Abstract
Background
Renal ischemia–reperfusion injury (IRI) is a major cause of acute kidney injury and often leads to multi-organ dysfunction and systemic inflammation. Volatile anesthetics have potent antiinflammatory effects and we aimed to determine whether the representative volatile anesthetic isoflurane protects against acute kidney injury-induced liver and intestinal injury and the mechanisms involved in this protection.
Methods
Mice were anesthetized with pentobarbital and subjected to 30 min of left renal ischemia after right nephrectomy followed by exposure to 4 h of equi-anesthetic doses of pentobarbital or isoflurane. Five hrs after renal IRI, plasma creatinine and alanine aminotransferase were measured. Liver and intestine tissues were analyzed for pro-inflammatory mRNAs, histology, sphingosine kinase-1 (SK1) immunoblotting, SK1 activity, and sphingosine-1-phosphate levels.
Results
Renal IRI with pentobarbital led to severe renal, hepatic, and intestinal injury with focused peri-portal hepatocyte vacuolization, small intestinal apoptosis and pro-inflammatory mRNA upregulation. Isoflurane protected against renal IRI and reduced hepatic and intestinal injury via induction of small intestinal crypt SK1 mRNA, protein and enzyme activity and increase in S1P. We confirmed the importance of SK1 as mice treated with a selective SK inhibitor (SKI-II) or mice deficient in SK1 enzyme were not protected against hepatic and intestinal dysfunction with isoflurane.
Conclusions
Taken together, we demonstrate that isoflurane protects against multi-organ injury after renal IRI via induction of the SK1/sphingosine-1-phosphate pathway. Our findings may help to unravel the cellular signaling pathways of volatile anesthetic-mediated hepatic and intestinal protection and lead to new therapeutic applications of volatile anesthetics during the perioperative period.
Acute kidney injury (AKI) continues to be a major clinical problem during the perioperative period. 1 Development of AKI often leads to multi-organ dysfunction and systemic inflammation and contributes to significant morbidity and mortality. In particular, hepatic dysfunction occurs frequently in patients with AKI and leads to other complications such as intestinal barrier dysfunction, respiratory failure, and multi-organ failure frequently complicated by sepsis. 2 We recently demonstrated that AKI leads to rapid hepatic and intestinal injury in mice with increased inflammation, apoptosis, and necrosis. 3 This hepatic and intestinal injury was mediated by upregulation of the pro-inflammatory cytokines TNF-α, IL-17A, and IL-6 after AKI.
Volatile anesthetics are an integral part of the perioperative period as they are administered to virtually all patients undergoing general anesthesia in the United States. We previously reported that volatile anesthetics protected against renal ischemia–reperfusion injury (IRI) in vivo 4 and had direct antiinflammatory and antinecrotic effects in cultured human kidney proximal tubule cells. 5 Most volatile anesthetics are lipophilic molecules 6 and have been shown to increase membrane fluidity and activate sphingomyelin hydrolysis in the renal cortex. 7 The lysophospholipid sphingosine-1-phosphate (S1P) is a product of sphingomyelin hydrolysis and functions as both an extracellular ligand for specific G protein-coupled receptors as well as an intracellular second messenger in promoting cell growth and survival and the inhibition of apoptosis. 8
In this study, we questioned whether volatile anesthetics would provide protection of the liver and intestine after renal IRI. We hypothesized that volatile anesthetics activate the sphingosine kinase-1 (SK1)/S1P pathway in the small intestine to protect against renal IRI-induced hepatic and intestinal injury. We demonstrate rapid hepatic and intestinal injury following renal IRI with protective effects mediated by the representative volatile anesthetic, isoflurane. Isoflurane reduced hepatic and intestinal pro-inflammatory cytokine upregulation and intestinal apoptosis via induction of SK1 in small intestinal crypts.
Materials and Methods
Materials
Isoflurane (2-chloro-2-[difluoromethoxy]-1,1,1-trifluoro-ethane) was purchased from Abbott Laboratories (North Chicago, IL). SKI-II; 4-[4-{4-Chlorophenyl}-2-thiazolyl]amino phenol) was purchased from Tocris Bioscience (Ellisville, MO). Unless otherwise specified, all other reagents were purchased from Sigma, St. Louis, MO.
Murine model of renal IRI
All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University (New York, NY). We utilized male C57BL/6 (Harlan, Indianapolis, IN; 20 to 25 g) or SK1KO (kindly provided by R. L. Proia, PhD, Chief, Genetics of Development and Disease Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA; 20 to 25g) mice. The generation and initial characterization of SK1KO mice has been described previously. 9 These mice were backcrossed to C57BL/6 mice for more than 10 generations.
In our model of renal IRI, mice were initially anesthetized with intraperitoneal pentobarbital (Henry Schein Veterinary Co., Indianapolis, IN; 50 mg/kg body weight, or to effect) and subjected to right nephrectomy and 30 min of left renal ischemia. After closure of the abdomen in two layers, the mice were then exposed to an additional 4 h of equipotent doses of either pentobarbital via intermittent intraperitoneal administration or isoflurane (1.2% or minimum alveolar concentration MAC, defined as the concentration of volatile anesthetic in the lungs that is needed to prevent movement in 50% of subjects in response to a painful stimulus) as described previously. 4 Briefly, mice were placed in an airtight 10 l chamber on a warming blanket with inflow and outflow hoses located at the top and bottom of the chamber, respectively. Isoflurane was delivered in room air at 5 l/min using an isoflurane-specific vaporizer (Datex-Ohmeda, Madison, WI). The vaporizer was set to maintain chamber isoflurane concentration at 1.2% monitored by an infrared analyzer sampling gas at the outflow hose. The mice were placed on a heating pad under a warming light to maintain body temperature ~37°C.
To test the effects of SK inhibition, the SK inhibitor SKI-II was administered to mice undergoing renal IRI. SKI-II (50 mg/kg) was administered s.c. 15 min preischemia and 4 h postreperfusion. SKI-II is an SK-selective inhibitor with minimal effects on other kinases 10 and this dose was shown to have effective inhibition of activity without significant toxicity. 11
For experiments involving sham operation or renal IRI, all samples (including plasma and tissue) were collected from mice 24 h after sham operation or renal IRI. For experiments involving sham exposure to pentobarbital or isoflurane (SK mRNA and protein expression, SK1 immunofluorescence, SK activity, and S1P measurement), mice were exposed to 4 h of pentobarbital or isoflurane (without sham operation or renal IRI) and allowed to recover for 1 h. Samples were collected 5 h after initial anesthetic exposure.
Plasma alanine aminotransferase (ALT) activity and creatinine level
Plasma ALT activity and creatinine levels were measured using the Infinity ALT assay kit and an enzymatic creatinine reagent kit, respectively, according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA).
Histologic analysis of liver and small intestine injury
For histologic preparations, liver or small intestine (jejunum and ileum) tissues collected from mice were washed in ice-cold PBS and fixed overnight in 10% formalin. After automated dehydration through a graded alcohol series, tissues were embedded in paraffin, sectioned at 5 μm and stained with hematoxylin-eosin (H&E). Liver H&E sections were graded for renal IRI-induced vacuolization injury (score range 0–4) by a pathologist (V.D.D.) blinded to the samples.
Detection of small intestinal apoptosis after renal IRI
We detected small intestinal apoptosis with terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) staining. In situ labeling of fragmented DNA was performed with TUNEL stain (green fluorescence) using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer.
Assessment of liver and small intestine inflammation and SK expression
Hepatic and intestinal inflammation was determined by measuring mRNA encoding markers of inflammation, including IL-6, IL-17A, intercellular adhesion molecule 1 (ICAM-1), monocyte chemoattractive protein 1 (MCP-1), macrophage inflammatory protein 2 (MIP-2), and tumor necrosis factor-α (TNF-α) (table 1). In addition, SK1 and SK2 mRNA levels were measured. Semiquantitative real-time RT-PCR was performed as described. 12
Table 1.
Primers used to amplify mRNAs encoding SK1, SK2, GAPDH, and proinflammatory cytokines based on published GenBank sequences for mice.
| Primers | Accession Number | Sequence (Sense/Antisense) | Product Size (bp) | Cycle Number | Annealing Temp (°C) |
|---|---|---|---|---|---|
| Mouse SK1 | NM_011451 (variant 1) | 5'–GATGCATGAGGTGGTGAATG–3' | 337 | 22 | 64 |
| NM_025367 (variant 2) | 5'–GCCCACTGTGAAACGAATC–3' | ||||
| Mouse SK2 | NM_203280 (variant 1) | 5'–ACTGCTCGCTTCTTCTCTGC–3' | 437 | 23 | 68 |
| NM_020011 (variant 2) | 5'–ACCATTGAGGGACAGGTCAG–3' | ||||
| Mouse MIP-2 | X53798 | 5'–CCAAGGGTTGACTTCAAGAAC–3' | 282 | 28 | 60 |
| 5'–AGCGAGGCACATCAGGTACG–3' | |||||
| Mouse ICAM-1 | X52264 | 5'–TGTTTCCTGCCTCTGAAGC–3' | 409 | 21 | 60 |
| 5'–CTTCGTTTGTGATCCTCCG–3' | |||||
| Mouse TNF-α | X02611 | 5'–TACTGAACTTCGGGGTGATTGGTCC–3' | 290 | 24 | 65 |
| 5'–CAGCCTTGTCCCTTGAAGAGAACC–3' | |||||
| Mouse MCP-1 | NM_011333 | 5'–ACCTGCTGCTACTCATTCAC–3' | 312 | 22 | 60 |
| 5'–TTGAGGTGGTTGTGGAAAAG–3' | |||||
| Mouse IL-6 | NM_031168 | 5'–CCGGAGAGGAGACTTCACAG–3' | 421 | 30 | 62 |
| 5'–GGAAATTGGGGTAGGAAGGA–3' | |||||
| Mouse IL-17 | NM_010552 | 5'–TCCAGAAGGCCCTCAGACTA–3' | 248 | 32 | 66 |
| 5'–ACACCCACCAGCATCTTCTC–3' | |||||
| Mouse GAPDH | M32599 | 5'–ACCACAGTCCATGCCATCAC–3' | 450 | 15 | 65 |
| 5'–CACCACCCTGTTGCTGTAGCC–3' |
bp, base pairs; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ICAM-1, intercellular adhesion molecule-1; MCP-1, monocyte chemoattractant protein 1; MIP-2, macrophage inflammatory protein 2; SK, sphingosine kinase; TNF-α, tumor necrosis factor alpha. Respective anticipated RT-PCR product size, PCR cycle number for linear amplification and annealing temperatures used for each primer are also provided.
Vascular permeability of liver and small intestine after renal IRI
Changes in liver and small intestine vascular permeability were assessed by quantitating extravasation of Evans blue dye (EBD) into the tissue as described previously. 13
Immunoblotting analyses of small intestine
Small intestinal tissues in mice were collected and homogenized in lysis buffer (20 mM Hepes (pH 7.4), 2 mM EGTA, 1 mM DTT, 1% Triton X-100, 10% glycerol, and protease inhibitor cocktail (Calbiochem, La Jolla, CA)) on ice with a glass homogenizer. The homogenates were centrifuged for 20 min at 16,000×g. The supernatant was collected and used for immunoblotting as described previously. 14 The samples (50–100 μg protein/lane) were separated on 9 or 12% SDS-PAGE and then transferred to Immobilon membranes (Millipore, Bedford, MA). The membranes were blocked with blocking buffer (5% nonfat dry milk in TBS containing 0.1% Tween 20) and incubated overnight with polyclonal anti-SK1 (3297; 1:1000 dilution; Cell Signaling, Beverly, MA), anti-SK2 (ab37977; 1:2000 dilution; Abcam, Cambridge, MA), or monoclonal anti-β-actin (A5316; 1:5000 dilution; σ) antibodies diluted in blocking buffer at 4°C. After being washed, the membranes were incubated with horseradish peroxidase-conjugated donkey antirabbit or sheep antimouse (1:5000 dilution; ECM Bioscience, Versailles, KY) antibodies for 1 h at room temperature. Finally, the membranes were detected with enhanced chemiluminescence immunoblotting detection reagents (Amersham, Piscataway, NJ) with subsequent exposure to a CCD camera coupled to a UVP Bio-imaging System (Upland, CA). The band intensities of the immunoblots were within the linear range of exposure for all experiments.
Immunofluorescence staining for SK1 in small intestine
Immunofluorescence to detect SK1 was performed as described. 15 Small intestinal tissues in mice were collected and embedded in oxytetracycline compound and frozen, and cryosections were incubated with anti-SK1 antibody (AP7237; Abgent, San Diego, CA) overnight at 4°C. After washing with PBS, the sections were incubated with Texas Red-conjugated goat antirabbit IgG (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. For nuclear staining, 4[prime],6-diamidino-2-phenylindole (DAPI; blue) was placed on sections for 1 min. The sections were mounted with Prolong Gold antifade reagent (Invitrogen, Carlsbad, CA) and observed under an Olympus IX81 (Center Valley, PA) fluorescence microscope.
In vivo intestine enzyme preparation from mice and SK activity assay
Small intestines were collected and homogenized mechanically in cell lysate buffer (100 mM sucrose, 1 mM EGTA, 20 mM MOPS, pH 7.4, 5% Percoll, 0.01% digitonin, protease (Calbiochem) and phosphatase inhibitors) on ice. After a 1,000×g spin for 15 min to pellet cellular debris, protein concentrations were determined. SK activity was measured as described by Vessey et al. 16 using 20 μg of protein, with some modifications as described. 17
High-pressure liquid chromatography (HPLC) detection of S1P
S1P levels were measured in the small intestines of mice using HPLC as described. 18
Protein determination
Protein content was determined with the Thermo Scientific bicinchoninic acid protein assay reagent with bovine serum albumin as a standard.
Statistical analysis
The data were analyzed with a two-tailed Student's t test when comparing means between two groups. One-way ANOVA plus Tukey post hoc multiple comparison test was used when comparing multiple groups. The ordinal values of the liver injury scores were analyzed by the Mann–Whitney nonparametric test. In all cases, a probability statistic less than 0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SEM.
Results
Isoflurane protects against acute renal and hepatic injury after renal IRI in mice
Twenty-four hrs after renal IRI, mice exposed to pentobarbital anesthesia developed significant renal dysfunction as indicated by a rise in plasma creatinine (Cr = 2.39 ± 0.05 mg/dL, N = 10, P less than 0.001 vs.. sham) above sham values (Cr = 0.47 ± 0.03 mg/dL, N = 6). Isoflurane exposure after renal IRI protected the kidneys as evidenced by a significant decline in plasma creatinine levels (Cr = 1.61 ± 0.17 mg/dL, N = 8, P less than 0.001 vs.. pentobarbital renal IRI).
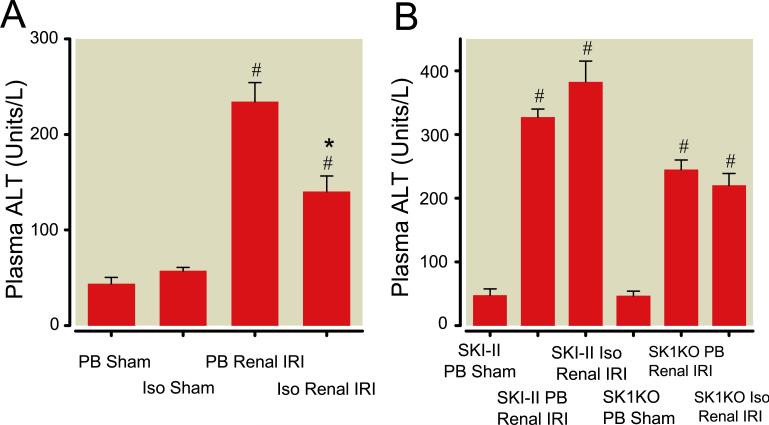
Twenty-four hrs after renal IRI, mice developed acute hepatic injury with pentobarbital exposure as indicated by a rise in plasma ALT above sham levels (fig. 1<pick;f1;0>A). In contrast, isoflurane exposure after renal IRI protected against liver injury with a significant reduction in plasma ALT levels (fig. 1A).
Fig. 1.
Plasma alanine aminotransferase (ALT, U/L) after renal ischemia–reperfusion injury (IRI). (A) Plasma ALT was measured in C57BL/6 mice exposed to 4 h of pentobarbital (PB) or 1.2% isoflurane (Iso) after sham operation (N = 7 for each group) or renal IRI (N = 8 for each group). (B) Plasma ALT from C57BL/6 mice treated with the sphingosine kinase (SK) inhibitor SKI-II or from SK1 knockout (SK1KO) mice after sham operation (PB anesthesia; N = 4 for each group) or renal IRI (N = 6 for each group) followed by exposure to 4 h of PB or Iso. In all cases (A and B), plasma ALT was measured 24 h after renal IRI. # P less than 0.05 versus. sham mice. * P less than 0.05 versus. PB Renal IRI group. Data presented as mean ± SEM.
To evaluate the role of SK in mediating the protective effects of isoflurane after renal IRI, we treated some animals with a selective SK inhibitor, SKI-II, before induction of renal IRI. SKI-II administration had no detrimental effects on renal function (Cr = 0.43 ± 0.04 mg/dL, N = 3) in sham-operated mice. There were no differences in plasma creatinine values with SKI-II treatment before renal IRI with pentobarbital exposure (Cr = 2.53 ± 0.18 mg/dL, N = 6) compared to isoflurane exposure (Cr = 2.32 ± 0.09 mg/dL, N = 5, P = 0.47 vs.. SKI-II pentobarbital renal IRI). Plasma ALT levels increased in SKI-II-treated mice exposed to pentobarbital after renal IRI compared to sham mice (fig. 1B). There was no reduction in plasma ALT levels after isoflurane exposure.
In addition, we utilized a strain of mice deficient in SK1 enzyme. There were no differences in plasma creatinine values in SK1KO mice after renal IRI with pentobarbital exposure (Cr = 2.33 ± 0.18 mg/dL, N = 8) compared to isoflurane exposure (Cr = 2.25 ± 0.38 mg/dL, N = 6, P = 0.82 vs.. SK1KO pentobarbital renal IRI). SK1KO mice exposed to pentobarbital after renal IRI had increased plasma ALT levels compared to sham-operated mice with no reduction with isoflurane exposure (fig. 1B).
Isoflurane exposure reduces hepatic vacuolization and small intestinal apoptosis
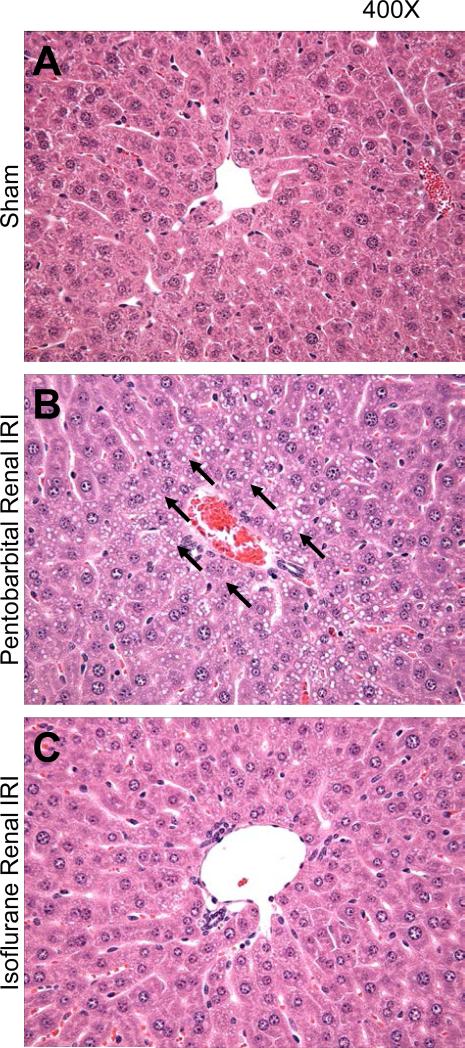
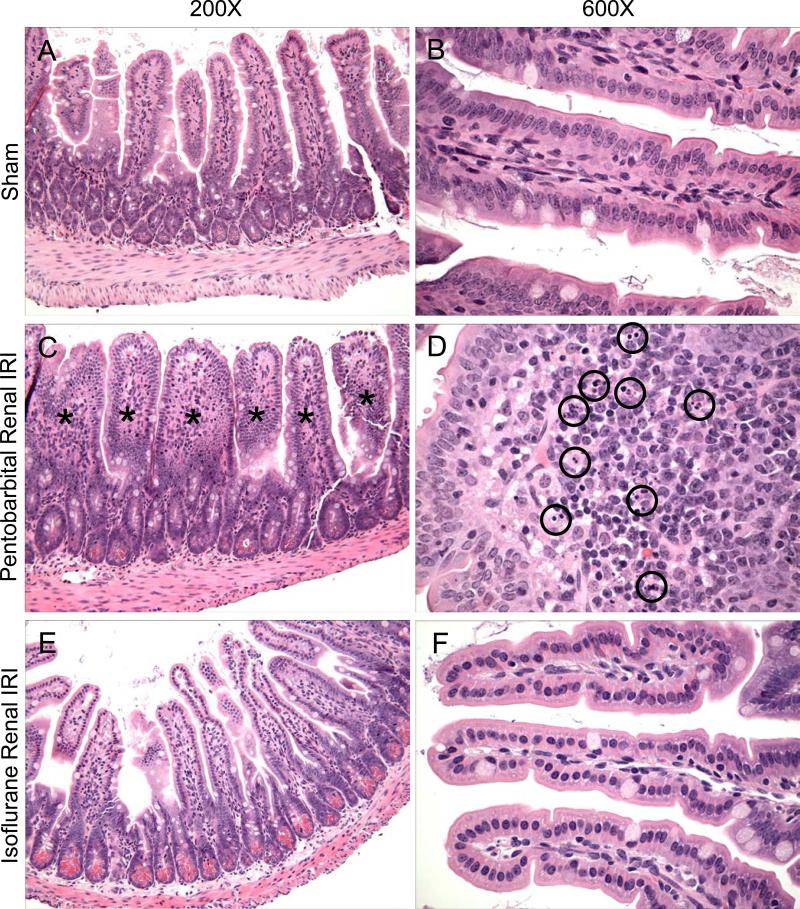
In figures 2<pick;f2;0> and 3<pick;f3;0>, the protective effects of isoflurane anesthesia are further supported by representative histologic slides. Pentobarbital exposure after renal IRI led to marked hepatocyte vacuolization (fig. 2B) and profound epithelial villous swelling and apoptosis in the small intestine (figs. 3C-D). Isoflurane exposure after renal IRI dramatically attenuated these injuries in the liver (fig. 2C) and small intestine (figs. 3E-F).
Fig. 2.
Isoflurane protects against liver injury after renal ischemia–reperfusion injury (IRI). Representative photomicrographs of liver from 4 experiments (hematoxylin and eosin staining, magnification of 400×) of mice subjected to sham operation (A) or to renal IRI followed by 4 h of pentobarbital (B) or 1.2% isoflurane (C). Tissues were collected 24 h after renal IRI. Arrows point to areas of significant hepatocyte vacuolization.
Fig. 3.
Isoflurane protects against small intestinal injury after renal ischemia–reperfusion injury (IRI). Representative photomicrographs of small intestine from 4 experiments (hematoxylin and eosin staining, magnifications of 200× and 600×). Sham-operated mice show normal intestinal morphology (A-B). The intestines of mice exposed to 4 h of pentobarbital after renal IRI demonstrate marked epithelial villous swelling (C, swollen villi highlighted by [lsqb]*[rsqb]), and an enlarged image of a single, swollen villous (D) shows numerous apoptotic bodies (circled). In contrast, the intestines of mice exposed to 4 h of 1.2% isoflurane after renal IRI were protected from severe injury (E-F). Tissues were collected 24 h after renal IRI.
After renal IRI, the predominant component of hepatic injury was vacuolization. Pentobarbital exposure after renal IRI resulted in severe hepatic vacuolization as demonstrated by the vacuolization score (3.4 ± 0.2, N = 5, score ranges from 0 to 4). In contrast, hepatic vacuolization was less severe with isoflurane exposure after renal IRI (1.6 ± 0.4, N = 4, P less than 0.05 vs.. pentobarbital renal IRI). Sham mice had normal hepatic histology as demonstrated by a vacuolization score of (0.0 ± 0.0, N = 4).
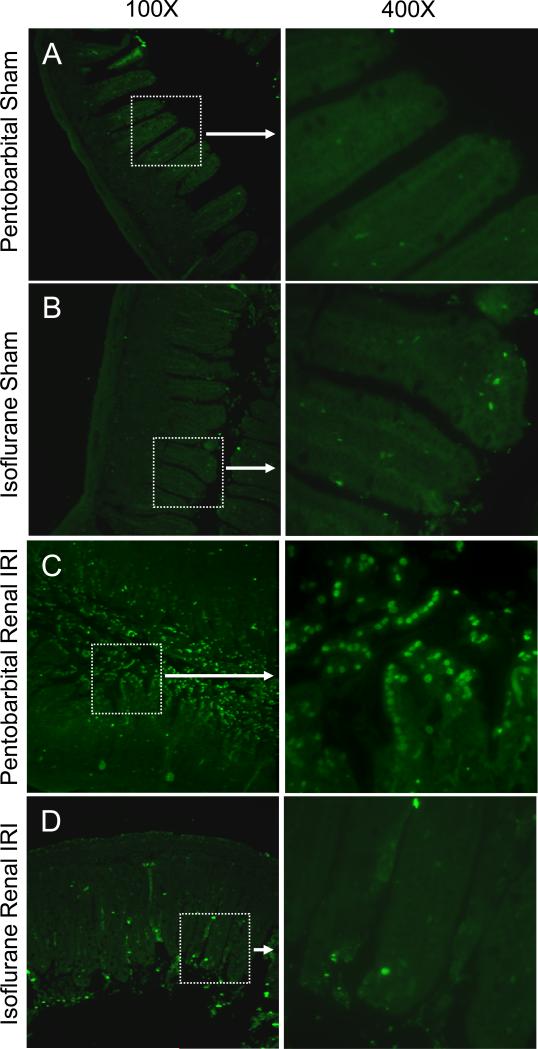
We failed to detect significant TUNEL-positive cells in small intestinal sections from sham-operated mice exposed to pentobarbital (fig. 4A) or isoflurane (fig. 4B). Mice exposed to pentobarbital after renal IRI (fig. 4C) showed many TUNEL-positive cells in the small intestine (representative of 4 experiments). Mice exposed to isoflurane after renal IRI (fig. 4D) had a reduction in TUNEL-positive cells in the small intestine.
Fig. 4.
Isoflurane protects against intestinal apoptosis after renal ischemia–reperfusion injury (IRI). Representative fluorescence photomicrographs (of 4 experiments) illustrating apoptotic nuclei (TUNEL fluorescence staining, green) in the small intestine. The left side of each panel depicts a 100× fluorescence photomicrograph with a highlighted area (white box) enlarged to 400× on the right side the panel. Mice were exposed to 4 h of pentobarbital after sham operation (A) or renal IRI (C) or to 4 h of 1.2% isoflurane after sham operation (B) or renal IRI (D). Tissues were collected 24 h after renal IRI.
Mice exposed to isoflurane after renal IRI show reduced pro-inflammatory gene expression in the liver and small intestine
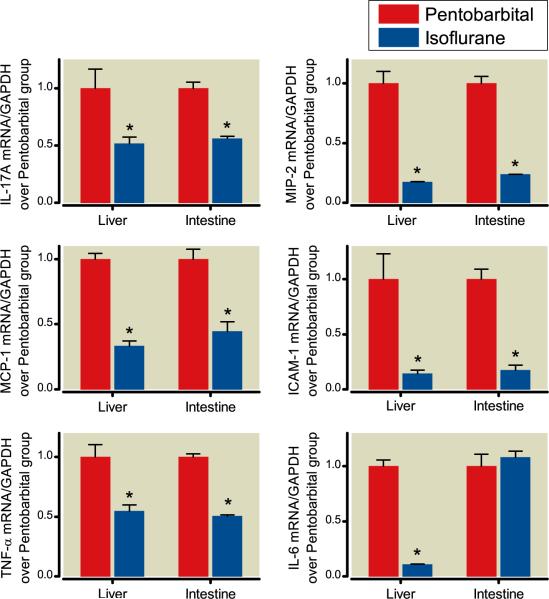
We found increased mRNA expression of TNF-α, ICAM-1, IL-17A, MCP-1, MIP-2, and IL-6 in the livers and small intestines of mice exposed to pentobarbital after renal IRI compared to sham mice. 3 When mice were exposed to isoflurane after renal IRI, there were significantly reduced expressions of most pro-inflammatory mRNAs (TNF-α, IL-17A, MCP-1, ICAM-1, and MIP-2) in both the liver and intestine compared to pentobarbital exposure (fig. 5). Isoflurane exposure decreased IL-6 expression in the liver, but not intestine, after renal IRI.
Fig. 5.
Isoflurane protects against renal ischemia–reperfusion injury (IRI) mediated hepatic and intestinal pro-inflammatory messenger RNA (mRNA) upregulation. Mice were subjected to renal IRI followed by exposure to 4 h of pentobarbital (open bars) or 1.2% isoflurane (closed bars). Liver and small intestine tissues were collected 24 h after renal IRI. Densitometric quantifications of band intensities relative to GAPDH from RT-PCR reactions. N = 4 per group. * P less than 0.05 versus. appropriate pentobarbital group. Data presented as mean ± SEM.
Isoflurane exposure decreases hepatic and small intestinal vascular permeability after renal IRI
We measured liver and small intestinal vascular permeability using EBD which binds to plasma proteins and its appearance in extravascular tissues reflects an increase in vascular permeability. 19 Renal IRI caused significant increases in vascular permeability as measured by increased EBD content compared to sham mice in the liver, jejunum, and ileum (fig. 6). Vascular permeability was significantly decreased with isoflurane exposure after renal IRI in the liver, jejunum, and ileum.
Fig. 6.
Isoflurane reduces vascular permeability after renal ischemia–reperfusion injury (IRI). Quantification of Evans blue dye (EBD) extravasation as an index of vascular permeability of liver, jejunum, and ileum tissues in mice 24 h after sham operation (sham, pentobarbital (PB) anesthesia) or renal IRI followed by exposure to 4 h of PB or 1.2% isoflurane (Iso). N = 3 per group. Data are presented as means ± SEM # P less than 0.05 versus. PB sham group. * P less than 0.05 versus. PB renal IRI group.
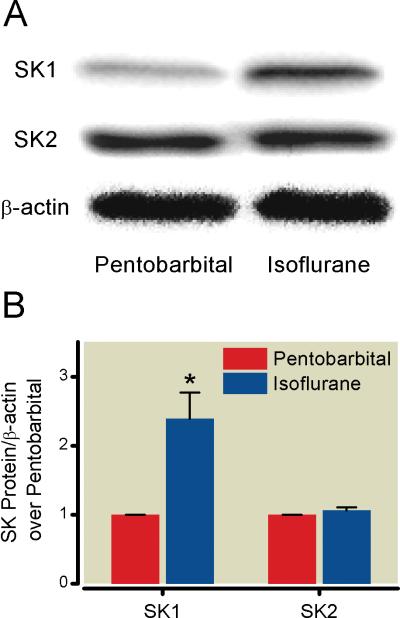
Isoflurane increases small intestinal SK1, but not SK2, expression
Isoflurane anesthesia increased small intestinal SK1 mRNA expression (fig. 7) and protein expression (fig. 8) in sham mice compared to mice anesthetized with pentobarbital. However, there were no changes in SK2 mRNA or protein expression in the small intestine after isoflurane exposure (figs. 7 and 8).
Fig. 7.
Isoflurane activates intestinal sphingosine kinase (SK) 1, but not SK2, messenger RNA (mRNA) expression. (A) Representative gel images of RT-PCR (of 4 experiments) of SK1, SK2, and GAPDH from the small intestines of sham mice exposed to 4 h of pentobarbital or 1.2% isoflurane. (B) Densitometric quantifications of band intensities relative to GAPDH from RTPCR reactions. * P less than 0.05 versus. Pentobarbital sham group. Data presented as mean ± SEM.
Fig. 8.
Isoflurane increases intestinal sphingosine kinase (SK) 1, but not SK2, protein expression. (A) Representative immunoblot images (of 4 experiments) of SK1, SK2, and β-actin from the small intestines of sham mice exposed to 4 h of pentobarbital or 1.2% isoflurane. (B) Densitometric quantifications of band intensities relative to β-actin from immunoblot images. * P less than 0.05 versus. Pentobarbital sham group. Data presented as mean ± SEM.
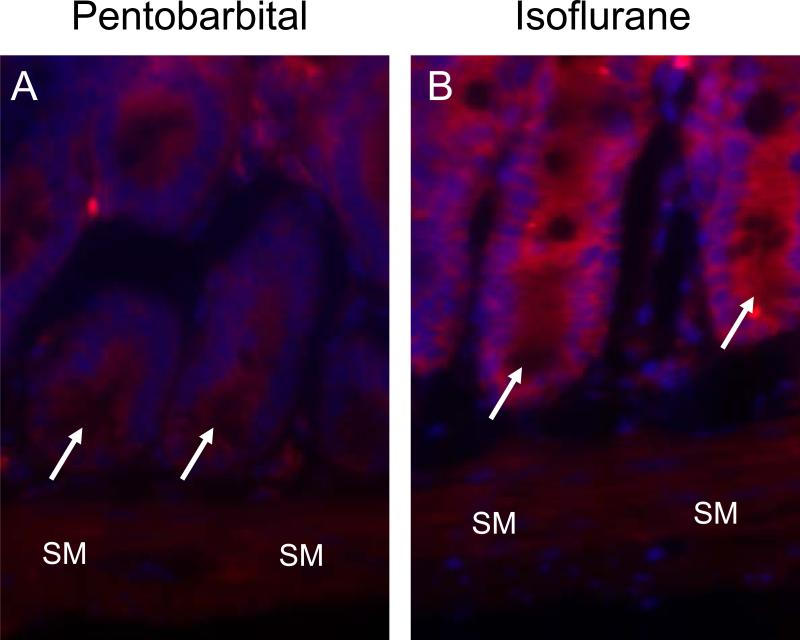
Isoflurane increases small intestinal crypt SK1 expression
Compared to pentobarbital anesthesia (fig. 9A), isoflurane anesthesia increased staining for SK1 in the small intestine, specifically in intestinal crypts (red fluorescence, fig. 9B, representative of 4 experiments).
Fig. 9.
Isoflurane increases sphingosine kinase (SK) 1 in small intestinal crypts. Representative immunofluorescence images (of 4 experiments) for SK1 (red) and nuclear staining (blue) from sham mice exposed to 4 h of pentobarbital (A) or 1.2% isoflurane (B). SM designates smooth muscle layer. Arrows point to small intestinal crypts.
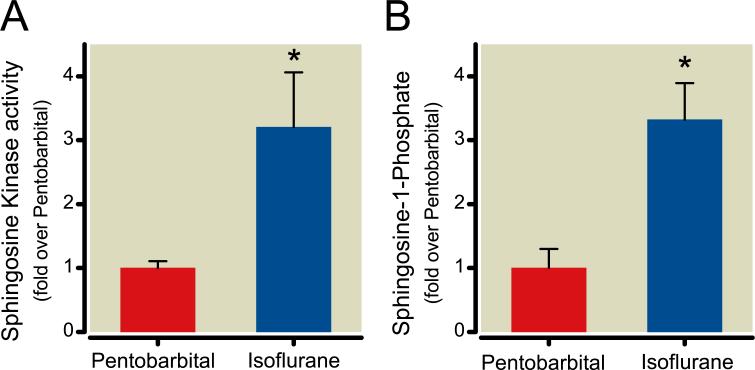
Isoflurane increases small intestinal SK activity and S1P levels
The small intestines of sham mice anesthetized with isoflurane demonstrated higher SK activity compared to mice anesthetized with pentobarbital (fig. 10A). Correspondingly, small intestinal S1P levels were higher in mice after isoflurane anesthesia than after pentobarbital anesthesia (fig. 10B).
Fig. 10.
Isoflurane exposure increases sphingosine kinase (SK) 1 activity and sphingosine-1-phosphate (S1P) formation. (A) Relative SK1 activity (fold over pentobarbital group) from intestines of sham mice exposed to 4 h of pentobarbital or isoflurane (N = 6 per group). (B) Formation of S1P (fold over pentobarbital group) from the intestines of sham mice exposed to 4 h of pentobarbital or isoflurane (N = 4 per group). * P less than 0.05 versus. Pentobarbital group. Data presented as mean ± SEM.
Discussion
The major finding of this study is that a clinically relevant concentration of isoflurane (1 MAC) administered after renal IRI reduced the degree of renal and hepatic dysfunction as well as hepatic and intestinal injury by reducing inflammation and apoptosis while improving vascular permeability. The protective effects of isoflurane were mediated by the SK1/S1P pathway as isoflurane failed to protect mice treated with an inhibitor of SK activity (SKI-II) or in mice lacking SK1 enzyme. Moreover, isoflurane induced small intestinal SK1 mRNA, protein expression, and enzymatic activity leading to higher S1P levels in the small intestine.
AKI continues to be a significant clinical problem in the perioperative period and development of postoperative AKI requiring renal replacement therapy carries a 60% mortality rate. 20 Extra-renal dysfunction associated with AKI, including liver and respiratory failure, was predictive of in-hospital mortality. 21 AKI is an inflammatory process involving multiple cellular and systemic responses, including activation of pro-inflammatory cytokines and chemokines 22 and infiltration by leukocytes such as neutrophils, macrophages, and T cells. 23 Indeed, modulation of the inflammatory cascade (e.g., with adenosine generation and signaling modulation via ecto-5[prime]-nucleotidase 24 as well as the A2B-adenosine receptor 25) provides powerful protection against AKI after renal IRI. There is growing interest in the extra-renal manifestations of AKI as it is becoming clear that AKI leads to a systemic inflammatory state affecting distant organs. AKI caused increases in pulmonary capillary leak and interstitial neutrophil infiltration via IL-6, 26 led to worsening of cardiac function and induced cardiomyocyte apoptosis, 27 and increased neuronal pyknosis and microgliosis in the brain and increased the pro-inflammatory cytokines KC and G-CSF. 28
Previous studies have shown that the gut plays an important role in mediating the hyperdynamic response early in sepsis. 29 Hepatic dysfunction after AKI has been described 30 and we recently identified Paneth cells, located in small intestinal crypts, as the source of the inflammatory mediator, IL-17A, seen in mice following AKI. 31 Release of IL-17A from Paneth cells led to hepatic dysfunction and a cascade of inflammation, including generation of TNF-α and IL-6. Furthermore, mice deficient in TNF-α, IL-17A, or IL-6 or mice treated with antibodies to TNF-α, IL-17A, or IL-6 had attenuation of hepatic and small intestinal inflammation. 3
In our model, isoflurane decreased the expression of IL-17A, TNF-α, MIP-2, MCP-1, and ICAM-1 in the liver and small intestine after renal IRI, reflecting the direct antiinflammatory effects of isoflurane. The antiinflammatory effects of volatile anesthetics are well described, as they decreased TNF-α mediated release of heme oxygenase-1 and IL-8 in human monocytic THP-1 cells 32 as well as reduced KC and MIP-2 in a model of endotoxin-induced lung injury. 33
IL-17A is involved in innate immune defense and inflammation and is mainly produced by a subset of T cells known as Th17 cells. 34 Th17 cells can be found in the intestinal lamina propria of healthy mice 35 but rarely in the spleen, mesenteric lymph nodes, or Peyer's patches. 36 IL-17A derived from intestinal Paneth cells was shown to mediate shock induced by TNF-α 37 and we demonstrated that AKI induces production of IL-17A in Paneth cells, causing increased levels of additional cytokines, including TNF-α, 31 a potent mediator of the inflammatory cascade seen after AKI. 38 Here, we show that isoflurane upregulated SK1 in intestinal crypts, revealing a potential mechanism for the decrease in IL-17A and TNF-α, both produced in intestinal crypts, seen after isoflurane exposure. MIP-2 is a chemokine involved in inflammation and immunoregulation and is a potent regulator of neutrophil chemotaxis. 39 MCP-1 mediates inflammation 40 and ICAM-1 regulates neutrophil retention 41 after AKI. Isoflurane exposure reduced IL-6 expression after renal IRI in the liver, but not in the small intestine suggesting a divergent role of this cytokine in the liver and intestine. Indeed, IL-6 plays a role in liver inflammation and progression to hepatocellular carcinoma, 42 but protects enterocytes against cell death and apoptosis and protected mice against intestinal IRI. 43
The protective effects of volatile anesthetics have been shown in multiple organ systems including the brain, 44 heart, 45 and lung. 33 In our model, mice were protected from AKI-induced liver and intestine injury with exposure to isoflurane after renal IRI (i.e., postconditioning). Clinically, volatile anesthetics can be administered outside of the operating room in the intensive care unit 46 and this may potentially have therapeutic benefits in patients suffering from AKI. We demonstrated that the protective effects of volatile anesthetics on the kidney and cultured human proximal tubule cells were mediated via the SK/S1P pathway 17,18 and recently, isoflurane postconditioning was shown to protect neonatal rats from hypoxicischemic brain injury via a mechanism dependent on SK/S1P signaling. 44 Given this knowledge, we tested whether there was a role for SK/S1P signaling in protecting against extra-renal organ dysfunction following renal IRI. We found that administration of the SK inhibitor, SKI-II, reversed the isoflurane-mediated protection from renal IRI. In addition, we demonstrated that mice lacking SK1 enzyme were not protected against liver and intestinal injury after renal IRI with isoflurane. Due to the inherent concerns regarding the use of genetic knockout mice (e.g., alterations in the expression of unrelated proteins), we used both a pharmacologic inhibitor as well as genetic knockout mice to study the role of SK in renal IRI. Using immunofluorescence imaging, we determined that the small intestinal crypts had the greatest increase in staining for SK1. Taken together, these data indicate that activation of the SK1/S1P pathway in small intestinal crypts is involved in mediating the protective effects of isoflurane on the liver and intestine after renal IRI.
The lysophospholipid S1P has multiple roles in cellular signaling and balances against the proapoptotic effects of sphingosine and ceramide via the “sphingolipid rheostat.” 47 S1P protected intestinal cells from apoptosis via Akt activation 48 and mediated the protective effects of glucocorticoids on renal mesangial cells. 49 S1P reduced IRI injury in a rat model of lung transplantation 50 and protected against renal IRI injury in mice. 51 S1P binds to specific G protein-coupled receptors, of which five are known (S1P1–5). 8 FTY720, a sphingosine analog that is phosphorylated in vivo, has been shown to produce lymphopenia through its actions on the S1P1-receptor by reducing lymphocyte egress from lymph nodes and has shown protection in models of liver ischemia, 52 bowel ischemia, 53 and renal ischemia. 54
SK, the enzyme catalyzing the conversion of sphingosine to S1P, is an important regulator of the sphingolipid rheostat. Many agents are known to stimulate SK activity, including agonists of growth factor receptors (e.g., PDGF, VEGF, NGF, and EGF), TGF-β, and TNF-α. 55 Of the two known isoforms, SK1 mediates cytoprotection while SK2 is generally thought to be proapoptotic. 56 Indeed, SK1 was shown to mediate ischemic postconditioning in isolated mouse hearts 57 and to be protective via down-regulation of JNK activity. 58 Mice lacking SK1 enzyme had poor recovery from anaphylaxis and delayed histamine clearance, while mice lacking SK2 enzyme had rapid recovery from anaphylaxis. 59 In certain models such as Crohn's disease, SK was found to have a deleterious effect as SK inhibition 60 or mice lacking SK1 enzyme 61 had reduced inflammation and colon damage.
Anesthetics can cause hemodynamic changes and regional disturbances in blood flow. We previously demonstrated that compared to pentobarbital, volatile anesthetics did not significantly alter systemic blood pressure or renal blood flow. 17 Certain volatile anesthetics, such as methoxyflurane, undergo renal metabolism with direct nephrotoxic effects due to inorganic fluoride. However, isoflurane is minimally metabolized and has not been linked to fluoride nephrotoxicity. 62
A limitation of our study is that volatile anesthetics may have both local and systemic effects to reduce the severity of hepatic and intestinal injury following renal IRI, so it is difficult to determine in an in vivo study whether volatile anesthetics mediate their protective effects directly on small intestinal crypts or via systemic effects on the migration and infiltration of lymphocytes and neutrophils. The effects are likely multifactorial, including both direct cytoprotective effects on hepatocytes and intestinal epithelial cells with activation of pro-survival signaling pathways, such as the ERK and Akt pathways, as well as systemic antiinflammatory mechanisms including peripheral lymphopenia, preservation of endothelial barrier integrity, and reduction of pro-inflammatory cytokines.
In conclusion, we demonstrated that isoflurane activates the SK1/S1P signaling pathway in small intestinal crypts to reduce hepatic and intestinal injury, apoptosis, and pro-inflammatory mRNA upregulation after renal IRI. Further elucidation of the mechanisms of protection may lead to advancements in the treatment of extra-renal organ dysfunction following renal IRI.
Isoflurane protects against acute renal, hepatic and intestinal dysfunction following renal ischemia-reperfusion injury via activation of sphingosine kinase 1/sphingosine-1-phosphate signaling in the small intestine.
MS #201008004 Final Boxed Summary Statement.
What we already know about this topic
* Acute kidney injury results in a systemic inflammatory condition that injures other organs, including the intestine and the liver.
*Potent volatile anesthetics have anti-inflammatory effects and protect against renal ischemia-reperfusion injury.
What this article tells us that is new
* The volatile anesthetic isoflurane protects the intestine and the liver after renal ischemia-reperfusion injury by attenuating pro-inflammatory cytokine upregulation and intestinal apoptosis through induction of the sphingosine kinase-1/sphingosine-1-phosphate pathway
Acknowledgments
This work was supported by the grants R01 DK-058547 and R01 GM-067081 from the National Institutes of Health, Bethesda, MD
References
- 1.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007:326–31. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 3.Park SW, Chen SWC, Kim M, Brown KM, Kolls JK, D'Agati VD, Lee HT. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2010 doi: 10.1038/labinvest.2010.151. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–24. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 6.Antkowiak B. How do general anaesthetics work? Naturwissenschaften. 2001;88:201–13. doi: 10.1007/s001140100230. [DOI] [PubMed] [Google Scholar]
- 7.Lochhead KM, Zager RA. Fluorinated anesthetic exposure “activates” the renal cortical sphingomyelinase cascade. Kidney Int. 1998;54:373–81. doi: 10.1046/j.1523-1755.1998.00022.x. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–4. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 9.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–92. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 10.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–9. [PubMed] [Google Scholar]
- 11.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 12.Lee HT, Park SW, Kim M, D'Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009;89:196–208. doi: 10.1038/labinvest.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SW, Kim M, Chen SW, D'Agati VD, Lee HT. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2010;33:31–42. doi: 10.1097/SHK.0b013e3181c02c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo JD, Kim M, D'Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006;17:3115–23. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Chen SW, Park SW, Kim M, D'Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;75:809–23. doi: 10.1038/ki.2008.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Anal Biochem. 2005;337:136–42. doi: 10.1016/j.ab.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Kim N, Kim M, D'Agati VD, Emala CW, Sr., Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–35. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Kim M, Park SW, Pitson SM, Lee HT. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–62. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr., Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–24. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 20.Lin YF, Ko WJ, Chu TS, Chen YS, Wu VC, Chen YM, Wu MS, Chen YW, Tsai CW, Shiao CC, Li WY, Hu FC, Tsai PR, Tsai TJ, Wu KD. The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am J Surg. 2009;198:325–32. doi: 10.1016/j.amjsurg.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–7. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 22.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–64. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 23.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 24.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5[prime]-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–45. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 25.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–9. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 27.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–58. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Koo DJ, Chaudry IH, Wang P. The important role of the gut in initiating the hyperdynamic response during early sepsis. J Surg Res. 2000;89:31–7. doi: 10.1006/jsre.1999.5807. [DOI] [PubMed] [Google Scholar]
- 30.Golab F, Kadkhodaee M, Zahmatkesh M, Hedayati M, Arab H, Schuster R, Zahedi K, Lentsch AB, Soleimani M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009;75:783–92. doi: 10.1038/ki.2008.683. [DOI] [PubMed] [Google Scholar]
- 31.Park SW, Kim M, Chen S, Brown K, Kolls J, Lee HT. Paneth cell activation after acute kidney injury causes liver and intestine injury and systemic inflammation in mice [lsqb]Abstract[rsqb]. J Immunol. 2010;184:35.9. [Google Scholar]
- 32.Boost KA, Leipold T, Scheiermann P, Hoegl S, Sadik CD, Hofstetter C, Zwissler B. Sevoflurane and isoflurane decrease TNF-alpha-induced gene expression in human monocytic THP-1 cells: Potential role of intracellular IkappaBalpha regulation. Int J Mol Med. 2009;23:665–71. doi: 10.3892/ijmm_00000178. [DOI] [PubMed] [Google Scholar]
- 33.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–7. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Vanlaere I, de Rycke R, Cauwels A, Joosten LA, Lubberts E, van den Berg WB, Libert C. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med. 2008;205:1755–61. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: The role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 39.Driscoll KE. Macrophage inflammatory proteins: Biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–90. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 40.Zager RA, Johnson AC. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol. 2009;296:F1032–41. doi: 10.1152/ajprenal.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: Role of oxygen metabolites and ICAM 1. Kidney Int. 1995;48:1584–91. doi: 10.1038/ki.1995.451. [DOI] [PubMed] [Google Scholar]
- 42.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 43.Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186–96. doi: 10.1136/gut.2008.151175. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–7. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–14. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Soukup J, Scharff K, Kubosch K, Pohl C, Bomplitz M, Kompardt J. State of the art: Sedation concepts with volatile anesthetics in critically Ill patients. J Crit Care. 2009;24:535–44. doi: 10.1016/j.jcrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 48.Greenspon J, Li R, Xiao L, Rao JN, Marasa BS, Strauch ED, Wang JY, Turner DJ. Sphingosine-1-phosphate protects intestinal epithelial cells from apoptosis through the Akt signaling pathway. Dig Dis Sci. 2009;54:499–510. doi: 10.1007/s10620-008-0393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forster A, Emmler T, Schwalm S, Ebadi M, Heringdorf DM, Nieuwenhuis B, Kleuser B, Huwiler A, Pfeilschifter J. Glucocorticoids protect renal mesangial cells from apoptosis by increasing cellular sphingosine-1-phosphate. Kidney Int. 2010;77:870–9. doi: 10.1038/ki.2010.62. [DOI] [PubMed] [Google Scholar]
- 50.Okazaki M, Kreisel F, Richardson SB, Kreisel D, Krupnick AS, Patterson GA, Gelman AE. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant. 2007;7:751–8. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 51.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–8. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 52.Kaudel CP, Frink M, van Griensven M, Schmiddem U, Probst C, Bergmann S, Krettek C, Klempnauer J, Winkler M. FTY720 application following isolated warm liver ischemia improves long-term survival and organ protection in a mouse model. Transplant Proc. 2007;39:493–8. doi: 10.1016/j.transproceed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Sugito K, Inoue M, Ikeda T, Hagiwara N, Koshinaga T, Kusafuka T. Effect of FTY720 and ex vivo graft irradiation in rat small bowel transplantation: Apoptosis of crypt cells and lymphocytes. Transplant Proc. 2007;39:3432–5. doi: 10.1016/j.transproceed.2007.07.083. [DOI] [PubMed] [Google Scholar]
- 54.Fuller TF, Hoff U, Kong L, Naether M, Wagner P, Nieminen-Kelha M, Nolting J, Luft FC, Hegner B, Dragun D. Cytoprotective Actions of FTY720 Modulate Severe Preservation Reperfusion Injury in Rat Renal Transplants. Transplantation. 2010;89:402–8. doi: 10.1097/TP.0b013e3181caa499. [DOI] [PubMed] [Google Scholar]
- 55.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–26. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Cybulsky AV, Takano T, Papillon J, Khadir A, Bijian K, Chien CC, Alpers CE, Rabb H. Renal expression and activity of the germinal center kinase SK2. Am J Physiol Renal Physiol. 2004;286:F16–25. doi: 10.1152/ajprenal.00144.2003. [DOI] [PubMed] [Google Scholar]
- 57.Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–40. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 58.Di A, Kawamura T, Gao XP, Tang H, Berdyshev E, Vogel SM, Zhao YY, Sharma T, Bachmaier K, Xu J, Malik AB. A novel function of sphingosine kinase 1 suppression of JNK activity in preventing inflammation and injury. J Biol Chem. 2010;285:15848–57. doi: 10.1074/jbc.M109.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, Watford W, Meylan F, Diesner SC, Li L, Schnermann J, Proia RL, Rivera J. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–40. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, Smith CD. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn's disease. Inflammopharmacology. 2010;18:73–85. doi: 10.1007/s10787-010-0032-x. [DOI] [PubMed] [Google Scholar]
- 61.Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2009;23:143–52. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cousins MJ, Skowronski G, Plummer JL. Anaesthesia and the kidney. Anaesth Intensive Care. 1983;11:292–320. doi: 10.1177/0310057X8301100402. [DOI] [PubMed] [Google Scholar]