is noteworthy that the previous studies of learned nonuse did not directly quantify frequency of spontaneous use of the affected limb in a free situation as was done in the more current work. Ogden and Franz (1917) reported that the impaired hand of animals with extensive motor cortex damage returned to normal motor function within 3 weeks of the lesion if the less impaired arm was constrained and the impaired arm subjected to various “therapies” to induce the animal to move the affected limb. This return to normal function included continued use of the impaired hand for fine motor tasks after the less impaired hand was removed from the restraint.
To our knowledge, the possibility that a less intense intervention could stimulate spontaneous use of the impaired hand following motor cortex injury in a dexterous motor task has not been investigated. We investigated this possibility in monkeys with surgically induced lesions of frontal lobe motor areas that initially caused paresis of the contralesional arm. We studied macaque monkeys because of their similarity to humans in terms of cortical structure and highly developed hand function (Heffner and Masterton 1975, 1983; Zilles et al. 1995; Picard and Strick 1996; Roland and Zilles 1996; Geyer et al. 2000; Lemon and Griffiths 2005; Schieber 2007). Lesion size was varied to involve arm areas of primary motor cortex only (M1), M1 + lateral premotor cortex (LPMC), and M1 + LPMC + supplementary motor cortex (M2) to study the effects of progressively larger lesions on contralesional hand motor recovery (Darling et al. 2009b). In the current work, we focused specifically on post-lesion duration of nonuse and amount of use of the contralesional hand when the monkey could choose which hand to use. The intervention involved testing of impaired limb motor function with a limited number of movements only once every week for the first 2 months after injury and then once every 2 weeks thereafter. Specifically, we used two simple experimental devices that allow controlled testing of each hand without constraint applied to either limb as the monkey roamed freely about the cage (Darling et al. 2006; Pizzimenti et al. 2007). Collectively, both testing devices allowed a maximum of 40 opportunities to use the impaired limb to acquire small food targets. Despite food restriction for 18–24 h prior to testing, most monkeys did not make any attempts to acquire the food targets with the contralesional hand for 2–4 weeks after the lesion. This suggests that the motor cortex lesions effectively disrupt the anatomical projections converging upon or leaving this cortex, probably induced learned nonuse for variable post-lesion durations, even when motivation to acquire the food targets would be high (Darling et al. 2009b). Spontaneous use of the hand was tested using a third apparatus in which the monkey could use either hand to use to acquire small food targets. We hypothesized that larger lesions of motor cortex, especially those also affecting supplementary motor area (SMA) (Rostomily et al. 1991), would induce a longer duration of nonuse of the impaired hand in a fine motor task. A second hypothesis was that recovery of spontaneous use of the impaired hand toward pre-lesion levels would depend on the level of recovery of skill in dexterous manipulation of small objects.
Methods
Experimental animals
Nine monkeys (Macaca mulatta) served as subjects for these experiments (Table 1). The monkeys were housed and maintained in a US Department of Agriculture (USDA) approved and inspected facility. All behavioral protocols were approved by the University of South Dakota Institutional Animal Care and Use Committee and performed according to US Department of Agriculture, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of animals. Prior to testing, each monkey was evaluated by a primate veterinarian and judged to be healthy and free of any neurological deficit. One monkey (SDM55) had a physical defect in the third digit of the preferred hand, which was in a permanently extended position at the interphalangeal joints. However, this did not interfere with his ability to perform precision grasping/manipulation of small objects with digits one and two. To minimize training effects, all monkeys that were enrolled in training/testing procedures did not have manual cage enrichment post lesion (i.e., apparati which required use of dexterous movements—e.g., perforated ball, hanging mirror, foraging boards, etc.). To compensate for this, visual and auditory enrichment programs were enhanced and daily contact with staff was increased.
Table 1.
Characteristics and experimental parameters of monkeys
| Monkey | Agea (years) | Sex | HI2 | Les. Cat. | Areas lesioned | Post-lesion dur. (mo.) | GMLV (mm3) | WMLV (mm3) |
|---|---|---|---|---|---|---|---|---|
| SDM38 | 20.7 | F | 6R | F1 | M1 | 12 | 101.96 | 9.62 |
| SDM55 | 11.8 | M | 20L | F2 | Ml + LPMC | 12 | 207.67 | 20.51 |
| SDM49 | 3.7 | M | 65R | F2 | M1 + LPMC | 1 | 202.91 | 53.64 |
| SDM64 | 13.6 | F | 95.3L | F2 | M1 + LPMC | 6 | 217.92 | 43.04 |
| SDM70 | 7.2 | M | 4.4R | F2 | M1 + LPMC | 6 | 143.27 | 7.76 |
| SDM50 | 8.7 | F | 79R | F3 | M1 + LPMC +M2 | 12 | 430.91 | 53.66 |
| SDM56 | 9.2 | M | 39.3L | F3 | M1 + LPMC +M2 | 6 | 407.54 | 64.10 |
| SDM67 | 13.8 | F | 1.1R | MF | M1 + PMC + M2 + pSMA | 12 | 236.19 | 135.37 |
| SDM46 | 7.1 | M | 96L | MF | M1 + LPMC + pSMA + M2 + M3 + PFC | 3 | 811.07 | 190.26 |
HI handedness index, Les Cat lesion category: F1 (M1 primary motor cortex), F2 (M1 + LPMC lateral premotor cortex), F3 (M1 + LPMC + M2 supplementary area), MF multi-focal lesion, M3 rostral cingulate motor area, pSMA pre-supplementary motor area, PFC prefrontal cortex, GMLV gray matter lesion volume, WMLV white matter lesion volume
Age at time of lesion
Experimental apparati
Three devices were used for fine hand motor testing in these experiments. They included a standard dexterity board (sDB), modified dexterity board (mDB), and modified movement assessment panel (mMAP). The sDB used in our study is similar to that used in many previous studies of fine motor control in monkeys (Nudo et al. 1992; Nudo and Milliken 1996; Kermadi et al. 1997; Mason et al. 1998). This device permitted the monkey choice of which hand to use to acquire small food pellets using precision grasp. Specifically, it was used to test for hand preference before the brain lesion (Nudo et al. 1992) and for spontaneous use of each hand after the lesion for testing of learned nonuse. The mDB (Pizzimenti et al. 2007) and mMAP (Darling et al. 2006) devices were used for motor testing of each hand before and after the lesion. These two devices allow the experimenter to control which hand the monkey is able to use to acquire food targets. Specifically, this is accomplished by opening or closing right and left portal doors in addition to mechanical constraints placed on the hand path to allow for controlled testing of each hand without the need for restraints on one limb.
Behavioral procedures for handedness index, learned nonuse, and motor performance
Video recording of hand preference testing and spontaneous hand use post-lesion was used to acquire data on the number of reaches with each hand to the food targets. During pre-lesion hand preference testing with a standard dexterity board (sDB), the monkey had opportunities to retrieve ten food pellets from each of the four wells and from the flat surface (50 pellets in total, random order of pellet placements). Three separate testing sessions totaling 150 trials were conducted for each animal. Data recorded included the number of reaches with each hand and number of successful acquisitions. These were used to compute a handedness index which reflects strength of hand preference (Nudo et al. 1992). During post-lesion nonuse testing, the food pellets were placed centrally on the flat surface of the sDB to make grasping as easy as possible. For these tests we simply recorded the number of reaches with each hand over 20 trials (note that if the monkey first reached with one hand and was unsuccessful and then reached with the other, it was counted as two reaches—one with each hand). We also recorded whether the monkey was successful in acquiring the food pellet. Post-lesion testing began within 2 weeks after the lesion and was carried out weekly or biweekly in most monkeys (2 monkeys were tested less often).
Prior to all experimental sessions the monkey was food restricted for 18–24 h. Full testing sessions with the mDB included five retrieval attempts for each of the wells (A–E) for both limbs proceeding from the easiest well (E) to the most difficult (A), thereby giving the monkey 50 opportunities to retrieve pellets (25 with each hand). During post-lesion tests, the more impaired hand (contralateral to the surgically induced lesion) was always tested first to ensure high motivation. Full testing sessions with the mMAP included blocks of five trials at each difficulty level (i.e., flat surface, straight rod and curved rod) with each hand, thereby giving the monkey 30 opportunities to retrieve carrot chips (15 with each hand). The monkeys’ exposure to the tasks of the mDB and the mMAP were limited to the experimental testing sessions and they had limited opportunities to use fine motor control at other times (e.g., only during feeding as there were no apparati available to encourage fine movement control). Post-lesion nonuse testing involved 20 trials with the food pellets placed on the central part of the flat surface of the sDB. As previously mentioned, the animal could use either hand to retrieve the pellets in this test.
Data acquisition
Quantitative measurements of movement kinematics and kinetics were made in several testing and training sessions before the lesion and at regular intervals after the lesion using the mDB and mMAP devices as described previously (Darling et al. 2006, 2009a, b; Pizzimenti et al. 2007). For the mDB, video recording at 100 frames/s with 4 cameras in calibrated 3-dimensional (3D) space was used to identify the following parameters of the movement: time hand exited cage, time of first touch of any part of the hand to the mDB (touchdown) or food pellet, duration of manipulation of the food pellet and number of lost contacts between the food pellet and the digit used to dislodge the food pellet and 3D coordinates of the thumb and fingertip at touchdown to compute accuracy of the reach (relative to the pellet target) and grip aperture.
Surgical procedure
These procedures were carried out as described previously (Pizzimenti et al. 2007; Darling et al. 2009b; McNeal et al. 2010) and will be presented only briefly here. All lesions were made in the cortical hemisphere contralateral to the preferred limb. The planned surgical lesions included the arm areas of M1 (category F1 lesion); M1 + the adjacent LPMC (category F2 lesion); and M1 + LMPC + the supplementary motor cortex (M2) (category F3 lesion). The purpose of studying these lesions was to evaluate whether progressively larger lesions of arm motor areas induced greater duration and magnitude of hand motor deficits and whether recovery was associated with different mechanisms in terms of plasticity of corticospinal tract connections for control of hand movement (Darling et al. 2009b; McNeal et al. 2010). Two additional cases were also included in which there was an initial surgical removal of motor cortex areas, but the lesion spread rostrally to involve medial pre-frontal cortex (multifocal or MF lesion—Table 1). These unique cases provide additional important information concerning recovery of ipsilesional hand function (Darling et al. 2009b). After aseptic cortical exposure under isoflurane anesthesia, the animal was transferred to intravenous ketamine anesthesia and intracortical microstimulation (ICMS) was used to localize the arm areas of M1, LPMC, and M2 (Morecraft et al. 2001, 2002, 2007a, b; McNeal et al. 2010). Following intracortical microstimulation mapping, the animal was transferred back to isoflurane anesthesia. Subpial aspiration was then used to remove the gray matter of the arm area(s) and avoid regions controlling face/head and leg function identified by ICMS. The dura was then sutured closed, the bone flap replaced and anchored to the cranium, and the skin closed with sterile sutures. In all cases, a second surgery was performed 33–34 days prior to sacrifice to inject neural tract tracers into arm areas of spared, intact motor cortices. These tract tracing experiments were designed to examine potential neuroplasticity in intact neuronal projection systems. Results of these studies will be reported in other manuscripts (e.g., McNeal et al. 2010). Tissue obtained from these experiments is also being used to track cellular and molecular mechanisms that accompany motor recovery (Nagamoto-Combs et al. 2007, 2009). Complete anatomical descriptions of the lesions in all these monkeys are presented in two previous studies (Darling et al. 2009b; McNeal et al. 2010).
Data analysis
The mDB tests were analyzed as described previously to compute performance scores on individual trials in each test for analysis of post-lesion recovery relative to pre-lesion levels of performance (Darling et al. 2006, 2009b; Pizzimenti et al. 2007). For the post-lesion nonuse tests we recorded: (1) week of first attempt with the impaired hand, (2) week of first successful acquisition with the impaired hand, and (3) percentage of attempts and successful acquisitions with the impaired hand. Similarly, on the mDB task we obtained the post-lesion week of first attempt on any well and first successful acquisition and first test session on which there were successful acquisitions on all attempts on the well on which they performed best during the pre-lesion phase. Note that one monkey, SDM46, had no post-lesion successes on the mDB task. We also computed a measure of recovery of impaired hand motor skill as described previously (Pizzimenti et al. 2007; Darling et al. 2009b). Briefly, skill was defined as the mean performance score divided by the standard deviation of performance scores over five consecutive test sessions (25 trials) on the mDB and MAP tasks. Pre-lesion skill was computed over the last five testing sessions before the lesion. The maximum post-lesion skill was computed as the highest skill over five consecutive testing sessions during post-lesion testing. Recovery was defined as the ratio of post-lesion maximum skill divided by pre-lesion skill. We used maximum post-lesion skill because this measure provides the best estimate of potential for recovery of skill. Multiple and single linear regression analyses were used to examine whether spontaneous use of the impaired hand on the nonuse tests depended on skillful use of the hand and recovery of fine motor skill in the mDB tasks and on volume of gray and white matter lesions.
Results
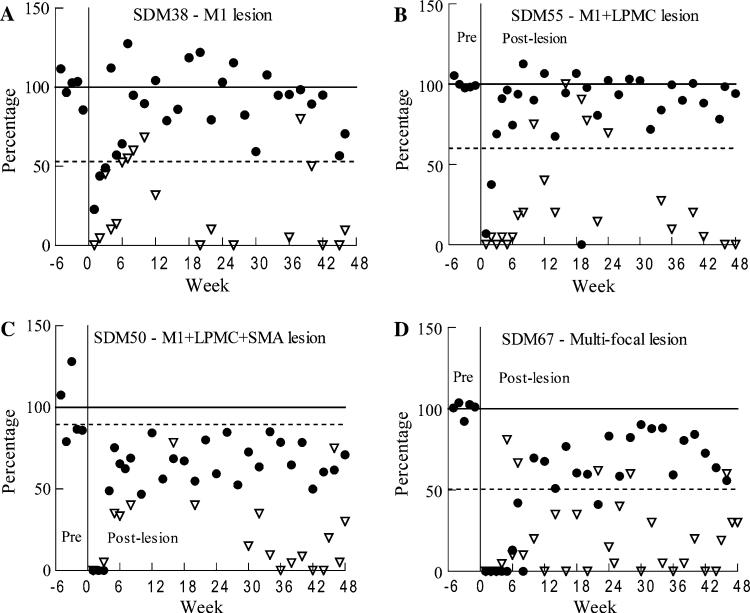
There was clear evidence of nonuse in the first days to weeks following surgically induced motor cortex lesions. For example, observations of behavior in the cage in the first few days following the lesion indicated that most monkeys did not use the contralesional hand for gross activities (postural support, climbing) or fine motor activities (e.g., grasping food, even if it was placed near the impaired hand). Consistent with this observation, most monkeys also did not make an attempt with the contralesional hand in the first post-lesion motor tests 1 week after the lesion (Table 2, Fig. 1). The first post-lesion nonuse test was performed within 2 weeks of the lesion, during which most monkeys did not use the contralesional hand (Table 2). The percentage of impaired hand use on the nonuse tests increased over the first 8 weeks after the lesion in most monkeys, but showed considerable variation within and among the subjects (Table 3, Fig. 1). Relatively high percentage use of the contralesional hand in the learned nonuse test generally occurred after return of good manipulation ability (i.e., when the monkey was successful on all trials in the mDB task for the best well) over the first 8 weeks of recovery (e.g., Fig. 1a, b, Tables 2, 3). Surprisingly, two monkeys with large multi-focal lesions (SDM46, SDM67) began using the impaired hand in the nonuse task before attempts were made with the same hand in the mDB task (e.g., Figs. 1d, 2a—filled squares, Tables 1, 2). Also notable is that three monkeys used the contralesional hand in the nonuse test on at least one occasion more often than in the pre-lesion hand preference testing (Table 3).
Table 2.
Recovery in the nonuse and mDB tasks
| Monkey | Post-lesion week of first attempt |
Post-lesion week of irst success |
Post-lesion week of all success | mDB manip skill recovery ratio |
|||
|---|---|---|---|---|---|---|---|
| mDB | Nonuse | mDB | Nonuse | mDB—Best well | First well | Second well | |
| SDM38 | 1 | 2 | 1 | 2 | 4 | 0.89 | 0.88 |
| SDM55 | 1 | 2 | 2 | 7 | 4 | 1.09 | 1.31 |
| SDM70 | 1 | 4 | 1 | 4 | 2 | 1.09 | 1.28 |
| SDM49 | 2 | 4 | 2 | 4 | 3 | NA | NA |
| SDM64 | 4 | 6 | 4 | 6 | 6 | 0.88 | 0.81 |
| SDM50 | 2 | 3 | 4 | 3 | 22 | 0.85 | 1.48 |
| SDM67 | 7 | 4 | 8 | 4 | 19 | 0.43 | 0.47 |
| SDM46 | 3 | 2 | ns | 10 | ns | 0.00 | 0.00 |
| SDM56 | 2 | na | 2 | na | 6 | 0.50 | 0.48 |
NA not available (only four post-lesion testing sessions), ns no successful target acquisitions during post-lesion period, na no attempts during post-lesion period
Fig. 1.
Recovery of contralesional hand performance in the mDB and nonuse tasks in four monkeys. Recovery of performance in the mDB task (solid circles) was computed as the percentage ratio of post-lesion performance score/average pre-lesion performance score (over the last five pre-lesion tests) on the well with highest pre-lesion skill. Recovery of spontaneous use of the contralesional hand (open triangles) is plotted as the percentage use of that hand in the post-lesion tests. The solid horizontal line represents average pre-lesion performance scores over the last five pre-lesion tests. The dashed horizontal line represents pre-lesion use of the preferred hand in the handedness test (note that the lesion was always made in the hemisphere contralateral to the preferred hand). Note that post-lesion performance score ratios of zero indicate no attempts in the testing session
Table 3.
Percentage use of the contralesional (pre-lesion preferred) hand in the nonuse task and hand preference task
| Monkey | Highest % use in first 8 weeks after lesion | Highest % use observed | Avg. % use | % Use on pre-lesion HP testa |
|---|---|---|---|---|
| SDM38 | 60.0 | 80 | 24.8 | 53.0 |
| SDM55 | 20.0 | 100 | 28.6 | 60.0 |
| SDM70 | 17.4 | 40 | 8.3 | 52.2 |
| SDM49 | 70.8 | 70.8 | 35.4 | 82.5 |
| SDM64 | 5.0 | 45.0 | 9.3 | 97.6 |
| SDM50 | 40.0 | 78.3 | 24.1 | 89.5 |
| SDM67 | 80.9 | 80.9 | 25.7 | 50.6 |
| SDM46 | 13.0 | 14.8 | 4.1 | 98.0 |
| SDM56 | 0 | 0 | 0 | 69.7 |
Percentage use of the preferred (contralesional) hand in the pre-lesion hand preference test
Fig. 2.
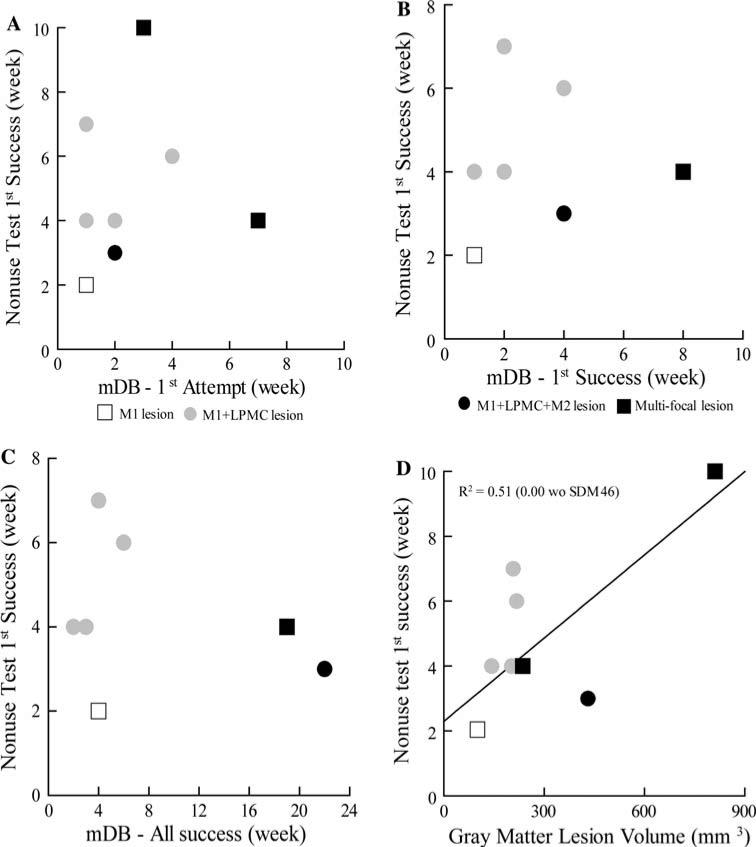
Scatter graphs showing post-lesion week of first attempt by the contralesional hand on the nonuse task versus post-lesion week of first attempt (a), first success (b) and five successes (c) on the best well of the mDB task. Post-lesion week of first attempt on the nonuse task is also plotted against total (gray matter + white matter) lesion volume in d. Each plotted symbol is data from a single monkey. Note that SDM56 was excluded from a, b, c and all regression analyses because he had no attempts on the nonuse task. SDM46 had no successes on the mDB task and thus is not included in b and c
Post-lesion duration until the first attempt with the contralesional hand on the nonuse task was not correlated with strength of hand preference, recovery on the mDB task, or lesion volume. The first attempt with the impaired hand on the nonuse test occurred within 4 weeks of the lesion in seven of nine monkeys (Table 2, Fig. 2a). Five monkeys made attempts on the nonuse task before they were consistently successful (i.e., on all attempts for the best well) with the contralesional hand in the mDB task (Table 2, Fig. 2b). However, one monkey (SDM56) never chose to use the impaired hand in the nonuse task. Regression analyses showed that the post-lesion week when the first attempt occurred on the nonuse task was not correlated with lesion volume (Fig. 2d, Table 4, p > 0.3), handedness index (R = 0.233, p > 0.4) or with the post-lesion week on which the: first attempt (Fig. 2a, p > 0.05), first success (Fig. 2b, p > 0.1), or successes on all attempts occurred on the mDB (best well) task (Fig. 2c, p > 0.4).
Table 4.
Coefficients of determination for single linear regression analyses of recovery in the nonuse task versus lesion volume [with (w) and without (wo) SDM46]
| First attempt (week) |
First success (week) |
Avg. % use |
Highest % use |
|||||
|---|---|---|---|---|---|---|---|---|
| wSDM46 | woSDM46 | wSDM46 | woSDM46 | wSDM46 | woSDM46 | wSDM46 | woSDM46 | |
| GM | 0.114 | 0.003 | 0.507* | 0.000 | 0.215 | 0.075 | 0.295 | 0.116 |
| WM | 0.018 | 0.091 | 0.361* | 0.002 | 0.068 | 0.013 | 0.126 | 0.000 |
| TV | 0.092 | 0.022 | 0.505* | 0.001 | 0.189 | 0.039 | 0.270 | 0.082 |
GM gray matter lesion volume as independent variable, WM white matter lesion volume as independent variable, TV total (gray matter + white matter) lesion volume as independent variable
p < 0.05
Post-lesion duration until the first success on the nonuse task was also not correlated with recovery on the mDB task, but was correlated with lesion volume. All eight monkeys that made attempts on the nonuse task with the impaired hand had their first success within 10 weeks following the lesion (Table 2, Fig. 3). The first success on the nonuse task occurred after the first success on the mDB task in five monkeys and before the first success on the mDB task in three monkeys (SDM50, SDM67, SDM46). Regression analysis showed that post-lesion week of first success on the nonuse task was not correlated with post-lesion week of first attempt, first success, or consistent success on the mDB task (Fig. 3a–c; p > 0.4), but was positively correlated with lesion volume (Fig. 3d, Table 4, p < 0.05). It is important to note, however, that two monkeys with large lesions (SDM50—M1 + LPMC + M2 lesion, SDM67—multi-focal lesion) had success on the nonuse task in the same post-lesion week or 2 and 3 weeks before some monkeys with smaller lesions (SDM70, 55, 64: M1 + LPMC lesions). Moreover, if we exclude the monkey with the largest lesion (SDM46) from this regression analysis, no significant relationship exists between post-lesion week of first success and gray matter lesion volume (Table 4, p > 0.4).
Fig. 3.
Scatter graphs showing post-lesion week of first attempt by the contralesional hand on the nonuse task versus post-lesion week of first attempt (a), first success (b) and five successes (c) on the best well of the mDB task. Post-lesion week of first attempt on the nonuse task is also plotted against total (gray matter + white matter) lesion volume in d. Each plotted symbol is data from a single monkey. Note that SDM56 was excluded from a, b, c and all these regression analyses because this monkey had no attempts on the nonuse task. SDM46 had no successes on the mDB task and thus is not included in b and c
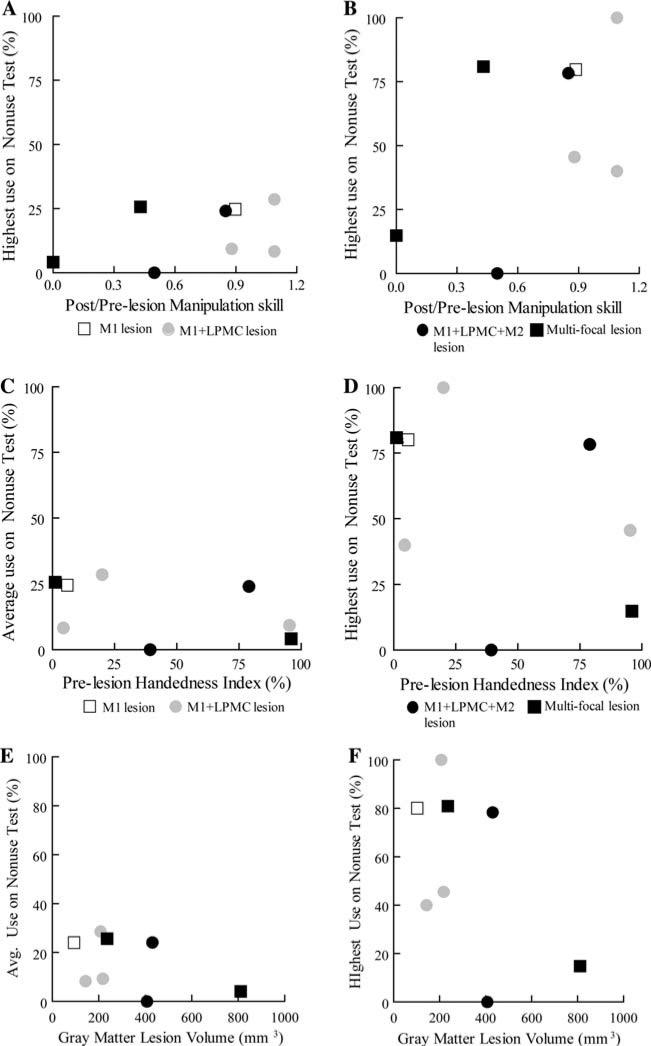
Percentage use of the contralesional hand in the nonuse task was not correlated with recovery of skilled use of that hand. Five of the monkeys had individual nonuse testing sessions in which the contralesional hand was used in greater than 50% of trials in the nonuse task, indicating a reversion to this hand as the preferred hand on those test days. However, neither average (over all post-lesion nonuse test sessions) nor maximum (in a single test session) percentage use of the contralesional hand in the nonuse task were correlated with the ratio of post-lesion to pre-lesion manipulation skill in the mDB task (Fig. 4a, b; p > 0.05). There was also no evidence that average or maximum percentage use of the contralesional hand in the nonuse task depended on strength of pre-lesion handedness (Fig. 4c, d, p > 0.05) or lesion volume (Fig. 4e, f, Table 4, p > 0.05).
Fig. 4.
Scatter graphs showing average and highest percentage use on the nonuse test plotted against the ratio of post to pre-lesion manipulation skill (a, b), pre-lesion handedness index (c, d) and gray matter (GM) lesion volume (e, f). Each plotted symbol is data from a single monkey. Note that SDM49 was not included in these graphs or the associated regression analyses due to the short post-lesion observation period
Discussion
Our findings clearly demonstrate that lesions to the arm areas of motor cortex, and additional frontal lobe areas in two monkeys, affecting the preferred hand can cause learned nonuse of that hand for a period of up to 6 months for a fine motor task. All but one of the nine monkeys recovered some spontaneous use of the contralesional hand in the nonuse task. Forced use of the impaired hand in food acquisition tasks similar to those used to test for nonuse may have stimulated recovery of fine motor function in that hand and return of spontaneous use of the hand to varying degrees. Indeed, it is remarkable that over time the contralesional hand became the preferred hand again (i.e., used more often than the ipsilesional hand) in some nonuse testing sessions. Also remarkable was that many of the monkeys began spontaneous use of the contralesional hand in the nonuse task before they were consistently successful with that hand in the similar mDB task.
Our findings show convincingly that occasional forced use of the impaired contralesional hand for food acquisition is sufficient to overcome learned nonuse following localized frontal lobe lesions in macaque monkeys. Previous work suggested that full-time constraint of the minimally impaired (ipsilesional) hand for 2–3 weeks and daily contralesional training is needed to ensure contralesional limb use following (comparatively) larger motor cortex lesions (Ogden and Franz 1917), smaller lesions limited to M1 digit areas (Murata et al. 2008), or after deafferentation of one upper limb (Taub et al. 1977). However, less intense interventions were not attempted in these previous studies. Although the devices we used for motor testing were designed to overcome the need for restraining the ipsilesional limb for purposes of monitoring recovery of contralesional hand motor function, it now seems clear that use of these devices occasionally (once per week or once every 2 weeks for a maximum of 40 movements) can stimulate recovery of spontaneous use of the contralesional limb even when the monkey could use the ipsilesional limb to perform a task. However, one monkey (SDM56) that recovered good function in the contralesional hand on the mDB task never chose to use that hand in the nonuse task. Thus, occasional forced use was not sufficient in all monkeys to stimulate use of an impaired hand when either hand can be used for a fine motor task. It is not clear whether constraint of the ipsilesional limb would have successfully reinstated spontaneous use of the contralesional limb in this monkey, although it seems highly likely. These findings are consistent with the idea that limb constraint is not a necessary part of therapy designed to promote recovery of hand function (Taub et al. 2006; Brogardh et al. 2009), but it is possible that constraint of the less impaired limb is necessary in some patients and would hasten recovery.
An important question is whether recovery of spontaneous use of the contralesional limb occurred because the brain lesions were smaller than in past work. Ogden and Franz (1917) indicated that surgical damage in their cases was restricted to lateral areas of motor cortex, which appeared to include most of M1 and lateral premotor cortex but did not extend onto the medial wall of the hemisphere. Thus, arm, leg, trunk, and facial movements contralateral to the lesion were affected by these large lesions. They also indicated that the cortex located deep in the central sulcus was damaged by inserting the cautery into cortex lining the anterior bank of the sulcus. However, this procedure may have also damaged parts of the postcentral gyrus somato-sensory areas as indicated from appearance of the brain, but this was not confirmed with histological analyses. In contrast, in the present work we avoided damage to the posterior bank of the central sulcus to ensure no damage to primary somatosensory cortex, and the lesions were limited to arm areas of M1 and lateral premotor cortex. In four cases, the lesion extended to include motor (and other) regions on the medial wall of the hemisphere (Table 1). Thus, only the upper limb was paretic following lesions in the present work in comparison to the entire side of the body and head in previous work. These differences in the lesions could be an important factor as the monkeys studied in the present work were less incapacitated immediately after the lesion due to sparing of leg, trunk and face motor areas, and somatosensory cortex. Partial sparing of the anterior bank of the central sulcus may also be an important factor in recovery of contralesional fine hand motor function because neurons in this region are excitatory to digit muscles with low currents (Lemon et al. 1986; Sato and Tanji 1989). Moreover, it is likely that direct connections between motor cortex neurons of this region and spinal motor neurons innervating digit muscles play an important role in control of coordinated digit movements for grasping (Heffner and Masterton 1975, 1983; Kuypers 1981; Lemon and Griffiths 2005; Schieber 2007). Notably, however, it was recently shown that good recovery of fine hand motor function is possible with extensive forced daily practice even with damage to the M1 region deep in the central sulcus (Murata et al. 2008). It is likely that damage to this area would prolong the post-lesion period over which the monkeys would not spontaneously use the impaired hand when the other hand could be used, but it appears unlikely that this would completely prevent recovery of spontaneous use of the contralesional hand. The finding that three of four monkeys with large lesions affecting the medial wall (M2 and other areas) recovered some spontaneous use of the contralesional hand is consistent with the idea that minimal forced use can promote recovery of spontaneous use of the hand for fine motor tasks even with quite large frontal cortex lesions.
Pre-lesion training with both hands on the mMAP and mDB tasks may be an important factor in recovery of spontaneous use of the contralesional hand. That is, the monkeys were experienced with being forced to use each hand to perform fine motor tasks while highly motivated due to short term food deprivation. The monkeys are likely aware that to obtain food rapidly after a period of food deprivation they must be able to use both hands, thereby stimulating attempts to rapidly recover use of the contralesional hand. This could explain the post-lesion findings of attempts and successful use of the contralesional hand in the nonuse task before the mDB task in some monkeys (Fig. 1, Table 2), as the required hand and digit movements are similar to those used in the mDB task. Indeed, the nonuse task could be regarded as a simpler version of the mDB task because the monkey's hand does not have to follow a constrained path during the reaching movement and the hand/digit motions can occur without the monkey's arm being extended far from the body. It is also possible that no pre-lesion training or forced use of the contralesional hand is necessary to cause eventual use of the contralesional hand to grasp food. Travis (1955) reported that after unilateral ablation of precentral cortex rhesus monkeys do recover precise ability to pick up small pieces of food with the contralesional hand. However, up to 3 months post-operatively the monkey used only the ipsilesional hand when food was presented directly in front, but would use the contralesional hand if food was presented directly to that hand.
Early post-lesion tests of motor performance using the ipsilesional hand, coupled with opportunities to use the contralesional hand in a motivated forced-use situation, may also be important in recovery of use of the contralesional hand. Training or practice of a task with one hand is known to cause some transfer of learning to influence initial performance with the other hand, suggesting that there may be a stored representation of the task that is not effectorspecific (e.g., Morton et al. 2001; Sainburg and Wang 2002; Wang and Sainburg 2003). Thus, practice of the task with the ipsilesional hand may help restore a representation that can be used by the contralesional limb and perhaps cause earlier use of the contralesional limb after the lesion. Moreover, we have recently shown that the ipsilesional limb recovers to produce more highly skilled hand movements than were observed pre-lesion in these monkeys (Darling et al. 2009a). Improved control of movements by the ipsilesional hand may create a better task representation that can also be accessed by brain areas controlling the contralesional hand to promote recovery of its function. However, regular use of the ipsilesional limb may also reinforce learned nonuse of the contralesional hand (Taub et al. 2006) and enhance inhibition of the damaged hemisphere (Duque et al. 2007). We minimized testing of ipsilesional hand motor performance with a relatively small number of trials at weekly and every other week intervals during post-lesion testing, but of course did not limit use of the ipsilesional hand in the cage.
Cerebral hemispheric dominance, as indicated by pre-lesion strength of hand preference, may also play a role in recovery of spontaneous use of the contralesional hand in the nonuse task. It has been reported that dominant (left) hemisphere damage due to stroke can create greater impairments in hand motor function and in learning of complex hand motor tasks than strokes affecting the right hemisphere in humans (Kimura and Archibald 1974; Kimura 1982), although a recent study did not confirm this (Harris and Eng 2006). Humans generally exhibit rather strong hemispheric dominance with the majority being left hemisphere dominant and right handed (e.g., Knecht et al. 2000), whereas rhesus monkeys exhibit weaker handedness that can vary with each task and probably have much weaker hemispheric dominance (Lehman 1980, 1989). Given this likely weaker hemispheric dominance in rhesus monkeys, one might expect low motivation to recover function of an impaired hand, especially since rhesus monkeys often demonstrate excellent skill with both hands in a variety of fine motor tasks, although they may exhibit a preference for one hand in a particular task (Lehman 1980, 1989). Moreover, damage to the more dominant hemisphere may remove inhibition to the undamaged hemisphere which may now dominate and perhaps inhibit use of the contralesional hand (Mansur et al. 2005; Fregni et al. 2006) since frontal motor cortex gives rise to homotopic callosal projections (Pandya and Vignolo 1971; Liu et al. 2002; Boussaoud et al. 2005). Such effects might lead to an inverse relationship between strength of pre-lesion hand preference and frequency of use of the contralesional hand in the nonuse task. Alternatively, one might expect greater motivation to recover use of a hand that was preferred to a greater extent before the lesion because the monkey is accustomed to using that hand for this type of fine motor tasks. Such high motivation was especially obvious in the case of SDM46 as this monkey had a high pre-lesion hand preference (handedness index of 96) and attempted to use the contralesional hand on the nonuse task at 2 weeks post-lesion despite having by far the largest lesion volume and greatest impairment of hand function. Thus, a direct relation between strength of pre-lesion hand preference and frequency of spontaneous use of the contralesional hand might be observed. A third alternative is that use of the contralesional hand in a task when either hand could be used occurs randomly and does not depend on pre-lesion hand preference, lesion volume, or recovery of skill but may depend solely on motivation of the monkey. Indeed, the finding that the post-lesion duration until first attempt in the nonuse task was not correlated with lesion volume or with post-lesion duration until first attempt, first success or consistent success in the mDB task supports this idea in terms of the first attempt at spontaneous use. However, post-lesion duration until first success on the nonuse task was correlated with lesion volume (Fig. 3d, Table 4), suggesting that this was not a random event. This conclusion is preliminary, however, because there was no relationship between these variables when SDM46 (the monkey with the largest lesion) was excluded from the analysis.
There was no evidence to support the idea that use of the contralesional hand in the nonuse task is related to either a desire to recover use of a previously more strongly preferred hand (expected direct relationship) or to greater post-lesion inhibition of the damaged hemisphere in monkeys with stronger hand preference (expected inverse relationship). No significant relationships were observed between pre-lesion handedness index and either maximum or average percentage use of the contralesional hand on post-lesion nonuse tests (Fig. 4c, d). It is possible that these two mechanisms counteract each other in different monkeys, thereby causing the poor relationship observed. For example, SDM50 and SDM56 had very similar lesions, but SDM50 had a much stronger hand preference (Table 1) and used the contralesional hand regularly in the nonuse task, whereas SM56 never used the contralesional hand in the nonuse task (Table 2). In contrast, SDM64 and SDM55 also had similar lesions, but SDM64 had much stronger hand preference and used the contralesional hand much less often in the nonuse task than SDM55. Variations in lesion size, which motor areas were lesioned and recovery of manipulation skill probably also contribute to recovery of spontaneous use of the contralesional hand. However, simple linear regression analyses did not show significant relationships between contralesional hand use in the nonuse task (highest use in a single session or average use over the entire post-lesion period) and recovery of skill (Fig. 4a, b), or lesion volume (Fig. 4e, f, Table 4). These factors probably also interact with intrinsic motivation level of the monkey as there was clearly very high motivation to recover ability to use the contralesional hand in the nonuse task in three monkeys with large lesions (SDM46, SDM67 with multi-focal lesions and SDM50 with a M1 + LPMC + M2 lesion). In the post-lesion recovery period, these monkeys all had substantial impairments of contralesional hand motor function but made attempts in the nonuse task before the mDB task. Other monkeys with small versus large lesions showed relatively low motivation to use the contralesional hand in the nonuse task despite good recovery of skill (e.g., SDM56 with a M1 + LPMC + M2 lesion never made an attempt and SDM70 with a M1 + LPMC lesion rarely used the impaired hand in the nonuse task although both monkeys had good recovery of skill). Thus, high motivation can overcome the effects of a large lesion in attempts to use the impaired hand spontaneously. Thus, it is doubtful that spontaneous contralesional hand use in the nonuse task is random, but there is no strong evidence against this possibility in this group of nine monkeys with variations in lesion size and involvement of different cortical motor areas.
The finding that recovery of spontaneous hand use was not dependent on lesion volume and the motor areas that were damaged probably reflects the good recovery of hand motor skill in all except the largest lesions. All monkeys recovered good to excellent contralesional hand motor skill except SDM46. Our recent work suggests that this recovery is related to intraspinal sprouting of CST axons from the arm area of M2 in spinal cord laminae involved in control of contralesional upper limb muscles following localized injury to M1 and LPMCd (McNeal et al. 2010). Such a mechanism might also explain the recovery of other monkeys in which the arm area of M2 was spared (e.g., SDM38, SDM67 had only minor damage to M2), but cannot explain recovery in monkeys in which M2 was damaged (e.g., SDM50, SDM56, SDM46). It is likely that intraspinal sprouting from tracts originating in other supraspinal motor areas such as M1 of the undamaged hemisphere (ipsilateral CST) and, perhaps, cingulate motor areas, and brainstem motor nuclei might explain their recovery.
In conclusion, the present findings show that surgically induced lesions of motor cortex in monkeys produce a form of learned nonuse of the contralesional impaired hand that lasts for variable periods after the injury. Following isolated frontal cortex injury, this learned nonuse can be alleviated by a relatively small amount of forced use of the contralesional hand during the post-lesion recovery period in most monkeys. Indeed, some monkeys began to use the contralesional hand when either hand could be used before any forced use of the contralesional hand occurred in a similar task (i.e., before any attempts in the mDB task). This indicates a strong motivation to regain function in that hand, likely due to short-term food restriction and previous experience with required use of that hand to obtain the food reward.
There are many issues to consider when comparing these findings in monkeys to the human condition following brain injury. One major issue is the need for pre-lesion training of the monkeys in the motor tasks used after the lesion, which of course does not occur directly in humans. However, the types of motor tasks used to test human hand motor skill after brain injury are typically rather simple tasks which healthy humans of the same age perform quite easily with minimal (if any) training. As such, we feel that these findings have some implications for human rehabilitation following brain injury. A second major issue is that many brain injuries in humans affect multiple areas (motor, sensory and higher order processing areas including cognitive areas) whereas the lesions in most monkeys were limited to motor areas (including higher order processing areas for the motor system). Clearly, lesions affecting sensory areas are likely to have a large impact on recovery as already discussed and lesions affecting more cognitive areas might also produce greater deficits in skill reacquisition (as indicated by the poor hand motor recovery of SDM46, the monkey with a lesion extending into medial prefrontal cortex). It is noteworthy that the injury model used here is most closely related to effects of neurosurgical procedures in humans, which also produces effects similar to other types of brain injuries on limb movement (e.g., Laplane et al. 1977a, b; Mikuni et al. 2005; Chamoun et al. 2007).
Overall, our findings provide strong support for the idea that inducing high motivation to recover use of a neurologically impaired limb is likely to improve recovery. Indeed, recent reviews suggest that motivation is a major factor in rehabilitation of upper limb use in hemiplegic stroke patients and that use of computer gaming technology may be effective in the rehabilitation of hand movement control in human stroke patients because of the possibility of enhancing motivation during therapy (Levin et al. 2009; Sivak et al. 2009; Timmermans et al. 2009). Moreover, a recent model of stroke rehabilitation suggests that if therapy is sufficient to encourage spontaneous movements by the patient, continued improvement in function may occur without further therapy (Han et al. 2008). It seems likely that the weekly post-lesion motor testing under conditions of high motivation in the present study was sufficient to encourage spontaneous use of the contralesional hand (as indicated by its use in the nonuse task) and further improvement of hand function in monkeys with brain lesions affecting motor areas. Strong evidence supporting this hypothesis comes from a recent report that 2 weeks of intensive constraint induced therapy led to continued improvements in upper limb function (especially strength) for 24 months post-therapy due to greater use of the impaired hand (Wolf et al. 2008). Collectively, these findings underscore the importance of a systematically applied therapeutic intervention with high motivation to inhibit the induction of learned nonuse phenomena and improve the motor recovery process after brain injury.
Acknowledgments
Supported by National Institutes of Health grant NS 046367 and The South Dakota Spinal Cord Injury and Traumatic Brain Injury Research Council.
Contributor Information
Warren G. Darling, Motor Performance Laboratory, Department of Integrative Physiology, The University of Iowa, Iowa City, IA 52242, USA warren-darling@uiowa.edu
Marc A. Pizzimenti, Department of Anatomy and Cell Biology, Carver College of Medicine, The University of Iowa, Iowa City, IA 52242, USA
Diane L. Rotella, Motor Performance Laboratory, Department of Integrative Physiology, The University of Iowa, Iowa City, IA 52242, USA
Stephanie M. Hynes, Motor Performance Laboratory, Department of Integrative Physiology, The University of Iowa, Iowa City, IA 52242, USA
Jizhi Ge, Laboratory of Neurological Sciences, Division of Basic Biomedical Sciences, Sanford School of Medicine, The University of South Dakota, Vermillion, SD 57069, USA.
Kimberly S. Stilwell-Morecraft, Laboratory of Neurological Sciences, Division of Basic Biomedical Sciences, Sanford School of Medicine, The University of South Dakota, Vermillion, SD 57069, USA
Tyler Vanadurongvan, Laboratory of Neurological Sciences, Division of Basic Biomedical Sciences, Sanford School of Medicine, The University of South Dakota, Vermillion, SD 57069, USA.
David W. McNeal, Laboratory of Neurological Sciences, Division of Basic Biomedical Sciences, Sanford School of Medicine, The University of South Dakota, Vermillion, SD 57069, USA
Kathryn M. Solon-Cline, Laboratory of Neurological Sciences, Division of Basic Biomedical Sciences, Sanford School of Medicine, The University of South Dakota, Vermillion, SD 57069, USA
Robert J. Morecraft, Laboratory of Neurological Sciences, Division of Basic Biomedical Sciences, Sanford School of Medicine, The University of South Dakota, Vermillion, SD 57069, USA
References
- Boussaoud D, Tanne-Gariepy J, Wannier T, Rouiller EM. Callosal connections of dorsal versus ventral premotor areas in the macaque monkey: a multiple retrograde tracing study. BMC Neurosci. 2005;6:67. doi: 10.1186/1471-2202-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogardh C, Vestling M, Sjolund BH. Shortened constraint-induced movement therapy in subacute stroke–no effect of using a restraint: a randomized controlled study with independent observers. J Rehabil Med. 2009;41:231–236. doi: 10.2340/16501977-0312. [DOI] [PubMed] [Google Scholar]
- Chamoun RB, Mikati MA, Comair YG. Functional recovery following resection of an epileptogenic focus in the motor hand area. Epilepsy Behav. 2007;11:384–388. doi: 10.1016/j.yebeh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Crocker MD, MacKay-Lyons M, McDonnell E. Forced use of the upper extremity in cerebral palsy: a single-case design. Am J Occup Ther. 1997;51:824–833. doi: 10.5014/ajot.51.10.824. [DOI] [PubMed] [Google Scholar]
- Darling WG, Peterson CR, Herrick JL, McNeal DW, Stilwell-More-craft KS, Morecraft RJ. Measurement of coordination of object manipulation in non-human primates. J Neurosci Methods. 2006;154:38–44. doi: 10.1016/j.jneumeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, Hynes SM, Rotella D, Headley G, Ge J, McNeal DW, Stilwell-Morecraft KS, Vanadurongvan T, More-craft RJ. Recovery of ipsilesional hand fine motor function following motor cortex lesions in rhesus monkeys. Society for Neuroscience. 2009a2009 Abstract Viewer #768.12. [Google Scholar]
- Darling WG, Pizzimenti MA, Rotella DL, Peterson CR, Hynes SM, Ge J, Solon K, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Volumetric effects of motor cortex injury on recovery of dexterous movements. Exp Neurol. 2009b;220:90–108. doi: 10.1016/j.expneurol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol. 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLoS Comput Biol. 2008;4:e1000133. doi: 10.1371/journal.pcbi.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. 2006;20:380–389. doi: 10.1177/1545968305284528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner R, Masterton B. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain Behav Evol. 1975;12:161–200. doi: 10.1159/000124401. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain Behav Evol. 1983;23:165–183. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Tempini A, Rouiller EM. Effects of reversible inactivation of the supplementary motor area (SMA) on unimanual grasp and bimanual pull and grasp performance in monkeys. Somatosens Mot Res. 1997;14:268–280. doi: 10.1080/08990229770980. [DOI] [PubMed] [Google Scholar]
- Kimura D. Left-hemisphere control of oral and brachial movements and their relation to communication. Philos Trans R Soc Lond B Biol Sci. 1982;298:135–149. doi: 10.1098/rstb.1982.0077. [DOI] [PubMed] [Google Scholar]
- Kimura D, Archibald Y. Motor functions of the left hemisphere. Brain. 1974;97:337–350. doi: 10.1093/brain/97.1.337. [DOI] [PubMed] [Google Scholar]
- Knapp HD, Taub E, Berman AJ. Conditioned response following deafferentation in the monkey. Trans Am Neurol Assoc. 1959;84:185–187. [PubMed] [Google Scholar]
- Knapp HD, Taub E, Berman AJ. Movements in monkeys with deafferented forelimbs. Exp Neurol. 1963;7:305–315. doi: 10.1016/0014-4886(63)90077-3. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kuypers H. Anatomy of the descending motor pathways. In: Brooks VB, editor. Handbook of physiology section 1, the nervous system, vol II. motor control, pt I. American physiological society; Bethesda: 1981. pp. 567–666. [Google Scholar]
- Laplane D, Talairach J, Meininger V, Bancaud J, Bouchareine A. Motor consequences of motor area ablations in man. J Neurol Sci. 1977a;31:29–49. doi: 10.1016/0022-510x(77)90004-1. [DOI] [PubMed] [Google Scholar]
- Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977b;34:301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- Lehman RA. The handedness of rhesus monkeys. III. Consistency within and across activities. Cortex. 1980;16:197–204. doi: 10.1016/s0010-9452(80)80055-4. [DOI] [PubMed] [Google Scholar]
- Lehman RA. Hand preferences of rhesus monkeys on differing tasks. Neuropsychologia. 1989;27:1193–1196. doi: 10.1016/0028-3932(89)90102-4. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the cortico-spinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GW, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol. 1986;381:497–527. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MF, Knaut LA, Magdalon EC, Subramanian S. Virtual reality environments to enhance upper limb functional recovery in patients with hemiparesis. Stud Health Technol Inform. 2009;145:94–108. [PubMed] [Google Scholar]
- Liu J, Morel A, Wannier T, Rouiller EM. Origins of callosal projections to the supplementary motor area (SMA): a direct comparison between pre-SMA and SMA-proper in macaque monkeys. J Comp Neurol. 2002;443:71–85. doi: 10.1002/cne.10087. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Mason CR, Miller LE, Baker JF, Houk JC. Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. J Neurophysiol. 1998;79:537–554. doi: 10.1152/jn.1998.79.2.537. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon K, Hynes SM, Pizzimenti MA, Rotella D, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni N, Ikeda A, Yoneko H, Amano S, Hanakawa T, Fukuyama H, Hashimoto N. Surgical resection of an epileptogenic cortical dysplasia in the deep foot sensorimotor area. Epilepsy Behav. 2005;7:559–562. doi: 10.1016/j.yebeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. J Comp Neurol. 2007a;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007b;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morton SM, Lang CE, Bastian AJ. Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res. 2001;141:438–445. doi: 10.1007/s002210100889. [DOI] [PubMed] [Google Scholar]
- Murata Y, Higo N, Oishi T, Yamashita A, Matsuda K, Hayashi M, Ya-mane S. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J Neurophysiol. 2008;99:773–786. doi: 10.1152/jn.01001.2007. [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs C. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J Neurotrauma. 2009 doi: 10.1089/neu.2009.0966. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo R, Jenkins W, Merzenich M, Prejean T, Grenda R. Neuro-physiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden R, Franz SI. On cerebral motor control: the recovery from experimentally produced hemiplegia. Psychobiology. 1917;1:33–47. [Google Scholar]
- Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Res. 1971;26:217–233. [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Pizzimenti MA, Darling WG, Rotella DL, McNeal DW, Herrick JL, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]
- Quencer K, Okun MS, Crucian G, Fernandez HH, Skidmore F, Heilman KM. Limb-kinetic apraxia in Parkinson disease. Neurology. 2007;68:150–151. doi: 10.1212/01.wnl.0000250331.35912.a5. [DOI] [PubMed] [Google Scholar]
- Roland PE, Zilles K. Functions and structures of the motor cortices in humans. Curr Opin Neurobiol. 1996;6:773–781. doi: 10.1016/s0959-4388(96)80027-4. [DOI] [PubMed] [Google Scholar]
- Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62–68. doi: 10.3171/jns.1991.75.1.0062. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res. 2002;145:437–447. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato KC, Tanji J. Digit-muscle responses evoked from multiple intracortical foci in monkey precentral motor cortex. J Neurophysiol. 1989;62:959–970. doi: 10.1152/jn.1989.62.4.959. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Chapter 2 comparative anatomy and physiology of the corticospinal system. Handb Clin Neurol. 2007;82:15–37. doi: 10.1016/S0072-9752(07)80005-4. [DOI] [PubMed] [Google Scholar]
- Sivak M, Mavroidis C, Holden MK. Design of a low cost multiple user virtual environment for rehabilitation (MUVER) of patients with stroke. Stud Health Technol Inform. 2009;142:319–324. [PubMed] [Google Scholar]
- Taub E, Ellman SJ, Berman AJ. Deafferentation in monkeys: effect on conditioned grasp response. Science. 1966;151:593–594. doi: 10.1126/science.151.3710.593. [DOI] [PubMed] [Google Scholar]
- Taub E, Goldberg IA, Taub P. Deafferentation in monkeys: pointing at a target without visual feedback. Exp Neurol. 1975;46:178–186. doi: 10.1016/0014-4886(75)90040-0. [DOI] [PubMed] [Google Scholar]
- Taub E, Heitmann RD, Barro G. Alertness, level of activity, and purposive movement following somatosensory deafferentation in monkeys. Ann N Y Acad Sci. 1977;290:348–365. doi: 10.1111/j.1749-6632.1977.tb39737.x. [DOI] [PubMed] [Google Scholar]
- Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- Timmermans AA, Seelen HA, Willmann RD, Kingma H. Technology-assisted training of arm-hand skills in stroke: concepts on reacquisition of motor control and therapist guidelines for rehabilitation technology design. J Neuroeng Rehabil. 2009;6:1. doi: 10.1186/1743-0003-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res. 2003;149:520–526. doi: 10.1007/s00221-003-1392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D, for the EI Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, Morris D, Blanton S, Nichols-Larsen D, Clark PC. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qu M, Dabringhaus A, Seitz R, Roland PE. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat. 1995;187(Pt 3):515–537. [PMC free article] [PubMed] [Google Scholar]