Abstract
The objective of these studies was to provide detailed analyses of the time course of sulfur mustard (SM) vapor-induced clinical, histological, and biochemical changes following cutaneous exposure in hairless guinea-pigs. Three 6cm2 sites on the backs of each guinea-pig were exposed to SM vapor (314 mg3) for 6 minutes (low dose) or 12 minutes (high dose). Animals were killed at 6, 24, and 48 hours, or 2 weeks postexposure. Erythema, edema, histopathology, and analysis of matrix metalloproteinase (MMP)-2 and -9 content were evaluated. Erythema was observed by 6 hours, and edema by 24 hours postexposure. Vapor exposure caused epidermal necrosis with varying degrees of dermatitis, ulceration, hemorrhage, and separation of the dermis from the epidermis. Later changes included epidermal regeneration with hyperplasia and formation of granulation tissue in the dermis with loss of hair follicles and glandular structures. Relative amounts of pro and active MMP-2 and MMP-9 were significantly increased in the high-dose SM group at 2 weeks. Erythema, edema, and histologic changes are consistent with findings among human victims of SM attack. This model, with observations to 2 weeks, will be useful in assessing the efficacy of countermeasures against SM.
Sulfur mustard (SM), a highly reactive electrophilic bifunctional alkylating agent, has been used as a chemical warfare agent in several conflicts, beginning in World War I, and more recently, in the Iran–Iraq War.1 Its relative ease of production and stockpiling and difficulty verifying its storage, along with its multiple incapacitating health effects, make mustard gas a continuing threat. Identification of effective therapies for SM-induced injuries is the focus of research worldwide.
SM primarily attacks the respiratory tract, skin, and eyes. Effects on human skin have recently been reviewed by Ghanei et al.2 and described for Iranian victims of attack.3 Clinical signs include erosion, erythema, edema, vesicle and bulla formation, hyper- and hypopigmentation, and ulceration. Lesions are often slow to heal, and may increase susceptibility to secondary infection.4 Epidermal histologic changes observed in human victims of SM attack included hyperkeratosis, parakeratosis, hyperganulosis, hyperplasia, atrophy, necrosis, and vesicles or bulla containing fluid and cells. Apoptosis, ancantholytic cells, multinucleated giant cells, and cells with various morphologic atypia, disruption of the basement membrane, and hyperpigmentation were also observed. Changes within the dermis included vascular dilatation, perivascular edema with mononuclear infiltration, endothelial swelling, and extravasated erythrocytes. Changes in sweat glands included increased thickness of the basal membrane, hypertrophy and sometimes atrophy of luminal cells. Hair follicles showed increased thickness of the basal membrane, perifollicular fibrosis, and regional mono-nuclear inflammatory cell infiltration with focal destruction.
The potential mechanisms underlying SM-induced dermal injury are complex, and not fully defined.4–6 Because SM is a highly reactive alkylating agent, it binds readily to DNA, proteins, and small molecules such as glutathione that protect against free radical-induced injury. DNA binding ultimately results in apoptosis or cellular necrosis, depending on SM dose.6 Exposure also results in the release of inflammatory cytokines, recruitment of inflammatory cells, and enhanced expression of tissue matrix metalloproteinases (MMPs). These latter enzymes can degrade collagen type IV and cell matrix adhesion structures epidermal/dermal junction and are believed to mediate the formation of the documented SM-induced blisters.7
The purpose of these studies was to define a time course of cutaneous lesion development in hairless guinea-pigs exposed to SM vapors. Our period of observation extends to 2 weeks in order to characterize longer term effects of SM exposure on the skin. Vapor exposures were used to more accurately mimic exposure of people during an SM attack. Hairless guinea-pigs were used because the anatomy of their skin closely mimics that of human skin.8 The data obtained will aid in future assessments of the efficacy of potential therapeutics to reduce longer term SM-induced lesions and promote wound healing.
MATERIALS AND METHODS
Chemicals
SM (bis [2-chloroethyl] sulfide) was synthesized by reaction of thiodiglycol with hydrochloric acid under reflux. The reaction product was washed with a saturated solution of sodium chloride and dried over magnesium sulfate. SM was purified by vacuum distillation. The product was characterized by gas chromatography/mass spectroscopy (GC/MS; Agilent, Santa Clara, CA), proton nuclear magnetic resonance (1H-NMR; Bruker, Billerica, MA), and GC/flame ionization detection (FID, mini Continuous Air Monitoring System [miniCAMS]; OI Analytical, Pelham, AL). The GC/MS ionization pattern and the 1H-NMR spectral pattern were consistent with the SM structure. The compound was determined to be > 99% pure by GC. Measurement of vapor concentrations achieved in these studies was made using a Mini Continuous Air Monitoring System (CAMS).
Animals
All procedures were conducted under protocols approved by the Institutional Animal Care and Use Committee at the Lovelace Respiratory Research Institute. The Lovelace facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Male IAF hairless guinea-pigs (Crl:HA-Hrhr) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed in shoebox cages with hardwood chip bedding and microisolator tops. The animal rooms were maintained at 21 ± 1°C with 30–70% humidity. A light cycle of 12-hour light–dark was implemented with light starting at 06:00 hours. Food (Harlan Teklad-certified guinea-pig diet, Harlan Teklad, Madison, WI) and water were provided ad libitum, except during exposures. Animals were randomized by weight into dose groups using a computerized data acquisition system (Path-Toxs® Version 4.4.2; Xybion, Cedar Knolls, NJ). The guinea-pigs were identified by unique alphanumeric number encoded onto a transponding microchip (BMDS®; Bio Medic Data Systems Inc., Seaford, DE) injected subcutaneously. Animals were approximately 18 weeks old at the time of exposure.
Experimental design
Early dose-range finding pilot studies in the guinea-pig were conducted using vapors generated using 6 μL SM dispensed within Teflon cups and exposure times from 1 to 16 minutes. Results of these pilot studies indicated that exposure durations of 6 and 12 minutes provided lesions whose clinical and histological severities were clearly dose dependent and of a magnitude (not too mild or severe) that could be useful in assessing the efficacy of potential therapeutics.
Groups of 16 guinea-pigs were exposed via vapor cup to SM vapor for 6 minutes (low dose) or 12 minutes (high dose). Subgroups of 2 animals/dose were sacrificed 6, 24, 48 hours, and 2 weeks postexposure. One sham-exposed guinea-pig was also included at each sacrifice time. Endpoints included assessment of skin exposure sites for erythema and edema, MMP content, and histological changes.
Vapor generation and characterization
Exposures to SM vapors occurred within a glove box maintained at negative pressure to the exposure laboratory. Vapors were generated within Teflon exposure cups (21.1 cm3 volume; 6.6 cm2 skin-exposed surface area) with a ~ 2 mm hole drilled into the base. These cups were fabricated in house from preexisting materials. A filter paper disk was affixed to the inside base of the cup. For animal exposures, cups were inverted and sealed onto the guinea-pig. Vapors were generated by dispensing 6 μL neat SM via a positive displacement pipette through the hole in the cup base onto the filter paper disk. Once the SM was dispensed, the hole in the cup was sealed using an adhesive-backed paper disk, thus initiating the exposure. For quantitation of vapor concentration associated with an animal exposure, vapors were generated as described above in two separate cups sealed to a stainless steel substrate before and after the animal exposures. Vapor was extracted from the cup via a gas-tight Hamilton syringe and injected directly into a calibrated MiniCAMS GC/FID. The measured vapor concentrations in the four cups were averaged. The vapor concentration was not measured in cups on the animals during animal exposures because vapor absorption by skin reduces measured vapor concentration in air.9
Animal exposures
The guinea-pigs were anesthetized by subcutaneous injection of 10mg/kg ketamine (Phoenix Pharmaceuticals, St. Joseph, MO) and 2.5 mg/kg xylazine (LLOYD Laboratories, Shenandoah, IA). Buprenorphine (0.05 mg/kg; Hospira Inc., Lake Forest, IL) was also administered subcutaneously before the SM exposure, and as needed postexposure, to provide analgesia.
The dorsum was cleaned using a 70% alcohol wipe. Vapor cups were affixed to the back of the guinea-pigs.6 Two 2.5 cm diameter holes were cut into 5cm wide pieces of double-sided tape (Harrison Bros. Inc., Chapel Hill, NC). Two such pieces were affixed to the skin immediately lateral to the spine of each guinea-pig providing four skin-exposed circles aligned in a rectangular configuration on each animal’s back. A vapor cup was centered over each skin site and secured via the adhesive surface of the tape. The SM was dispensed onto the filter disk in three of the four cups, and the cup base was sealed. The fourth cup provided a sham exposure site for each animal. After the exposure, the cups were removed, and the guinea-pigs were transferred to shoebox cages and observed until they were awake and mobile.
In-life observations
The animals were observed for up to 2 weeks postexposure. Erythema and edema were scored subjectively for the first 3 days postexposure (until study day 4) dosing using a modified Draize scoring system as described by Mershon et al.10 Briefly, edema and erythema were scored separately on an ordinate scale from 0 (no erythema or edema) to 4 (severe erythema or edema).
Skin reflectance measurements were also made on all sites using a Konica Minolta Chroma Meter® (Model CR-400; Konica Minolta, Ramsey, NJ) for the first 3 days postexposure. The chromameter uses reflected light and reads color in a three-dimensional format L* (measure of “lightness”), a* (measure of red/green light) and b* (measure of blue/yellow light). The a* value is the parameter used as an indicator of erythema. The instrument made four replicate readings at each site. The a* reading for the control site on each animal was subtracted from that of values, resulting in a net erythema value, Δa*.
Necropsy
The guinea-pigs were euthanized with an intraperitoneal injection of a barbiturate-based euthanasia solution (Euthasols®; Virbac Co., Fort Worth, TX), followed by induction of the pneumothorax. Square pieces of skin encompassing the 2.5 cm diameter circular skin exposure sites were excised. The square was sliced craniocaudal to generate two halves. One half was pinned flat to dissection wax and immersion-fixed in neutral buffered formalin (NBF). Two mediolateral slices (4–5 mm wide) were removed from the center of the remaining half and frozen in liquid nitrogen after removal of the subcutaneous adipose and muscle tissues. Cranial and caudal to these slices, mediolateral slices were embedded and frozen in optimal cutting temperature compound for other purposes.11
For histology, serial mediolateral slices were made from the NBF-fixed skin specimens to produce ~ 5 sections for each specimen. Specimens were routinely processed, embedded in paraffin, sectioned at 5 μm from the cranial side of the skin slices, and stained with hematoxylin and eosin for evaluation by light microscopy.
Histopathology
Animals were evaluated in a randomized order without attention being paid to SM exposure or time postexposure. Up to 17 different findings per skin site were subjectively graded for severity and classified into one of four hierarchical histopathologic categories including inflammatory, degenerative/necrotizing, fluid dysregulatory, and hyperplastic changes. These lesions are described in detail as results are presented below. Severity grades of absent, minimal, mild, moderate, and marked corresponded to the ordinal scores of 0–4, respectively.
Relative MMP Content
Skin samples were homogenized by sonication in a buffer consisting of 0.3% Triton X-100 in phosphate-buffered saline, maintained on ice for the duration of the sonication. The lysates were then centrifuged at 4 °C. An aliquot of the supernatant was reserved for analysis of protein concentration using a bicinchoninic acid assay (Pierce Chemical Co., Rockford, IL) and the remaining supernatants were stored at −80°C until use. Tissue extraction was performed in the absence of protease inhibitors. Supplementary experiments indicated that the presence or absence of inhibitors had no effect on the intensity or pattern of MMPs in control skin extracts.
Zymography was performed as described previously,12 using a 7.5% acrylamide gel containing 1.5mg/mL molecular grade gelatin (BioRad Laboratories, Hercules, CA). Samples (75 μL containing 75 μg total protein) were combined with 25 μL of 4 × Laemmli sample buffer without reducing agents, and 20 μL were loaded onto mini-gels (BioRad Protean IV system). Molecular weights were assessed using PageRuler Plus Pre-stained Protein Ladder (Fermentas Life Sciences, Glen Burnie, MD), with at least one empty lane between this standard and samples containing MMPs. Under these conditions, the dithiotreitol in the ladder does not affect detection of the MMPs. An internal standard of MMP-2 and MMP-9 from human H1080 fibrosarcoma cells (a gift from Dr. Paul McGuire) was included on each gel. The MMP-2 and MMP-9 bands in skin lysates were identified by parallel migration with major bands produced by this fibroscarcoma cell line.13
Following resolution by polyacrylamide gel electrophoresis, the gels were washed in 2.5% Triton X-100 and then placed in development buffer consisting of 100 mM Tris buffer, pH 7.4 with 5 mM CaCl2 and 1 μM ZnCl2. Following continued incubation on a rotating platform for 1 hour, the gels were incubated at 37°C for 22–23 hours, then fixed with 40% methanol, 7% acetic acid, and stained in the same solution containing 2mg/mL Coomassie Brilliant Blue R250 overnight. The gels were destained in distilled water. Digital images were taken with a GS-800 Calibrated Densitometer system (PowerLook2100XL, BioRad Laboratories) and analyzed using Quantity One (version 4.5.1; BioRad Laboratories).
Four samples from each of three groups: untreated control skin, skin from animals exposed to SM for 6 minutes, and skin from animals exposed for 12 minutes were included on each gel. To control for variability in responses among gels, the intensity of all gelatinase bands in each control skin sample on each gel was summed, and the average total band intensity for the four control samples was taken as a basal level of total gelatinase protein expression. This value was used to normalize the intensity of each band in the control and SM-exposed skin sample on that gel as in indicator of the relative change in its expression as a function of SM treatment. Average relative intensities for pro- and active forms of MMP-2 and MMP-9 bands are reported as a function of time and dose group.
Data analysis and statistics
At each time point examined, there were four skin samples from an untreated animal, an unexposed sample from each of the 6- or 12-minute-exposed animals, respectively, and six samples from animals treated with SM for 6 or 12 minutes.
Erythema and edema scores for each of the three SM-exposed sites on each animal were averaged for each observation time as a function of SM dose. Scores for control sites were treated similarly. For erythema measured using a chromameter, control site values for a* were subtracted from values obtained at SM-exposed sites on that animal. Then, scores for each of the three SM-exposed sites on each animal were averaged for each observation time as a function of SM dose. Results are provided as the mean and standard deviation of six SM-exposed sites per time point.
Histologic severity scores for the hierarchical categories of skin lesions were summed per specimen, and means and standard deviations of severity scores for each category are presented in order to compare differences between the exposure groups.
For analysis of MMP content in skin, group means and standard deviations of relative band intensities were calculated using Microsoft Excels® software and the data were examined for SM treatment effects. An analysis of variance for an unbalanced design was performed using the SAS general linear models procedure (PROC GLM; SAS Institute Inc., Cary, NC). The analyses of variance were followed with multiple comparison analyses of the least square means for treatment groups segregated by time. Significance level was p ≤ 0.05.
RESULTS
Vapor concentration
The measured SM vapor concentration (at 25.3°C) in vapor cups placed on stainless steel substrates before and after animal exposures was 314 ± 38.4mg/m3. The corresponding C × t was 0.44 and 0.88gminute/m3 for the low- and high-dose animals, respectively.
Clinical signs
Baseline Draize scores for erythema and edema for all animals were zero (data not shown). Following SM exposure, scores for the control site on each animal at each observation time were also zero (data not shown). Exposure to SM vapor for 6 and 12 minutes resulted in moderate to severe erythema beginning within 6 hours of exposure on study day 1 and persisting through 72 hours postexposure (study day 4, Table 1). Erythema scores for both dose groups were significantly greater than zero at all time points, and the scores for the high-dose group were significantly greater than for the low-dose group 24–72 hours postexposure. Baseline erythema Δa* scores for the low- and high-SM–dose groups were 0.12 ± 1.70 (n = 24) and −3.9 ± 1.54 (n = 24), respectively. SM exposure significantly increased the Δa* scores for low- and high-dose groups at all time points examined. Values for the low- and high-dose groups were comparable until 72 hours postexposure where the values for the low dose group were significantly less than for the high dose group. Edema was observed in both 6- and 12-minute exposure groups 24 hours postexposure, being severe among the 12-minute exposure group from 6 to 72 hours postexposure and slight to moderate in severity among animals in the 6-minute exposure group. Values for the low- and high-dose groups were significantly increased compared with control beginning at 48 hours, and values for the high-dose group were significantly greater than the low-dose group from 24–72 hours postexposure.
Table 1.
SM-induced erythema and edema as a function of sulfur mustard (SM) dose and time postexposure*
| 6 hours
|
24 hours
|
48 hours
|
72 hours
|
|||||
|---|---|---|---|---|---|---|---|---|
| Low SM | High SM | Low SM | High SM | Low SM | High SM | Low SM | High SM | |
| Erythema–Draize† | 3.42 ± 0.72‡ | 3.83 ± 0.38‡ | 3.78 ± 0.43‡ | 4.00 ± 0.00*,‡ | 2.83 ± 1.03‡ | 4.00 ± 0.00*,‡,§ | 3.50 ± 0.55‡ | 4.00 ± 0.00‡ |
| Erythema Δa* | 8.69 ± 1.45‡ | 8.73 ± 1.94‡ | 5.44 ± 2.43‡ | 5.67 ± 1.56‡ | 5.31 ± 2.79‡ | 7.24 ± 1.65‡ | 3.83 ± 1.12‡ | 8.59 ± 2.67*,‡,§ |
| Edema–Draize¶ | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.39 ± 0.78 | 4.00 ± 0.00*,‡,§ | 1.75 ± 1.42‡ | 4.00 ± 0.00*,‡,§ | 2.33 ± 1.37‡ | 4.00 ± 0.00*,‡,§ |
Results are the mean ± SD of values from 18 skin sites, except for day 1 where n = 24.
Scores: 1, very slight erythema; 2, well-defined erythema; 3, moderate to severe erythema; 4, severe erythema.
Significantly different from mean air control value.
Significantly different from mean low dose value.
Scores: 1, very slight edema; 2, slight edema; 3, moderate edema; 4, severe edema.
Histologic changes
Controls
Histopathologic changes in the control specimens were minimal to mild, i.e., a low background level of inflammation was present in the skin (Figure 1A). Data compiled from control animal specimens, where all four skin sites were exposed to air, and control sites (site #1) on animals exposed to SM (sites 2–4) were combined for comparison purposes. Control site histology from SM-exposed animals closely resembled that from animals exposed only to air. Further, there were no discernible differences in the skin that were dependent on the site at which exposures were conducted, i.e., control sites were relatively the same regardless of whether they came from the cranial or caudal aspect of the animal.
Figure 1.
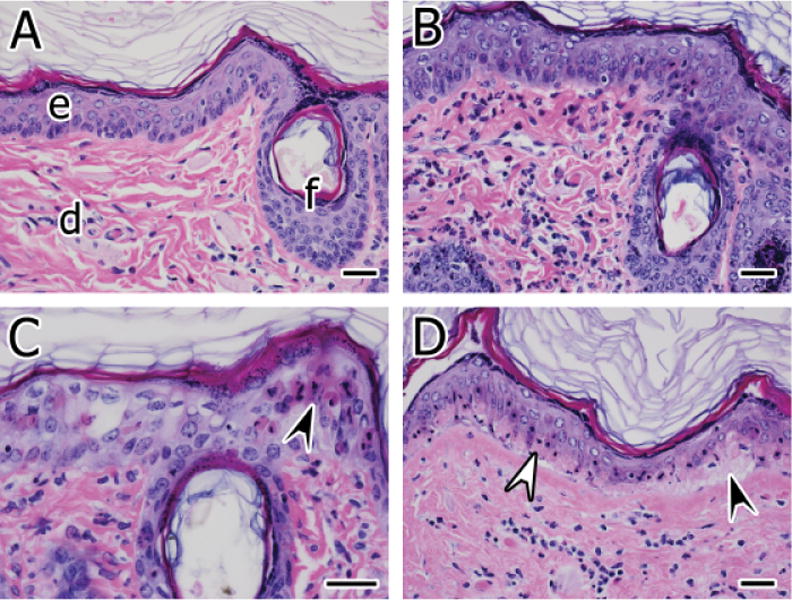
(A) Normal skin from an air-exposed control site. The dermis (d) contains a few scattered, mostly mononuclear leukocytes. The epidermis (e) and a follicular infundibulum (f) are also indicated. (B) Skin from a site exposed to sulfur mustard (SM) for 6 minutes at 6 hours postexposure. Inflammatory cells composed of heterophils and mononuclear leukocytes are scattered in the superficial dermis. Some of the superficial acanthocytes are mildly swollen and nuclei are vesicular (intracellular edema). (C) Epidermal epithelial apoptosis (arrowhead) and intracellular edema are progressively more severe at 24 hours postexposure to SM for 6 minutes. (D) Basal epithelial necrosis characterized by hypereosinophilic cytoplasm, nuclear pyknosis, and karyorrhexis is present in the epidermis (white arrowhead) at 24 hours postexposure to SM for 12 minutes. Most of the acanthocytes superficial to the basal cells retain viable features. The affected epidermis is minimally separated from the underlying dermis (black arrowhead). Hematoxylin and eosin stain, bars = 30 μm.
SM-induced lesions
General
Exposure to SM caused epidermal necrosis with varying degrees of dermatitis, and ulceration that was accompanied by the formation of granulation tissue in the dermis and epidermal regeneration at the later time points. Exposure to SM for 12 minutes caused lesions of greater severity than exposure for 6 minutes, regardless of which postexposure time point was considered. However, differences in the dose response were observed at different time points when histopathology scores were broken down by class of lesion (Figure 2). The classification was somewhat arbitrary in that most findings were interdependent, were coexistent, or existed in a duration or spatial continuum with each other. They included the broad category of: (1) inflammatory findings (superficial dermatitis, epidermal exocytosis, serocellular crust formation); (2) degenerating and/or necrotizing changes (basal epithelial hydropic degeneration, epidermal epithelial apoptosis/dyskeratosis, basal epithelial necrosis, full-thickness epidermal necrosis, follicular necrosis, ulceration, dermoepidermal cleft formation); (3) fluid dysregulatory changes (epidermal/follicular intracellular edema, spongiosis, acantholysis); and (4) hyperplastic findings (acanthosis, hypergranulosis, hyperkeratosis, and regenerative epidermal or follicular epithelium with dysplastic features). Among low-dose specimens, the severity of inflammatory findings decreased slightly with time, while the severity of fluid dysregulatory and degenerative/necrotizing changes remained constant. In contrast, severity scores for inflammation, fluid dysregulation, and degenerative/necrotizing lesions among high-dose skin samples increased with time to 48 hours and then declined somewhat by 2 weeks. Some of the finer differences observed in SM-exposed skin as a function of exposure level and time are detailed below.
Figure 2.
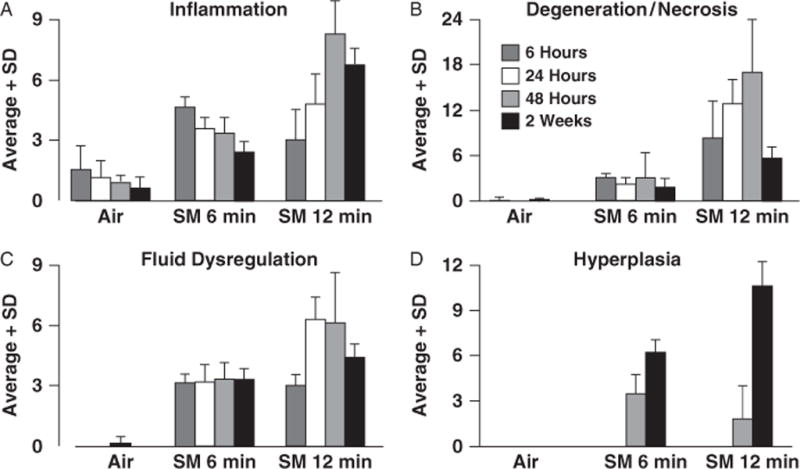
Break-down of histopathology scores in hairless guinea-pig skin specimens exposed to sulfur mustard (SM) for 6 (n = 5–6 skin sites) or 12 minutes (n = 6 skin sites). Control sites (n = 8) were exposed to air. Up to 17 lesions identified and scored for severity were grouped into hierarchical categories of inflammatory (A), degenerative/necrotizing (B), fluid dysregulatory (C), and hyperplastic changes (D). Lesion severity scores in each of the categories were summed for each of the skin sites and averaged for the exposure/treatment/time-point groups. Note that there were no hyperplastic changes evident at 6 and 24 hours postexposure.
6 and 24 hours postexposure
The SM exposure caused a number of acute changes evident at 6 and 24 hours postexposure. These changes included dermatitis characterized by infiltrates of heterophils and mononuclear leukocytes into perivascular and periadnexal foci of the superficial dermis (Figure 1B). Dermatitis and epidermal exocytosis at 6 hours was slightly more severe in specimens exposed to the low-dose SM compared with those exposed to the high dose, while at 24 hours postexposure, the severity of inflammatory findings was slightly greater in the high-dose SM (Figure 2). Apoptosis in the epidermal epithelium was common at 6 hours in the low-dose–exposed specimens and worsened by 24 hours (Figure 1C). Apoptosis was also present in specimens exposed to the high dose of SM. However, compared with the low dose, the high dose caused more serious cell death as characterized by extensive coagulative necrosis of the epidermal basal epithelial cell layer (Figure 1D). Rarely, the degenerated and necrotic epidermis was slightly separated from the dermis by variably sized clefts of clear space (dermoepidermal cleft formation) containing cell debris, inflammatory cells, and proteinaceous material; more frequently this change was evident at later time points.
48 hours and 2 weeks postexposure
By 48 hours postexposure, regenerative and hyperplastic changes became evident, and these continued to be prominent out to 2 weeks postexposure (Figures 2 and 3). Inflammatory and degenerative/necrotic changes continued to be evident and were increased in severity in the high-dose group. Full-thickness epidermal necrosis was also present in the high-dose group (Figure 3A and B). Necrosis and crusting were less prevalent and severe or absent in the low-dose specimens, but hyperplasia in the epidermis (acanthosis, hypergranulosis, hyperkeratosis) was a feature and often times was accompanied by dysplastic changes.
Figure 3.
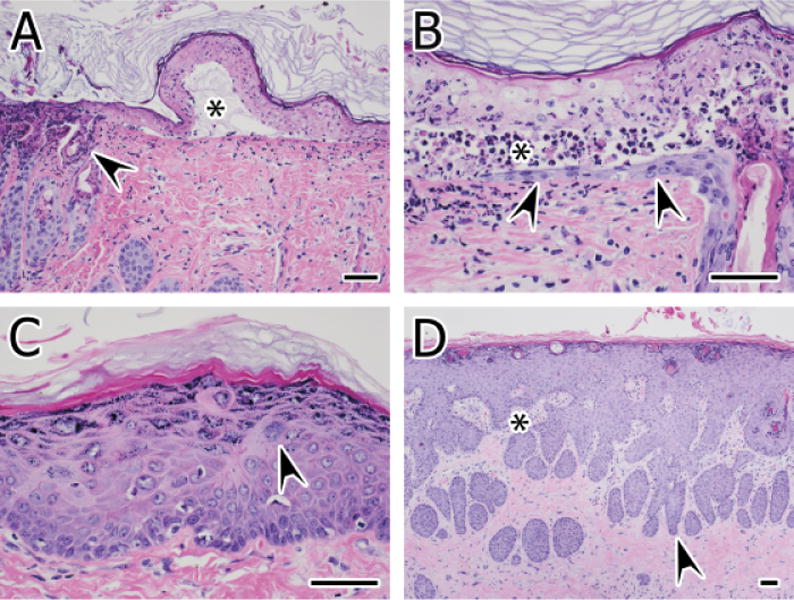
(A) Necrotic epidermis overlies a microvesicular space (*) formed by dermoepidermal separation (cleft formation) at 48 hours postexposure to SM for 12 minutes. The space is filled with granular to finely fibrillar eosinophilic proteinaceous material, cell debris, and inflammatory cells. Full-thickness necrosis of the adjacent epidermis and necrosis of follicular infundibula and ostia are also present (arrowhead). (B) Full-thickness epidermal necrosis in the epidermis at 48 hours postexposure to high-dose SM is separated from a regenerating epithelium (arrowheads) extending from a subjacent hair follicle by a cleft filled with necrotic cell debris and heterophils (*). (C) Acanthosis (hyperplasia) is present at 2 weeks postexposure to SM for 6 minutes. The granular cell layer is also thickened (hypergranulosis), minimal hyperkeratosis is present, and the epidermis displays mild dysplasia characterized by dyskeratosis, slightly disorganized cell layers, and large cells with karyomegaly (arrowhead). (D) Marked acanthosis with features of pseudocarcinomatous hyperplasia is present at 2 weeks postexposure to SM for 12 minutes. The dermis is devoid of adnexae but contains irregular pegs of granulation tissue (*) that interdigitate with the hyperplastic down-growths (arrowhead). Hematoxylin and eosin stain, bars = 60μm
Hyperplastic changes persisted and sometimes worsened in specimens of both dose groups at 2 weeks postexposure (Figure 3C and D). Dermal granulation tissue was sometimes evident at 2 weeks in the high dose but not in the low-dose specimens (Figure 3D). The overlying epidermis was sometimes ulcerated, but in some instances these early scar formations were accompanied by marked hyperplastic changes in the epidermis. In some foci, pseudocarcinomatous hyperplasia was evident (Figure 3D). Occasionally, the beds of granulation tissue overlaid a dermis with markedly hyperplastic and hypertrophic endothelium and fibroblasts.
Overall, the low-dose SM exposure was associated with less follicular and epidermal necrosis than that evident in specimens exposed to the high dose, and recovery of the epidermis, albeit associated with hyperplastic change, was more complete by 2 weeks in the low-dose specimens. The high-dose SM exposure caused some separation of the epidermis from underlying dermis, and this was always associated with necrotic and degenerative changes in the epidermal epithelium. Some of the early scar formation at 2 weeks in the high-dose specimens also had persistent surface crusts and epidermal ulceration. The high-dose SM effect on the epithelium, particularly that of the superficial portions of hair follicles, may have precluded regeneration of the epidermis after extensive loss of stem cells needed for replacement.
Relative MMP content
Proteins with gelatinase activity under zymography conditions were identified at > ~ 200, 180, ~ 140, 95, 90, 70, 65, and 55 kD (Figure 4). The identities of bands at > ~ 200 and 55 kD are not known. The band at 180 kD is likely a homodimer of active MMP-9 and the band at ~ 140kD is likely pro-MMP-9-bound neutrophil gelatinase-associated lipocalin.14
Figure 4.
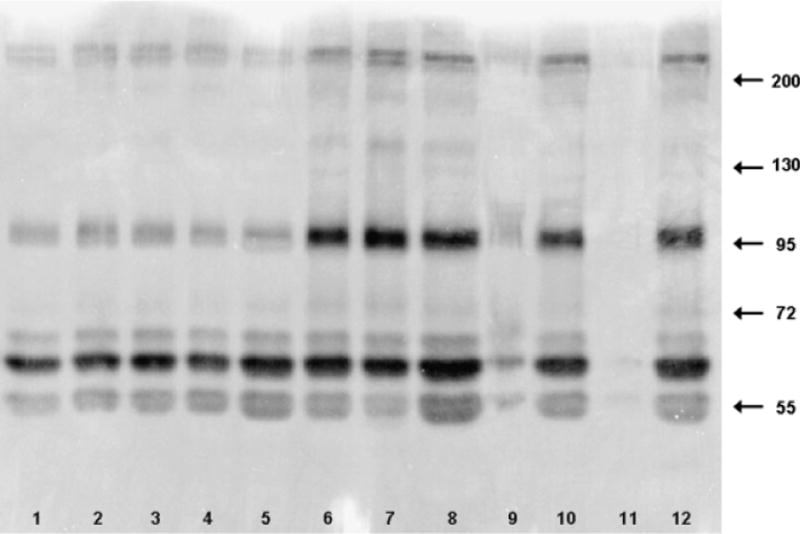
Example zymogram of matrix metalloproteinases detected in skin lysates from the 24-hour time points. Lanes 1–4 are four sites from an unexposed animal. Lanes 5–8 are from a low-dose animal, with lane 5 the control site. Lanes 9–12 are from a high-dose animal, with lane 9 the control site. Molecular weight standards were run in lane 14. Contrast of the clear lanes on blue gel has been reversed for clarity.
MMP-9 and MMP-2 were identified based on comigration with the MMPs from HT1080-cell–conditioned medium.
The relative quantities of pro-MMP-9 (~ 95 kD), active MMP-9 (~ 90 kD), pro-MMP-2 (~ 70 kD), and active MMP-2 (~ 65 kD) normalized to the average total gelatinase signal in untreated skin samples are shown in Figure 5. Constitutive levels of pro-MMP-2 and -9 as well as active MMP-2 were present in skin from untreated animals as well as from the control sites on SM-treated guinea-pigs. Significant increases in pro- and active forms of MMP-2 and -9 occurred only among high-dose animals at 2 weeks postexposure. Pro-MMP-9 was also significantly increased at 48 hours.
Figure 5.
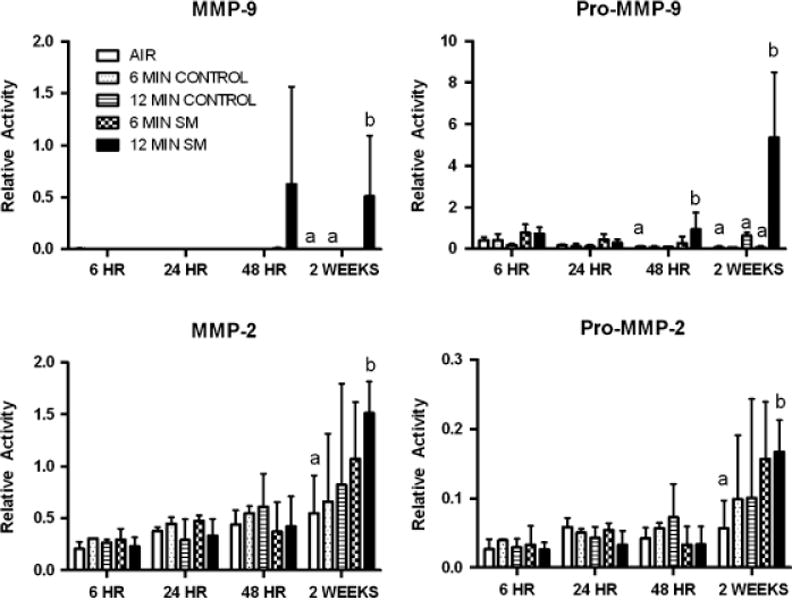
Matrix metalloproteinase (MMP) levels in animals exposed to sulfur mustard (SM) for 6 or 12 minutes. Results are expressed as the relative signal from pro-enzyme and active MMP-2 and -9 in each sample compared with the average total signal (integrated intensity of all bands) in skin from animals not treated with SM, that is, the sum of all bands detected in the control animals would be 1. The fractions of the signal represented by pro-MMP-2, MMP-2, pro-MMP9, and MMP-9 in control sites was 0.19 ± 0.15, 0 ± 0, 0.05 ± 0.01, and 0.39 ± 0.14, respectively. Results are the mean ± SD of four values per time point for untreated controls, two values per time point for control sites from SM-treated animals, and six values per time point for SM-treated sites. Bars within a different letter designations (a, b) within each time point are significantly different from each other (ANOVA, p≤ 0.05).
DISCUSSION
SM vapor-induced skin lesions occurring within 24 hours postexposure have been described previously,10,15–17 and to the best of our knowledge, only Dachir et al.18 have examined the histological changes for longer periods postexposure. The study reported here is unique in that detailed histological evaluations and effects on pro- and active forms of MMPs-2 and -9 are described to 2 weeks postexposure.
The SM vapor concentration measured in our vapor cup exposure system was 314 mg/m3, and is approximately one quarter of the saturated vapor concentration of SM (1.3 g/m3) reported by Dalton et al.,9 indicating that the SM liquid had not completely vaporized within the cup. The SM concentrations used in these studies (0.44–0.88g min/m3 are two to four times greater than the minimum concentration reported by Sidell et al.19 to cause skin erythema (0.2 g min/m3). It is not possible to relate these concentrations directly to those encountered by victims of SM exposure for several reasons, including the high variability of human exposure scenarios, lack of corresponding exposure assessment, and differential sensitivity of skin sites within a given individual.1,19
Severe erythema, assessed by Draize score, was present by 6 hours postexposure in both the 6 and 12-minute exposure groups. This is consistent with erythema reported at 4–8 hours postexposure by Mershon et al.10 and Yourick et al.15 for guinea-pigs exposed to vapors generated from 10 μL SM for 6 or 8 minutes. Reflectance measurement of erythema in our study (Δa*) peaking at 6 hours postexposure and dropping somewhat by 24 hours exposure are also consistent with findings of Snider et al.17 in guinea-pigs exposed to SM vapor (generated from 10 μL in a vapor cup) for 6 and 12 minutes. In contrast to Mershon et al.10 who reported slight edema by 6 hours postexposure, edema was first scored in our study at 24 hours postexposure in both the 6- and 12-minute exposure groups.
Due to differences in experimental design, it is difficult to make direct comparisons of histologic changes reported by others in SM-exposed animals. However, during the first 24 hours postexposure in our study, we identified inflammatory changes (dermatitis, epidermatitis), fluid dysregulatory changes (edema, acantholysis), and degenerative changes (epidermal necrosis, follicular necrosis, and epidermal/dermal blister formation) consistent with early change reported in guinea-pigs (Table 2)10,15,17 and man.3 Apoptosis, mostly observed 6 and 24 hours among the 6-minute exposure group in our study, was not reported in the above SM studies but was observed in skin biopsy samples of individuals approximately 5 days after being exposed to SM during the Iran–Iraq war.3 At 48 hours postexposure, regenerative changes were evident in skin samples in our study, consistent with the findings of Dachir et al.18 However, by 48 hours, hyperplastic and dysplastic changes were also present in our low-dose animals and degenerative and necrotic changes persisted with increased severity in the high-dose group.
Table 2.
Summary of histologic findings in hairless guinea-pigs exposed to sulfur mustard (SM) vapor via vapor cup
| Author | Experimental design | Histology |
|---|---|---|
| Mershon et al.10 | 1–16 minutes of exposure to 10 μL of SM vapor. Qualitative histology assessed at 24 hours | 1 and 2 minutes of exposure: Minimal to mild epidermal intracellular edema 4 and 8 minutes of exposure: Intra- and/or intercellular edema, epidermolysis, acantholysis, pustular epidermatitis, epidermal–dermal separation, and epidermal necrosis Virtually no microvesicles with 4 minute exposure (1/39 skin sites), 8-minute exposure-induced microvesicles (31/40 skin sites) Increasing exposure time and severity of dermal damage associated with increased numbers of inflammatory cells (neutrophils/eosinophils) and increasing congestion and edema |
| Snider et al.17 | 3–12-minute exposure to 10μL of SM vapor Histology assessed at 24 hours. Report incidences and severity scores |
Epidermal necrosis incidence: 92% (severity score: 0.9) at 3 minutes of exposure; 100% incidence (3.6) at 12 minutes of exposure Follicular necrosis: 85% incidence (0.9) at 4 minutes, 100% incidence (2.2) at 12 minutes Pustular dermatitis: incidence < 20%, severity < 0.5 at all time points Microblisters: 6% incidence (0.1) at 5 minutes, 96% incidence (2.6) at 12 minutes |
| Yourick et al.15 | Sacrifice 2–24 hour post 8-minute exposure to 10μL SM. Incidence and severity (in some cases) reported |
Intracellular edema—First seen 2 hours postexposure, highest incidence at 24 hours Follicular necrosis—First seen at 4 hours, more pronounced at 8 hours. No severity score reported Pustular epidermatitis—First seen at 8 hours, peaked at 16 hours, remained high through 24 hours. No severity score reported Epidermal necrosis first observed at 8 hours, incidence peaked at 16 hours. Severity scores of 3–3.5 |
| Dachir et al.18 | Exposures 1, 5, 10, 15 and 30 minutes, 5μL SM Qualitative assessment of histopathology for sacrifices at 1, 24, 48 hours, 1 and 2 weeks following exposure. Incidence and severity not reported |
After a 15-minute SM exposure: At 1 hour postexposure: Morphological changes in nuclei in basal layer and congestion of blood vessels At 24 hours postexposure: Vesication and epithelial damage At 48 hours postexposure: Regenerative process began, consisting of a scab and new epidermis At 1 week postexposure: Epithelial coverage of the wound was only partial and new epidermis not fully attached to basement membrane After a 10 minute exposure: At 2 weeks: Epithelial hyperplasia, thickening of epidermis evident |
At 2 weeks postexposure, ulcerative changes in high-dose guinea-pigs suggested that wound healing was not complete. Granulation tissue in the dermis with an associated loss of hair follicles and glands is consistent with reports of alteration in hair follicles and glands among SM-exposed individuals.3 The presence of dysplasia and hyperplasia in animals at 48 hour and 2 weeks postexposure suggest the potential long-term development of skin cancer in SM-exposed skin. An increased incidence of skin cancers has been reported among individuals occupation-ally exposed to high levels of SM;20,21 however, strong evidence does not exist for an increase among Iranians with SM-induced dermal injuries incurred during the Iran–Iraq war.22,23
For this study, we focused on SM-induced activation of MMPs-2 and -9 because both are present in skin and because the expression of MMP-9 has been up regulated in skin fibroblasts in vitro,24 in cultured human skin,7 and in animals following SM exposure.18,25,26 MMP inhibition has reduced SM-induced toxicity in cultured human skin7 and rabbit eye.27 Therefore, MMP inhibition may be a viable therapeutic strategy for minimizing SM lesions provided the therapeutic agents reach the target tissues.
The patterns of SM-induced changes in MMPs in skin in this study differ substantially from changes reported in the literature for guinea-pig (gelatinase activity),18 mouse (gelatinase activity, RT-PCR, and Western blot),25 and weanling pig skin (reverse transcription real-time PCR, specific transcript PCR).26 Primarily, only increases in MMP-9 relative gelatinase activity or mRNA production have been reported previously, and these changes were observed within days of SM exposure. We report increases in relative amounts of pro-and active forms of MMP-2 and MMP-9 at 2 weeks postexposure. Our clinical and histologic findings suggest MMP levels would be increased before the 2-week time point, as reported by others. The reasons for these differences in MMP responses are not clear, but may include differences in animal species, dose, assay procedures/sensitivity, as well as methods of data analysis. The significantly increased relative levels of pro- and active forms of MMP-2 and -9 in the high-dose group may be contributing to the continued presence of degenerative and inflammatory lesions in the animals observed at 2 weeks.
In summary, this study provides a detailed analysis of the time course of lesion development and resolution in a hairless guinea-pig model of SM cutaneous exposure. Early clinical and histologic findings are similar to those reported previously in hairless guinea-pigs and man. The clinical, histologic analysis, and associated MMP-2 and -9 profiles may prove useful in assessing the efficacy of therapies targeting SM-induced injury.
Acknowledgments
This work, conducted by the CounterAct Research Center of Excellence at Lovelace Respiratory Research Institute, was funded by NIH NINDS #5U54NS058185-03. The authors are grateful to Edward Mormando, Sonia Lopez, Genevieve Chavez, Carolyn Elliott, Brad Tibbetts, Lois Herrera, and the necropsy and histology staff for excellent technical assistance. We thank Dr. John Graham of the US Army Medical Research Institute of Chemical Defense, Aberdeen Proving Ground, MD, for initial guidance on Draize and light reflectance scoring of lesions.
References
- 1.Reuter R. Hazards of chemical weapons release during war. Environ Health Perspect. 1999;107:985–90. doi: 10.1289/ehp.99107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghanei M, Poursaleh Z, Harandi AA, Emandi SE, Emandi SN. Acute and chronic effects of sulfur mustard on the skin: a comprehensive review. Cutan Ocul Toxicol. 2010;29:269–77. doi: 10.3109/15569527.2010.511367. [DOI] [PubMed] [Google Scholar]
- 3.Naraghi ZS, Mansouri P, Mortazavi M. A clinicopathological study on acute cutaneous lesions induced by sulfur mustard gas (yperite) Eur J Dermatol. 2005;15:140–5. [PubMed] [Google Scholar]
- 4.Paromov V, Suntres Z, Smith M, Stone WL. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. J Burns Wounds. 2007;7:e7. [PMC free article] [PubMed] [Google Scholar]
- 5.Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–9. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Mol MA, van den Berg RM, Benschop HP. Involvement of caspases and transmembrane metalloproteases in sulphur mustard-induced microvesication in adult human skin in organ culture: directions for therapy. Toxicology. 2009;258:39–46. doi: 10.1016/j.tox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Sueki H, Gammal C, Kudoh K, Kligman AM. Hairless guinea pig skin: anatomical basis for studies of cutaneous biology. Eur J Dermatol. 2000;10:357–64. [PubMed] [Google Scholar]
- 9.Dalton CH, Maidment MP, Jenner J, Chilcott RP. Closed cup vapor systems in percutaneous exposure studies: what is the dose? J Anal Toxicol. 2006;30:165–70. doi: 10.1093/jat/30.3.165. [DOI] [PubMed] [Google Scholar]
- 10.Mershon MM, Mitcheltree LW, Petrali JP, Braue EH, Wade JV. Hairless guinea pig bioassay model for vesicant vapor exposures. Fundam Appl Toxicol. 1990;15:622–30. doi: 10.1016/0272-0590(90)90046-m. [DOI] [PubMed] [Google Scholar]
- 11.Mishra NC, Rir-sima-ah J, March T, Weber W, Benson J, Jaramillo R, Seagrave JC, Schultz G, Grotendorst G, Sopori M. Sulfur mustard induces immune sensitization in hairless guinea pigs. Int Immunopharmacol. 2010;10:193–9. doi: 10.1016/j.intimp.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res. 2004;30:1–15. doi: 10.1080/01902140490252858. [DOI] [PubMed] [Google Scholar]
- 13.Yan C, Han R. Effects of genistein on invasion and matrix metalloproteinase activities of HT1080 human fibrosarcoma cells. Chin Med Sci J. 1999;14:129–33. [PubMed] [Google Scholar]
- 14.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 15.Yourick JJ, Dawson JS, Benton CD, Craig ME, Mitcheltree LW. Pathogenesis of 2,2′-dichlorodiethyl sulfide in hairless guinea pigs. Toxicology. 1993;84:185–97. doi: 10.1016/0300-483x(93)90116-a. [DOI] [PubMed] [Google Scholar]
- 16.Petrali JP, Oglesby SB, Hamilton TA, Mills KR. Comparative morphology of sulfur mustard effects in the hairless guinea pig and a human skin equivalent. J Submicrosc Cytol Pathol. 1993:113–8. [PubMed] [Google Scholar]
- 17.Snider TH, Matthews MC, Braue EH., Jr Model for assessing efficacy of topical skin protectants against sulfur mustard vapor using hairless guinea pigs. J Appl Toxicol. 1999;19(Suppl 1):S55–8. doi: 10.1002/(sici)1099-1263(199912)19:1+<s55::aid-jat616>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Dachir S, Cohen M, Fishbeine E, Sahar R, Brandies R, Horwitz V, Kadar T. Characterization of acute and long-term sulfur mustard-induced skin injuries in hairless guinea-pigs using non-invasive methods. Skin Res Technol. 2010;16:114–24. doi: 10.1111/j.1600-0846.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- 19.Sidell FR, Urbanetti JS, Smith WJ, Hurst CG. Vesicants. In: Zajtchuk R, Bellamy R, editors. Medical aspects of chemical and biological warfare. Chapter 7. Office of the Surgeon General, Department of the Army; Washington, DC: 1997. pp. 197–228. [Google Scholar]
- 20.IARC. Monographs on the evaluation of carcinogenic risks to humans: overall evaluations of carcinogenicity: an updating of IARC monographs. Lyon: IARC. 1987;1–42(Suppl):259–60. [PubMed] [Google Scholar]
- 21.Klehr NW. Spätmanifestationen bei ehmaligen Kampfgasarbeitern. Z Hautkr. 1983;59:1161–70. [PubMed] [Google Scholar]
- 22.Wattana M, Bey T. Mustard gas or sulfur mustard: an old chemical agent as a new terrorist threat. Prehosp Disaster Med. 2009;24:19–29. doi: 10.1017/s1049023x0000649x. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 23.Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Ries C, Popp T, Egea V, Kehe K, Jochum M. Matrix metalloproteinase-9 expression and release from skin fibroblasts interacting with keratinocytes: upregulation in response to sulphur mustard. Toxicology. 2009;263:26–31. doi: 10.1016/j.tox.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Shakarjian MP, Bhatt P, Gordon MK, Chang YC, Casbohm SL, Rudge TL, Kiser RC, Sabourin CL, Casillas RP, Ohman-Strickland P, Riley DJ, Gerecke DR. Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure. J Appl Toxicol. 2006;26:239–46. doi: 10.1002/jat.1134. [DOI] [PubMed] [Google Scholar]
- 26.Sabourin CL, Danne MM, Buxton KL, Casillas RP, Schlager JJ. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. J Biochem Mol Toxicol. 2002;16:263–72. doi: 10.1002/jbt.10050. [DOI] [PubMed] [Google Scholar]
- 27.Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, Turetz J, Gutman H, Buch H, Brandeis R, Horwitz V, Solomon A, Amir A. Ocular injuries following sulfur mustard exposure–pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]


