Abstract
Quantity and timing of protein ingestion are major factors regulating myofibrillar protein synthesis (MPS). However, the effect of specific ingestion patterns on MPS throughout a 12 h period is unknown. We determined how different distributions of protein feeding during 12 h recovery after resistance exercise affects anabolic responses in skeletal muscle. Twenty-four healthy trained males were assigned to three groups (n= 8/group) and undertook a bout of resistance exercise followed by ingestion of 80 g of whey protein throughout 12 h recovery in one of the following protocols: 8 × 10 g every 1.5 h (PULSE); 4 × 20 g every 3 h (intermediate: INT); or 2 × 40 g every 6 h (BOLUS). Muscle biopsies were obtained at rest and after 1, 4, 6, 7 and 12 h post exercise. Resting and post-exercise MPS (l-[ring-13C6] phenylalanine), and muscle mRNA abundance and cell signalling were assessed. All ingestion protocols increased MPS above rest throughout 1–12 h recovery (88–148%, P < 0.02), but INT elicited greater MPS than PULSE and BOLUS (31–48%, P < 0.02). In general signalling showed a BOLUS>INT>PULSE hierarchy in magnitude of phosphorylation. MuRF-1 and SLC38A2 mRNA were differentially expressed with BOLUS. In conclusion, 20 g of whey protein consumed every 3 h was superior to either PULSE or BOLUS feeding patterns for stimulating MPS throughout the day. This study provides novel information on the effect of modulating the distribution of protein intake on anabolic responses in skeletal muscle and has the potential to maximize outcomes of resistance training for attaining peak muscle mass.
Key points
A single bolus of ∼20 g of protein after a bout of resistance exercise provides a maximal anabolic stimulus during the early post-exercise recovery period (∼5 h), but the effect of various protein feeding strategies on skeletal muscle protein synthesis during an extended recovery period (12 h) is unknown.
We compared three different patterns of ingestion of 80 g of protein during 12 h recovery after resistance exercise and the associated anabolic response in human skeletal muscle. Protein was ingested in 10, 20 or 40 g feedings using a pulsed, intermediate or bolus ingestion regimen, respectively.
Our results indicate that repeated ingestion of 20 g of protein was superior for stimulating muscle protein synthesis during the 12 h experimental period.
The three dietary treatments induced differential phosphorylation of signalling proteins and changes in mRNA abundance.
This study shows that the distribution of protein intake is an important variable to promote attainment and maintenance of peak muscle mass.
Introduction
Skeletal muscle protein synthesis is stimulated by exercise (Chesley et al. 1992; Biolo et al. 1995; Phillips et al. 1997) and protein feeding (Rennie et al. 1982; Cuthbertson et al. 2005). When feeding follows exercise the effect is enhanced (Moore et al. 2009a,b) and promotes positive net protein balance in skeletal muscle (Phillips et al. 1997). Although the majority of studies of exercise and protein ingestion have characterized the response to a single protein feeding over 4–6 h of recovery (Tipton et al. 1999; Dreyer et al. 2008; Moore et al. 2009a,b; West et al. 2011), it is important to note that the enhanced sensitivity of myofibrillar protein synthesis (MPS) to protein ingestion following resistance exercise is sustained for at least 24 h (Burd et al. 2011). However, the paucity of studies measuring MPS beyond the traditional 4–6 h recovery window precludes our ability to prescribe an optimized spacing and amount of protein intake throughout the day or during a prolonged (i.e. 12 h) recovery period after resistance exercise. Such information could be important for enhancing skeletal muscle mass with the potential for use in a variety of populations including the elderly and athletes (Layman, 2009; Paddon-Jones & Rasmussen, 2009; Phillips & Van Loon, 2011).
Investigations during early recovery after exercise provide some insight into the potentially beneficial effects of longer term feeding strategies. It is known that there is a dose–response in muscle protein synthesis to the intensity (Kumar et al. 2009) and volume (Burd et al. 2010) of exercise, as well as to the amount of protein consumed after exercise (Moore et al. 2009a). Specifically, muscle protein synthesis can be maximally stimulated by the intake of 20 g of high quality protein (Moore et al. 2009a), with lower doses resulting in suboptimal rates of muscle protein synthesis, and protein intakes above this level stimulating irreversible oxidative amino acid (AA) catabolism (Moore et al. 2009a). Furthermore, rates of MPS 1–5 h after a bout of resistance exercise are higher when rapid aminoacidaemia is achieved by ingesting a bolus (25 g) of whey protein compared to a slow, moderate but sustained rise in blood amino acids elicited by an identical protein source consumed in a pulsed feeding pattern (West et al. 2011). Thus, the interplay of the quantity and timing of protein ingestion is a major factor regulating the capacity of the muscle protein synthetic machinery to respond to repeated anabolic stimuli. Importantly, elevated rates of muscle protein synthesis return to basal values in the resting state after 2 h of AA provision (either orally or intravenously) despite a sustained AA availability (Bohe et al. 2001; Atherton et al. 2010), a phenomenon called the ‘muscle full’ effect (Atherton et al. 2010). How rapidly the protein synthesis machinery recapitulates its capacity to respond to the provision of nutrients and whether there are differences in the optimal timing and quantities of protein necessary to repeatedly stimulate maximal skeletal muscle protein synthesis after a bout of resistance exercise have not been determined. There may also be potential for exceptions to the ‘muscle full’ effect under specific physiological or experimental conditions (Glover et al. 2008). Another important factor to consider is the capacity of exercise to extend the duration of the anabolic stimulus induced by feeding (Moore et al. 2009b). Collectively, this suggests that increased aminoacidaemia after exercise is beneficial for the muscle hypertrophy response but the optimal pattern of aminoacidaemia remains to be established.
Therefore, the aim of the present study was to determine how the quantity and timing of protein ingestion after a single bout of resistance exercise influence the muscle anabolic response throughout the entire day. Previous studies have shown muscle protein synthesis following increased availability of amino acids peaks within ∼2 h (Bohe et al. 2001; Atherton et al. 2010) but cyclical oscillation of aminoacidaemia appears to be required to prevent a potential ‘muscle full’ effect (Atherton et al. 2010). Therefore, in the present study we hypothesized that 20 g of protein ingested every 3 h would be the optimal intervention for repeated stimulation of MPS due to provision of sufficient (Moore et al. 2009a), but not excessive, protein to stimulate MPS.
Methods
Ethical approval
Subjects were informed of any potential risks involved in the study before providing their written informed consent. The study was approved by the Australian Institute of Sport Ethics Committee and conformed to the standards set by the latest revision of the Declaration of Helsinki.
Subjects
Twenty-four young, healthy non-smoking males with at least 2 years of high-intensity resistance training experience (≥2 times per week) were recruited for this study (Table 1). Technical error (i.e. incorrect protein administration) necessitated that one subject was excluded from the final analysis. Body composition was measured using a whole-body scan narrowed fan-beam dual energy X-ray analysis (DXA Lunar Prodigy, GE Healthcare, Madison, WI, USA) with GE Encore 13.60 software (GE Healthcare). Anthropometric measurements and one repetition maximum (RM) were assessed 1–2 weeks before experimental trials.
Table 1.
Subjects' characteristics
| Group | |||
|---|---|---|---|
| Bolus | Intermediate | Pulse | |
| n | 8 | 7 | 8 |
| Age (years) | 25 ± 5 | 25 ± 3 | 25 ± 5 |
| Body mass (kg) | 83.6 ± 10.5 | 80.5 ± 11.1 | 82.0 ± 6.4 |
| Percentage fat | 16.7 ± 5.2 | 14.6 ± 6.9 | 15.1 ± 5.8 |
| Lean body mass (kg) | 66.2±5.4 | 65.3±6.4 | 66.5±5.3 |
| 1 RM (kg) | 125 ± 9 | 128 ± 21 | 137 ± 18 |
| 1 RM/body mass (kg) | 1.51 ± 0.18 | 1.59 ± 0.16 | 1.68 ± 0.21 |
Data are mean ± SD. RM, repetition maximum.
Diet and exercise control
Subjects were each provided with individualized prepacked meals for the 72 h prior to an experimental trial. The standardized diets were based on achieving daily energy availability (intake minus energy cost of exercise) of 45 kcal (kg fat-free mass)−1, and provided a daily intake of 1.5 g protein (kg body mass)−1 and 4 g CHO (kg body mass)−1 with the remaining energy coming from fat. No exercise was allowed during the 48 h period prior to a trial, but subjects were allowed to undertake their habitual training session during the first day of the dietary standardization, with an appropriate snack being provided in their prepackaged meals to account for the energy cost of the session. Subjects were instructed to refrain from any alcohol or caffeine consumption 48 h prior to a trial and to consume the last meal of their diets prior to 21.00 h the night before a trial. A food and exercise checklist was completed by each subject to note the compliance to these instructions.
Experimental trials
Subjects were matched for body mass before being randomly assigned to one of three different experimental groups using a parallel group design. An overview of the experimental procedure is shown in Fig. 1. Subjects reported to the laboratory at ∼07.00 h after a 10–12 h overnight fast and a Teflon catheter was inserted in the antecubital vein of each arm for blood sampling and tracer infusion. A primed, continuous (0.05 μmol·kg−1·min−1; 2 μmol·kg−1 prime) infusion of l-[ring-13C6] phenylalanine (Cambridge Isotopes Laboratories, Woburn, MA, USA) commenced immediately after the first (baseline) blood sample was drawn. After a resting period of 2 h, the first muscle biopsy was taken and subjects undertook the resistance exercise bout (described subsequently). After exercise and during the following 12 h recovery period, subjects consumed 80 g of a commercially available whey protein isolate (ISO8 WPI; 82.9 g protein, 2.1 g fat, 3.4 g carbohydrates per 100 g; Musashi, Australia), enriched with 5%l-[ring-13C6] phenylalanine, and mixed with water to a total volume of one litre. Drinks were consumed within the 12 h period after the resistance exercise bout according to the following schedule: pulsed feeding consisting of 8 × 10 g in 125 ml every 1.5 h (PULSE, n= 8); intermediate feeding consisting of 4 × 20 g in 250 ml every 3 h (INT, n= 7); or bolus feeding consisting of 2 × 40 g in 500 ml every 6 h (BOLUS, n= 8). Water was provided ad libitum with 30 min restrictions before and after each protein drink to avoid any potential influence on gastric emptying.
Figure 1.
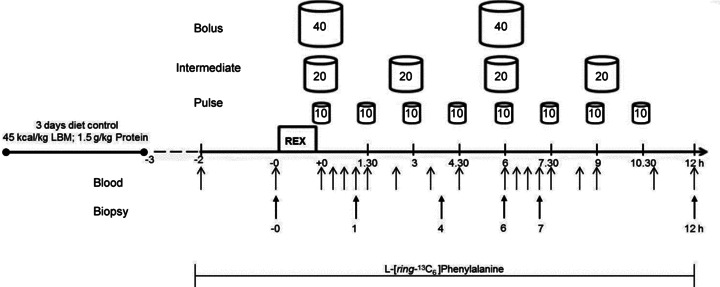
Schematic representation of the experimental protocol. Negative time points indicate before exercise, positive time points indicate after exercise. LBM, lean body mass; REX, resistance exercise.
Biological samples
Blood samples (4 ml) were taken before the exercise bout and at repeated time points throughout recovery (Fig. 1). Muscle biopsy samples were taken from the vastus lateralis using 5 mm Bergström needles adapted for manual suction immediately before exercise and at 1, 4, 6, 7 and 12 h post exercise. The initial three biopsies were taken from a randomly selected leg and the final three biopsies were taken from the contralateral leg. Distance between sampling points was ∼0.5 cm and biopsies were taken from distal to proximal. Muscle was cleaned with saline to remove excess blood and immediately frozen in liquid N2. Muscle and plasma samples were stored at −80°C until subsequent analysis.
Exercise
Bilateral leg extension 1 RM strength test was completed by each subject a minimum of 1 week prior to the experimental protocol using a plate-loaded machine. After a warm-up of two sets of five moderate intensity repetitions the 1 RM was determined as the highest successfully lifted weight during a maximum of six attempts. On the day of a trial subjects completed two warm-up sets of five repetitions at ∼50 and ∼60% 1 RM with 2 min rest between sets. The resistance exercise training bout incorporated four sets of 10 repetitions at ∼80% 1 RM with 3 min rest between sets. Exercise range of motion was ∼85 deg, with leg extension endpoint set at ∼5 deg from full extension.
Analytical procedures
Insulin and amino acid concentration
Plasma insulin concentration was measured using an automated enzyme amplified chemiluminescence Immulite 1000 system (Siemens Diagnostics, Australia) according to the manufacturer's guidelines. Plasma amino acids were analysed by high performance liquid chromatography to determine amino acid concentrations as described previously (Wilkinson et al. 2007; West et al. 2011). Estimates of the precision of the method were determined by assessing the repeatability of standards (1.5, 2.5, 3.5, 5, 10 μl) injected to generate a standard curve. Coefficients of variation for the volumes listed above were 5–15%.
Western blot
Intracellular signalling proteins were extracted, isolated and quantified as previously described (Coffey et al. 2011). The amount of protein loaded in each well was 50 μg. Polyclonal anti-phospho mammalian target of rapamycin (mTOR) Ser2448 (no. 2971), monoclonal anti-phospho-Akt Ser473 (no. 9271), tuberin sclerosis complex-2 (TSC2) Thr1462 (no. 3617), ribosomal protein S6 (rpS6) Ser235/6 (no. 4856), 4E-BP1 Thr37/46 (no. 2855), eEF2 Thr56 (no. 2331), and anti-α-tubulin control protein (no. 3873) were purchased from Cell Signaling Technology (Danvers, MA, USA). Polyclonal anti-phospho-PRAS40 Thr246 (no. 07–888), p70 S6K Thr389 (no. PK1015) were from Millipore (Temecula, CA, USA).
Fractional synthetic rate
Pre-infusion plasma sample proteins as first measurement time point (Burd et al. 2012b) were extracted in acetonitrile. Muscle tissue was processed as previously described (Moore et al. 2009b).
Calculations
The fractional synthetic rate (FSR; % h−1) of myofibrillar proteins was calculated using the standard precursor–product method:
where Ep2–Ep1 represents the change in bound protein enrichment between two biopsy samples; Eic is the average enrichment of intracellular phenylalanine between the two biopsy samples; and t is the time between biopsies. The utilization of ‘tracer-naive’ subjects allowed us to use the pre-infusion blood sample (i.e. mixed plasma protein fraction) as the baseline enrichment (Ep1) for the calculation of resting MPS (Burd et al. 2010). All measurements of enrichment, whether made using gas chromatography–mass spectrometry (GC-MS) or gas chromatography combustion isotope ratio mass spectrometry (GC-C-IRMS) were validated using external standard curves with standards of known composition. Plasma-free, muscle intracellular, and protein-bound enrichments fell within the linear range and coefficients of variability on repeat measurements never exceeded 5–6%.
RNA extraction, reverse transcription and RT-PCR
Skeletal muscle (∼20 mg) tissue RNA extraction, reverse transcription and real-time polymerase chain reaction (RT-PCR) was performed as previously described (Camera et al. 2012; West et al. 2012). TaqMan-FAM-labelled primer/probes for atrogin-1 (Hs01041408_m1), MuRF-1 (Hs00822397_m1), SLC3A8 (Hs00255854_m1) and BCAT2 (Hs01553550_m1) primers (Applied Biosystems, Carlsbad, CA, USA) were used. Glycerald-ehyde-3-phosphate dehydrogenase (GAPDH, HS9999-9905_m1) was used as the housekeeping gene. The relative amounts of mRNAs were calculated using the relative quantification (ΔΔCT) method (Livak & Schmittgen, 2001). Analysis of mRNA was restricted to 1 h, 7 h and 12 h post exercise due to limited muscle sample at various time points.
Statistical analysis
Data were analysed using two-way analysis of variance (ANOVA) with Student–Newman–Keuls post hoc analysis (SigmaStat for Windows; Version 3.10). Data for western blotting were log-transformed prior to analysis. All data are presented as mean ± standard deviation (SD) and the level of statistical significance was set at P < 0.05.
Results
Plasma insulin concentration
There was a time × group interaction for plasma insulin concentration (P < 0.001). Plasma insulin concentration was increased above resting levels only in BOLUS, peaking 40 min after exercise (Fig. 2). Plasma insulin concentration in BOLUS was also increased above rest at 20 min, 40 min, 1 h, 1 h 30 min, (∼3- to 8-fold P < 0.05) and 6 h 40 min, 7 h and 7 h 30 min (∼2- to 4-fold; P < 0.05) post exercise after the first and second drink respectively. BOLUS was greater than INT and PULSE conditions at the corresponding time points (∼2- to 4-fold; P < 0.05).
Figure 2.
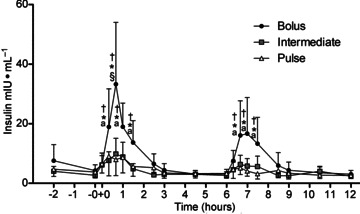
Plasma insulin concentration following a bout of leg extension resistance exercise (4 sets × 10 repetitions at 80% one repetition maximum) and post-exercise ingestion of 80 g whey protein consumed using a BOLUS (2 × 40 g every 6 h), INT (4 × 20 g every 3 h) or PULSE (8 × 10 g every 1.5 h) ingestion protocol during a 12 h recovery period. −0 and +0 h are pre- and post exercise, respectively. Data were analysed using a 2-way ANOVA with Student–Newman–Keuls post hoc analysis. Values are mean ± SD. Different vs.§, all other time points within treatment; a, rest; †, Intermediate and *, Pulse, at equivalent time point (P < 0.05).
Plasma amino acids
There was a time × group interaction (P < 0.001) for plasma EAA, BCAA and leucine concentrations. There were robust increases in AA concentration during the early (1–6 h) recovery period following BOLUS compared to more moderate changes for INT and PULSE (Fig. 3). EAA concentration increased above resting levels after feeding in BOLUS peaking 40 min post exercise (135%; P < 0.001). BCAA and leucine concentrations were different from rest 40 min after ingestion of BOLUS (37 and 57%, respectively; P < 0.05) and remained elevated 3.5 h post exercise. After the initial INT feeding there was a trend towards difference from rest for leucine (P= 0.06) but not for BCAA or EAA. Changes in AA concentration with PULSE did not increase above resting values during the 1–6 h recovery period.
Figure 3.
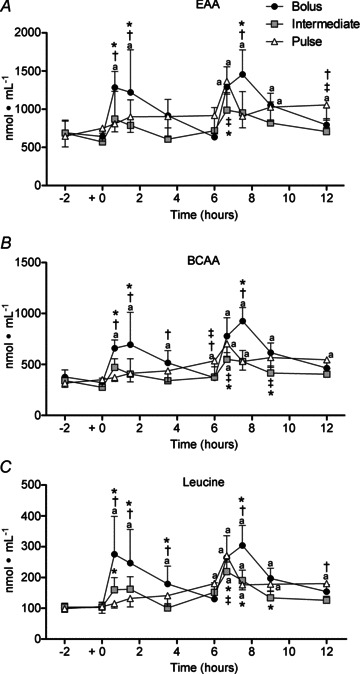
Plasma essential amino acids (EAA; A), branched-chain amino acids (BCAA; B), and leucine (C) concentration following a bout of leg extension resistance exercise and post-exercise BOLUS, INT or PULSE ingestion protocol during a 12 h recovery period, as described in Fig. 2. Data were analysed using a 2-way ANOVA with Student–Newman–Keuls post hoc analysis. Values are mean ± SD. Different vs. a, rest; *, Pulse; †, Intermediate; and ‡, Bolus, at equivalent time point (P < 0.05).
During the late recovery period (6–12 h) the different AA sub-fractions increased above resting levels in response to all feeding protocols. However, peak EAA, BCAA and leucine concentrations were higher for BOLUS (87–205%; P < 0.001) compared with INT (110–39%; P < 0.04) and PULSE (67–174%; P < 0.004). Notably, PULSE sustained the increase in AA concentration above resting values throughout the 6–12 h recovery period (64–83%; P < 0.03) and remained above resting values at 12 h (42–83%; P < 0.005). The concentration of each AA sub-fraction with BOLUS remained elevated at 9 h (52–87%; P < 0.001) and BOLUS and INT returned to resting values at 12 h and were lower compared to PULSE at the end of recovery for EAA (19–50%; P < 0.05). The increase in AA concentration with INT was restricted to the 6–7 h period (i.e. 6 h 40 min) and was lower in INT than PULSE and BOLUS at the equivalent time point (21–41%; P < 0.04).
Intracellular and plasma tracer enrichments
Intracellular free phenylalanine enrichments showed a stable precursor pool throughout the 14 h infusion in all groups (Supplemental Fig. 1a, available online only). Linear regression line slopes of plasma enrichments were not significantly different from zero in BOLUS, INT and PULSE groups (P= 0.96, 0.98, 0.99, respectively; Supplemental Fig. 1b).
Myofibrillar fractional synthetic rate
Each ingestion protocol resulted in increased myofibrillar FSR above resting values throughout 1–12 h of recovery (88–148%; P < 0.02; Fig. 4B) with values of 0.06, 0.079 and 0.053%·h−1 for PULSE, INT and BOLUS, respectively. There were corresponding increases in FSR during the early 1–4 h recovery period regardless of timing or quantity of protein ingestion (Fig. 4A). Thereafter, INT generated a superior FSR compared to PULSE and BOLUS during the 4–6 and 6–12 h periods (47–78% and 34–42%, respectively; P < 0.02). Consequently, INT elicited a greater rate of myofibrillar FSR during the entire 1–12 h period compared to both PULSE and BOLUS (31 and 48% difference, respectively; P < 0.02).
Figure 4.
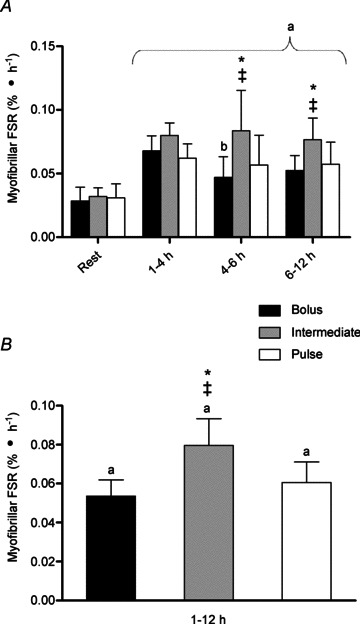
Myofibrillar fractional synthetic rate (FSR) between time points (A) and mean FSR throughout 1–12 h (B) following a bout of leg extension resistance exercise and post-exercise BOLUS, INT or PULSE ingestion protocol during a 12 h recovery period, as described in Fig. 2. Data were analysed using a 2-way ANOVA with Student–Newman–Keuls post hoc analysis. Values are mean ± SD expressed as %· h−1. Different vs. a, Rest; b, 1–4 h; ‡, Bolus and *, Pulse at equivalent time point (P < 0.05).
Signalling responses
Akt/TSC2
AktSer473 phosphorylation during recovery from exercise increased above rest in BOLUS at 1 h (∼4-fold) and was higher than all other time points (P < 0.05; Fig. 5A). Phosphorylation of Akt at 1 h was also greater in BOLUS compared to INT and PULSE (∼2-fold difference; P < 0.03) whereas at 7 h it was higher in both BOLUS and INT compared to PULSE (∼3- and ∼2-fold difference, respectively; P < 0.02). Similar to AktSer473 the phosphorylation of TSC2Thr1462 increased above resting values only in BOLUS at 1 and 7 h (∼4- and 3-fold, respectively; P < 0.002; Fig. 5B). At the 4 h time point until the end of the recovery (12 h) TSC2 phosphorylation in INT was generally lower than BOLUS and PULSE (∼3- to 2-fold; P < 0.05).
Figure 5.
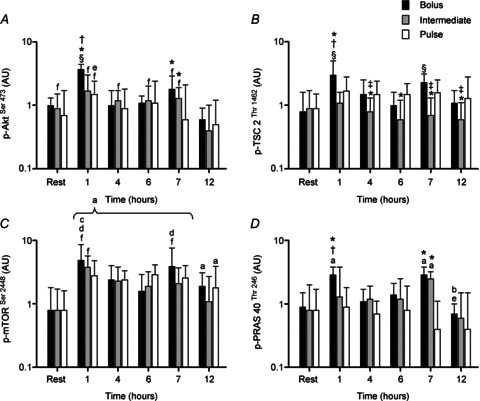
Phosphorylation of AkrSer473 (A), TSC2Thr1462 (B), mTORSer2448 (C), PRAS40Thr246 (D), following a bout of leg extension resistance exercise and post-exercise BOLUS, INT or PULSE ingestion protocol during a 12 h recovery period, as described in Fig. 2. Data were analysed using a 2-way ANOVA with Student–Newman–Keuls post hoc analysis. Values are mean ± SD expressed as arbitrary units. Different vs.§, all time points within treatment; a, rest; b, 1 h post exercise; c, 4 h post exercise; d, 6 h post exercise; e, 7 h post exercise; f, 12 h post exercise; *, Pulse; †, Intermediate; and ‡, Bolus at equivalent time point (P < 0.05).
mTOR/PRAS40
The protein ingestion protocols generated similar increases in phosphorylation of mTORSer2448 throughout 12 h recovery (Fig. 5C). Generally, all time points for each group were higher than rest (∼2- to ∼6-fold, P < 0.05). Peak mTOR phosphorylation occurred in BOLUS at 1 and 7 h post exercise (∼6- and ∼5-fold, respectively) but was not different from INT or PULSE at the equivalent time point. There was a significant increase in PRAS40Thr246 phosphorylation above resting values in BOLUS at 1 and 7 h (∼3-fold; P < 0.05) and INT at 7 h (∼3-fold; P < 0.05) after cessation of exercise but the phosphorylation state of PRAS40Thr246 was unchanged with PULSE (Fig. 5D). Phosphorylation of PRAS40Thr246 after 1 h of recovery was higher in BOLUS compared with INT and PULSE (∼2- and ∼3-fold, respectively, P < 0.05) whereas at 7 h both BOLUS and INT were different from PULSE (∼7- and ∼6-fold, respectively, P < 0.001).
p70 S6K/rpS6
p70 S6SKThr389 phosphorylation was elevated above resting values during the 12 h of recovery for all ingestion protocols (∼3- to ∼26-fold; P < 0.02) but with different magnitudes in the response between interventions (Fig. 6A). BOLUS elicited the highest phosphorylation at 1 h (∼26-fold; P < 0.001) with a second lower peak at 7 h (12-fold; P < 0.001). Phosphorylation in INT peaked at 1 h (12-fold; P < 0.001) and 4 h (7-fold; P < 0.001) but was similar throughout the remainder of the recovery period. Phosphorylation of p70 S6K above rest in PULSE showed little variation between time points (3- to 6-fold). The p70 S6SKThr389 phosphorylation response at 1 h was higher in BOLUS and INT compared to PULSE (∼5- and ∼2-fold, respectively; P < 0.02) and at 7 h was higher in BOLUS than INT and PULSE (∼2- and ∼3-fold, respectively; P < 0.02).
Figure 6.
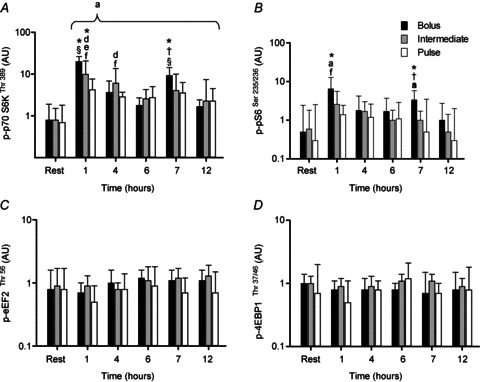
Phosphorylation of p70 S6KThr389 (A), rpS6Ser235/236 (B), eEF2Thr52 (C) and 4E-BP1Thr37/46 (D) following a bout of leg extension resistance exercise and post-exercise BOLUS, INT or PULSE ingestion protocol during a 12 h recovery period, as described in Fig. 2. Data were analysed using a 2-way ANOVA with Student–Newman–Keuls post hoc analysis. Values are mean ± SD expressed as arbitrary units. Different vs.§, all time points within treatment; a, rest; d, 6 h post exercise; f, 12 h post exercise; *, Pulse; and †, Intermediate; at equivalent time point (P < 0.05).
rpS6Ser235/236 phosphorylation was only higher than resting levels during recovery in BOLUS at 1 and 7 h post exercise (12- and ∼6-fold, respectively; P < 0.01; Fig. 6B). Phosphorylation at the 1 h time point was higher in BOLUS compared to PULSE (∼5-fold; P < 0.03), and at 7 h phosphorylation was higher in BOLUS than both INT and PULSE (∼3- and ∼7-fold, respectively; P < 0.05).
mRNA responses
The mRNA abundance of MuRF-1 increased at 1 h post exercise compared to all other time points in INT and PULSE (2- to 3-fold compared to rest; P < 0.001) but MuRF-1 mRNA did not change during the recovery period in BOLUS. Consequently, MuRF-1 expression was higher in INT and PULSE compared to BOLUS at 1 h (P < 0.001; Fig. 7A). There were no differences in the abundance of atrogin-1 mRNA within or between groups during the entire recovery period (data not shown).
Figure 7.
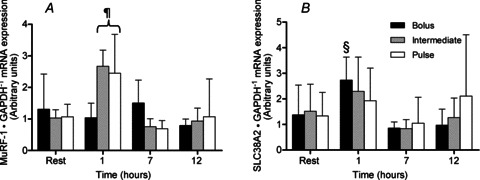
MuRF-1 (A) and SLC38A2 (B) mRNA expression relative to GAPDH following a bout of leg extension resistance exercise and post-exercise BOLUS, INT or PULSE ingestion protocol during a 12 h recovery period, as described in Fig. 2. Data were analysed using a 2-way ANOVA with Student–Newman–Keuls post hoc analysis. Values are mean ± SD. Different vs.§, all time points within group; ¶, all time points within group and Bolus at equivalent time point (P < 0.05).
SLC38A2 (SNAT2) mRNA expression was increased at 1 h post exercise compared to all other time points within BOLUS (∼2-fold compared to rest; P < 0.05), but there was no change after either INT or PULSE protocols (Fig. 7B). There were no changes in mRNA expression of BCAT2 during the recovery period (data not shown).
Discussion
This study provides novel information demonstrating that the regulation of muscle protein synthesis can be substantially modulated by the timing and distribution of 80 g of protein intake during prolonged (12 h) recovery from a single bout of resistance exercise. Specifically, we show for the first time that rates of myofibrillar protein synthesis (MPS) remain elevated above rest throughout 12 h of recovery when a single bout of resistance exercise is followed by the partitioned ingestion of 80 g of high quality protein. Furthermore, we show that daily rates of protein synthesis were highest with regular (i.e. every 3 h) intake of a moderate (20 g) quantity of rapidly digested whey protein. We also show that the nutrient- and contraction-sensitive intracellular signalling network regulating translation for protein synthesis was stimulated in response to all feeding protocols but in a hierarchical manner where BOLUS was higher than INT and PULSE.
Previous studies examining the effects of protein availability on mixed muscle protein synthesis at rest have shown that despite prolonged hyperaminoacidaemia, rates of muscle protein synthesis return to resting levels within 2 h after peak aminoacidaemia (Bohe et al. 2001; Atherton et al. 2010). As might be expected, the provision of exogenous protein post exercise elevated MPS above basal levels regardless of the feeding pattern. While the INT bound incorporation values appear systematically lower they are, in fact, not statistically different and even when using a mean intracellular enrichment from the other groups the differences in FSR persist. Therefore, intracellular enrichments over the 12 h feeding period are not the reason for the higher FSR values in INT. Consequently, the superior MPS in INT was possibly due to an optimized interplay between resistance exercise, time between ingestion, and quantity of each (whey) protein feeding; this probably resulted in a cyclical plasma AA profile that might be considered ideal to stimulate MPS. We have previously reported that there appears to be a minimum threshold for elevation of blood aminoacidaemia to generate maximal rates of muscle protein synthesis and that a sufficient quantity of rapidly digested protein is required to transiently induce an optimal protein synthesis response (Moore et al. 2009a; West et al. 2011). While the temporal resolution of time points selected for quantifying plasma amino acid concentrations failed to clearly show distinct amino acid profiles throughout the 12 h post-exercise period a hierarchical response was observed for leucine concentration early in recovery indicative of an initial divergence in branch-chain amino acid availability between feeding patterns (Fig. 3). Thereafter, the continuous hyperaminoacidaemia or lack of postprandial periods of relative hypoaminoacidaemia over the 12 h period with PULSE feeding most likely resulted in some suboptimal stimulation of the protein synthesis machinery similar to that observed with continuous amino acid infusion. In contrast, larger boluses of protein (i.e. >20 g) are sufficient to maximally stimulate muscle protein synthesis, but represent a suboptimal ingestion pattern due to irreversible amino acid oxidation of the excess protein (Moore et al. 2009a). Given that the fraction of protein that appears in the systemic circulation seems to be constant regardless of the size of the protein meal (Pennings et al. 2012), the fate of the amino acids ingested may be in tissues other than skeletal muscle with PULSE and BOLUS feeding patterns. A limitation of the current study is that our results may only be valid for individuals of average body mass (i.e. 70–80 kg). Similar ingestion regimens should be assessed in other populations (e.g. obese) and represent an area for future studies.
The present study did not include an exercise-only intervention but the additive effect of protein ingestion on the anabolic response following resistance exercise is well established and rates of MPS would be superior compared with resistance exercise in isolation regardless of the ingestion protocol (Biolo et al. 1995, 1997; Moore et al. 2009a). We chose to examine 12 h post-exercise recovery to be representative of multiple daily feeding opportunities. We purposely designed the study to isolate the effects of protein intake and avoid interference by carbohydrate or fat ingestion, as protein alone has been shown to robustly stimulate muscle protein synthesis after exercise (Biolo et al. 1997; Tipton et al. 1999, 2003; Moore et al. 2009a,2009b; Burd et al. 2010; West et al. 2011). While individuals in the present study were presumably in negative energy balance over the day of the trial, the majority of studies involving protein feedings are performed after an overnight fast, yet still result in robust increases in resting and exercise-induced myofibrillar protein synthesis (Biolo et al. 1997; Tipton et al. 1999; Bohe et al. 2001; Moore et al. 2009a,b; Atherton et al. 2010; Burd et al. 2010, 2011; West et al. 2011). As such, in conditions of energy balance or positive energy balance we would probably have observed a similar, if not greater, MPS response.
The present data extend the findings of previous studies with the novel observation that the pattern of protein intake can modify the extent of muscle protein synthesis during the late (i.e. 6–12 h) period of recovery. In the present study, the cumulative protein intake by the 1–4 h period post exercise was 40 g for BOLUS and INT, and only 30 g for PULSE. Yet despite these different patterns of intake, we and others (Cuthbertson et al. 2005; Moore et al. 2009a; Pennings et al. 2012) have shown that >20 g of protein is sufficient to induce maximal rates of protein synthesis in the early recovery period. The divergence in the MPS response after 4 h, with superior outcomes for INT, suggests that the timing and distribution of protein intake becomes a critical factor for optimizing MPS late in recovery. Our results indicate that 20 g of rapidly digested high-quality protein every 3 h provides both sufficient amino acids and an adequate latency period for repeated stimulation of muscle protein synthesis. The implication is that 40 g of protein every 6 h, which simulates a daily pattern of ‘three square meals’, or 10 g of protein every 1.5 h simulating a ‘grazing’ eating pattern, are inferior in their capacity to stimulate MPS. However, it should be noted that our results apply to rapidly digested whey protein and mixed meal consumption of whole foods, or a slowly digested protein such as casein, would probably reduce the magnitude of plasma aminoacidaemia and subsequent MPS response but intuitively the relative effect of the pattern and timing of protein ingestion would probably remain. Indeed, our findings indicate that individuals who have the goal of maximally stimulating muscle anabolism may benefit from strategies that regularly isolate rapidly digested, high quality protein ingestion from other daily nutrient intakes. Regardless, we suggest that the current population recommendations for protein intake, which are provided as a total daily target without consideration of the pattern of protein intake, should be reassessed in view of the likely impact on muscle protein metabolism and, subsequently, lean body mass. Such a concept has been raised previously when protein metabolism has been estimated from the traditional nitrogen balance techniques (Leverton & Gram, 1949).
Results from our cell signalling data provide new information regarding nutrient–exercise interactions regulating translation initiation events. As might be expected, as a critical node in the insulin signalling network (Taniguchi et al. 2006), phosphorylation of AktSer473 and its downstream targets TSC2Thr1472 and PRAS40Thr246 corresponded closely to changes in plasma insulin concentration. The mTORC1 regulates metabolic activity and is a focal point for integrating nutrient (i.e. amino acid) and exercise signal transduction (Dreyer et al. 2006, 2008; Rivas et al. 2009; Camera et al. 2010; Terzis et al. 2010; Zoncu et al. 2011). Interestingly, phosphorylation of Ser2448 was ∼2 to ∼6-fold above resting values during the entire recovery period independent of the protein ingestion protocol. This is the first study to show prolonged (12 h), sustained increases in phosphorylation of mTORSer2448 above rest consistent with elevated muscle protein synthesis above rest. Similarly, p70 S6KThr389 phosphorylation increased above rest throughout the recovery period in all ingestion protocols, although the magnitude of the response differed and was dependent on the pattern of protein ingestion (BOLUS>INT>PULSE at the 1 and 7 h recovery time points). As a signalling kinase proximal to translation initiation, p70 S6K is promoted as a physiological marker closely associated with muscle protein synthesis (Baar & Esser, 1999; Kumar et al. 2009; Burd et al. 2010; Fry et al. 2011). We found no correlation between the degree of phosphorylation of S6K and the myofibrillar FSR response (r= 0.2). The lack of change in 4E-BP1 Thr37/46 phosphorylation following exercise and protein ingestion was unexpected but has been observed previously (Breen et al. 2011; Coffey et al. 2011; Burd et al. 2012a) and might be attributable to insufficient volume and/or intensity of exercise or possibly due to competitive interaction with p70 S6K as a raptor substrate (Dennis et al. 2013). Similarly, we also failed to see a nutrient-sensitive response for eEF2 phosphorylation which has also been shown by others (Breen et al. 2011; Moore et al. 2011; Burke et al. 2012). Accordingly, our results support the premise that the snapshot provided by quantification of mTOR-S6K phosphorylation is indicative of elevated MPS compared to baseline but does not accurately reflect the magnitude or duration of the MPS response.
Modulating intake of macronutrients also generates changes in transcriptional activity of the muscle cell (Drummond et al. 2010; Borgenvik et al. 2012). The BOLUS condition induced an early post-exercise increase in mRNA expression of the amino acid transporter SLC38A2/SNAT2 while there was a repressed expression of MuRF-1 compared with INT and PULSE feeding protocols. The increase in amino acid transporter and blunting of ubiquitin ligase expression has been shown previously (Drummond et al. 2010; Borgenvik et al. 2012) but the physiological significance of such changes has yet to be clearly defined. Nonetheless, our results indicate that a large bolus feeding of amino acids, or possibly high plasma leucine concentrations (Churchward-Venne et al. 2012), may be necessary to substantially alter the transcriptional activity in muscle. However, this effect was no longer evident following a second bolus ingestion 6 h later and may indicate that an exercise–nutrient interaction is required to induce changes in transcription of specific gene targets (Drummond et al. 2010, 2011). Nonetheless, the possibility exists that a large bolus of protein is advantageous for enhancing AA transporter expression and/or suppressing catabolic activity compared with moderate–small quantities of protein intake during the early post-exercise recovery period.
In conclusion, the results from the current study provide new information demonstrating that the timing and distribution of protein ingestion is a key factor in maximally stimulating rates of MPS throughout an entire day. During the 12 h recovery period after a single bout of resistance exercise 20 g of whey protein ingested every 3 h was the optimal feeding pattern for promoting enhanced rates of MPS in the present study. These results highlight the importance of the interplay between timing and quantity of protein ingestion on MPS over the course of each day and represent an important dietary message that merits consideration for population recommendations for daily protein intake. The chronic effects and practicalities of incorporating such an ingestion strategy within the total daily eating plan including whole foods represent areas for future study. In the meantime, this study emphasizes that the timing of protein intake is a separate variable and a crucial factor in the development of optimal nutritional strategies to maintain and/or enhance peak muscle mass in humans.
Acknowledgments
This study was funded by Australian Research Council Linkage Project grant (LP100100010). The authors thank Greg Shaw, Alisa Nana, Joanne Mirtschin, Christine Dziedzic, Siobhan Moran, Lynne Mercer, Felicity Galvez, Eric Haakonsen, Jesse Featonby and Tracy Rerecich for technical assistance during experimental trials and laboratory analysis. D.R.M. and T.S. were employed by Nestlé Research Center in Lausanne, Switzerland for the duration of the study. All the other authors report no conflict of interest.
Glossary
- AA
amino acid
- BCAA
branched chain amino acid
- EAA
essential amino acid
- FSR
fractional synthetic rate
- MPS
myofibrillar protein synthesis
- mTOR
mammalian target of rapamycin
- RM
repetition maximum
- rpS6
ribosomal protein S6
- TSC2
tuberin sclerosis complex-2
Authors contributions
J.L.A., L.M.B., D.R.M., T.S., S.M.P., J.A.H. and V.G.C. designed the research; J.L.A., D.M.C., L.M.B., E.M.B., M.L.R., D.W.D.W., N.A.J. and V.G.C. conducted the research; J.L.A., D.M.C., D.W.D.W., S.M.P., J.A.H. and V.G.C. analysed the data; J.L.A., L.M.B., J.A.H., S.M.P., T.S., D.R.M. and V.G.C. wrote the paper; J.L.A., J.A.H. and V.G.C. have primary responsibility for the final content. All authors have read and approved the final manuscript.
Supplementary material
Supplemental Fig. 1a
Supplemental Fig. 1b
References
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgenvik M, Apró W, Blomstrand E. Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am J Physiol Endocrinol Metab. 2012;302:E510–E521. doi: 10.1152/ajpendo.00353.2011. [DOI] [PubMed] [Google Scholar]
- Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, Baar K, Tipton KD. The influence of carbohydrate–protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol. 2011;589:4011–4025. doi: 10.1113/jphysiol.2011.211888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, Andrews RJ, West DWD, Little JP, Cochran AJR, Hector AJ, Cashaback JGA, Gibala MJ, Potvin JR, Baker SK, Phillips SM. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Physiol. 2012a;590:351–362. doi: 10.1113/jphysiol.2011.221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, Groen BB, Beelen M, Senden JM, Gijsen AP, van Loon LJ. The reliability of using the single-biopsy approach to assess basal muscle protein synthesis rates in vivo in humans. Metabolism. 2012b;61:931–936. doi: 10.1016/j.metabol.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Burd NA, Holwerda AM, Selby KC, West DWD, Staples AW, Cain NE, Cashaback JGA, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010;588:3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DWD, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- Burke LM, Hawley JA, Ross ML, Moore DR, Phillips SM, Slater GR, Stellingwerff T, Tipton KD, Garnham AP, Coffey VG. Preexercise aminoacidemia and muscle protein synthesis after resistance exercise. Med Sci Sports Exerc. 2012;44:1968–1977. doi: 10.1249/MSS.0b013e31825d28fa. [DOI] [PubMed] [Google Scholar]
- Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc. 2010;42:1843–1852. doi: 10.1249/MSS.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- Camera DM, West DWD, Burd NA, Phillips SM, Garnham AP, Hawley JA, Coffey VG. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J Appl Physiol. 2012;113:206–214. doi: 10.1152/japplphysiol.00395.2012. [DOI] [PubMed] [Google Scholar]
- Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey V, Moore D, Burd N, Rerecich T, Stellingwerff T, Garnham A, Phillips S, Hawley J. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol. 2011;111:1473–1483. doi: 10.1007/s00421-010-1768-0. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dennis MD, Kimball SR, Jefferson LS. Mechanistic target of rapamycin complex 1 (mTORC1)-mediated phosphorylation is governed by competition between substrates for interaction with raptor. J Biol Chem. 2013;288:10–19. doi: 10.1074/jbc.M112.402461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–E1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr Metab. 2009;6:12. doi: 10.1186/1743-7075-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverton RM, Gram MR. Nitrogen excretion of women related to the distribution of animal protein in daily meals. J Nutr. 1949;39:57–65. doi: 10.1093/jn/39.1.57. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCTMethod. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 2011;201:365–372. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009a;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009b;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JMG, van Loon LJC. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E992–E999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Van Loon LJ. Dietary protein for athletes: from requirements to optimum adaptation. J Sports Sci. 2011;29(Suppl. 1):S29–38. doi: 10.1080/02640414.2011.619204. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Rivas DA, Lessard SJ, Coffey VG. mTOR function in skeletal muscle: a focal point for overnutrition and exercise. Appl Physiol Nutr Metab. 2009;34:807–816. doi: 10.1139/H09-073. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E. The dergree of p70S6K and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol. 2010;110:835–843. doi: 10.1007/s00421-010-1527-2. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003;284:E76–E89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- West DWD, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, Hawley JA, Coffey VG, Phillips SM. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol. 2012;112:1805–1813. doi: 10.1152/japplphysiol.00170.2012. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Tarnopolsky MA, MacDonald MJ, MacDonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


